Abstract
Renal tubular dysfunction could be involved in the increased sodium and water reabsorption in chronic heart failure (CHF). The goal of the present study was to examine the molecular basis for the increased renal sodium and water retention in CHF. We hypothesized that dysregulation of renal epithelial sodium channels (ENaC) could be involved in the pathogenesis of CHF. The left coronary ligation-induced model of heart failure in the rat was used. Real-time PCR and Western blot analysis indicated that the mRNA and protein abundance of α-, β-, and γ-subunits of ENaC were significantly increased by in the cortex (mRNA: α-ENaC Δ104 ± 24%, β-ENaC Δ47 ± 16%, γ-ENaC Δ55 ± 18%; protein: α-ENaC Δ114 ± 28%, β-ENaC Δ150 ± 31%, γ-ENaC Δ39 ± 5% compared with sham rats) and outer medulla (mRNA: α-ENaC Δ52 ± 18%, β-ENaC Δ38 ± 8%, γ-ENaC Δ39 ± 13%; protein: α-ENaC Δ88 ± 16%, β-ENaC Δ94 ± 28%, γ-ENaC Δ45 ± 9% compared with sham rats) of CHF compared with sham-operated rats. Immunohistochemistry microscopy confirmed the increased labeling of α-, β-, and γ-ENaC subunits in the collecting duct segments in rats with CHF. Furthermore, there was a significant increase in diuretic (7-fold compared with sham) and natriuretic responses (3-fold compared with sham) to ENaC inhibitor benzamil in the rats with CHF. Absence of renal nerves produced a greater contribution of ENaC in sodium retention in rats with CHF. These results suggest that the increased expression of renal ENaC subunits may contribute to the renal sodium and water retention observed during CHF.
Keywords: sodium and water retention, renal function
one hallmark of chronic heart failure (CHF) is fluid retention. An impaired ability to excrete a sodium load is commonly seen in CHF (20, 26, 29). Although CHF can be defined as an abnormal physiological state in which the heart cannot pump enough blood to satisfy the metabolic demands of the body, altered renal function has been suggested to contribute in the pathogenesis of CHF (20, 29). The renal defects could range from the altered glomerular hemodynamics to abnormal regulation of renal tubular sodium and water transport. Four basic ways have been suggested to account for the sodium retention that is observed in CHF: 1) a reduction in glomerular filtration rate (GFR), 2) activation of the renal nerves, 3) activation of the renin-angiotensin-aldosterone pathway, and 4) inhibition of natriuretic factors. Each of these mechanisms contributes to some extent in patients with CHF.
It has been previously shown that renal nerves contribute to the retention of sodium and water in rats with CHF (7, 25). However, we also have demonstrated that although renal denervation normalized the blunted renal diuretic and natriuretic responses to acute volume expansion, the results from the renal denervated kidneys from CHF were still significantly different from the renal denervated control rats. These results suggest that in addition to the renal nerves, there also may be other factor(s) that are involved in sodium retention during CHF. Moreover, there may be an interaction between the renal nerves and other tubular factor(s). In models of CHF, the renal involvement may be presented, at least in part, by inappropriate sodium and water retention. The excessive retention of sodium in CHF may result from either a reduced GFR or an enhanced tubular reabsorption, or both (1). However, the roles of tubule segments and the molecular basis for the inappropriate sodium and water retention remain largely undefined in CHF. Particularly, there is very little, if any, information regarding the abundance and functional role of epithelial sodium channels (ENaC) in the kidneys of rats with CHF and their interactions with the tonic renal nerve activity.
ENaC represents the rate-limiting step in sodium reabsorption and thus plays an important role in the maintenance of sodium balance and extracellular fluid volume (10). ENaC is composed of at least three subunits: α, β, and γ. ENaC localization in the kidney appears to be confined to the distal convoluted tubules, cortical collecting ducts, and medullary collecting ducts (5, 31) of the distal nephron. The activity of ENaC is regulated by angiotensin, aldosterone, and vasopressin, which markedly increase the apical permeability of the collecting duct to sodium (2, 3, 8). In CHF, the levels of angiotensin, aldosterone, and vasopressin are all reported to be elevated (27). It is plausible that this increased neurohumoral drive during CHF (25) may alter the expression and function of ENaC. The purpose of this study was to investigate the possible changes in the message (mRNA) and protein abundance of the subunits of the amiloride-sensitive ENaC expressed along the renal tubule that would functionally limit the salt and water losses during CHF.
Therefore, the hypothesis we tested in this study is that dysregulation of renal ENaC subunits may contribute in the pathogenesis of CHF, independently or possibly by interactions with tonic renal nerve activity. We addressed this hypothesis by posing three questions: 1) Is there an increased abundance of ENaC subunits in the kidneys of rats with CHF? 2) Does blockade of ENaC with benzamil produce enhanced diuretic and natriuretic responses in the rats with CHF? 3) Does renal denervation enhance or augment the renal excretory responses to blockade of ENaC with benzamil?
METHODS
Induction of heart failure.
All the procedures on animals in this study were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee. The experiments were conducted according to the American Physiological Society's “Guiding Principles for Research Involving Animals and Human Beings” and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Male Sprague-Dawley rats weighing 200–220 g were obtained from Sasco Breeding Laboratories (Omaha, NE) and were randomly assigned to a sham-operated group and a CHF group. CHF was produced by coronary artery ligation, as previously described (41). Each rat was caged individually in an environment with ambient temperature maintained at 22°C and humidity at 30–40%. Laboratory chow and tap water were available ad libitum.
