Abstract
The P2Y2 receptor (P2Y2-R) antagonizes sodium reabsorption in the kidney. Apart from its effect in distal nephron, hypothetically, P2Y2-R may modulate activity/abundances of sodium transporters/channel subunits along the nephron via antagonism of aldosterone or vasopressin or interaction with mediators such as nitric oxide (NO), and prostaglandin E2 (PGE2) or oxidative stress (OS). To determine the extent of the regulatory role of P2Y2-R in renal sodium reabsorption, in study 1, we fed P2Y2-R knockout (KO; n = 5) and wild-type (WT; n = 5) mice a high (3.15%)-sodium diet (HSD) for 14 days. Western blotting revealed significantly higher protein abundances for cortical and medullary bumetanide-sensitive Na-K-2Cl cotransporter (NKCC2), medullary α-1-subunit of Na-K-ATPase, and medullary α-subunit of the epithelial sodium channel (ENaC) in KO vs. WT mice. Molecular analysis of urine showed increased excretion of nitrates plus nitrites (NOx), PGE2, and 8-isoprostane in the KO, relative to WT mice, supporting a putative role for these molecules in determining alterations of proteins involved in sodium transport along the nephron. To determine whether genotype differences in response to aldosterone might have played a role in these differences due to HSD, in study 2 aldosterone levels were clamped (by osmotic minipump infusion). Clamping aldosterone (with HSD) led to significantly impaired natriuresis with elevated Na/H exchanger isoform 3 in the cortex, and NKCC2 in the medulla, and modest but significantly lower levels of NKCC2, and α- and β-ENaC in the cortex of KO vs. WT mice. This was associated with significantly reduced urinary NOx in the KO, although PGE2 and 8-isoprostane remained significantly elevated vs. WT mice. Taken together, our results suggest that P2Y2-R is an important regulator of sodium transporters along the nephron. Pre- or postreceptor differences in the response to aldosterone, perhaps mediated via prostaglandins or changes in NOS activity or OS, likely play a role.
Keywords: purinergic signaling, extracellular nucleotides, urinary concentration, arginine vasopressin
signaling via the P2Y2 receptor (P2Y2-R), by virtue of its ability to antagonize the actions of arginine vasopressin (AVP) and aldosterone, regulates water and sodium absorption in the distal nephron (20, 22, 23, 37, 46, 49, 50). Besides these distal sites, P2Y2-R is expressed to varying degrees in several segments of proximal nephron, such as the proximal convoluted tubule, and the loop of Henle (2, 6, 29, 32, 44), which play critical roles in urinary concentration. Accordingly, our recent studies and those conducted by Vallon and associates documented that signaling through P2Y2-R plays an overarching role in balancing the effect of AVP on the urinary concentration mechanism (24, 36, 54). This overarching role is uncovered in P2Y2-R knockout (KO) mice as increased sensitivity of the kidney to circulating AVP levels, resulting in significantly increased protein abundances of the aquaporin-2 (AQP2) water channel, Na-K-2Cl cotransporter (NKCC2), and UT-A isoforms of the urea transporter in the medulla.
The role of P2Y2-R-mediated signaling in antagonizing the actions of aldosterone by inhibiting amiloride-sensitive sodium transport via the apical epithelial sodium channel (ENaC) in the distal nephron is well documented (22, 23, 30, 31, 38, 50). However, a role for P2Y2-R in the regulation of thiazide-sensitive Na-Cl cotransporter (NCC), another aldosterone-stimulated protein, has not been reported. On the other hand, we (35) and others (47) have demonstrated decreased expression of NCC as a major regulatory change associated with the combination of aldosterone infusion and a high-NaCl diet (the aldosterone-escape model), compared with rats infused with aldosterone, but maintained on a low-NaCl diet. We have also demonstrated reduced medullary protein levels of NKCC2, increased eNOS (endothelial nitric oxide synthase) protein, and over a fivefold increase in urinary NOx (nitrates plus nitrites excretion) in these high-NaCl/aldosterone-infused rats (35). An increase in endothelial-derived nitric oxide (NO) production is known to inhibit expression of major sodium transporters, such as the sodium/hydrogen exchanger isoform 3 (NHE3), NKCC2, NCC, and Na- K-ATPase (19). Taken together, these findings suggest that the natriuresis of aldosterone escape is accompanied by an increase in medullary NO production, perhaps leading to decreased expression/activity of NKCC2 and NCC. Furthermore, it has been shown that cyclooxygenase (COX) inhibitors enhance urinary concentration ability by increasing the protein abundance of NKCC2 (14). Thus both NO and prostaglandin E2 (PGE2) modulate the expression and/or activity of sodium transporters/channels (14, 19). Also, interestingly, P2Y2-R is known to interact with both NO and prostanoid systems (8, 15, 20), thus potentially expanding the ability of purinergic signaling to regulate major sodium transporters/channels along the nephron by altering NO and prostanoid production, independently of its direct regulatory effect on ENaC in the distal nephron. Finally, a growing body of evidence suggests that the damaging effects of aldosterone excess on the kidney and cardiovascular system, particularly in the presence of high salt intake, are mediated through its ability to induce “oxidative stress,” inflammatory, and fibrotic processes (4). Therefore, if P2Y2-R is involved in attenuating aldosterone actions it might also hypothetically affect renal oxidative stress.
