Abstract
It has been reported that the action of infiltration anesthesia on the jawbone is attenuated significantly by elevation of the periosteal flap with saline irrigation in clinical studies; however, the reason is unclear. Therefore, the lidocaine concentration in mandibular bone after subperiosteal infiltration anesthesia was measured under several surgical conditions. The subjects were 48 rabbits. Infiltration anesthesia by 0.5 mL of 2% lidocaine with 1 : 80,000 epinephrine (adrenaline) was injected into the right mandibular angle and left mandibular body, respectively. Under several surgical conditions (presence or absence of periosteal flap, and presence or absence of saline irrigation), both mandibular bone samples were removed at a fixed time after subperiosteal infiltration anesthesia. The lidocaine concentration in each mandibular bone sample was measured by high-performance liquid chromatography. As a result, elevation of the periosteal flap with saline irrigation significantly decreased the lidocaine concentration in the mandibular bone. It is suggested that the anesthetic in the bone was washed out by saline irrigation. Therefore, supplemental conduction and/or general anesthesia should be utilized for long operations that include elevation of the periosteal flap with saline irrigation.
Key Words: Periosteal flap, Lidocaine concentration, Jawbone, Infiltration anesthesia.
In clinical research, it has been reported that the action of infiltration anesthesia on the jawbone was reduced significantly by elevation of the periosteal flap with irrigation by water or saline (EPFI) compared with nonelevation of the periosteal flap (NEPF).1 In that report, significant differences were detected in the initial dose of local anesthetics (EPFI: 4.3 ± 1.4 mL; NEPF: 1.8 ± 0.9 mL) and in the duration of anesthesia action (EPFI: 38 ± 26 minutes; NEPF: 65 ± 27 minutes). These results suggested that the duration of infiltration anesthesia action in EPFI decreases by half compared within NEPF, even if the anesthetic was infiltrated at a double dose. However, it is uncertain why the action of infiltration anesthesia is reduced markedly in EPFI. The aim of this study was to assess the lidocaine concentration in mandibular bone after subperiosteal infiltration (infiltration) anesthesia under several surgical conditions, ie, presence or absence of periosteal flap, and presence or absence of saline irrigation.
METHODS
Subjects
This study was approved by the animal research committee of Ohu University. Subjects were 48 Japan white rabbits (weight 2.92 ± 0.21 kg; 16 weeks old; male) (Table 1). The rabbits were housed in room air (23°C, relative humidity 65%) with solid feed and tap water.
Table 1.
Characteristics of Subjects
General Anesthesia
General anesthesia was induced with 100% oxygen and 5% sevoflurane (Figure 1). After intubation via tracheotomy, anesthesia was maintained with 100% oxygen and 4% sevoflurane under spontaneous respiration.
Figure 1.
Method of general anesthesia.
Infiltration Anesthesia and Characteristics of Sample Bone
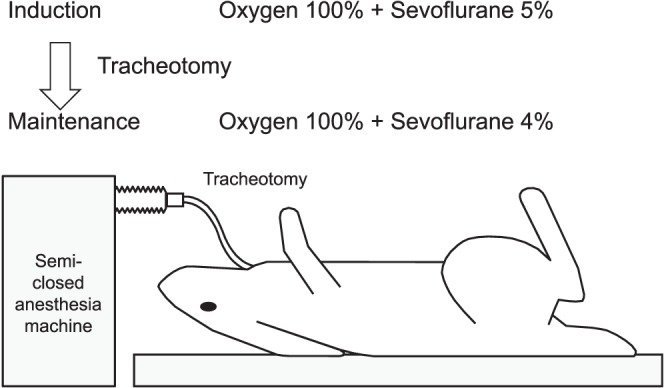
After general anesthesia, local anesthesia was administered by a metered-dose injector (Citoject, Heraeus Kulzer Co., Hanau, Germany) with a 33-guage × 21-mm needle. Infiltration anesthesia of 0.5 mL of 2% lidocaine with 1 : 80,000 adrenaline was injected over 20 seconds into the central area of the right mandible angle (A) and the last molar apical area of the left mandible body (B), respectively (Figure 2). The infiltration anesthesia areas in both A and B became the bone sample areas under several experimental variables that were completed. Area A was selected as an area with thin cortical bone and low bone density (width 3 mm, cortical bone width 0.6mm, bone density 140 mg/cm2). Area B was selected as an area with thick cortical bone and high bone density (width 6 mm, cortical bone width 1.5 mm, bone density 420 mg/cm2). These cortical bone and bone density data were measured previously in one mandibular sample of a rabbit. The width of the cortical bone was measured by X-ray computed tomography (3DX-multi image micro CT type-F; Morita Co, Tokyo, Japan), and bone density was measured by the dual-energy X-ray absorptiometry method using a densitometer (DCS600, Aloka Co, Tokyo, Japan).