The degrees of left ventricular dysfunction and heart failure were determined using both hemodynamic and anatomic criteria. Left ventricular end-diastolic pressure (LVEDP) was measured using a Mikro-Tip catheter (Millar Instruments, Houston, TX) at the time of the terminal experiment. To measure infarct size, the heart was dissected and the atria and right ventricle were removed. A digital image of the left ventricle was captured using a digital camera (Kodak, Rochester, NY). The percentage of infarct area to total left ventricle area was quantified using SigmaScan Pro (Aspire Software International, Ashburn, VA). Rats with both LVEDP >15 mmHg and infarct size greater than approximately 30% of total left ventricular wall were considered to be in CHF.
RNA extraction and protein isolation.
Two groups of rats (sham, n = 11; CHF, n = 11) were anesthetized with pentobarbital sodium (50 mg/kg ip). The renal artery and vein were ligated, and the kidneys were removed, weighed, dissected, frozen on dry ice, and then stored at −70°C. The cortical and medulla samples were put in 0.5 ml of TRI Reagent and homogenized. The total RNA in the homogenate was extracted according to the TRI Reagent manufacturer's instructions (MRC, Cincinnati, OH).
Cortical and medulla samples were homogenized in 20% (wt/vol) ice-cold buffer containing 10 mM Tris·HCl, pH 7.4, 1% SDS, 1 mM sodium vanadate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 100 μl/ml protease cocktail inhibitor (BD Biosciences). The samples were kept on ice for 30 min. Next, samples were centrifuged at 12,000 g for 20 min at 4°C, and protein concentration in supernatant was determined using a BCA protein assay kit (Pierce, Rockford, IL).
Real-time quantitative RT-PCR measurement for ENaC subunit mRNA.
Real-time RT-PCR assays were performed to assess relative mRNA levels. RNA was isolated, followed by reverse transcription for 40 min at 37°C in the presence of 1.5 μM of random hexamers and 400 units of Maloney murine leukemia virus reverse transcriptase. Each 1.5-μl aliquot of the RT product was used for ENaC cDNA amplification. The cDNA was amplified by real-time quantitative PCR with the Bio-Rad Icycler IQ system (Bio-Rad Laboratories, Hercules, CA) using the TaqMan probe labeled with FAM dye and Black Hole quencher. All gene-specific primer pairs were designed using BeconDesign 4.0 (Bio-Rad Laboratories) (Table 1). Relative expression of ENaC mRNA was normalized to the expressed reference gene, ribosomal protein L19 (RPL19). The data were analyzed using the 2−ΔΔCt method.
Table 1.
PCR primer sequences
| Name | Accession No. | Sequence |
|---|---|---|
| α-ENaC | U54700 | Forward: 5′-CATGCAAGGACTGGGGAAGG-3′ |
| Backward: 3′-TGGTCATGATCCTGCTGCTTAG-5′ | ||
| Probe: 3′-TGCCCAACTCGCCTCGCTTCCTACT-5′ | ||
| β-ENaC | U35175 | Forward: 5′-AGAAGAAGGCCATGTGGTTCC-3′ |
| Backward: 3′-GCTCAGGTAGGTCTGGATGAAG-5′ | ||
| Probe: 5′-CACGCTGCTCTTCGCCTGCCTGG-3′ | ||
| γ-ENaC | U37540 | Forward: 5′-CCATCCAGGAGTGGTACAAGC-3′ |
| Backward: 3′-GGCATCACAGGACATCCCATC-5′ | ||
| Probe: 3′-AGCAGGTCACCAGCAGTTCTTCGGC-5′ | ||
| RPL19 | NM031103 | Forward: 5′-CTGAAGGTCAAAGGGAATGTGTTC-3′ |
| Backward: 3′-TTCGTGCTTCCTTGGTCTTAGAC-5′ | ||
| Probe: 3′-TGCGAGCCTCAGCCTGGTCAGCC-5′. |
ENaC, epithelial sodium channel; RPL19, ribosomal protein L19.
Western blot analysis of ENaC subunit protein.
Protein extraction was used for Western blot analysis in the samples obtained as described above. The protein sample was mixed with an equal volume of 2× 4% SDS sample buffer. The sample was then loaded onto the 7.5% SDS-PAGE gel for electrophoresis at 40 mA per gel for 60 min. The fractionated proteins on the gel were electrophoretically transferred onto the polyvinylidene difluoride membrane at 300 mA for 90 min. The membrane was incubated with 5% milk-Tris-buffered saline-Tween solution for 30 min at room temperature. The membrane was incubated with primary antibody (rabbit anti-rat ENaC subunits, 1:2,000; provided by Dr. U. S. Rao; rabbit anti-rat GAPDH, 1:1,000; Santa Cruz Biotechnology) at 4°C overnight. A description and characterization of anti-ENaC antibodies has been presented previously (28). The previous studies showed that these antibodies are highly specific and do not exhibit any cross-reactivity. After being washed, the membrane was incubated with secondary antibody (goat anti-rabbit IgG, peroxidase conjugated, 1:5,000; Pierce) for 40 min at room temperature. The signals were visualized using an enhanced chemiluminescence substrate (Pierce) and detected using the UVP digital imaging system (Upland, CA).
ENaC subunit immunohistochemistry.