In view of the above, we hypothesized that P2Y2-R, by virtue of its known interactions with NO and prostanoid systems, may influence the expression of sodium transporters/channels along the nephron induced by a high-sodium diet with/without aldosterone infusion. Hence, to address this hypothesis, in this study we determined the protein abundances of sodium transporters or channels in the cortex and medulla of P2Y2-R KO and wild-type (WT) mice in two different series of experiments, namely, high-sodium diet feeding for 14 days, or high-sodium diet feeding for 4 days in the background of aldosterone infusion. Furthermore, to probe the mechanisms underlying P2Y2-R-mediated effects, we evaluated the production of NO and PGE2 by assaying their urinary content and/or by determining the renal expression of enzymes involved in their biosynthesis, namely, NOS and COX isoforms. Finally, to uncover any potential link between P2Y2-R and oxidative stress, we determined the urinary excretion of 8-isoprostane (8-iso prostaglandin F2α), a marker of oxidative stress (33).
METHODS
Experimental Animals
The animal protocols describing the procedures in this study were approved by the Institutional Animal Care and Use Committees of the Veterans Affairs Salt Lake City Health Care System and the University of Utah. We obtained breeders of P2Y2-R gene KO and the corresponding WT mice from Dr. Beverly Koller (University of North Carolina at Chapel Hill, Chapel Hill, NC). These mice were generated by targeted mutagenesis of the P2Y2-R gene in mouse embryonic stem cells. Chimeric mice thus generated were bred into a genetic background of B6D2 (7, 16). Breeding and genotyping of the mice were performed as described previously (54).
Study Protocols
Two studies were conducted. In the first study, we examined the effect of high-sodium (3.51% Na) diet in WT and P2Y2-R KO mice. Groups of WT and P2Y2-R KO mice were fed a high-sodium diet (n = 5 mice/genotype) for 14 days and then euthanized. The high-sodium diet was fed as dry chow pellets (TestDiets, Richmond, IN), with ad libitum access to drinking water. In the second study, we evaluated the effect of a high-sodium diet in a background of aldosterone infusion in WT and P2Y2-R KO mice. Briefly, after collection of baseline 24-h urine samples, on day −3 under isofluorane anesthesia groups of WT and P2Y2-R KO mice (n = 5 mice/genotype) were subcutaneously implanted with osmotic minipumps (Alzet model 1002; Durect, Cupertino, CA) preloaded with aldosterone (Sigma, St. Louis, MO). Aldosterone was delivered throughout the experimental period at a rate of 20 μg/day. Immediately following the implantation of the minipumps, the mice were fed a low-sodium diet (0.03% Na; TestDiets) for 3 days (days −3 to 0). On day 0, all the mice were switched to a high-sodium diet that continued for another 4 days (days 0 to +4) before euthanasia on day +4. Throughout the experimental period, all mice had ad libitum access to drinking water.
Sample Collection and Analysis
Twenty-four-hour consumption of food and water, urine output, and urine osmolality were monitored by placing the mice in individual plastic metabolic cages. At the end of the experimental periods, the mice were euthanized and blood was collected. Kidneys were removed, and cortices and medullas were dissected out, flash frozen in liquid nitrogen, and then stored at −80°C for analysis. Aliquots of urine samples were centrifuged to obtain clear supernatants. Osmolality of the clear urine supernatants and serum were determined by the vapor pressure method (Wescor, Logan, UT). Urinary sodium levels were measured on an AVL 9180 Electrolyte Analyzer (AVL Scientific, Roswell, GA). Total nitrate/nitrite contents of urine samples were determined by a commercial colorimetric kit (Cayman Chemical, Ann Arbor, MI). Urinary excretion of PGE2 was determined using a prostaglandin E metabolite EIA kit (Cayman Chemical) as described previously (56, 57). Urinary 8-isoprostane was quantified by an EIA kit from Oxford Biomedical Research (Oxford, MI).
Western Blot Analysis of Tissue Samples
Cortical and medullary tissue samples were processed separately, and Western blotting was performed by the methods established in our laboratories (10, 43, 45). Briefly, samples were prepared by homogenizing the frozen tissues in a buffer containing protease inhibitors. After determination of the protein concentrations, the homogenates were solubilized in Laemmli sample buffer. The quality of tissue sample preparation was assessed by staining loading gels with Coomassie blue (Gelcode Blue, Pierce Endogen, Rockford, IL) and then examining the sharpness of the bands. To assess the alterations in the protein abundances of different sodium transporters, semiquantitative immunoblotting was performed. For immunoblotting, 10–30 μg of protein from each sample was loaded into individual lanes of minigels of 7, 10, or 12% polyacrylamide (precast, Bio-Rad, Hercules, CA). Blots were probed with our own rabbit peptide-derived polyclonal antibodies against NHE3, NKCC2, NCC, sodium-phosphate cotransporter (NaPi-2), and the three subunits of ENaC (α, β, and γ), as previously described (39). We used a commercial monoclonal antibody against the α-1 subunit of Na-K-ATPase (Millipore, Temecula, CA). Loading accuracy was evaluated by reprobing nitrocellulose membranes with β-actin monoclonal antibody (Sigma).