Figure 2.
Method of infiltration anesthesia and characteristics of bone.
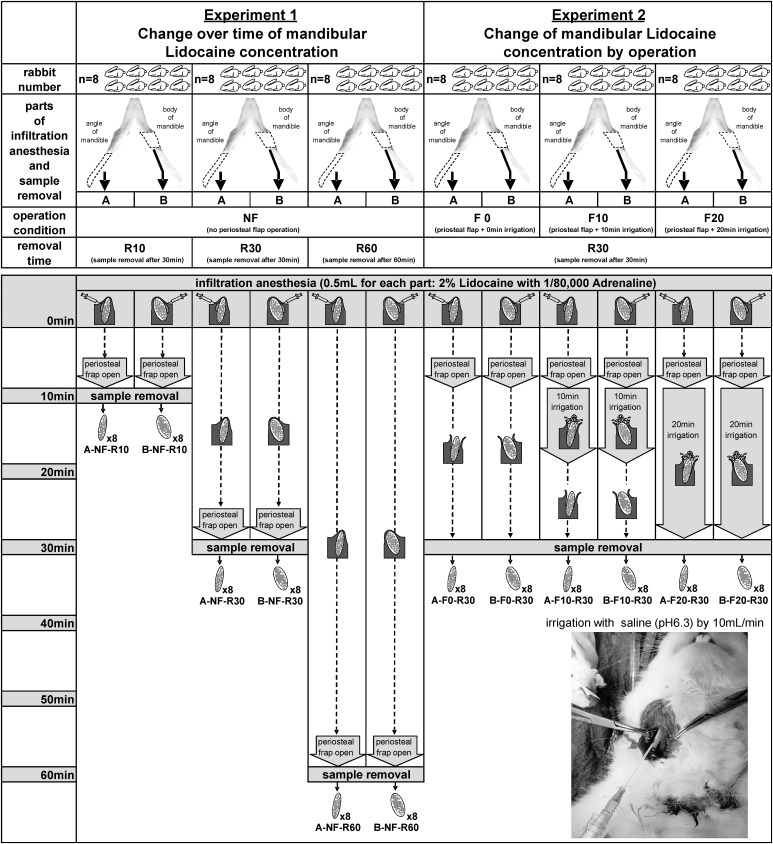
Experimental Conditions
The four surgical conditions (Figure 3) were set as no elevation of the periosteal flap (NF), elevation of the periosteal flap with no irrigation (F0), 10-minute irrigation (F10), or 20-minute irrigation (F20). In addition, bone samples were removed at 10 minutes (R10), 30 minutes (R30), or 60 minutes (R60) after infiltration anesthesia. All experimental conditions were described as a combination of area, surgical condition, and sample removal time (eg, A-NF-R10).
Figure 3.
Each condition, procedure, and sample of this study.
Experiment 1: Change Over Time of Lidocaine Concentration in Mandibular Bone
Twenty-four rabbits were used to confirm the time-dependent changes of lidocaine concentration in mandibular bone under no elevation of the periosteal flap (NF) (Figure 3). Both bone samples (A, B) were removed at 10 minutes, 30 minutes, or 60 minutes (R10, R30, or R60) after infiltration anesthesia. Therefore, the 6 experimental conditions were A-NF-R10 (n = 8), B-NF-R10 (n = 8), A-NF-R30 (n = 8), B-NF-R30 (n = 8), A-NF-R60 (n = 8), and B-NF-R60 (n = 8).
Experiment 2: Change of Lidocaine Concentration in Mandibular Bone by Operation
The other 24 rabbits were used to confirm the change of lidocaine concentration in mandibular bone according to the surgical procedure after infiltration anesthesia (Figure 3). Elevation of the periosteal flap with 0 minutes, 10 minutes, or 20 minutes of irrigation (F0, F10, or F20) was applied to A and B, and these samples were removed 30 minutes after infiltration anesthesia. Therefore, the 6 experimental conditions were A-F0-R30 (n = 8), B-F0-R30 (n = 8), A-F10-R30 (n = 8), B-F10-R30 (n = 8), A-F20-R30 (n = 8), and B-F20-R30 (n = 8).
