Abstract
Chronic inflammation is essential for cancer growth and metastasis. It follows that factors reducing inflammation would abrogate cancer and restore tissue health. However, roles for anti-inflammatory CD41 regulatory cells (TREG) in cancer are enigmatic and controversial. Our recent data reveal that TREG may function in cancer similarly to inflammatory bowel disease or multiple sclerosis, whereby TREG accumulate but lack potency to restore tissue homeostasis under inflammatory conditions. Interestingly, early life exposures to diverse environmental organisms reinforce a protective TREG phenotype that inhibits cancer. In contrast, hygienic individuals with few exposures earlier in life suffer from a dysregulated TREG feedback loop. Consequently, hygienic subjects have increased risk of malignancy later in life. This cancer condition is reversible by blocking underlying inflammation. Taken together, these data help explain increased inflammation-associated cancer rates in hygienic societies and identify targets to abrogate cancer and restore overall health.
Keywords: gut bacteria, inflammation, TREG cells, cancer
The link between chronic inflammation and cancer has been recognized for many years.1 However, the precise interaction between inflammatory cells and their elaborated factors and tumor cells remains unknown. Originally, inflammation was believed to be primarily a beneficial host response, representing the body’s fight against invading tumor cells. More recent data however suggest just the opposite: that inflammation may be the cause of some cancers and a powerful stimulus for tumor growth and invasion.1–4 Indeed, there are numerous data supporting the association of chronic long-standing inflammation and increased risk of tumors of the gastrointestinal (GI) tract.5–8 In addition, systemic non-steroidal anti-inflammatory drug (NSAID) use has been linked with a significant decrease in cancer of the bowel, lung, liver and prostate.9 However, therapies aimed at restoring homeostasis involve numerous challenges including immune suppression with increased risk of pathogenic infections, as well as removing beneficial antitumor effects of immune surveillance.
Balanced Activities of T Cells are Essential for Health
Overall health with ability to control inflammation and restore homeostasis requires adaptation to diverse challenges throughout life.10,11 Pivotal roles for T lymphocytes in this process of homeostasis were first discovered in mouse models more than a decade ago.12 A major component of immune tolerance and homeostasis is performed by CD4+ cells expressing CD25, namely regulatory T cells (TREG). TREG prevent immune disorders by suppressing proliferation of reactive T lymphocytes and other destructive immune responses.11,13–15 Ability of CD4+ TREG to offer protection from other destructive inflammatory sequelae has been convincingly demonstrated during inflammatory bowel disease (IBD) in humans and in mice.10 However, maintenance of lower bowel homeostasis actually also depends on differentiation and specialization of proinflammatory T-effector cells (TEFF). Along with corresponding anti-inflammatory TREG cells, the CD4+ subsets function together to allow for effective immune responses to diverse GI tract insults in naïve animals. In the bowel, prior exposures to bacteria appear to impart increased TEFF and TREG efficiency to downregulate subsequent infectious challenges and IBD.11,14–19 TREG functions to inhibit pathology have also been well characterized in autoimmune disorders such as multiple sclerosis (MS).20 Mouse models of experimental allergic encephalitis (EAE) mimic MS in human patients and show that equilibrium between activities of proinflammatory TEFF cells and anti-inflammatory TREG is needed to sustain overall health. Taken together, TREG are widely viewed as essential to maintain immune homeostasis and restore tissue and whole body health.
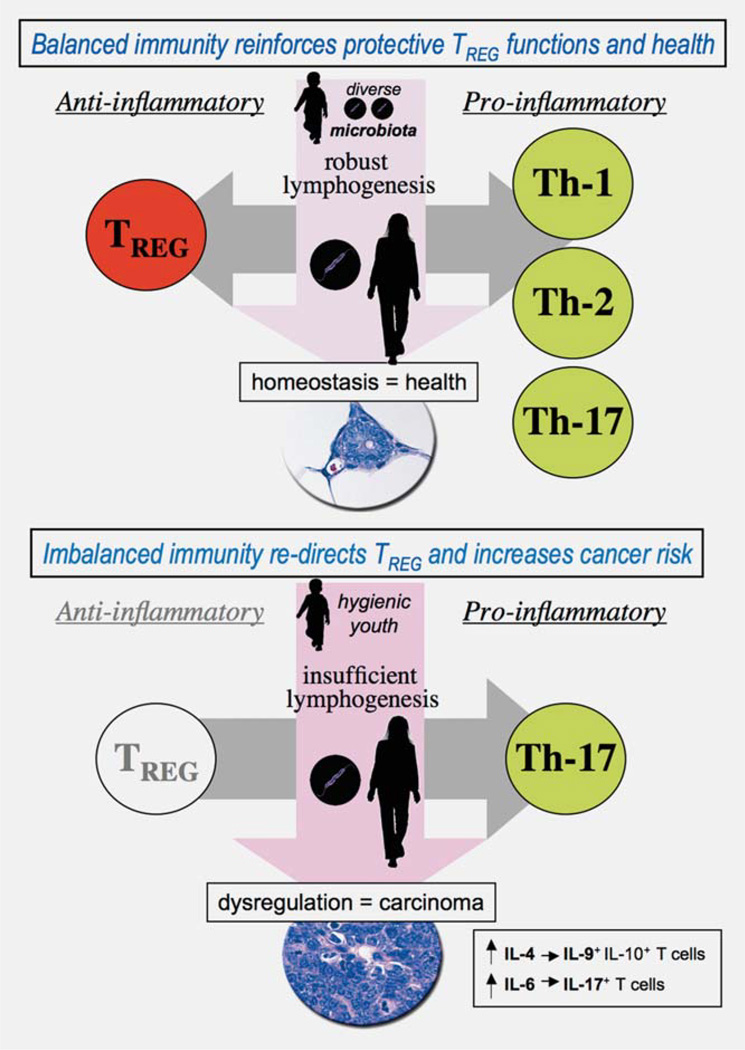
TREG are generally regarded as a homogeneous population of cells. However, recent data suggest that the same transcriptional factors involved in TEFF antigen specific polarization may also be involved in programming of matching TREG.21 According to this paradigm, context-dependent TREG suppress the corresponding type of host TEFF responses, whether these may be dedicated to T helper (Th) type-1 for self or intracellular pathogens, to Th-2 for extracellular pathogens, to Th-17 for self or pathogens, or, finally, to lymphoid follicle stimulating events.21 Thus, TREG primed toward diverse classes of organisms earlier in life will more efficiently downregulate corresponding inflammation later in life, whether that inflammation is driven by Th-1, Th-2 or Th-17 (Fig. 1). This raises the possibility that TREG may be re-directed and applied therapeutically during conditions involving uncontrolled inflammation such as IBD, MS and some types of inflammation-associated cancer.