Quantitative Real-Time RT-PCR Assay of Tissue Samples
These were performed as per the methods established in our laboratory (52, 53). Briefly, total RNA from cortical or whole medullary tissue samples was extracted by the TRIzol method (Invitrogen, Carlsbad, CA), and traces of genomic DNA were removed. Clean RNA samples were processed by SuperScript Reverse Transcriptase II (Invitrogen) to obtain cDNA samples. cDNAs for target genes were quantified by real-time amplifications in the Applied Biosystems 7500 Real-Time PCR system (Foster City, CA) with AmpliTaq gold, and SYBR Green was used for detection. cDNAs were amplified for 40 cycles. Table 1 shows the sequences of the primers, annealing temperatures, and amplicon sizes. Specificity of amplifications was assessed by sequencing the PCR products in the DNA core facility at the University of Utah, and blasting them in the National Center for Biotechnology Information (NCBI) nucleotide database. Expression of target genes was computed relative to the expression levels of the housekeeping gene β-actin.
Table 1.
Nucleotide sequences of primer pairs used in PCR
| Gene | Accession No. | Primer Position | Primer Sequence | Annealing Temperature, °C | Amplicon Size, bp | Reference Source |
|---|---|---|---|---|---|---|
| iNOS | NM_010927.2 | 2563–2582 | ACTGTGTGCCTGGAGGTTCT | 60 | 176 | 17 |
| 2720–2739 | TCTCTGCCTATCCGTCTCGT | |||||
| eNOS | NM_008713.3 | 513–533 | GAGAGCGAGCTGGTGTTTG | 60 | 188 | 17 |
| 682–701 | TGATGGCTGAACGAAGATTG | |||||
| β-Actin | NM_007393.2 | 1034–1054 | GCTCTGGCTCCTAGCACCATG | 60 | 73 | 17 |
| 1089–1108 | GCCACCGATCCACACAGAGT | |||||
| COX-1 | NM_008969.3 | 1406–1429 | ACTGGTGGATGCCTTCTCTC | 60 | 150 | Designed by us |
| 1536–1556 | GCCAAACCTCTTTCGGTATTC | |||||
| COX-2 | NM_011198.3 | 1389–1408 | TACAAGCAGTGGCAAAGGCC | 60 | 282 | 3 |
| 1692–1671 | CAGTATTGAGGAGAACAGATGG |
iNOS and eNOS, inducible and endothelial nitric oxide synthase, respectively; COX, cyclooxygenase.
Statistical Analysis
Quantitative data are expressed as means ± SE. Differences between the means of two groups were determined by an unpaired or paired t-test. P values <0.05 were considered significant.
RESULTS
Effect of a High-Sodium Diet
Water intake and urine parameters.
The body weights of WT and P2Y2-R KO mice used in the this study were 25.5 ± 0.2 vs. 22.0 ± 0.2 g, respectively, which were significantly different (P < 0.001 by unpaired t-test). Hence, the urinary parameters in each mouse, except for urine osmolality, were normalized to 20 g body wt before determination of the group mean values. In both WT and P2Y2-R KO mice, water intake and urine output increased and urine osmolality decreased as a function of time (Fig. 1). Water intake on days 3, 6, and 14, and urine output on day 14, were modestly, but significantly higher in the P2Y2-R KO mice compared with the WT mice. No genotype differences in urine osmolalities were observed with a high-sodium diet.
Fig. 1.

Effect of feeding a high-sodium diet on water intake (A), urine output (B), and urine osmolality (C) in wild-type (WT) and P2Y2 receptor (P2Y2-R) knockout (KO) mice. Groups of WT and P2Y2-R KO mice (n = 5 mice/genotype) were fed a high-sodium diet (3.51% Na) for 14 days. Mice were placed in metabolic cages to monitor water intake and urine output. Mean values obtained on days 0, 1, 3, 6, and 14 of the experimental period are presented. To compensate for differences in body weight of individual mice, the values of water intake and urine output were adjusted to 20 g body wt. *P < 0.05 compared with the corresponding time point in WT mice by unpaired t-test.
Sodium exchanger, transporter, and channel subunit expression.
Figure 2A shows immunoblots from WT and P2Y2-R KO mice fed a high-sodium diet. Figure 2B summarizes the densitometry values showing changes in sodium transporter/channel protein abundances in the cortex and medulla in P2Y2-R KO mice, relative to their respective WT controls. When fed a high-sodium diet, the protein abundances of NKCC2 in the cortex and medulla of P2Y2-R KO mice were significantly increased compared with WT mice. P2Y2-R KO mice also showed significant increases in protein abundances of α-ENaC and α-1-Na-K-ATPase in the medulla compared with the WT mice. NCC abundance was not regulated differentially between the genotypes under high sodium intake.