Methods of Elevation of Periosteal Flap and Irrigation
Each elevation of the periosteal flap was started 5 minutes after infiltration anesthesia because clinical operations in humans usually begin after waiting for about 5 minutes after infiltration anesthesia (Figure 3). Each periosteal flap was elevated from the inferior edge of the mandible because a rabbit's mouth is narrow for surgery. It took about 5 minutes for elevation of the periosteal flap because it was elevated around the whole circumference of the mandibular bone. Normal saline (0.9%, pH 6.3) was used for each irrigation of the mandibular bone surface via a 20-gauge intravenous indwelling catheter, and the infusion rate was adjusted to 10 mL/min. To reduce experimental technical errors and variation, the infiltration anesthesia, elevation of the periosteal flap, and irrigation were performed by the same researcher.
Methods for Analysis of Lidocaine Concentration in Mandibular Bone
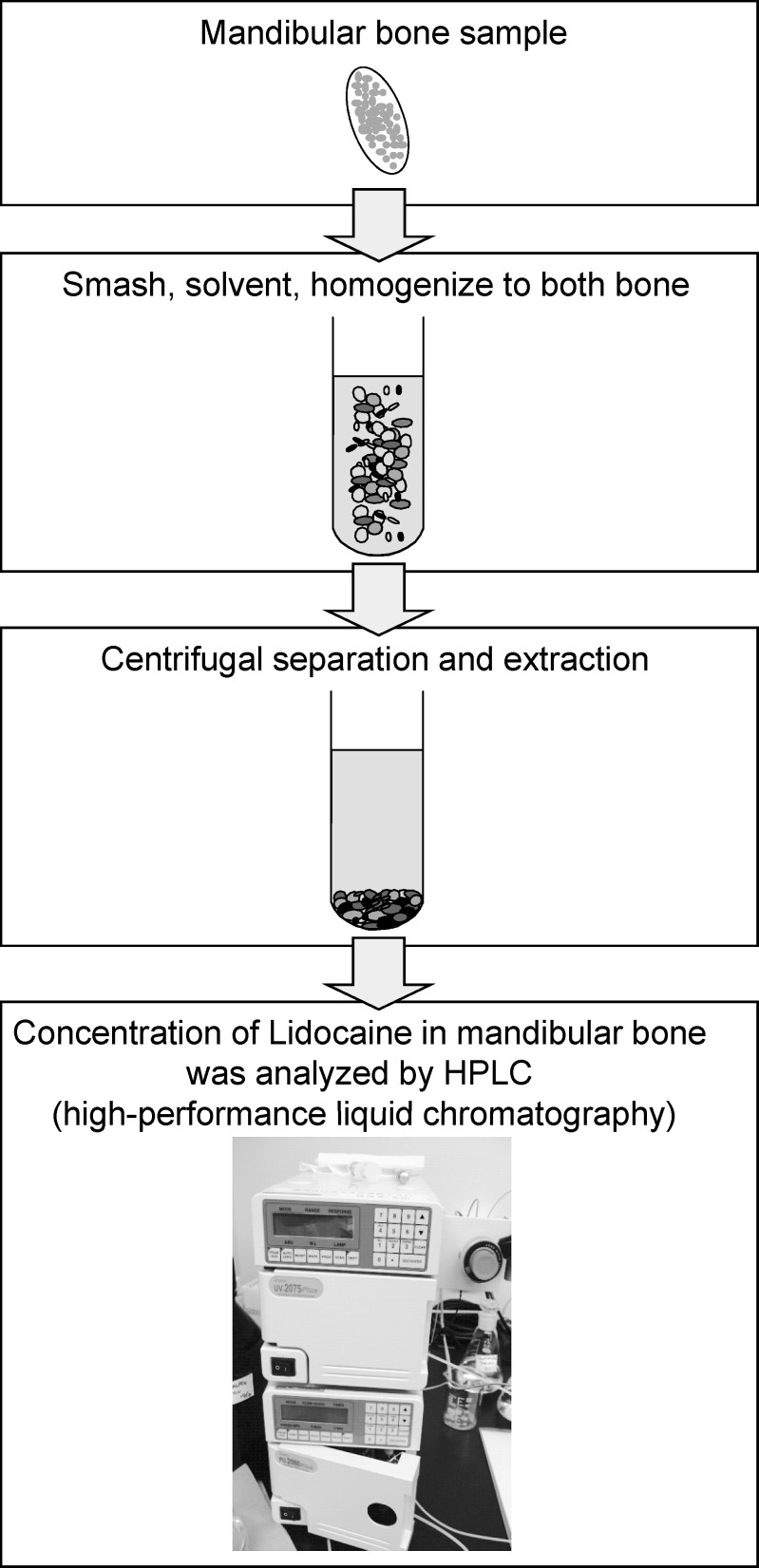
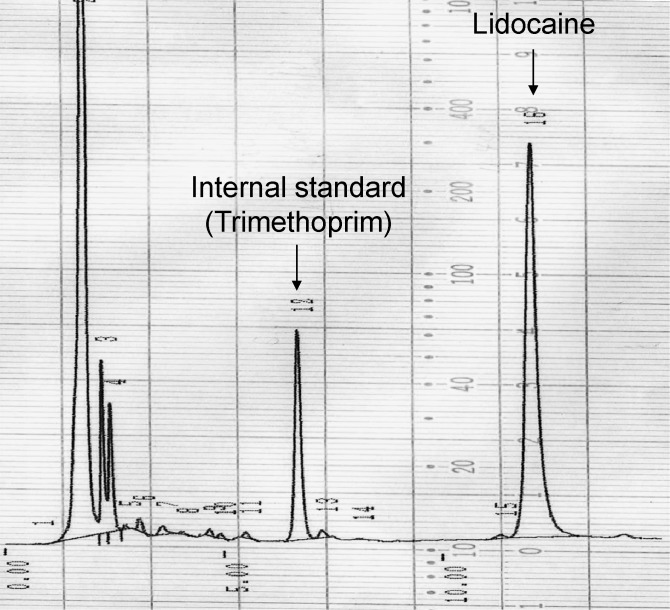
At the time of measurement, the cryogenically-preserved mandibular bone sample at −80°C was crushed by a mill (TK-CM20S, Tokken Co, Tokyo, Japan) (Figure 4). The crushed bone sample was homogenized by a