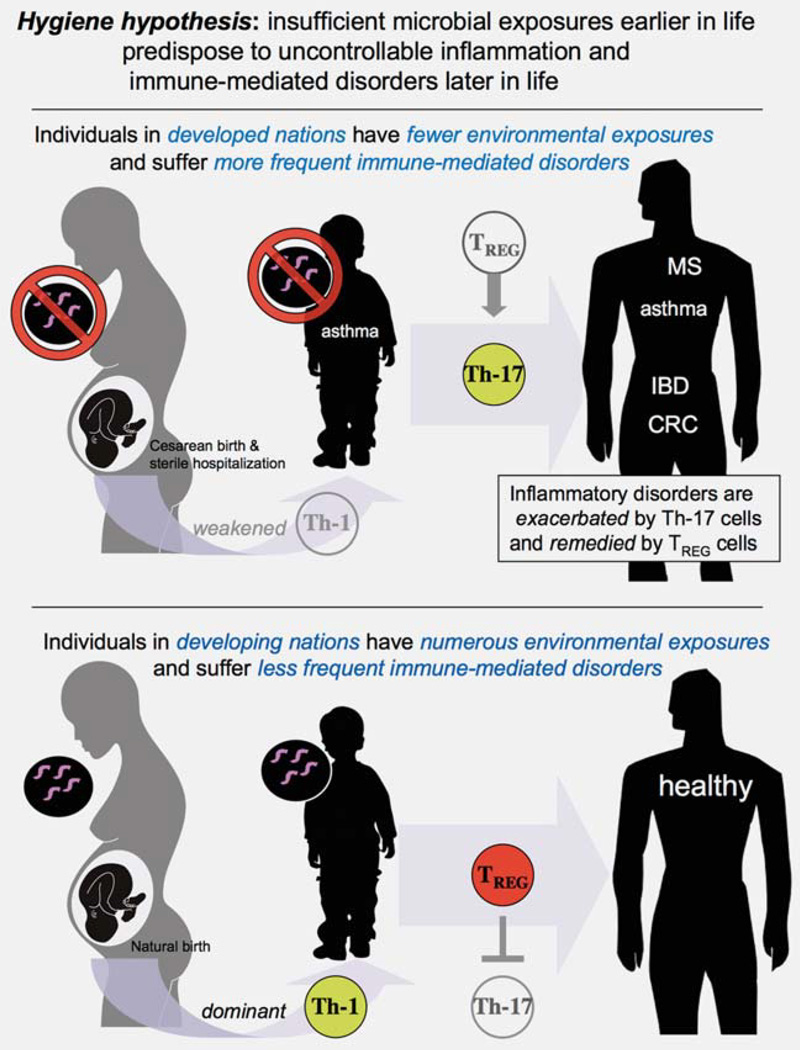
Figure 1.
Immunological profile is pivotal in cancer risk and outcome. Epidemiologic studies have shown that by midlife multiple preneoplastic lesions exist throughout the human body. However, only a few lesions will become cancerous. Data from mouse models suggest that interactions between infectious agents alter immunological profile and modulate cancer outcomes. Microbial exposures may either inhibit or exacerbate carcinogenesis by triggering immune cell activation or secretion of factors. The “hygiene hypothesis” theorizes that exposures to diverse microbiota reinforce innate immunity and T helper (Th)-1 host responses that lead to balanced and efficient immune responses later in life. By contrast, stringent hygiene conditions deprive the immune system of essential priming rendering TREG cells ineffectual and readily directed toward a carcinogenic Th-17 host response.
Foxp3+ TREG Cells Accumulate But Fail to Protect During Inflammatory Disorders
A large body of literature exists demonstrating the efficacy of TREG to suppress inflammation in humans. However, an enigma arises: the presence of large numbers of TREG cells does not assure anti-inflammatory potency, tissue restoration or health, even though properly functioning TREG are clearly protective in humans and in mice. To address this paradox, molecular and cellular mechanisms for TREG have been studied using animals models with antibody-mediated blockade or adoptive transfer of TREG.22 One challenge to better understand TREG biology has been the lack of TREG identity markers. Transcription factor Foxp3 is critical for TREG functions and therefore has been widely used for phenotyping and localization,23 albeit with some limitations.
Although adoptive transfer of TREG cures IBD in mice, Foxp3+ TREG cells accumulate in colon and secondary lymphoid organs during IBD with low TREG potency to restore tissue balance (Fig. 2).24 This observation may be explained by insufficiently low levels of anti-inflammatory cytokine interleukin (IL)-10 in bowel that predispose to a break in immune tolerance. This undermines TREG function and favors proinflammatory Th-17 TEFF cells.25,26 Likewise, in autoimmune disorders Viglietta et al.27 found a significant decrease in functional efficacy of TREG from blood of human patients with MS. Bettelli et al.20 subsequently showed that Th-17 was crucial in the break in tolerance during MS. Large numbers of TREG accumulate during MS but fail to suppress disease in humans or mice with chronically elevated levels of TNF-α and IL-6.28
Under normal physiological conditions, TREG responses are beneficial to the host to reinforce protective acute inflammation. Afterward, TREG regain anti-inflammatory functions and restore tissue homeostasis and health.
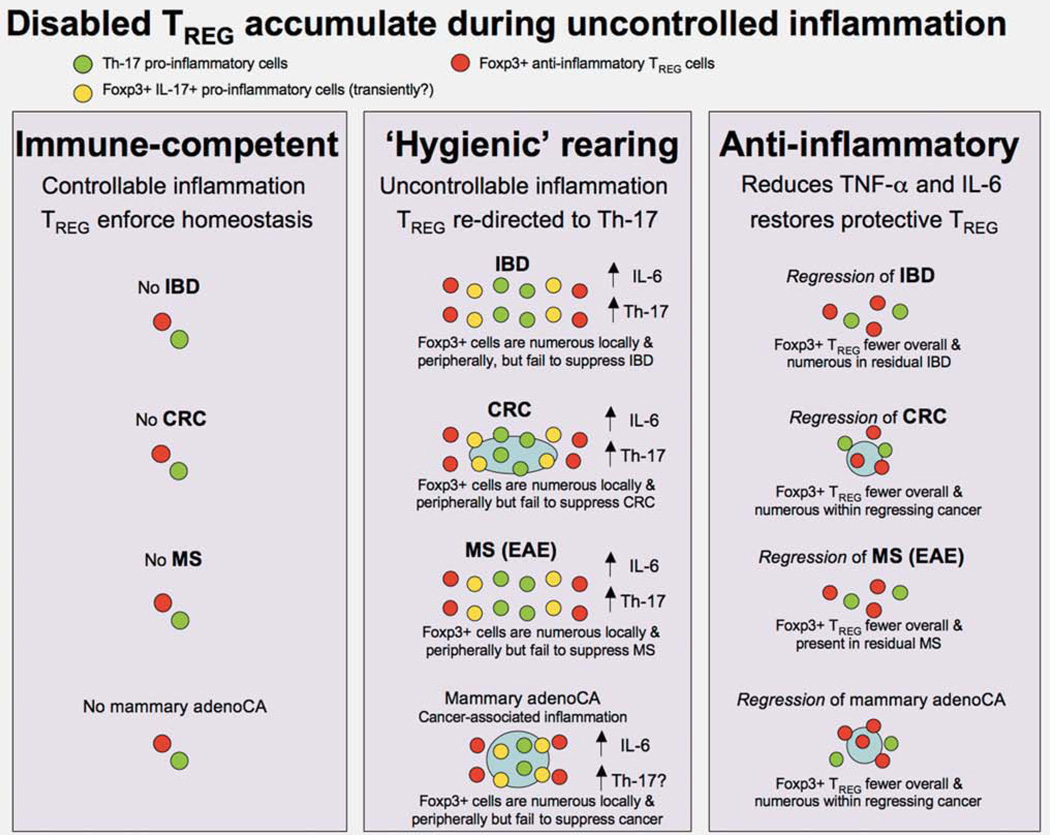
Figure 2.
Reciprocal roles of Th-17 and TREG cells emerge in seemingly diverse diseases: multiple sclerosis (MS), inflammatory bowel disease (IBD) and some types of cancer. Improvements in societal cleanliness have reduced many serious infections but led to an increase in allergies, asthma and autoimmune diseases including multiple sclerosis (MS) and inflammatory bowel disease (IBD). Research using mouse models with experimental allergic encephalitis (EAE), mimicking patients with MS, displays an interleukin (IL)-6-mediated shift in T cells toward pathogenic Th-17. EAE is remedied by downregulation of inflammation and restoration of properly functioning TREG cells. Some types of inflammation-associated cancers such as colorectal carcinoma (CRC) demonstrate similar immunologic underpinnings. Most surprising were our discoveries that cancers lacking overt inflammation in extraintestinal sites, such as prostate and mammary tissue, display similar TREG cell distribution and clinical responses after anti-inflammatory therapy. Broader relevancy of these findings is highlighted by observations that non-steroidal anti-inflammatory drugs (NSAIDs) lower the risk of many cancers including nor only CRC but also cancers of prostate, lung and breast in humans. Hygienic rearing increases risk of uncontrollable Th-17 host response.