Under pentobarbital sodium anesthesia (50 mg/kg ip), kidneys were perfused via the abdominal aorta with 150 ml of heparinized saline, followed by 250 ml of 4% paraformaldehyde in 0.1 M sodium phosphate buffer. Kidneys were dissected and fixed in Bouin's solution and then dehydrated in 70% ethanol. Paraffin-embedded tissue sections from rats were processed according to standard histochemical methods. Paraffin slides were washed in xylene before rehydration in a series of ethanol washes. Antigen retrieval was performed by boiling slides in 10 mM calcium citrate, followed by permeabilization and blocking. The primary antibody (rabbit anti-rat ENaC subunits, 1:5,000; provided by Dr. U. S. Rao) was incubated with the sections overnight, and the next day the sections were washed and incubated with Cy3-conjugated donkey anti-rabbit secondary antibody (1:500; Jackson ImmunoResearch, West Grove, PA) for 2 h at room temperature. The sections were cover slipped with fluoromounting-G (SouthernBiotech, Birmingham, AL). Distribution of ENaC immunofluorescence within the cortex and medulla was viewed using an Olympus fluorescence microscope (Japan) equipped with a digital camera (Qimaging; Surrey, BC, Canada).
Renal function studies.
On the day of the experiment, rats were anesthetized with inactin (100 mg/kg ip). Body temperature was maintained at 36–38°C by a heated stage. After tracheal intubation, the animals were allowed to breathe independently. The left femoral artery was cannulated with PE-50 polyethylene tubing and connected to a pressure transducer for the continuous recording of arterial pressure. The left femoral vein was cannulated with PE-50 tubing for administration of supplemental anesthesia and drug.
The kidneys were exposed through a retroperitoneal flank incision, and left renal denervation was performed by stripping the sheath and adventitia from the exposed left renal artery and vein. To destroy any remaining nerve fibers, the renal vessels were painted with 95% ethanol. Previously, this technique has been shown to decrease renal norepinephrine concentration to <5% of endogenous levels (24). Subsequently, both ureters were cannulated with PE-10 tubing. Surgery was completed within 30 min, and an additional 30-min stabilization period was allowed before the start of the first urine collection.
Urine was collected in preweighed tubes from both left and right kidney via ureteral catheters, and urine volume was measured gravimetrically. Two urine collections (10 min each) were obtained before a bolus dose of ENaC inhibitor benzamil (0.7 mg/kg iv; Sigma, St. Louis, MO) in 0.5 ml of 0.9% saline. After injection of benzamil, urine was collected at 5, 10, 15, 20, 30, and 40 min. Sodium concentration (ion-selective electrode; Beckman ion analyzer) of each of the urine samples was also analyzed.
Data analysis.
Data were subjected to two-way ANOVA followed by a multiple range (for multiple comparisons) or Student Newman Keuls test. P values <0.05 were considered to indicate statistical significance.
RESULTS
Baseline data (group characteristics).
Table 2 summarizes the salient characteristics of sham and CHF rats utilized in the present study. Heart weight, body weight, and wet lung weight were significantly higher in CHF rats compared with sham rats (P < 0.05). This indicates that retention of water occurs in CHF rats.
Table 2.
Characteristics of sham and CHF rats
| Sham | CHF | |
|---|---|---|
| Body weight, g | 386 ± 16 | 431 ± 24* |
| Heart weight, g | 1.3 ± 0.2 | 1.7 ± 0.2* |
| Wet lung weight, g | 1.8 ± 0.1 | 2.1 ± 0.2* |
| Infarct size, % of epicardial LV | 0 | 34 ± 6* |
| LVEDP, mmHg | 1 ± 1 | 20 ± 4* |
| +dP/dtmax, mmHg/s | 7,538 ± 385 | 5,827 ± 319* |
| −dP/dtmax, mmHg/s | −5,328 ± 641 | −4,082 ± 281* |
Values are means ± SE; n = 15 for each group of sham-operated (Sham) and chronic heart failure (CHF) rats.
LV, left ventricle; LVEDP, left ventricular end-diastolic pressure; ±dP/dtmax, maximum rate of rise and fall in pressure over time.
P < 0.05 compared with respective sham group.
The CHF group displayed an average myocardial infarct size over 30% of left ventricle. Sham rats had no observable damage to the myocardium. LVEDP was significantly elevated in CHF rats compared with sham rats (P < 0.05). The maximum rise in pressure over time was significantly decreased in CHF rats, indicating a decreased contractility that was manifest as an increase in LVEDP. The maximum decrease in pressure over time had a similar trend in rats with CHF. These data suggest that the rats in the CHF group had decreased cardiac contractile and diastolic function.
Real-time quantitative RT-PCR measurement for ENaC subunit mRNA.
In both cortex and outer medulla, CHF increased the abundance of ENaC subunits at the mRNA level. In CHF, α-ENaC mRNA level in the cortex was 1.73 ± 0.21 vs. 0.85 ± 0.11 (sham), significantly increased by 104 ± 24% compared with sham-operated rats (P < 0.05). In the outer medulla, α-ENaC mRNA level was 1.49 ± 0.20 (CHF) vs. 0.98 ± 0.18 (sham). α-ENaC was significantly increased 52 ± 18% compared with sham-operated rats (P < 0.05). β-ENaC mRNA was significantly increased by 47 ± 16% (cortex) and 38 ± 8% (outer medulla) in the CHF group (P < 0.05). γ-ENaC mRNA was also significantly increased by 55 ± 18% (cortex) and 39 ± 13% (outer medulla) in the CHF group (P < 0.05) (Fig. 1).
Fig. 1.