Polytron (PT-2100, Kinematica Co., Luzern, Switzerland) after mixing and suspending with borate (pH 9.18, 0.01M). Subsequently, 0.5 mL supernatant liquid was mixed with 100 μL trimethoprim (1.25 μg/mL) and 2.5 mL chloroform-methanol mixture (8 : 2). The liquid was centrifuged at 3000 rpm (1000g) for 10 minutes after rehomogenization for 2 minutes. After centrifugation, 1.5 mL organic fraction was isolated and depressurized for 10 minutes (40°C) to dry and fix by rotary evaporator (EYELA, Tokyo Science Material Co, Tokyo, Japan). It was dissolved in 200 μL mobile phase (50 mM KH2PO4 : CH3CN = 4 : 1). After 0.22 μm filtration (MILLEX-GV, Takara Biotechnology Co, Tokyo, Japan), another 70 μL mobile phase was added. The lidocaine concentration in mandibular bone was measured by high-performance liquid chromatography (HPLC) (LC-2000 Plus, Japan Spectrum Co, Tokyo, Japan).2 The condition of HPLC for this measurement is shown in Table 2. A typical example of the peak lidocaine concentration measured by HPLC is shown in Figure 5.
Figure 4.
Methods for analysis of lidocaine concentration in mandibular bone.
Table 2.
Condition for High-Performance Liquid Chromatography Analysis of Lidocaine
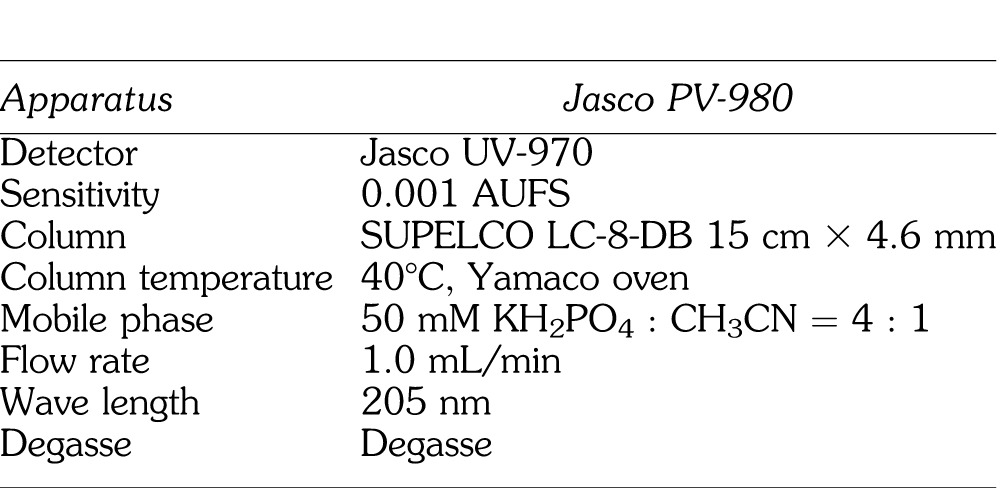
Figure 5.