Recent work from our laboratory found robust accumulations of Foxp3+ TREG surrounding colonic polyps as well as rapidly growing mammary tumors in mice (Fig. 2).29 In those mice, Foxp3+ cells accumulated in large numbers in the desmoplastic periphery and local lymph nodes, but failed to protect and instead exacerbated pathology. Blocking inflammation using anti-TNF-α antibody or by supplementation with gut bacteria-primed TREG resulted in rapid tumor regression with commensurate reduction and redistribution of Foxp3+ TREG to intratumoral locations.29 Remarkably, Salama et al.30 found that patients bearing colonic tumors with a high intratumoral density of FOXP3+ TREG showed a significant improvement in survival. A better understanding of this process to harness potency of TREG to restore normal tissue architecture and homeostasis may provide insight into novel therapies for inflammation- associated cancers.
IL-10-Dependent Functions of TREG Are Pivotal at Environmental Interfaces Such as Colon, Lung and Skin
Although Foxp3+ TREG are best known for ability to suppress TEFF proliferation,31,32 cytokine-dependent suppression by IL-10 and transforming growth factor (Tgf)-β also appear essential to reduce inflammation and facilitate wound repair. IL-10 production by TREG serves to maintain epithelial normalcy in tissues such as GI tract and lungs,22 despite continual interactions with diverse infectious agents.16,21 In addition, mice lacking IL-10 are also unable to sustain normal TREG functions needed to mount tolerance.33 Inter-related roles between TREG, IL-10 and Tgf-β in vivo appear to be complexly interdependent and essential for epithelial integrity. Tgf-β normally induces functionally suppressive Foxp3+ TREG from naive T cells in the periphery. However, under conditions of uncontrollable inflammation, IL-6 together with Tgf-β may instead inhibit TREG and re-direct CD4+ cells toward a pathogenic Th-17 phenotype.20 Likewise, uncontrollable elevations in other inflammatory cytokines such as IL-4 (Th-2) also block the generation of Tgf-β-induced TREG and instead induce a population of TEFF that produce IL-9; interestingly, the IL-9+ T cells demonstrate no regulatory properties despite producing abundant IL-10.34 Instead, IL-9 subverts IL-10 antineoplastic functions. Thus, it appears that resident T cells—including resident TREG—may be re-directed toward proinflammatory phenotypes for “rescue” whenever Th-1 or Th-2 fails to restore balance. Taken together, these data from animal models show that uncontrollable inflammation, as in the developing cancer microenvironment, may modulate TREG function. This may help explain apparent TREG accumulation without restoration of immune or epithelial homeostasis.
TREG Are Sufficient to Protect from Inflammation-Associated Carcinogenesis
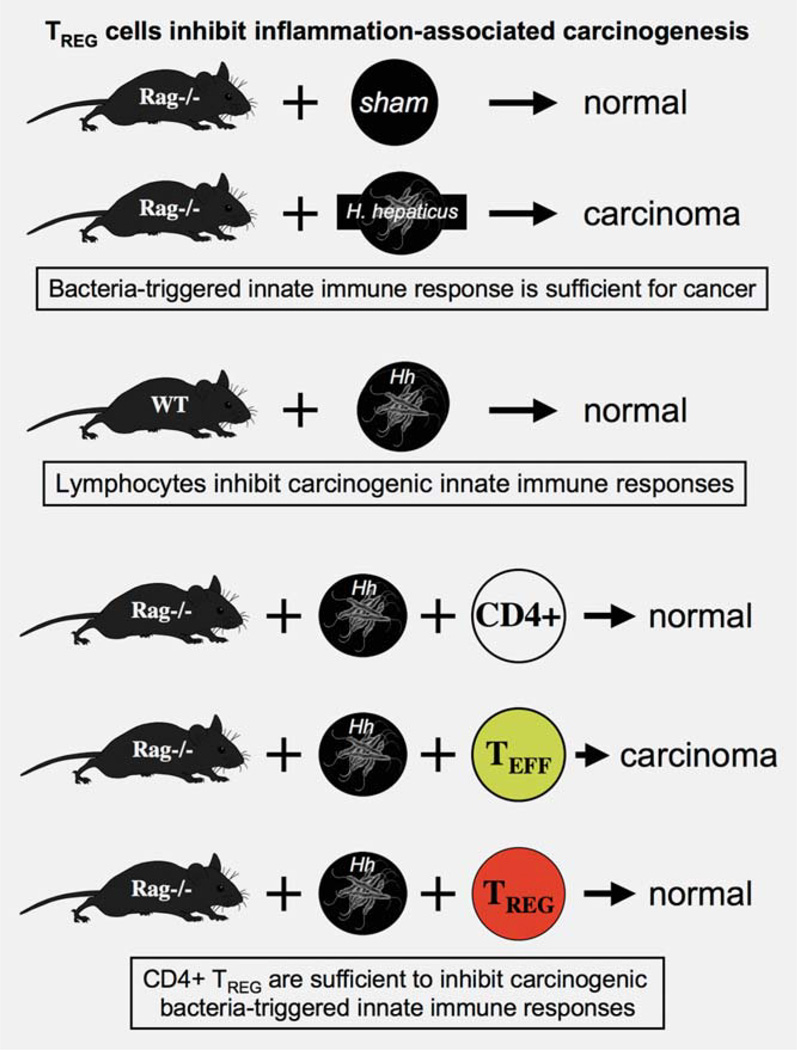
Relationships between GI tract bacteria and cancer have been studied using mutant mice mimicking human cancers. Recombinase activating gene 2 (Rag2)-deficient mice lack functional lymphocytes35 and are highly susceptible to microbial infections resulting in IBD.10,36–38 IBD eventually progresses to colorectal carcinoma (CRC) in those animals37 (Fig. 3). One well-established bacterial etiology for IBD in mice is Helicobacter hepaticus39— a bacterium also linked with carcinogenic inflammatory responses in both the liver and lower bowel of mice.37 A closely related bacterial organism, Helicobacter pylori, is classified as a carcinogen by the World Health Organization (WHO) for stomach cancer in humans.6–8 Taken together, H. hepaticus infection and innate immune inflammatory events were sufficient for carcinoma. However, strain-matched wild-type (WT) mice did not develop carcinoma after H. hepaticus infection, showing that effects of lymphocytes were sufficient to protect from cancer (Fig. 3).37 In these models, IBD and carcinoma arose only when TREG failed to suppress inflammation and restore epithelial health.
IL-10-dependent activities of TREG cells were essential to maintain mucosal integrity in the lower bowel. IL-10 served to normalize oncogenic K-ras within epithelia.
Figure 3.
TREG cells suppress deleterious immune-mediated pathology and afterward restore epithelial homeostasis. Similarities in roles for TREG in inflammation and carcinogenesis emerge during bacteriatriggered inflammatory diseases of the gastrointestinal (GI) tract. Gastritis-associated stomach cancer in humans, for example, is caused by infection with Helicobacter pylori, which is classified as a carcinogen by the World Health Organization (WHO). Transgenic mouse models have been widely employed to mimic GI tract inflammation and cancer in humans. Rag2-deficient mice entirely lacking functional lymphocytes are highly susceptible to inflammation-associated carcinoma after infection with closely related bacteria, H. hepaticus. These data from mice indicated that innate immune inflammatory events were sufficient for carcinoma. However, wild-type animals did not develop carcinoma after H. hepaticus infection, showing that effects of lymphocytes were sufficient to protect from cancer. Adoptive transfer of highly purified TREG cells was sufficient to prevent cancer in Rag−/− mice. Inflammatory bowel disease (IBD) and carcinoma arose only when TREG failed to suppress inflammation and restore epithelial homeostasis.