A: relative mRNA expression of epithelial sodium channel (ENaC) α-, β-, and γ-subunits in the cortex of sham and chronic heart failure (CHF) rats as measured by real-time PCR. B: relative mRNA expression of ENaC subunits in the outer medulla of sham and CHF rats as measured by real-time PCR. *P < 0.05 compared with respective sham group.
Western blot analysis of ENaC subunit protein.
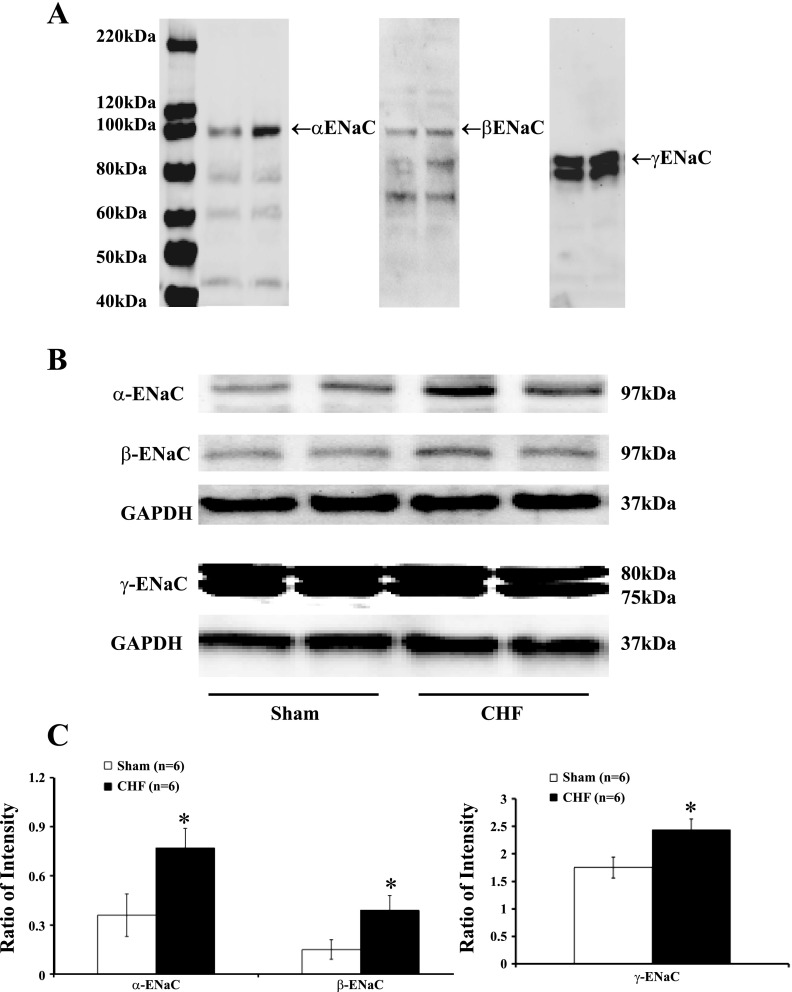
Western blot analysis showed α-ENaC (97 kDa), β-ENaC (97 kDa), and γ-ENaC (75 and 80 kDa) in the cortex and outer medulla of both sham and CHF rats. CHF rats had a significantly higher protein level of α-ENaC (0.77 ± 0.12 vs. 0.36 ± 0.13 in the cortex; 0.60 ± 0.05 vs. 0.32 ± 0.06 in the outer medulla) compared with sham-operated rats (P < 0.05). α-ENaC band intensity was significantly increased by 114 ± 28% (cortex) and 88 ± 16% (outer medulla) compared with sham-operated rats (P < 0.05). β-ENaC protein level was significantly increased by 150 ± 31% (cortex) and 94 ± 28% (outer medulla) in the CHF group (P < 0.05). Both bands associated with γ-ENaC were significantly increased by 39 ± 5% (cortex) and 45 ± 9% (outer medulla) in the CHF group (P < 0.05) (Figs. 2 and 3).
Fig. 2.
Immunoblotting for ENaC in the cortex of sham and CHF rats. A: an image of the bands detected from 40 to 220 kDa using α-, β-, and γ-ENaC antibodies in sham (first lane) and CHF rats (second lane). B: example of visualized electrophoresis bands of ENaC subunits and GAPDH in sham and CHF rats. C: mean data for band densities of ENaC subunits normalized by GAPDH in sham and CHF rats. *P < 0.05 compared with respective sham group.
Fig. 3.
Immunoblotting for ENaC in the outer medulla of sham and CHF rats. A: example of visualized electrophoresis bands of ENaC subunits and GAPDH in sham and CHF rats. B: mean data for band densities of ENaC subunits normalized by GAPDH in sham and CHF rats. *P < 0.05 compared with respective sham group.
ENaC subunit immunohistochemistry.
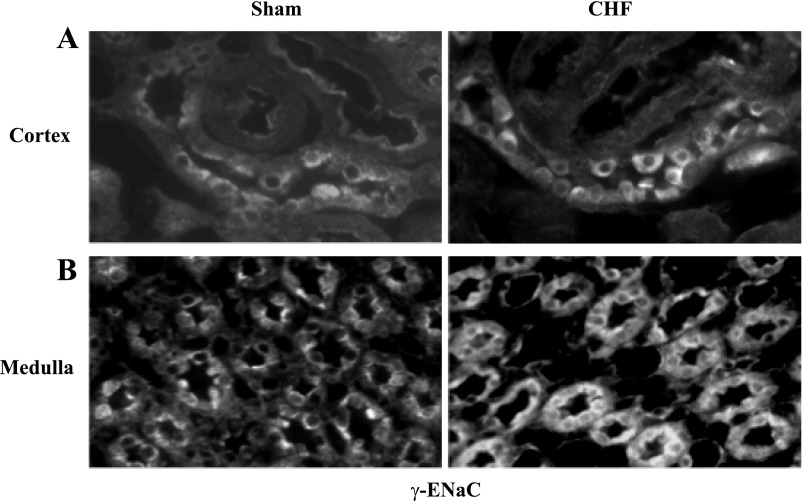
As an in situ confirmation of the alteration in ENaC subunits within the kidney, immunofluorescent staining for ENaC subunits in the collecting duct segments and medulla were higher in the kidneys from rats with CHF compared with sham rats (Figs. 4, 5, and 6).