Chromatogram of lidocaine from mandibular bone in rabbit.
Data Handling and Statistical Analysis
The lidocaine concentration in mandibular bone is shown in μg/g, the amount of lidocaine (μg) per 1 g mandibular bone. All data are expressed as the mean ± standard deviation. Each sample was regarded as unpaired data. Statistical analysis was performed by Friedman test, followed by Mann-Whitney U test with Bonferroni correction as the post hoc test. P < .05 was considered significant.
RESULTS
Experiment 1: Change Over Time of Lidocaine Concentration in Mandibular Bone
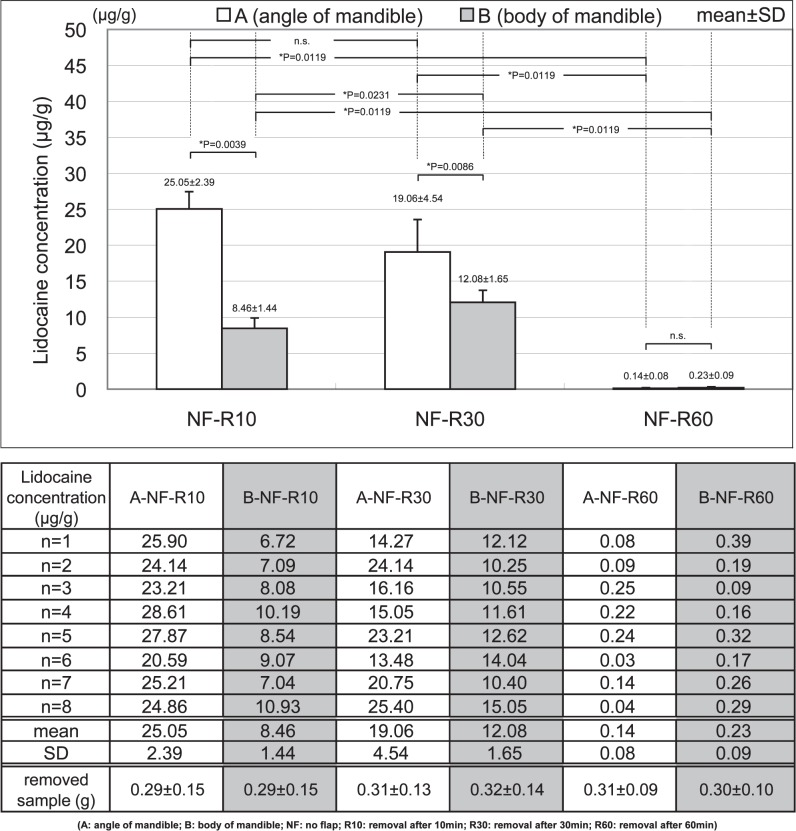
In the low bone density area (A), the lidocaine concentrations in A-NF-R10, A-NF-R30, and A-NF-R60 were 25.05 ± 2.39, 19.06 ± 4.54, and 0.14 ± 0.08 μg/g, respectively. There was no significant difference between A-NF-R10 and A-NF-R30. However, there were significant differences between other comparisons of the low bone density area (A), and the lidocaine concentration in mandibular bone decreased over time (Figure 6).
Figure 6.
Change over time of mandibular lidocaine concentration.
Figure 7.
Change of mandibular lidocaine concentration by operation.
In the high bone density area (B), the lidocaine concentrations in B-NF-R10, B-NF-R30, and B-NF-R60 were 8.46 ± 1.44, 12.08 ± 1.65, and 0.23 ± 0.09 μg/g, respectively. There were significant differences between all comparisons of the high bone density area (B); however, the lidocaine concentration at 30 minutes showed a significant increase compared to that at 10 minutes after infiltration anesthesia.