Poutahidis et al.40 demonstrated rapid reversibility of established neoplastic epithelial invasion. This work showed that an imbalance between IL-10, Tgf-β and IL-6 modulated TREG phenotype and predisposed to colorectal malignancy. Adoptive transfer of TREG collected from IL-10-deficient mice exacerbated neoplastic peritoneal invasion, whereas TREG from WT mice induced tumor remission. This loss of protection may have been due to decreased potency of IL-10 secretion by TREG, or to an inability to sustain TREG suppressive identity in the absence of IL-10.29,33,40 In any event, supplementation with exogenous IL-10 served to downregulate IL-6 and oncogenic K-ras expression within epithelial cells. Interestingly, administration of exogenous IL-10 in H. hepaticus-infected mice increased frequency of IFN-γ-bearing T cells (unpublished data), suggesting that IL-10 reinforced Th-1-mediated tolerance in these murine models. In addition, treatment with IL-10 also significantly reduced Gr-1+ 7/4+ (neutrophils) cells proven necessary for cancer, as neoplastic invasion was reversible using anti-Ly-6G (Gr-1) antibody.41 Taken together with earlier findings of Kullberg et al.,25,29 we concluded that IL-10 protected against a bacteria-triggered IL-6-mediated Th-17 host response contributing to IBD-associated cancer development and growth.
TREG Cells Suppressed Established Adenomatous Polyps—Even in Mice Lacking Overt IBD
In most human patients, CRC is not directly linked with IBD. Instead, sporadic CRC arises from intestinal adenomatous polyps that undergo a well-characterized series of genetic mutations42 rather than from IBD-associated premalignant foci.43 Mice with a heterozygous mutation in Apc (ApcMin/+) are predisposed to intestinal adenomas44 mimicking 85% of sporadic colon cancers in patients. In humans, inactivation of the Apc gene and alterations in β-catenin and the wnt signaling pathway lead to cancer.45–50 Amazingly, adoptive-transfer of highly purified CD4+CD25+ TREG abrogated adenomatous intestinal polyps in aged ApcMin/+ mice.29,51–53 This was a TREG-mediated effect, as transfer of purified TEFF cells under the same conditions did not suppress polyps and instead exacerbated invasive adenocarcinoma within polyps.53 TREG under these conditions express IFN-γ but require IL-10 for antineoplastic efficacy. These data supported that purified TREG may adopt hybrid phenotypes under conditions of uncontrollable inflammation.
Adoptive transfer of TREG cells induced regression of established intestinal polyps. However, TREG from “hygienic” donor animals failed to protect from cancer and instead increased IL-17 levels and carcinogenesis in tumor-prone tissues.
At least some of the antineoplastic benefits of TREG are attributable to reduced inflammation. Although Min mice entirely lacked overt IBD, intestinal polyp growth required proinflammatory cytokine TNF-α to sustain oncogenic c-myc levels and tumor growth.52,53 It is possible that TREG or IL-10 act directly to restore c-myc expression to baseline levels. Attempts at dissecting requirements for TREG utilized targeted depletion of CD25+ cells in young adult mice leading to increased bowel and prostate malignancy commensurate with increased levels of TNF-α, IL-6 and IL-9. Lesions lacked overt inflammation except for mast cells54 that were previously proven essential in tumorigenesis in Apc mutant mice.55,56 Mast cells may also be important systemically in regional lymph nodes following microbial infections, but subsequent coordination of lymphocyte progenitor and neutrophil recruitment and activation remain poorly understood (Fig. 4).57–59
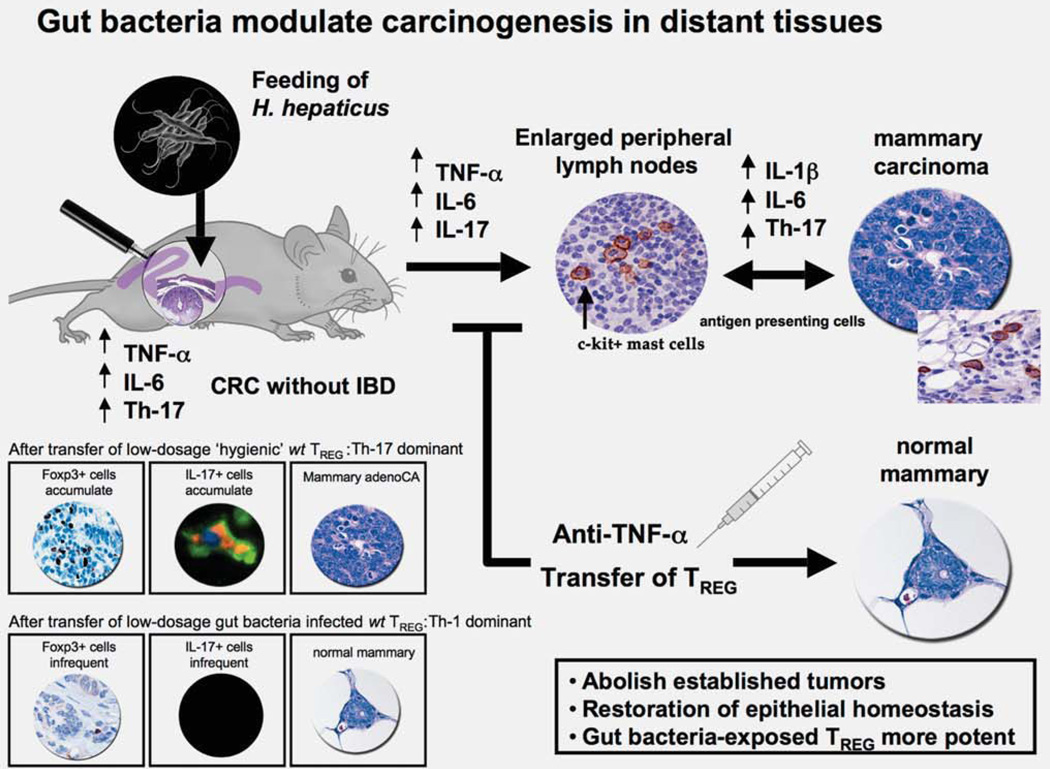
Figure 4.
Gut microbial infections modulate systemic immune events and outcome of carcinogenesis throughout the body. Helicobacter hepaticus colonizes the large intestine of C57BL/6 ApcMin\− mice genetically prone to adenomatous intestinal polyps without causing overt typhlocolitis. Nonetheless, malignant transformation of adenomatous polyps is enhanced after infection with H. hepaticus, even in the absence of overt IBD. Surprisingly, H. hepaticus colonization in the lower bowel greatly accelerated carcinogenesis in extraintestinal tissues such as the mammary gland. The tumorigenic effect coincided with a generalized enlargement of the lymph nodes and an elevation of proinflammatory cytokines including TNF-α, interleukin (IL)-6 and IL-17. Interestingly, innate immunity was sufficient for this carcinogenic effect. Mast cells were a feature of intestinal polyps, as well as mammary and prostate carcinogenesis in murine models.
Gut Bacteria-Triggered TREG Protect from Cancer in Extraintestinal Tissues
Surprisingly, earlier infection experiments with H. hepaticus in Min mice revealed that pathogenic gut bacteria significantly modulate carcinogenesis in extraintestinal tissues (Fig. 4). In those studies, H. hepaticus infection increased risk of mammary tumors in susceptible female Min mice when compared with uninfected counterparts. Mammary-associated lymph node enlargement after H. hepaticus infection may have been due to H. hepaticus-stimulated dendritic cells from either systemic activation or local exposure via teat mucosa. In this model, T cells from WT donors with earlier H. hepaticus infection had significantly increased potency to protect from not only adenomatous polyps but also mammary tumors.52 Based on our earlier data, TREG may require IL-10 exposure for cancer suppressive functions. Alternatively, secretion of IL-10 by TREG may be needed for epithelial balance. Kullberg et al.16 showed in vivo and in vitro that ability of T cells to produce IL-10 led to their enhanced anti-inflammatory potency after infection with H. hepaticus. Taken together, these data suggested that IL-10-dependent functions of TREG limited inflammation, restored epithelia and subsequently protected from cancer.
Pathogenic gut microbial infections trigger an IL-6 and Th-17-associated break in immune tolerance resulting in IBD. Diverse microbial infections in youth stimulate protective immunity and tolerance and lower likelihood of IBD later in life.