Fig. 2.
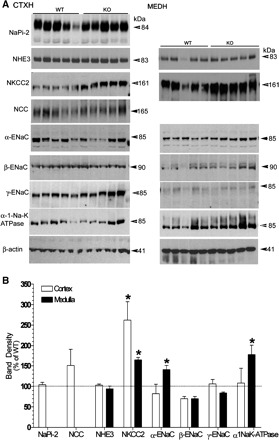
A: immunoblots for different sodium transporter and channel proteins in WT and P2Y2-R KO mice fed a high-sodium diet for 14 days (n = 5 mice/genotype). Blots in the left column correspond to cortical tissue samples, and those in the right column to medullary tissue samples. NaPi-2, NHE3, NKCC2, NCC, ENaC: Na-Pi type 2 cotransporter; sodium/phosphate exchanger isoform 3; Na-K-2Cl cotransporter; and epithelial Na channel, respectively. B: bar graph summary of the densitometric analysis of the immunoblots shown in A. Bars show percent change in band densities of different proteins in the cortex and medulla of P2Y2-R KO mice compared with the corresponding values in WT mice (100%). *Significant change (P < 0.05) in the protein band density in P2Y2-R KO mice vs. WT mice by unpaired t-test.
Urinary excretion of NO3/NO2 and PGE2.
Figure 3A shows urinary excretion of total nitrates/nitrites, a measure of NO production, in WT and P2Y2-R KO mice before (day 0) and after 14 days of high-sodium diet feeding (day 14). As shown, both WT and P2Y2-R KO mice showed marked increases in urinary nitrates/nitrates following high-sodium diet feeding. However, the increase in P2Y2-R KO mice was significantly higher than in WT mice. Figure 3B shows significant differences between WT and P2Y2-R KO mice in urinary excretion of PGE2 following 14 days of a high-sodium diet. As shown, both genotypes had significant elevations in urinary PGE2 excretion from their respective baseline (day 0) values. However, the mean urinary excretion of PGE2 in P2Y2-R KO mice on day 14 was about twofold higher compared with WT mice. Figure 3C shows urinary 8-isoprostane levels on days 0 and 14. Day 0 isoprostane levels, similar to day 0 PGE2 levels, were slightly higher in P2Y2-R KO mice vs. WT mice. However, following high-sodium diet feeding, P2Y2-R KO mice had about a threefold increase in the urinary 8-isoprostane. The modest increase in 8-isoprostane levels in WT mice on day 14 was not statistically significant from day 0 value.
Fig. 3.
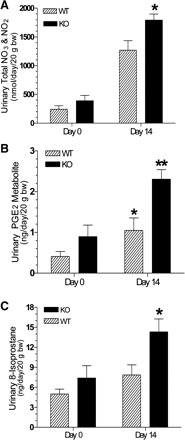
Effect of feeding a high-sodium diet for 14 days on urinary excretion of total nitrates (NO3) and nitrites (NO2), prostaglandin E2 (PGE2) metabolite, and 8-isoprostane in WT and P2Y2-R KO mice (n = 5 mice/genotype). A: urinary excretion of total NO3/NO2 (nmol·day−1·20 g body wt−1) on days 0 and 14. *Significantly different from the corresponding day value in WT mice (P < 0.02 by unpaired t-test). B: urinary excretion of PGE2 metabolite expressed as ng·day−1·20 g body wt−1. *Significantly different from corresponding day 0 value (P < 0.05 by paired t-test). **Significantly different from corresponding day 0 value (P < 0.01 by paired t-test) and day 14 value in WT mice (P < 0.01 by unpaired t-test). C: urinary excretion of 8-isoprostane expressed as ng·day−1·20 g body wt−1 on days 0 and 14. *Significantly different from the corresponding day value in WT mice (P < 0.02 by unpaired t-test).
Combined Effect of a High-Sodium Diet and Aldosterone Infusion
Water intake, urine parameters, and sodium.
The mean body weights of WT and P2Y2-R KO mice used in this study were not significantly different (20.0 ± 0.4 vs. 19.4 ± 0.5 g). Water intake, urine output, and urine osmolality in both WT and P2Y2-R KO mice were within normal limits and did not differ during the low-sodium period (data not shown). However, when the diet was switched to high sodium, both genotypes showed time-dependent increases in water intake and urine osmolality, associated with a decrease in urine osmolality. There were no significant differences between the genotypes (data not shown). However, when daily urinary excretion of sodium was monitored, significant differences between the two genotypes were noted toward the end of the experimental period. As shown in Fig. 4A, on days +3 and +4, the urinary excretion of sodium was significantly higher in WT, compared with P2Y2-R KO mice. Figure 4B shows the differences between genotypes in sodium excretion. During the low-sodium diet feeding, the differences were very low and were close to zero, indicating that both WT and P2Y2-R KO mice were able to excrete sodium to the same extent. However, after the switch to the high-sodium diet, the P2Y2-R KO mice showed significant impairment in their ability to excrete sodium from day +2 onward. The differences on days +3 and +4 were marked and were negative, indicating that P2Y2-R KO mice were not able to excrete sodium as efficiently as the WT mice.