In the comparison between the low bone density area (A) and high bone density area (B), the lidocaine concentration in A was significantly higher than in B at 10 minutes and 30 minutes after infiltration anesthesia. However, there was no significant difference between A and B at 60 minutes, because both lidocaine concentrations were markedly low.
Experimental 2: Change of Lidocaine Concentration in Mandibular Bone by Operation
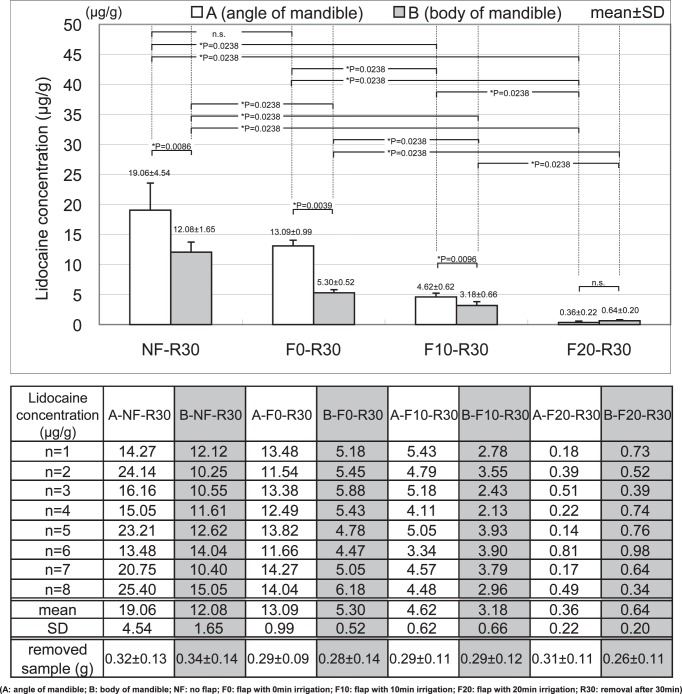
In the low bone density area (A), the lidocaine concentrations in A-NF-R30, A-F0-R30, A-F10-R30, and A-F20-R30 were 19.06 ± 4.54, 13.09 ± 0.99, 4.62 ± 0.62, and 0.36 ± 0.22 μg/g, respectively. There was no significant difference between A-NF-R30 (no periosteal flap) and A-F0-R30 (only periosteal flap with no irrigation); however, irrigation of the bone surface significantly reduced the lidocaine concentration in mandibular bone. Furthermore, the lidocaine concentration in mandibular bone was significantly more decreased after 20 minutes of irrigation than 10 minutes of irrigation.
In the high bone density area (B), the lidocaine concentrations in B-NF-R30, B-F0-R30, B-F10-R30, and B-F20-R30 were 12.08 ± 1.65, 5.30 ± 0.52, 3.18 ± 0.66, and 0.64 ± 0.20 μg/g, respectively. The lidocaine concentration in mandibular bone decreased in the order of B-NF-R30 (no periosteal flap), B-F0-R30 (only periosteal flap with no irrigation), B-F10-R30 (periosteal flap with 10 minutes irrigation), B-F20-R30 (periosteal flap with 20 minutes irrigation), and there were significant differences among all conditions in the high bone density area (B).
In the comparison between the low bone density area (A) and high bone density area (B), there was no significant difference between A and B under the condition of periosteal flap with 20 minutes irrigation, because both lidocaine concentrations were markedly low. On the other hand, the lidocaine concentration in the low bone density area (A) was significantly higher than in the high bone density area (B).
DISCUSSION
Infiltration Mechanisms of Local Anesthetic to Jawbone and the Bone Characteristics
The method of subperiosteal infiltration anesthesia in this study was to inject a local anesthetic (lidocaine) between the periosteum and bone surface after puncturing the periosteum with a needle.3 Subsequently, the local anesthetic which pools under the periosteum gradually infiltrates through the cortical bone to the bone marrow, enabling some local anesthetic to act on the target nerve or dental pulp.4 However, eventually, all residual local anesthetic is absorbed gradually from the capillary vessels into the systemic circulation and is metabolized by hepatic cytochrome P450.5,6
In this study, infiltration anesthesia was applied to the mandibular angle (A) at the thin cortical bone and low bone density areas, and the mandibular body (B) at the thick cortical bone and high bone density areas. In the mandibular angle (A), the cortical bone width and bone density were 0.6 mm and 140 mg/cm2, respectively. In the mandibular body (B), the cortical bone width and the bone density were 1.5 mm and 420 mg/cm2, respectively. Therefore, the cortical bone width and bone density of the mandibular angle (A) are about one third of the mandibular body (B) in the rabbit.