Fig. 4.
Immunofluorescent staining of renal sections for α-ENaC in cortex (A) and medulla (B) of a sham and a CHF rat. An increase in immunostaining is noted in the CHF rat (magnification, ×400).
Fig. 5.
Immunofluorescent staining of renal sections for β-ENaC in cortex (A) and medulla (B) of a sham and a CHF rat. An increase in immunostaining is noted in CHF rat (magnification, ×400).
Fig. 6.
Immunofluorescent staining of renal sections for γ-ENaC in cortex (A) and outer medulla (B) of a sham and a CHF rat. An increase in immunostaining is noted in CHF rat (magnification, ×400).
Diuretic and natriuretic responses to ENaC inhibitor.
There was no significant difference in mean arterial pressure in sham and CHF groups (basal and after benzamil injection). There were no significant differences in kidney weights between the groups of rats (Table 3). The basal urine flow and sodium excretion before benzamil injection were not significantly different between the two groups. Denervation caused basal urine flow and sodium excretion to significantly increase in both sham and CHF groups (P < 0.05).
Table 3.
Basal renal characteristics of sham and CHF rats
| Sham-Intact Kidneys | CHF-Intact Kidneys | Sham-Denervated Kidneys | CHF-Denervated Kidneys | |
|---|---|---|---|---|
| Kidney weight, g | 1.4 ± 0.1 | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.6 ± 0.3 |
| Urine flow, μl•min−1•g kidney wt−1 | 2.1 ± 0.2 | 2.7 ± 0.6 | 7.5 ± 0.9* | 8.3 ± 1.1* |
| Sodium excretion, μEq•min−1•g kidney wt−1 | 0.15 ± 0.04 | 0.17 ± 0.03 | 0.93 ± 0.21* | 1.25 ± 0.39* |
Values are means ± SE; n = 6 for each group of rats.
P < 0.05 compared with respective intact kidneys.
Benzamil injection produced diuresis and natriuresis from the intact and denervated kidneys in both groups of rats (Figs. 7 and 8). Both the diuresis and the natriuresis were significantly increased in CHF group compared with the corresponding sham rats after benzamil injection (P < 0.05). In the denervated kidneys, there was a significantly greater urine flow and sodium excretion compared with the intact kidney group (P < 0.05).
Fig. 7.
A: urine flow in response to benzamil injection in sham and CHF rats with intact renal nerves. B: urine flow in response to benzamil injection in sham and CHF rats with denervated kidneys. C: urine flow changes in response to benzamil injection. *P < 0.05 compared with respective sham group.
Fig. 8.
A: sodium excretion in response to benzamil injection in sham and CHF rats with intact renal nerves. B: sodium excretion in response to benzamil injection in sham and CHF rats with denervated kidneys. C: sodium excretion changes in response to benzamil injection. *P < 0.05 compared with respective sham group.
DISCUSSION
This study reports the novel findings that there is enhanced renal abundance and increased functional activity of ENaC subunits during CHF. This study demonstrated that the mRNA and protein abundance of α-, β-, and γ-subunits of ENaC were significantly increased in the cortex and outer medulla of the kidneys from rats with CHF. Immunohistochemistry microscopy confirmed the increased labeling of α-, β-, and γ-ENaC subunits in the collecting duct segments in rats with CHF. Furthermore, the diuretic and natriuretic responses to ENaC inhibitor benzamil were increased in the rats with CHF. Renal denervation unmasked a greater retentive role of ENaC in CHF. The observed increased expression of ENaC subunits associated with enhanced channel function suggests that dysregulation of renal ENaC subunits could be involved in the retention of sodium chloride and thus contribute to the pathogenesis and development of CHF.
In the present study, the CHF condition demonstrated an increased cardiac filling pressure, reduced left ventricular contractility, cardiac hypertrophy, and a large myocardial infarct over the left ventricle. These characteristics indicate that these rats were in heart failure. These rats also demonstrated an increased body weight and wet lung weight, which are indicative of peripheral and pulmonary edema. The excess water retention may be due to the limited salt and water losses during CHF.
Sodium reabsorption is carried out by various transporters that are present along the nephrons. These transporters appear to be localized to specific segments of the nephron and mediate the entry of sodium across the apical membrane. The α-, β-, and γ-subunits of ENaC are located in the distal nephron and the collecting duct (5, 31). The ENaC activity is regulated by a number of mechanisms, including aldosterone-stimulated intracellular trafficking of the ENaC α-, β-, and γ-subunits from the cytoplasm to the apical plasma membrane. Sodium transport could be expected to be proportional to the abundance of the α-ENaC protein levels. α-ENaC is sufficient to induce channel activity, whereas β- and/or γ-subunits allow for maximal expression of active sodium channels, since expression of all three subunits produces a current that is greater than a 100-fold potentiation over α-ENaC (5, 31). Although, the precise role of the β- and γ-subunits is uncertain, they appear to have a regulatory role in the stabilization and enhanced function of the channel (5, 31). The detection of abundant levels of hormone receptors and other regulatory molecules that control sodium reabsorption in the distal nephron, combined with physiological evidence (32, 40), strongly suggests that the fine control of sodium reabsorption is carried out in the distal nephron and collecting duct.