Fig. 4.
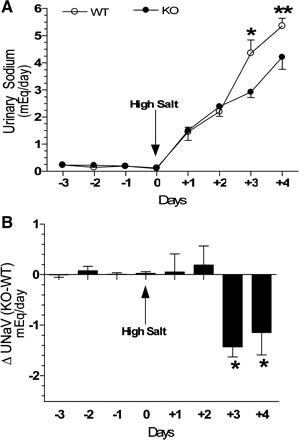
Effect of combination of a high-sodium diet and aldosterone infusion on urinary sodium excretion in WT and P2Y2-R KO mice. Groups of WT and P2Y2-R KO mice (n = 5 mice/genotype) were subcutaneously infused with aldosterone (20 μg/day) starting from day −3 and were fed a low-salt diet (0.03% Na) for 3 days. On day 0, the mice were switched to a high-salt (3.51% Na) diet and maintained on that diet 4 more days. All mice had ad libitum access to drinking water throughout the experimental period. A: daily urinary excretion of sodium in WT and P2Y2-R KO mice (mEq). On days +3 and +4, the daily urinary sodium excretion in WT mice was significantly higher compared with the P2Y2-R KO mice (*P < 0.02 and **P < 0.04 by unpaired t-test). B: differences in urinary excretion of sodium between the 2 genotypes by subtracting the mean values obtained in WT mice from the individual values obtained in P2Y2-R KO mice and then computing the means ± SE. *Significantly different from day +2 values (P < 0.03 or better by unpaired t-test).
Sodium exchanger, transporter, and channel subunit expression.
Figure 5A shows immunoblots from WT and P2Y2-R KO mice fed high-sodium diet in a background of aldosterone infusion. Figure 5B summarizes the densitometry values showing changes in sodium transporter and channel subunit protein abundances in the cortex and medulla in the P2Y2-R KO mice, relative to their respective WT controls. In the cortex, P2Y2-R KO mice had significantly higher levels of NHE3, but significantly lower levels of NKCC2 and α- and β-ENaC. In the medulla, we found significantly elevated NKCC2 in P2Y2-R KO (similar to what was observed with the high-sodium diet alone series). The protein abundances of no other transporter/ channel subunits shown were different between genotypes.
Fig. 5.

A: immunoblots for different sodium transporter and channel proteins in WT and P2Y2-R KO mice fed a high-sodium diet in a background of aldosterone infusion (n = 5 mice/genotype). Blots in the left column correspond to cortical tissue samples, and those in the right panel to medullary tissue samples. B: bar graph summary of the densitometric analysis of the immunoblots shown in A. Bars show percent change in band densities of different proteins in the cortex and medulla of P2Y2-R KO mice compared with the corresponding values in WT mice (100%). *Significant change (P < 0.05) in the protein band density in P2Y2-R KO mice vs. WT mice by unpaired t-test.
Urinary excretion of NO3/NO2 and expression of NOS isoforms in the kidney.
As shown in Fig. 6A, urinary excretion of NO3/NO2 on days −3 and 0 were low in WT and P2Y2-R KO mice and were not significantly different from each other. However, on day +4, the WT mice had a several-fold increase in the urinary excretion of nitrates/nitrites. The corresponding increase in P2Y2-R KO mice was significantly less. To gain insights into the probable cause for this observed difference on day +4, we determined the mRNA expression of inducible and endothelial NOS isoforms (iNOS and eNOS) relative to the mRNA expression of β-actin in the cortex and medulla of these two groups of mice. As shown in Fig. 6B, the relative expression of both NOS isoforms in the renal cortex was significantly lower in the P2Y2-R KO compared with the WT mice. Similarly, in the medulla the mRNA expression of iNOS was significantly lower in P2Y2-R KO, whereas the expression of eNOS showed a tendency for lower mean value, but was not significantly different (Fig. 6C).
Fig. 6.
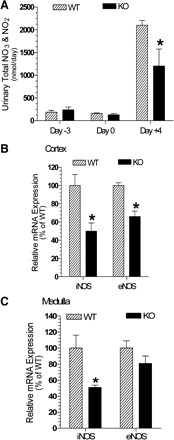
Effect of combination of a high-sodium diet and aldosterone infusion on urinary excretion of total NO3 and NO2 and relative mRNA expression of nitric oxide synthase (NOS) isoforms in the renal cortex and medulla of WT and P2Y2-R KO mice. A: urinary excretion of total NO3/NO2 as nmol/day on days −3, 0, and +4. *Significantly different from the corresponding value in WT mice (P < 0.05 by unpaired t-test). B: mRNA expression of NOS isoforms relative to the expression of β-actin in the cortex of WT and P2Y2-R KO mice on day +4. Results are plotted as percentage of respective mean values in WT mice (100%). *Significantly different from the corresponding value in WT mice (P < 0.01 unpaired t-test). C: mRNA expression of NOS isoforms relative to the expression of β-actin in the medulla of WT and P2Y2-R KO mice on day +4. Results are plotted as percentage of respective mean values in WT mice (100%). *Significantly different from the corresponding value in WT mice (P < 0.01 by unpaired t-test).