As a noninvasive condition (no periosteal flap elevation) in this study, bone samples were removed 10 minutes, 30 minutes, and 60 minutes after infiltration anesthesia. Accordingly, in the mandibular angle (A), the maximum lidocaine concentration was 25.05 μg/g at 10 minutes, subsequently decreasing over time. However, in the mandibular body (B), the lidocaine concentration maximally increased to 12.08 μg/g at 30 minutes. Moreover, the lidocaine concentration at the mandibular angle was consistently higher than at the mandibular body. These results suggest that the thick cortical bone and high bone density block and delay anesthetic infiltration into the bone marrow. Therefore, it is important to recognize the characteristics of the jawbone when infiltration anesthesia is applied in human clinical practice. If there is thick cortical bone and high bone density, more time should be spent after infiltration anesthesia before starting the operation.
Effect of Infiltration Anesthesia and Elevation of Periosteal Flap
In the mandibular angle (A), the lidocaine concentration did not change significantly between the noninvasive condition and the condition of periosteal flap elevation without irrigation. However, in the mandibular body (B), the lidocaine concentration in the case of periosteal flap elevation without irrigation decreased significantly compared to that under the noninvasive case. As above, thick cortical bone and high bone density block and delay anesthetic infiltration into the bone marrow. This means that residual local anesthetic remains in the subperiosteal space for a long time. If the periosteal flap is opened early after infiltration anesthesia to the thick cortical bone and high bone density area, there is a possibility that the unabsorbed residual local anesthesia will flow out. When infiltration anesthesia to the jawbone is applied in clinical practice, it is important to assess the bone condition and to wait an appropriate time until the lidocaine concentration in the mandibular bone has increased sufficiently.
Effect of Infiltration Anesthesia and Elevation of Periosteal Flap With Irrigation
In both the mandibular angle (A) with thin cortical bone and low bone density areas and the mandibular body (B) with thick cortical bone and high bone density areas, the lidocaine concentration in the mandibular bone decreased significantly with saline irrigation of the bone surface. In particular, the 20-minute irrigation significantly decreased the lidocaine concentration in the mandibular bone more than the 10-minute irrigation. This result suggests that irrigation of the bone surface reduces the local anesthetic action in the jawbone in a time-dependent manner. Moreover, the lidocaine concentration decreased to almost 0 μg/g with 20-minute irrigation in both the mandibular angle (A) and mandibular body (B). Although this 0 μg/g lidocaine concentration in the mandibular bone with 20 minutes of irrigation was about equal to that at 30 minutes after infiltration anesthesia, this was almost the same as the data 60 minutes after infiltration anesthesia with the noninvasive condition. Therefore, 20 minutes of irrigation of the bone surface halved the duration of infiltration anesthetic action. It was speculated that irrigation of the bone surface is one of the major factors in the attenuation of infiltration anesthesia action because there is a possibility that residual anesthetic in the jawbone is washed out by irrigation.
Operative Time and Duration of Action After Infiltration Anesthesia
The 2% lidocaine with 1 : 80,000 adrenaline that was used in this study is widely used in human clinical dental practice as a local anesthetic. This combination of lidocaine and adrenaline provides the appropriate local anesthetic action.7,8 In addition, this local anesthetic also excels in its hemostatic effects8 and lengthens the duration of anesthetic action to approximately 100 minutes.9 However, the anesthetic action of 2% lidocaine with 1 : 80,000 adrenaline in clinical practice often loses its effect significantly earlier than 100 minutes. It has been reported that the duration of anesthetic action of 2% lidocaine with 1 : 80,000 adrenaline decreased by half during operations involving elevation of a periosteal flap and irrigation in comparison with nonelevation of the periosteal flap.1 The results in this study agree with the finding of the previous report, which was done in human clinical practice. Almost all clinicians may think that the action of infiltration anesthesia is guaranteed for approximately 100 minutes regardless of the invasiveness of the surgical procedure because it is not recognized widely that the action of infiltration anesthesia on the jawbone can change with the invasiveness of the surgical procedure. However, these results suggest that the patient may feel pain earlier than predicted after infiltration anesthesia when impacted tooth extraction, cystectomy, or oral implant placement is performed under saline irrigation because the intraosseous concentration of local anesthetic falls more quickly compared to the less invasive procedure without flap irrigation. The painful surgical stress of the patient invites dangerous incidents and medical emergencies.10 To avoid such events, jaw surgery under infiltration anesthesia should be finished as fast as possible after waiting an appropriate time until the intraosseous concentration of local anesthetic has increased sufficiently. Specifically, such jaw surgery under infiltration anesthesia should be finished within a short time of less than 100 minutes. Normally if the anesthetic action unfortunately loses its effect earlier than predicted after infiltration anesthesia, one can inject additional drug by infiltration or conductive anesthesia. However, if the periosteal flap is already elevated, it is difficult to achieve adequate infiltration anesthetic in the jawbone, again due to anesthetic leakage. Even a psycho-sedation method cannot extend the local anesthetic action. Therefore, if there is a possibility that an operation with elevation of the periosteal flap will not finish within a specified short time, a nerve block or general anesthesia should be administered for clinical risk management.