We found that all three ENaC subunits are present in the cortex and medullary collecting ducts of the kidneys. The localization of the ENaC gene in the collecting duct makes it an important candidate gene for the transport of sodium and thus its involvement in the maintenance of sodium balance and extracellular fluid volume. The results show that the mRNA and protein abundance of α-, β-, and γ-subunits of ENaC were all significantly increased in both cortex and outer medulla of kidneys in rats with CHF. Consistent with these observations, examination of channel function using the ENaC inhibitor benzamil shows an enhanced diuretic and natriuretic response in rats with CHF. The changes in diuretic and natriuretic responses are consistent with the increased protein abundance of ENaC subunits in the kidneys of rats with CHF.
A more active form of ENaC channels is formed by undergoing a process of proteolysis. Proteolysis of ENaC subunits at specific sites within the extracellular domains of α- and β-subunits plays a key role in activating the channel by increasing its open probability and releasing the inhibitory fragments (14). In the present study there was an approximately twofold increase in the expression of ENaC channels in the kidneys of CHF rat compared with the three- to fourfold increase in natriuresis after blockade of ENaC channels with benzamil. These data can be interpreted as indicating that perhaps there were greater amounts of processed forms of ENaC in the CHF condition. This may be due to increased levels of proteases, such as plasmin, a serine protease that can cleave and activate ENaC and has been implicated in the pathogenesis of various volume abnormalities and disease conditions (23, 35). This possibility of increased proteolysis of the ENaC subunits in the kidneys of rats with CHF remains to be examined.
The pathophysiology of sodium and water retention in CHF is characterized by a complex interplay of hemodynamic and neurohumoral factors. The hemodynamic factors include vasoconstriction, tachycardia, and a reduced venous capacitance. These responses occur within minutes, whereas salt and water retention occur over days to weeks. The key element involved in renal sodium retention is activation of apical ENaC in the collecting tubule by aldosterone and vasopressin. Elevated basal plasma aldosterone and vasopressin levels with increased sodium intake have been linked to essential hypertension (42) and CHF (12, 27) in humans and animal models. Chronic treatment with aldosterone increases the α-ENaC subunit primarily and the β- and γ-subunits to a lesser extent (18, 34) at mRNA and protein levels. In contrast, vasopressin has quite a different effect from that observed with aldosterone; it primarily increases the expression of β- and γ-subunits, but not α-subunit to any great extent (8, 21). Interestingly, in the current study the expression of all three subunits was increased in rats with CHF. These results are consistent with the observations that both aldosterone and vasopressin are increased in rats with CHF (27). In isolated, perfused cortical collecting tubule, aldosterone and vasopressin both stimulate sodium reabsorption individually (6), but in combination, the reabsorption is greater than with either hormone alone (6, 30). This synergistic action of aldosterone and vasopressin may be important for the large increase in ENaC expression and action/function in the rats with CHF. In addition, other humoral substances such as endothelin and catecholamines are also elevated in CHF (19, 37). It is also possible that the activation of all ENaC subunits may be due to the combination of the multiple humoral activation at the same time that is observed during CHF.
Interestingly V2 vasopressin receptor antagonists have been suggested to be useful in treating sodium and water retention in CHF (4). Certainly the inhibition of the antidiuretic action that causes the frequent development of hyponatremia in patients with CHF is the obvious benefit of V2 receptor antagonists; however, they also relieve the extreme sodium retention that accompanies cirrhosis in the rat (15). Consistent with these observations, Lütken et al. (16) have recently demonstrated an increased abundance of aquaporin 2 (AQP2) in the medullary collecting duct principle cells of rats with CHF. They further showed that this increase in expression of AQP2 was mediated by angiotensin II (ANG II) via ANG II AT1 receptors, since treatment with candesartan abrogated the increase of AQP2 in rats with CHF (16). This study also reported an increased α-ENaC protein in rats with heart failure, consistent with the observations presented in the current study. There are also reports of increased tritransporter Na+-K+-2Cl− in the thick ascending limb of the loop of Henle in rats with CHF (22). These transporters are affected by vasopressin as well as ANG II and renal nerves. In other disease conditions where there are fluid balance abnormalities, such as diabetes mellitus, there is also upregulation of ENaC subunits (33). The increase in expression of these subunits is thought to be due, at least in part, to increased aldosterone and vasopressin activity in the streptozotocin-induced model of type 1 diabetes in rats (33). These changes are required for the maintenance of extracellular fluid volume in the face of a large osmotically driven fluid loss in the diabetic condition. In summary, the influence of these regulatory factors, aldosterone, vasopressin, ANG II, and renal nerves on various segments of the nephron contribute to the retention of sodium and water in the CHF condition as in other sodium and water retentive states. Although long-term increases in circulating aldosterone concentration has little effect on ENaC abundance, it does enhance the migration of ENaC into the apical membrane (18). Thus long-term exposure to high circulating vasopressin levels increases the ENaC abundance (9). These results suggest that increased aldosterone and vasopressin in CHF may contribute to the abnormal regulation of renal tubular sodium and water transport via ENaC system.