Anti-inflammatory drugs significantly reduce risk of cancer in breast,29 prostate,54 lung and skin, even though these tissues may lack overt inflammatory disease. Supplementation with TREG suppresses expression of COX-251 previously linked with several cancers in humans.60 Likewise, TREG also normalizes downstream expression of the c-myc53 oncogene previously linked with breast cancer in women.61 Indeed, uncontrolled inflammation increases relative risk for breast cancer in women.62–67 One possible factor that lowers risk for breast cancer in women is prior exposures to microbial products found in soil, untreated water and unpasteurized foods. Exposure to these factors is widely believed to trigger innate immune responses that, in turn, encourage the development of a competent immune responses and subsequently promote constructive host responses to diverse inflammatory triggers during adulthood.68–71 This is consistent with data from mouse models showing that gut bacteria-triggered TREG are highly potent to suppress extraintestinal carcinogenesis.52,54,72 In these studies protection from cancer was transferable between animals using purified TREG from IL-10 competent donor mice. These data raise the possibility that TREG may be modulated to protect from cancer.
Protective TREG Functions Are Unified in Seemingly Divergent Disease Phenotypes: Allergies, Autoimmune Disease and Cancer
While improvements in societal cleanliness have reduced the incidence of many serious infections, with increased hygiene there has been a concomitant increase in ailments including autoimmune diseases and some types of cancers.5,11,14,29,73 The “hygiene hypothesis” model suggests beneficial effects as a result of exposure to infectious agents earlier in life; those individuals have fewer aberrant immune reactions such as allergies and asthma later in life (Fig. 5).73 Recent evidence suggests survival benefit due to efficient induction of tolerance during infancy.74 This raises the possibility that periparturient and early life exposures denied during modern sanitization may increase vulnerability to some diseases later in life. Selectively re-introducing less pathogenic forms of microorganisms may protect against inflammation-associated pathology later in life due to infectious or noninfectious causes (Fig. 5).
Hygienic individuals with a dysregulated IL-10 and TREG feedback loop suffer from uncontrollable inflammation that redirects TREG cells toward a T helper (Th)-17-driven procarcinogenic process.
Thus, efficient immune tolerance limits subsequent tissue damage75,76 and prevents a host from “inflaming itself to death.” One possibility is that early life bacterial exposures result in more efficient IL-10-dependent TEFF polarization toward Th-1 and robust immune tolerance.21 A naïve host with insufficient exposures earlier in life has weakened Th-1, uncontrolled TNF-α, subsequent upregulation of IL-1β and IL-6 and exuberant Th-17 host responses.20,25,40 In this context, mice lacking IL-10 are unable to sustain Foxp3 expression and functional TREG and unable to mount immune tolerance.33 In modernized societies, hygienic individuals with a weakened IL-10 and TREG feedback loop consequently suffer uncontrollable inflammatory disorders that re-direct TREG toward a carcinogenic Th-17 phenotype. This cancer condition is readily reversible by blocking underlying inflammation.
IL-10 stimulated by bacteria and parasites serve to enhance protective immune balance and reinforce epithelial repair and health.
These hypotheses are supported by evidence that risks of inflammation-associated cancer, such as CRC, are increased in societies with rigorous hygiene practices.5 Murine models of colitis also support this “hygiene hypothesis” paradigm with dependency on IL-10 and TREG16,17,40,52,72 consistent with the observations of Belkaid and Rouse.11 Specifically, beneficial cancer-suppressing effects of microbial infections were dependent on IL-1040,51,52,77 similar to proposed roles for TREG in allergies and many types of autoimmune responses.14 This is displayed in mouse models of CRC whereby exogenous IL-10 administration is sufficient to restore TREG anti-inflammatory and antineoplastic functions.40,41 IL-10 arising after bacterial or parasitic challenges may serve to enhance protective immunity through-out the body via Th-1 versus Th-2.5,40,52,54,72 This process may involve antigenic crossreactivity as described with heterologous immunity.78,79 A robust IFN-γ response may explain greater TREG anticancer potency after gut bacterial infections in mice.52,72 Antigen specificity in T cells may be targeted against bacteria, even in extraintestinal sites. Using this strategy lifelong protection from cancer may be achieved using probiotic, non-pathogenic or sterile bacterial antigens. Probiotic Lactobacillus reuteri was previously shown to restore GI homeostasis in mice and humans.80,81
Figure 5.
Insufficient microbial exposures earlier in life predispose to uncontrollable inflammatory disorders later in life. Modern sanitization has reduced the incidence of many serious infections. However, individuals living in developed countries with more stringent hygiene practices also suffer from increased incidence of allergies, asthma, autoimmune disorders and some types of inflammation-associated cancer. This is due, at least in part, to dysregulated immune tolerance. Inability of TREG to inhibit autoimmune diseases such as MS depends on levels of IL-6 that redirect toward a Th-17 host response. Likewise, an IL-10 deficiency during IBD leads to break in immune tolerance and a proinflammatory Th-17 response. Hygienic individuals with a weakened IL-10 and TREG feedback loop suffer from uncontrollable inflammation that subsequently redirects TREG cells toward a Th-17-driven procarcinogenic process. In contrast, gut microbial exposures earlier in life may reinforce Th-1 host responses and homeostasis. Taken together, these observations link the immune system, gastrointestinal infections and seemingly divergent downstream phenotypes: IBD, MS and other autoimmune disease and cancer.
Plasticity and Reciprocal Relationships Between TREG and Th-17
The immune system is designed to recognize and destroy foreign pathogens while preserving immune tolerance to self. TREG cells in particular, play an essential role in maintaining immunological tolerance. A primary peripheral mechanism to adapt to novel immune challenges involves the Th-17 host response. Normally functioning TREG efficiently protect against tissue injury to self or microbes in mouse models. However, under overwhelming proinflammatory conditions, TREG may be redirected toward Th-17 response. Erdman et al.29 showed that prior microbial exposures reinforced immune tolerance conveyed by TREG; in contrast, hygienic rearing of donor mice yielded TREG that exacerbated IL-6 and IL-17 in target tissue. A key role for IL-17 is demonstrable using neutralization with anti-IL17A antibody to inhibit tumorigenesis in mice (data not shown), and this has been shown by others using Min mice.26 Targeting underlying IL-6 or STAT3 confers improved prognosis in colonic26,40,82 and breast malignancies (Fig. 4).83,84
Reciprocal roles exist between TREG and Th-17 cells. Chronic inflammatory conditions subvert TREG cell functions, recruit Th-17 cells and factors, and increase risk for malignancy.
Discovery of Foxp3 as a key transcription factor of TREG has provided new insights into TREG biology and revealed unexpected features of this lineage.85 Recent studies suggest that differences in the ability of TREG to suppress cancer may be due to downregulation of Foxp3. In hygienic donors, uncontrollable inflammation increases risk that TREG will lose regulatory activity and become ex-TREG. Under these conditions, TREG become capable of TEFF activities including the production of IL-17 (during high-ambient levels of IL-6) or perhaps IL-9 (with high levels of IL-4). Likelihood of these ex-TREG85 increases in hygienic, immunologically naïve animals. There is mounting evidence that the Foxp3-deficient ex-TREG survive with considerable biologic function and converts to Th-17 cells.
During health, Foxp3 appears to maintain the suppressor cell program that allows a relatively small subset of TREG to efficiently preserve immune tolerance.86 If TREG lose Foxp3 expression under inflammatory conditions, then these cells can develop into TEFF thus explaining massive peritumoral accumulations of Foxp3 cells with only rare Foxp3+ cells within actual tumor parenchyma.29 If TREG subsets based on T-bet or IRF4 may selectively suppress Th-1 and Th-2 effector subsets,87,88 then early life exposures to diverse microbiota may favor robust Th-1 that subsequently reduces likelihood of Th-17 or Th-2 responses associated with pathology later in life.