CONCLUSIONS
Lidocaine concentration in the mandibular bone after infiltration anesthesia was assessed in rabbits with elevation of the periosteal flap and/or saline irrigation. The findings are as follows:
-
(a)
It takes a long time for the anesthetic to infiltrate into the mandibular bone if the bone has a thick cortex and high density.
-
(b)
Lidocaine concentration in bone with a thick cortex and high density decreased significantly with elevation of the periosteal flap compared with noninvasive conditions.
-
(c)
Lidocaine concentration in mandibular bone decreased significantly in relation to the irrigation time of the bone surface in comparison with noninvasive nonirrigation conditions.
-
(d)
It is suggested that jaw surgery under infiltration anesthesia should be finished as fast as possible after waiting an appropriate time for the intraosseous concentration of local anesthetic to increase sufficiently.
REFERENCES
- 1.Yamazaki S, Seino H, Ogawa S, Ito H, Kawaai H. Elevation of periosteal flap with irrigation of the bone for minor oral surgery reduces the duration of action of infiltration anesthesia. Anesth Prog. 2006;53:8–12. doi: 10.2344/0003-3006(2006)53[8:EOAPFW]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piwowalska J, Kuczynska J, Pachecka J. Liquid chromatographic method for the determination of lidocaine and monoethylglycine xylidide in human serum containing various concentrations of bilirubin for the assessment of liver function. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;805:1–5. doi: 10.1016/j.jchromb.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Pompians-Miniac L, Grenier JP. Local-regional anesthesia in the mandible. Utilization review of technics for students as a function of current material [in French] Inf Dent. 1975;57:37–41. [PubMed] [Google Scholar]
- 4.Tang JP, Cha W. Clinical observation of pulpectomy under subperiosteal infiltration anesthesia [in Chinese] Shanghai Kou Qiang Yi Xue. 2001;10:186–187. [PubMed] [Google Scholar]
- 5.Tateno K, Inoue K, Sato T, Fukayama H. Differences in the degree of infiltration of local anesthesia according to the site of injection in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:6–10. doi: 10.1016/j.tripleo.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 6.Bargetzi MJ, Aoyama T, Gonzalez FJ, Meyer UA. Lidocaine metabolism in human liver microsomes by cytochrome P450IIIA4. Clin Pharmacol Ther. 1989;46:521–527. doi: 10.1038/clpt.1989.180. [DOI] [PubMed] [Google Scholar]
- 7.Kanaa MD, Whitworth JM, Meechan JG. A comparison of the efficacy of 4% articaine with 1 : 100,000 epinephrine and 2% lidocaine with 1 : 80,000 epinephrine in achieving pulpal anesthesia in maxillary teeth with irreversible pulpitis. J Endod. 2012;38:279–282. doi: 10.1016/j.joen.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Ahn J, Pogrel MA. The effects of 2% lidocaine with 1 : 100,000 epinephrine on pulpal and gingival blood flow. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:197–202. doi: 10.1016/s1079-2104(98)90426-7. [DOI] [PubMed] [Google Scholar]
- 9.Pitt Ford TR, Seare MA, McDonald F. Action of adrenaline on the effect of dental local anaesthetic solutions. Endod Dent Traumatol. 1993;9:31–35. doi: 10.1111/j.1600-9657.1993.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki S, Kawaai H, Sasaki S, Shimamura K, Segawa H, Saito T. Availability of a remote online hemodynamic monitoring system during treatment in a private dental office for medically high-risk patients. Ther Clin Risk Manag. 2008;4:721–726. doi: 10.2147/tcrm.s3227. [DOI] [PMC free article] [PubMed] [Google Scholar]