In the heart failure condition, excessive aldosterone production can be driven by an overactivation of the renin-angiotensin system. This increase in the renin-angiotensin system is further driven by increased renal nerve activity in rats with CHF (25). It has been shown that the levels of ANG II are increased in the heart failure condition (38). ANG II has important role in the control of blood pressure and as a regulator of aldosterone secretion. In addition, Giebisch and colleagues (11, 36) also have indicated that the effects of ANG II to stimulate volume reabsorption in the late distal nephron may act via sodium channels. They found that the effects of ANG II on volume reabsorption were inhibited by amiloride, an agent that blocks sodium channels. It has been established that ANG II directly acts on ENaC in the distal nephron/collecting duct segment (2). ANG II directly stimulates ENaC activity in the cortical collecting ducts. ANG II AT1 receptor blockade with candesartan or losartan prevented the stimulatory effects of ANG II. This distal nephron activation of renin-ANG II-ENaC system may also play an important role in the renal pathophysiology of CHF. It is of interest to note that the changes reported in the present study are specific to the kidney, since expression of ENaC in the lungs of rats with CHF is not altered (17).
In the present study, renal denervation by itself caused a significantly greater urine flow and sodium excretion in both sham and CHF groups. This is consistent with previous studies (43). The neural and hormonal changes may all contribute to the renal excretory responses. There is a large body of evidence from both animal and human studies that suggest defects in the central regulation of the sympathetic nervous system may play an important role in the sodium and fluid retention commonly observed in congestive heart failure (13, 26, 39). In the kidney with intact nerves, the neural influences on the renal diuretic and natriuretic responses cannot be neglected and definitely contribute to the retention of salt and water. However, in the absence of renal nerves, the blockade of ENaC with benzamil produced significantly greater diuresis and natriuresis in rats with CHF. These data can be interpreted in two ways: 1) that there is a greater influence of ENaC-mediated sodium retention in the absence of renal nerves, regardless of CHF, since there was a greater diuresis and natriuresis after ENaC blockade in both the sham and CHF groups; and/or 2) that there is a greater influence of ENaC in rats with CHF that is unmasked by renal denervation. A prudent conclusion would be that renal denervation does unmask the retentive role of ENaC in normal and CHF conditions and, furthermore, that this role is greater in rats with CHF, since the responses to blockade of ENaC produce a significantly greater diuresis and natriuresis in rats with CHF compared with sham-operated rats. In other words, the absence of renal nerves demonstrated a greater contribution of ENaC in sodium retention in rats with CHF. Conversely, the finding that denervation does not attenuate the natriuretic response to benzamil suggests that increased renal sympathetic nerve activity per se does not cause the increase in ENaC activity in the CHF model.
In summary, the present study demonstrated that the message (mRNA) and protein abundance of ENaC subunits were increased in the cortical and outer medulla of the kidneys from rats with CHF. There were significantly greater increases in diuretic and natriuretic responses to ENaC inhibitor benzamil in the rats with CHF compared with sham-operated controls. These results suggest that the increased expression of renal ENaC subunits may contribute to the renal sodium and water retention observed during CHF. Finally, getting insight into and an understanding of the physiological characteristics of ENaC regulation in disease conditions such as CHF will contribute to the development of new diagnostic and therapeutic tools for the clinical treatment of diseases depicted by edematous states.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Abildgaard U, Andersen JS, Daugaard G, Aldershvile J, Nielsen SL, Christensen NJ, Leyssac PP. Renal function in patients with untreated acute myocardial infarction. Scand J Clin Lab Invest 52: 689–695, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Beutler K, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension 41: 1143–1150, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Blazer-Yost BL, Liu X, Helman SI. Hormonal regulation of ENaCs: insulin and aldosterone. Am J Physiol Cell Physiol 274: C1373–C1379, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Burrell LM, Risvanis J, Johnston CI, Naitoh M, Balding LC. Vasopressin receptor antagonism: a therapeutic option in heart failure and hypertension. Exp Physiol 85: 259S–265S, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Canessa CM, Shild L, Buell L, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Chen L, Williams SK, Schafer JA. Differences in synergistic actions of vasopressin and deoxycorticosterone in rat and rabbit CCD. Am J Physiol Renal Fluid Electrolyte Physiol 259: F147–F156, 1990 [DOI] [PubMed] [Google Scholar]
- 7. DiBona GF, Sawin LL. Role of renal nerves in sodium retention of cirrhosis and congestive heart failure. Am J Physiol Regul Integr Comp Physiol 260: R298–R305, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol 279: F46–F53, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Ecelbarger CA, Kim GH, Wade JB, Knepper MA. Regulation of the abundance of renal sodium transporters and channels by vasopressin. Exp Neurol 171: 227–234, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev 77: 359–396, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na+-H+ exchange and Na+/HCO3− cotransport in the rabbit proximal tubule. Proc Natl Acad Sci USA 87: 7917–7920, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gheorghiade M, Niazi I, Ouyang J, Czerwiec F, Kambayashi J, Zampino M, Orlandi C. Vasopressin V2-receptor blockade with tovaptan in patients with chronic heart failure. Circulation 107: 2690–2696, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens 16: 1979–1987, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Hughey RP, Carattino MD, Kleyman TR. Role of proteolysis in the activation of epithelial sodium channels. Curr Opin Nephrol Hypertens 16: 444–450, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Jimenez W, Gal CS, Ros J, Cano C, Cejudo P, Morales-Ruiz M, Arroyo V, Pascal M, Rivera F, Maffrand JP, Rodes J. Long-term aquaretic efficacy of a selective nonpeptide V2-vasopressin receptor antagonist, SR121463, in cirrhotic rats. J Pharmacol Exp Ther 295: 83–90, 2000 [PubMed] [Google Scholar]