Urinary excretion of PGE2 and expression of COX isoforms in the kidney.
As shown in Fig. 7A, urinary excretion of PGE2 on days −3 and 0 were low in both WT and P2Y2-R KO mice and were not significantly different from each other. However, on day +4, both WT and P2Y2-R KO mice had a several-fold increase in the urinary excretion of PGE2. The increase in P2Y2-R KO mice was significantly higher than that in the WT mice. To gain insight into the probable cause for this observed difference on day +4, we determined the mRNA expression of COX-1 and COX-2 isoforms relative to the mRNA expression of β-actin in the cortex and medulla of these two groups of mice. As shown in Fig. 7B, relative expression of COX-2 in the cortex showed a significant twofold increase in the P2Y2-R KO mice compared with the WT mice. In addition, COX-1 expression in the cortex was modestly, but significantly lower in the P2Y2-R KO mice vs. the WT mice. No significant differences between the WT and P2Y2-R KO mice could be seen with respect to the relative expression of COX isoforms in the medulla (Fig. 7C).
Fig. 7.

Effect of combination of a high-sodium diet and aldosterone infusion on urinary excretion of PGE2 metabolite and relative mRNA expression of cyclooxygenases (COX) in the renal cortex and medulla of WT and P2Y2-R KO mice. A: urinary excretion of PGE2 metabolite on days −3, 0, and +4 (ng/day). *Significantly different from the corresponding value in WT mice (P < 0.05 by unpaired t-test). B: mRNA expression of COX-1 relative to the expression of β-actin in the cortex of WT and P2Y2-R KO mice on day +4. Results are plotted as percentage of respective mean values in WT mice (100%). *Significantly different from the corresponding value in WT mice (P < 0.01 by unpaired t-test). C: mRNA expression of COX-2 relative to the expression of β-actin in the medulla of WT and P2Y2-R KO mice on day +4. Results are plotted as percentage of respective mean values in WT mice (100%). *Significantly different from the corresponding value in WT mice (P < 0.02 by unpaired t-test).
Urinary excretion of 8-isoprostane.
Figure 8 shows urinary levels of 8-isoprostane in WT and P2Y2-R KO mice on days −3 and +4. As shown, the urinary 8-isoprostane levels in both groups were low on day −3, but the P2Y2-R KO mice had significantly higher levels compared with the WT mice. However, on day +4 the P2Y2-R KO mice had a marked increase, whereas the corresponding increase in WT mice is lower, resulting in about a twofold higher urinary excretion of 8-isoprostane in P2Y2-R KO mice vs. WT mice on day +4.
Fig. 8.
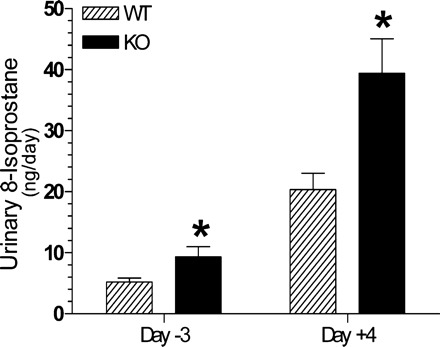
Effect of combination of a high-sodium diet and aldosterone infusion on urinary excretion of 8-isoprostane (ng/day) in WT and P2Y2-R KO mice. Result shown are for days −3 and +4. *Significantly different from the corresponding day value in WT mice (P < 0.04 for day −3 and P < 0.02 for day +4 by unpaired t-test).