Harnessing the Homeostatic Potency of TREG to Prevent or Treat Cancer
Underlying commonalities in diseases such as IBD and MS suggest that therapeutic approaches exploiting T-cell plasticity may be beneficial for a wide variety of immune-mediated diseases. Downregulating destructive inflammatory processes may be more effective and less toxic than more traditional chemotherapeutic approaches to cancer (Fig. 6). These approaches could be used collectively or alone:
-
Directly stimulate immune tolerance using GI bacteria
probiotic bacteria such as Lactobacillus reuteri
apathogenic commensal microbiota
synthetic, sterile microbial antigen preparations
-
Downregulate underlying inflammation
directly block key proinflammatory factors: TNF-α, IL-6 or STAT3 (chemotherapeutic or dietary)
stimulate IL-10 in GI tract: block inflammation, induce immune tolerance and restore epithelial oncogenes to baseline levels
-
Preferentially stimulate beneficial TREG in vivo
vitamin A derivatives, i.e., retinoic acid to induce TREG
vitamin D derivatives, i.e., to stimulate TREG
-
Re-program TREG ex vivo
“Condition” naïve CD4+ cells ex vivo and afterward return to host
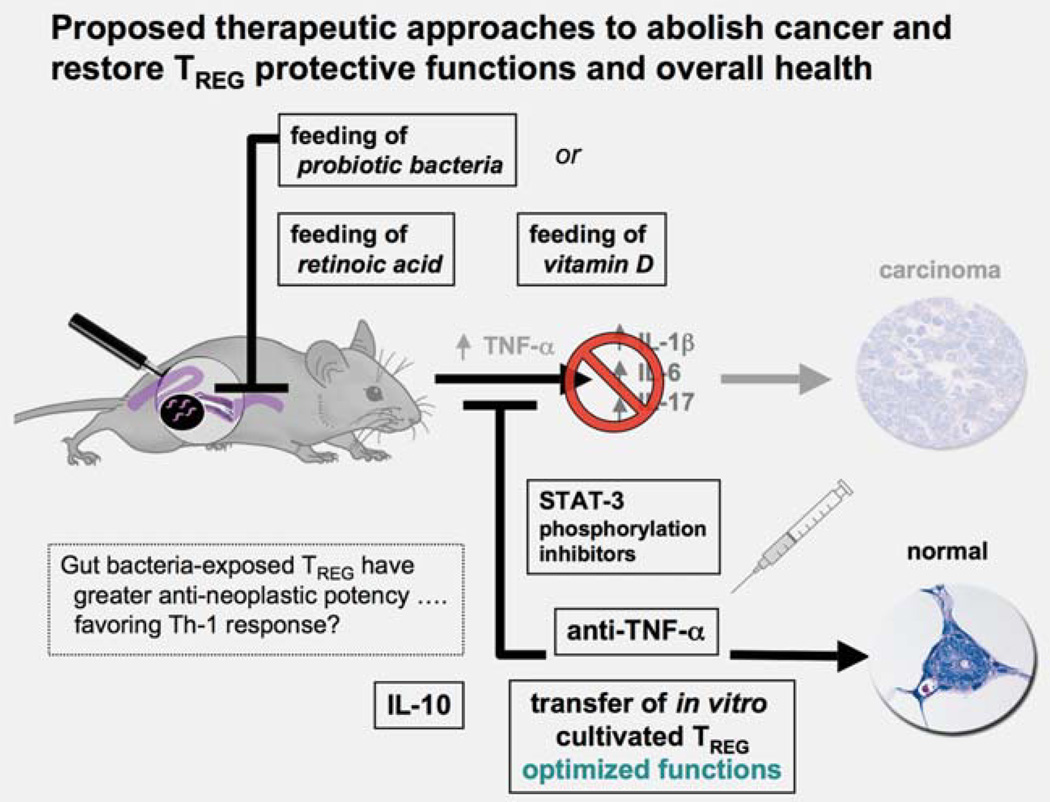
Figure 6.
Proposed strategies to reinforce protective TREG functions to abolish cancer and restore overall health. Cancer immunotherapy strategies to date focus primarily on boosting the antitumoral immunological responses of the cancer patient. In that way, TREG are viewed as an obstacle in tumor immunotherapy and a target for elimination. Recent data suggests that tumor survival and progression are instead vitally dependent on elevated and sustained local and systematic proinflammatory signaling. This raises the possibility that interrupting this proinflammatory loop of signals may be a more direct and constructive route to tumor eradication and restoration of overall health. Thus blocking TNF-α or the STAT3 inflammatory signaling pathway have been shown to rapidly and efficiently abrogate growing cancers in mouse models. Blocking these proinflammatory factors rapidly restores beneficial TREG functions and epithelial homeostasis. An emerging concept of overall health is systemic benefit of gut microbiota to reinforce beneficial effects of IL-10-dependent anti-inflammatory TREG cells. Adoptive-transfer studies in mice have shown the unsurpassed ability of gut bacteria-primed TREG to rapidly restore homeostasis in tissues of the GI tract and also in extraintestinal sites. It was surprising that transfer of TREG actually suppressed established carcinoma in mice. One therapeutic challenge will be to overcome redirection of TREG in the proinflammatory tumor environment. Harnessing antineoplastic potential may involve ex vivo cultivation, expansion and stabilization of human TREG for individualized therapies using autologous transfusions. Broader population-based reinforcement of TREG could be achieved by dietary habits undertaken earlier in life including probiotics or other bacterialbased modalities, or supplementation with retinoic acid and vitamin D.
Using gut microbiota to enforce an anti-inflammatory TREG phenotype serves to protect from a wide variety of immune-mediated diseases. Certain types of bacteria may uniquely coordinate the intestinal T-cell profile, and thus may be utilized to repair dysfunctional immunity arising from the bowel. Population-based strategies such as probiotic bacteria in foods81 may ultimately be used to reduce inflammation-associated cancer risk. Healthful benefits of routine yogurt consumption are well documented.
Directly targeting underlying inflammation would serve to block carcinogenic factors as well as to restore TREG to a cancer-protective phenotype. Humans with elevated TNF-α levels have poor cancer outcomes,1–3 and TNF-α is required for IBD-associated CRC and polyposis in mice.29,40,52,54,55 Highly efficacious monoclonal antibodies against TNF (infliximab) and TNF-binding fusion proteins (etanercept) have been routinely used in human patients with IBD and arthritis,89,90 albeit with reduced protection from serious bacterial infections, highlighting importance of overall immune balance for health. Restoration of homeostasis through suppression of TNF-α and reinforcement of TREG cells has been proposed for human patients suffering from IBD and other uncontrolled inflammatory disorders.89
Enhanced nutrition or therapeutic delivery of vitamin derivatives may also be used to facilitate induction and recruitment of TREG. Vitamin D promotes the differentiation of TREG and inhibits the differentiation of Th-17 cells.91 Increased serum levels of vitamin D have been associated with a decreased risk of MS, an autoimmune disorder characterized by a break in tolerance involving upregulation of Th-17. Vitamin D not only favors the induction of TREG but also enhances recruitment to inflammatory sites by modulating dendritic cells. A potent anti-inflammatory TREG phenotype could also be induced in resident T cells by administration of retinoic acid that favors anti-inflammatory Foxp3+ as described for IBD.92
Immediate benefits of TREG could be achieved in humans using ex vivo stimulation with bacterial antigens to educate and tolerize CD4+ cells.93 TREG may then afterward be returned to a patient. Many unanswered questions remain to optimize this process for therapeutic purposes, in particular regarding TREG identity and TREG recruitment with polarization toward Th-1 rather than toward Th-17 later in life.
Conclusions
Mouse models that mimic diseases in humans provide evidence unifying immune cell biology in IBD, autoimmune diseases, asthma and cancer. Under normal physiological conditions, TREG responses are beneficial to reinforce protective acute inflammation, and later regain protection from pathology.72 In immune-mediated diseases, normal homeostatic mechanisms become dysregulated leading to chronic inflammatory disease. During a break in immune tolerance, resident and recruited T cells are redirected toward a pro-inflammatory host response. This inducible proinflammatory condition creates a feedback loop involving STAT-3, IL-6 and IL-17 that further contributes to neoplastic invasion and metastases. Under these conditions, TREG accumulate locally, are not beneficial, and instead contribute to cancer growth. Interestingly, IL-10-dependent activities in the GI tract impart sustained protection from aforementioned events. Taken together, these observations connect GI tract infections, the immune system, and diverse immune-mediated diseases including autoimmune diseases and cancer, and help explain paradoxical aspects of TREG cell biology in these diseases.