- 16. Lütken SC, Kim SW, Jonassen T, Marples D, Knepper MA, Kwon TH, Frøkiaer J, Nielsen S. Changes of renal AQP2, ENaC, and NHE3 in experimentally induced heart failure: response to angiotensin II AT1 receptor blockade. Am J Physiol Renal Physiol 297: F1678–F1688, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maron MB, Luther DJ, Pilati CF, Ohanyan V, Li T, Koshy S, Horne WI, Meszaros JG, Walro JM, Folkesson HG. Adrenoceptor stimulation of alveolar fluid clearance is increased in rats with heart failure. Am J Physiol Lung Cell Mol Physiol 297: L487–L495, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McConnell PI, Olson CE, Patel KP, Blank DU, Olivari MT, Gallagher KP, Quenby-Brown E, Zucker IH. Chronic endothelin blockade in dogs with pacing-induced heart failure: possible modulation of sympathoexcitation. J Card Fail 6: 56–65, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Navas JP, Martinez-Maldonado M. Pathophysiology of edema in congestive heart failure. Heart Dis Stroke 2: 325–329, 1993 [PubMed] [Google Scholar]
- 21. Nicco C, Wittner M, Di Stefano A, Jounier S, Bankir L, Bouby N. Chronic exposure to vasopressin upregulates ENaC and sodium transport in the rat renal collecting duct and lung. Hypertension 38: 1143–1149, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Nogae S, Michimata M, Kanazawa M, Honda S, Ohta M, Imai Y, Ito S, Matsubara M. Cardiac infarcts increase sodium transporter transcripts (rBSC1) in the thick ascending limb of Henle. Kidney Int 57: 2055–2063, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Passero CJ, Hughey RP, Kleyman TR. New role for plasmin in sodium homeostasis. Curr Opin Nephrol Hypertens 19: 13–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel KP, Kline RL. Influence of renal nerves on noradrenergic responses to changes in arterial pressure. Am J Physiol Regul Integr Comp Physiol 247: R615–R620, 1984 [DOI] [PubMed] [Google Scholar]
- 25. Patel KP, Zhang K, Carmines PK. Norepinephrine turnover in peripheral tissues of rats with heart failure. Am J Physiol Regul Integr Comp Physiol 278: R556–R562, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Patel KP, Zhang PL, Carmines PK. Neural influences on renal responses to acute volume expansion in rats with heart failure. Am J Physiol Heart Circ Physiol 271: H1441–H1448, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Pedersen EB, Danielsen H, Jensen T, Madsen M, Sorensen SS, Thomsen OO. Angiotensin II, aldosterone and arginine vasopressin in plasma in congestive heart failure. Eur J Clin Invest 16: 56–60, 1986 [DOI] [PubMed] [Google Scholar]
- 28. Rao US, Mehdi A, Steimer RE. Expression of amiloride-sensitive sodium channel: a strategy for the coexpression of multimeric membrane protein in Sf9 insect cells. Anal Biochem 286: 206–213, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Rasool A, Palevsky PM. Treatment of edematous disorders with diuretics. Am J Med Sci 319: 25–37, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Reif MC, Troutman SL, Schafer JA. Sodium transport by rat cortical collecting tubule. Effects of vasopressin and desoxycorticosterone. J Clin Invest 77: 1291–1298, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rossier BC, Canessa CM, Schild L, Horisberger JD. Epithelial sodium channels. Curr Opin Nephrol Hypertens 3: 487–496, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Schafer JA, Hawk CT. Regulation of Na+ channels in the cortical collecting duct by AVP and mineralocorticoids. Kidney Int 41: 255–268, 1992 [DOI] [PubMed] [Google Scholar]
- 33. Song J, Knepper MA, Verbalis J, Ecelbarger CA. Increased renal ENaC subunit and sodium transporter abundances in streptozotocin-induced type I diabetic rats. Am J Physiol Renal Physiol 285: F1125–F1137, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Stokes JB, Sigmund RD. Regulation of rENaC mRNA by dietary NaCl and steroids: organ, tissue, and steroid heterogeneity. Am J Physiol Cell Physiol 274: C1699–C1707, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteris S, Krueger B, Stubbe J, Jensen ON, Thiesson HC, Uhrenholt TR, Jespersen B, Jensen BL, Korbmacher C, Skott O. Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol 20: 299–310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F143–F149, 1996 [DOI] [PubMed] [Google Scholar]
- 37. White CM. Catecholamines and their blockade in congestive heart failure. Am J Health Syst Pharm 55: 676–682, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Wollert KC, Drexler H. The renin-angiotensin system and experimental heart failure. Cardiovasc Res 43: 838–849, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Wyss JM, Carlson SH. The role of the nervous system in hypertension. Curr Hypertens Rep 3: 255–262, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Zeidel ML. Renal actions of atrial natriuretic peptide: regulation of collecting duct sodium and water transport. Annu Rev Physiol 52: 747–759, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol 282: R1006–R1015, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Zhang X, Hensen HW, Riegger GA, Schunkert H. Association of arginine vasopressin and arterial blood pressure in a population-based sample. J Hypertens 17: 319–324, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Zheng H, Li YF, Zucker IH, Patel KP. Exercise training improves renal excretory responses to acute volume expansion in rats with heart failure. Am J Physiol Renal Physiol 291: F1148–F1156, 2006 [DOI] [PubMed] [Google Scholar]