DISCUSSION
Several studies have already documented dietary sodium- and/or aldosterone-induced alterations in the expression and/or protein abundances of sodium transporters in the kidney (reviewed in Ref. 21). Hence, the major focus of the current study was to evaluate the role and extent of contribution of signaling through P2Y2-R for the alterations induced in the kidney by a high-sodium diet or a high-sodium diet plus aldosterone infusion. The use of P2Y2-R KO mice, with WT mice serving as controls, allowed us to evaluate the role of P2Y2-R independently of other variables, such as sodium intake or plasma aldosterone levels in the two series of experiments. Our study addressed three questions: 1) what is the effect of genetic deletion of P2Y2-R on the alterations in sodium transporter/channel expression induced by a high-sodium diet?; 2) what is the effect of genetic deletion of P2Y2-R on the alterations in natriuresis and sodium transporter/channel expression induced by a high-sodium diet plus aldosterone?; and 3) does genetic deletion of P2Y2-R influence the effect of NO and prostanoid systems or oxidative stress vis-à-vis natriuresis and sodium transporter/channel expression in the above two models? Our study revealed that genetic deletion of P2Y2-R had significant effects in both series of experiments, thus providing insights into the potential role of this receptor in maintaining sodium homeostasis. First, these two experimental conditions resulted in significant alterations in the protein abundances of some, but not all, major sodium transporters/channels/exchangers along the nephron in the P2Y2-R KO mice vs. WT mice. Second, when fed a high-sodium diet in the background of aldosterone infusion, P2Y2-R KO mice had impaired natriuresis compared with WT mice. Third, further probing in both series revealed significant differences in the urinary excretion of NO, PGE2, and 8-isoprostane in P2Y2-R KO mice vs. WT mice, suggesting that these signaling factors may play an important role, and genetic deletion of P2Y2-R had a profound influence on these effects. Finally, our study provided significant mechanistic insights by documenting changes in the expression of enzymes involved in the biosynthesis of NO or PGE2 in the kidneys of P2Y2-R KO mice vs. WT mice fed a high-sodium diet in the background of aldosterone infusion. Taken together, these observations underscore the potential sodium-homeostatic value of P2Y2-R, especially in sodium-retentive states, such as a high-sodium diet with/without high aldosterone.
Effect of a High-Sodium Diet
We challenged P2Y2-R KO and WT mice with a high-sodium diet for 14 days. The salient finding of this study was markedly increased protein abundance of NKCC2 in the cortex and medulla, as well as α-1-Na-K-ATPase in the medulla of P2Y2-R KO mice compared with the WT mice. In normal animals, the expression and activity of NKCC2 is regulated by AVP via its V2 receptor and the associated cAMP signaling pathway (11, 12). Agonist activation of P2Y2-R is known to antagonize the action of AVP by stimulating phospholipases and protein kinase C among other pathways (20, 37). It is possible that genetic deletion of P2Y2-R resulted in sensitization of the thick ascending limb to the action of AVP in the P2Y2-R KO mice fed a high-salt diet, resulting in increased protein abundance of NKCC2 compared with high-salt diet-fed WT mice. This, coupled with the significantly increased protein abundance of α-1-Na-K-ATPase in the medulla, might result in increased sodium reabsorption in the medullary thick ascending limb in the P2Y2-R KO mice.
Another important finding in our high-sodium diet series was significantly increased protein abundances of the α-ENaC subunit in the medulla of P2Y2-R KO mice. This may contribute to increased apical sodium reabsorption in the distal nephron. Aldosterone has been demonstrated to increase the abundance of this protein (27). Thus increased levels of this protein might be manifested as a result of altered sensitivity to aldosterone in the P2Y2-R KO mice. On the other hand, vasopressin has also been documented to increase the protein abundance and mRNA expression of the β- and γ-subunits of ENaC (9, 10). However, we did not see significant alterations in these subunits in the high-sodium diet-fed P2Y2-R KO mice in this study.
Thus our findings are, for the most part, in agreement with those of Vallon and associates (36) using short-period (2 h) pharmacological blockade in conscious P2Y2-R P2Y2-R KO and WT mice receiving a standard NaCl diet, who showed that furosemide, but not chlorthiazide or low-dose amiloride, resulted in significantly higher urine sodium excretion in P2Y2-R KO mice compared with the WT mice. However, this does not exclude the possibility of P2Y2-R-mediated fine-tuning of amiloride-sensitive sodium absorption in the distal nephron, especially when confronted with a high-sodium diet.
Effect of a High-Sodium Diet Plus Aldosterone
Since a high-sodium diet is usually accompanied by a fall in circulating levels of aldosterone, in the second series we sought to separate the effects due to differences in circulating aldosterone levels from all responses downstream of this, including differences in mineralocorticoid receptor sensitivity and/or ability to “escape” from the sodium-retaining actions of aldosterone (35, 47). This experimental protocol was designed to separate primary effects of aldosterone from the escape process by initiating the aldosterone infusion 3 days before a switch from a low-sodium diet to a relatively high-sodium intake. During the initial 3-day period, the high level of circulating aldosterone resulting from the infusion was appropriate for the low level of sodium intake. Only after the sodium intake was increased at day 0 there was a stimulus for the escape process (47). We used a dose approximately equivalent to what Knepper et al. or Riazi et al. used in rats (200 μg/day) (35, 47) based on direct body size comparisons, but lower than what Knepper or Riazi used when translated from rat to mouse species by computation based on body surface area and Km factor for these two species (34). However, mice in general have much lower plasma aldosterone levels compared with rats (1, 13, 18, 26). Thus we felt the dose was clearly adequate to increase sodium retention via aldosterone-sensitive pathways.