Roles for TREG cells in cancer appear contradictory on the surface.94–97 We postulate that TREG are beneficial—in fact, pivotal—in sustaining overall health and preventing cancer. Functional benefits of TREG depend on the age and prior health status of the host, and are context within the cancer microenvironment. In immune-competent animals, TREG constructively regulate inflammatory and epithelial growth factors that when uncontrolled lead to cancer growth. Thus, while it is true that TREG accumulate in the vicinity of many types of tumors, TREG also accumulate in large numbers during other uncontrolled inflammatory disease such as with IBD, but nonetheless may ultimately rescue pathology.24,98 Conditions such as IBD are curable after restoring immune balance and relevant TREG potency in mice. Under these conditions, TREG redistribute within tumors with anti-neoplastic effect.99–101 Likewise, supplementation with exogenous TREG induces regression of inflammation-associated neoplastic conditions of colon, breast and prostate tissues in our mouse models.40,52,53,72
The interplay of carcinogenic host factors is showcased in ApcMin/+ mice that are genetically susceptible to tumors arising from disruptions of the wnt signaling pathway. In these animals, growing intestinal tumor burden coincides with significantly increased levels of inflammatory cytokines IL-9, IL-6 and IL-17 predisposing to cancer growth.54 Accumulation of improperly functioning TREG under these conditions exacerbates cancer growth. These events accelerate a feedback loop that hastens cancer growth. In Apc mutant mice thymic depletion is coincident with polypogenesis102–104 may be a result and a cause of widespread immune dysfunction impeding TREG and further elevating cancer risk. Supplementary CD4+ TREG collected from immune competent donor animals would, at least initially, be untainted by high levels of inflammation and provide rescue of homeostasis. In support of this, experimental depletion of CD25+ cells in young ApcMin/+ mice rapidly induces intestinal and prostate carcinogenesis.54
The therapeutic strategy of blocking inflammation and redirecting TREG would directly target and inhibit factors required to sustain cancer growth. Permanent health benefits could be achieved using combinations of diet, anti-inflammatory agents and probiotics. Ex vivo stimulation of T cells with bacterial antigens or other factors that stabilize TREG phenotype may have utility to rescue individual cancer patients,93 as demonstrated using adoptive transfer in murine models with IBD or CRC. Many unanswered questions remain to optimize this process, especially regarding TREG identity and TREG recruitment later in life with underlying immune instability. As this field evolves, knowledge of factors that promote terminal differentiation of TREG may one day allow selective inhibition or exacerbation of polarized CD4+ T-cell responses in the clinics.
Acknowledgements
This work was supported by NIH (to S.E.E.), DOD Contract (to S.E.E.) and Pythagoras II Grant (to T.P.).
Grant sponsor: NIH; Grant number: R01CA108854; Grant sponsor: DOD Contract; Grant number: W81XWH-05-01-0460; Grant sponsor: Pythagoras II Grant; Grant number: 80860
Abbreviations
- CRC
colorectal cancer
- EAE
experimental allergic encephalitis
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- MS
multiple sclerosis
- TEFF
proinflammatory CD4+ effector T cell
- Th
T helper
- Tgf
transforming growth factor
- TNF
tumor necrosis factor
- TREG
anti-inflammatory CD4+ regulatory T cell
- Rag
recombinase activating gene (lacking functional lymphocytes)
- wt
wild type (genotype)
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 4.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–542. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 6.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 7.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correa P. Helicobacter pylori infection and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:238s–241s. [PubMed] [Google Scholar]
- 9.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powrie F, Maloy KJ. Immunology. Regulating the regulators. Science. 2003;299:1030–1031. doi: 10.1126/science.1082031. [DOI] [PubMed] [Google Scholar]
- 11.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 12.Taguchi O, Nishizuka Y. Experimental autoimmune orchitis after neonatal thymectomy in the mouse. Clin Exp Immunol. 1981;46:425–434. [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 14.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 15.Belkaid Y, Tarbell KV. Arming Treg cells at the inflammatory site. Immunity. 2009;30:322–323. doi: 10.1016/j.immuni.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 18.Chow J, Mazmanian SK. Getting the bugs out of the immune system: do bacterial microbiota “fix” intestinal T cell responses? Cell Host Microbe. 2009;5:8–12. doi: 10.1016/j.chom.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Mazmanian SK. Gut immune balance is as easy as S-F-B. Immunity. 2009;31:536–538. doi: 10.1016/j.immuni.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 21.Barnes MJ, Powrie F. Hybrid Treg cells: steel frames and plastic exteriors. Nat Immunol. 2009;10:563–564. doi: 10.1038/ni0609-563. [DOI] [PubMed] [Google Scholar]
- 22.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 24.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erdman SE, Rao VP, Olipitz W, Taylor CL, Jackson EA, Levkovich T, Lee CW, Horwitz BH, Fox JG, Ge Z, Poutahidis T. Unifying roles for regulatory T cells and inflammation in cancer. Int J Cancer. 126:1651–1665. doi: 10.1002/ijc.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 31.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 33.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, Khoury S, Oukka M, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 36.Erdman SE, Fox JG, Sheppard BJ, Feldman D, Horwitz BH. Regulatory T cells prevent non-B non-T colitis. Gastroenterology. 2001;120(Suppl 1):A524. [Google Scholar]
- 37.Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox JG, Dewhirst FE, Tully JG, Paster BJ, Yan L, Taylor NS, Collins MJ, Jr, Gorelick PL, Ward JM. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poutahidis T, Haigis KM, Rao VP, Nambiar PR, Taylor CL, Ge Z, Watanabe K, Davidson A, Horwitz BH, Fox JG, Erdman SE. Rapid reversal of interleukin-6-dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis. 2007;28:2614–2623. doi: 10.1093/carcin/bgm180. [DOI] [PubMed] [Google Scholar]
- 41.Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, Ge Z, Lee CW, Schauer DB, Wogan GN, Tannenbaum SR, Fox JG. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci USA. 2009;106:1027–1032. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 43.Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- 44.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 45.Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:119–126. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- 47.Schneikert J, Behrens J. The canonical Wnt signalling pathway and its APC partner in colon cancer development. Gut. 2007;56:417–425. doi: 10.1136/gut.2006.093310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- 49.Brewster SF, Browne S, Brown KW. Somatic allelic loss at the DCC. APC, nm23-H1 and p53 tumor suppressor gene loci in human prostatic carcinoma. J Urol. 1994;151:1073–1077. doi: 10.1016/s0022-5347(17)35186-8. [DOI] [PubMed] [Google Scholar]
- 50.Phillips SM, Morton DG, Lee SJ, Wallace DM, Neoptolemos JP. Loss of heterozygosity of the retinoblastoma and adenomatous polyposis susceptibility gene loci and in chromosomes 10p, 10q and 16q in human prostate cancer. Br J Urol. 1994;73:390–395. doi: 10.1111/j.1464-410x.1994.tb07602.x. [DOI] [PubMed] [Google Scholar]
- 51.Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 52.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Boussahmain C, Wang YY, Horwitz BH, Fox JG, Erdman SE. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395–7400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- 53.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Horwitz BH, Fox JG, Erdman SE. Proinflammatory CD4+ CD45RB(hi) lymphocytes promote mammary and intestinal carcinogenesis in Apc(Min/+) mice. Cancer Res. 2006;66:57–61. doi: 10.1158/0008-5472.CAN-05-3445. [DOI] [PubMed] [Google Scholar]
- 54.Poutahidis T, Rao VP, Olipitz W, Taylor CL, Jackson EA, Levkovich T, Lee CW, Fox JG, Ge Z, Erdman SE. CD4+ lymphocytes modulate prostate cancer progression in mice. Int J Cancer. 2009;125:868–878. doi: 10.1002/ijc.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, Rao VP, Poutahidis T, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA. 2007;104:19977–19982. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, Beckhove P, Gounari F, Khazaie K. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galli SJ, Nakae S. Mast cells to the defense. Nat Immunol. 2003;4:1160–1162. doi: 10.1038/ni1203-1160. [DOI] [PubMed] [Google Scholar]
- 58.Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109:3640–3648. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 60.Wang D, Dubois RN. Cyclooxygenase-2: a potential target in breast cancer. Semin Oncol. 2004;31:64–73. doi: 10.1053/j.seminoncol.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 61.Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- 62.Ness RB, Cauley JA. Antibiotics and breast cancer—what’s the meaning of this? JAMA. 2004;291:880–881. doi: 10.1001/jama.291.7.880. [DOI] [PubMed] [Google Scholar]
- 63.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 64.Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade. Oncol Rep. 2005;13:559–583. [PubMed] [Google Scholar]
- 65.Howe LR, Chang SH, Tolle KC, Dillon R, Young LJ, Cardiff RD, Newman RA, Yang P, Thaler HT, Muller WJ, Hudis C, Brown AM, et al. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005;65:10113–10119. doi: 10.1158/0008-5472.CAN-05-1524. [DOI] [PubMed] [Google Scholar]
- 66.Mazhar D, Ang R, Waxman J. COX inhibitors and breast cancer. Br J Cancer. 2006;94:346–350. doi: 10.1038/sj.bjc.6602942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Subbaramaiah K, Howe LR, Port ER, Brogi E, Fishman J, Liu CH, Hla T, Hudis C, Dannenberg AJ. HER-2/neu status is a determinant of mammary aromatase activity in vivo: evidence for a cyclooxygenase-2-dependent mechanism. Cancer Res. 2006;66:5504–5511. doi: 10.1158/0008-5472.CAN-05-4076. [DOI] [PubMed] [Google Scholar]
- 68.Arun B, Goss P. The role of COX-2 inhibition in breast cancer treatment and prevention. Semin Oncol. 2004;31:22–29. doi: 10.1053/j.seminoncol.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 69.Chang TW, Pan AY. Cumulative environmental changes, skewed antigen exposure, and the increase of allergy. Adv Immunol. 2008;98:39–83. doi: 10.1016/S0065-2776(08)00402-1. [DOI] [PubMed] [Google Scholar]
- 70.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu AH. Innate microbial sensors and their relevance to allergy. J Allergy Clin Immunol. 2008;122:846–858. doi: 10.1016/j.jaci.2008.10.002. quiz 58–60. [DOI] [PubMed] [Google Scholar]
- 72.Rao VP, Poutahidis T, Fox JG, Erdman SE. Breast cancer: should gastrointestinal bacteria be on our radar screen? Cancer Res. 2007;67:847–850. doi: 10.1158/0008-5472.CAN-06-3468. [DOI] [PubMed] [Google Scholar]
- 73.Weiss ST. Eat dirt—the hygiene hypothesis and allergic diseases. N Engl J Med. 2002;347:930–931. doi: 10.1056/NEJMe020092. [DOI] [PubMed] [Google Scholar]
- 74.Conrad ML, Ferstl R, Teich R, Brand S, Blumer N, Yildirim AO, Patrascan CC, Hanuszkiewicz A, Akira S, Wagner H, Holst O, von Mutius E, et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med. 2009;206:2869–2877. doi: 10.1084/jem.20090845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Segal BM, Glass DD, Shevach EM. Cutting edge: IL-10-producing CD4+ T cells mediate tumor Rejection. J Immunol. 2002;168:1–4. doi: 10.4049/jimmunol.168.1.1. [DOI] [PubMed] [Google Scholar]
- 77.Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, Feng Y, Tomczak M, Rogers AB, Horwitz BH, Fox JG. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63:6042–6050. [PubMed] [Google Scholar]
- 78.Page KR, Scott AL, Manabe YC. The expanding realm of heterologous immunity: friend or foe? Cell Microbiol. 2006;8:185–196. doi: 10.1111/j.1462-5822.2005.00653.x. [DOI] [PubMed] [Google Scholar]
- 79.Lemke LB, Ge Z, Whary MT, Feng Y, Rogers AB, Muthupalani S, Fox JG. Concurrent Helicobacter bilis infection in C57BL/6 mice attenuates proinflammatory H. pylori-induced gastric pathology. Infect Immun. 2009;77:2147–2158. doi: 10.1128/IAI.01395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 81.Pena JA, Rogers AB, Ge Z, Ng V, Li SY, Fox JG, Versalovic J. Probiotic Lactobacillus spp. diminish Helicobacter hepaticus-induced inflammatory bowel disease in interleukin-10-deficient mice. Infect Immun. 2005;73:912–920. doi: 10.1128/IAI.73.2.912-920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, Ito H, Nishimoto N, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 83.Berishaj M, Gao SP, Ahmed S, Leslie K, Al-Ahmadie H, Gerald WL, Bornmann W, Bromberg JF. Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res. 2007;9:R32. doi: 10.1186/bcr1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 87.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pereg D, Lishner M. Non-steroidal anti-inflammatory drugs for the prevention and treatment of cancer. J Intern Med. 2005;258:115–123. doi: 10.1111/j.1365-2796.2005.01519.x. [DOI] [PubMed] [Google Scholar]
- 91.Royal W, III, Mia Y, Li H, Naunton K. Peripheral blood regulatory T cell measurements correlate with serum vitamin D levels in patients with multiple sclerosis. J Neuroimmunol. 2009;213:135–141. doi: 10.1016/j.jneuroim.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 92.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 93.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- 94.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Degl’Innocenti E, Grioni M, Capuano G, Jachetti E, Freschi M, Bertilaccio MT, Hess-Michelini R, Doglioni C, Bellone M. Peripheral T-cell tolerance associated with prostate cancer is independent from CD4+CD25+ regulatory T cells. Cancer Res. 2008;68:292–300. doi: 10.1158/0008-5472.CAN-07-2429. [DOI] [PubMed] [Google Scholar]
- 96.Khazaie K, von Boehmer H. The impact of CD4+CD25+ Treg on tumor specific CD8+ T cell cytotoxicity and cancer. Semin Cancer Biol. 2006;16:124–136. doi: 10.1016/j.semcancer.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 97.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 98.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 99.Badoual C, Hans S, Fridman WH, Brasnu D, Erdman S, Tartour E. Revisiting the prognostic value of regulatory T cells in patients with cancer. J Clin Oncol. 2009;27:e5–e6. doi: 10.1200/JCO.2009.23.0680. author reply e7. [DOI] [PubMed] [Google Scholar]
- 100.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V, Laccourreye O, Bruneval P, Fridman WH, Brasnu DF, Tartour E. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 101.Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, Distel LV. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol. 2009;9:65. doi: 10.1186/1471-230X-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coletta PL, Muller AM, Jones EA, Muhl B, Holwell S, Clarke D, Meade JL, Cook GP, Hawcroft G, Ponchel F, Lam WK, MacLennan KA, et al. Lymphodepletion in the ApcMin/+ mouse model of intestinal tumorigenesis. Blood. 2004;103:1050–1058. doi: 10.1182/blood-2003-03-0707. [DOI] [PubMed] [Google Scholar]
- 103.Faubion WA, de Jong YP, Molina AA, Ji H, Clarke K, Wang B, Mizoguchi E, Simpson SJ, Bhan AK, Terhorst C. Colitis is associated with thymic destruction attenuating CD4+25+ regulatory T cells in the periphery. Gastroenterology. 2004;126:1759–1770. doi: 10.1053/j.gastro.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 104.Gounari F, Chang R, Cowan J, Guo Z, Dose M, Gounaris E, Khazaie K. Loss of adenomatous polyposis coli gene function disrupts thymic development. Nat Immunol. 2005;6:800–809. doi: 10.1038/ni1228. [DOI] [PMC free article] [PubMed] [Google Scholar]