After the mice were switched to a high-sodium diet, during the first 2 days both WT and P2Y2-R KO mice had comparable increases in urinary excretion of sodium. However, during the last 2 days of high-sodium diet feeding in the background of aldosterone infusion, the WT mice showed an abrupt and significant increase in urinary sodium excretion. The urinary excretion of sodium in P2Y2-R KO mice continued to increase in a linear fashion, but at a lower rate of increase than in the WT mice. We did not quantify sodium losses through feces and sweat from paw pads. Thus we could not definitively compute sodium balance.
Western blot analysis of kidney samples showed a marked (>2-fold) increase in the protein abundance of NHE3 in the cortex, and an associated significant increase in NKCC2 in the medulla of P2Y2-R KO mice vs. WT mice. However, the protein abundances of NaPi-2, NKCC2, and α- and β-ENaC in the cortex are modestly, but significantly lower in the P2Y2-R KO mice vs. WT mice. Hence, it is possible that markedly elevated cortical levels of NHE3 protein and/or modestly elevated medullary NKCC2 protein in the P2Y2-R KO mice vs. WT mice might have contributed for the impaired natriuresis observed in the P2Y2-R KO mice, a notion that was supported by the following mechanistic insights.
Alterations in NO and Prostanoid Systems or Oxidative Stress
In both series of experiments, we explored the potential influence of P2Y2-R on the NO and/or prostanoid systems or oxidative stress. The rationale for these studies is as follows. eNOS has been demonstrated to inhibit sodium transport by affecting apical membrane sodium channels in cultured collecting duct cells (41), and chronic inhibition of NO synthesis in rats has been shown to result in increased expression of major sodium transporters in the kidney. Thus endogenously derived NO may exert a tonic inhibitory effect on the expression of major sodium transporters, including Na- K-ATPase, NHE3, NKCC2, and NCC (19). Furthermore, it has been shown that extracellular nucleotides activate eNOS and increase NO generation, leading to eNOS phosphorylation at Ser-1177 in human umbilical vein endothelial cells. The latter apparently involved P2Y1-R, P2Y2-R, and P2Y4-R and was independent of adenosine, but dependent on intracellular calcium and PKC-δ (9). Thus there is a potential link between the P2Y2-R and NO system. Furthermore, COX inhibitors are known to enhance urinary concentrating ability especially by increasing the protein abundance of NKCC2 (14), one of the major sodium transporters in the kidney, and is responsible for the generation of medullary osmotic gradients. Also, we have demonstrated the interaction of P2Y2-R with the prostanoid system in the medullary collecting duct (20, 45, 52), the major site of PGE2 production in the kidney.
We observed that following high-sodium diet feeding, both WT and P2Y2-R KO mice had elevated urinary NO3/NO2 excretion, indicating increases in NO production in both genotypes. However, the elevation in P2Y2-R KO mice was significantly higher compared with the WT mice. On the other hand, when mice were fed a high-sodium diet in a background of aldosterone infusion, the urinary NO3/NO2 levels in WT mice increased even further, whereas the levels in P2Y2-R KO mice were significantly lower. This contrasting picture between the two studies suggests that in the absence of P2Y2-R signaling, with a high-sodium diet mice require greater NO production. The combination with aldosterone likely pushes them beyond the point where they can effectively compensate. This may explain the inability to excrete equivalent amounts of sodium by the two genotypes at that point. This notion was further supported by the observation of significantly lower relative expression of both iNOS and eNOS isoforms in the cortex, and iNOS in the medulla of P2Y2-R KO mice compared with WT mice. These observations were also consistent with the reported stimulatory effects of P2Y2-R, such as P2Y1-R, P2Y2-R, and P2Y4-R, on NOS isoforms (iNOS and eNOS) in the pulmonary system or human umbilical vein endothelial cells (8, 15). Furthermore, in agreement with an inhibitory effect of NO on expression of NHE3 and NKCC2, we did find reduced cortical NHE3 and medullary NKCC2 in the WT vs. P2Y2-R KO mice administered a high-sodium diet combined with aldosterone infusion.
As stated above, COX inhibitors enhance urinary concentration ability by increasing the abundance of NKCC2 (14). In this context, the enhanced production of PGE2 seen in high-sodium diet-fed plus aldosterone-infused P2Y2-R KO mice vs. high-sodium diet-fed P2Y2-R KO mice was perhaps a redundant mechanism to alleviate sodium retention, especially since NO generation may have decompensated at this point. Furthermore, increased PGE2 perhaps accounts for the lowering of cortical NKCC2 protein abundance in the P2Y2-R KO mice observed under this condition, but not seen in the high-sodium diet alone study. This notion was supported by the observed induction of COX-2 in the cortex, but not in the medulla of P2Y2-R KO mice fed a high-sodium diet in a background of aldosterone infusion.
Strikingly, our study provides evidence that enhanced oxidative stress induced by a high-sodium diet and/or aldosterone in P2Y2-R KO mice relative to WT mice may play a potential modulatory role in the outcome of the effect of NO and PGE2 on sodium transporters. The significant increases in urinary 8-isoprostane seen in P2Y2-R KO mice vs. WT mice in both series of experiments were an indication that in the absence of P2Y2-R signaling the oxidative stress induced by a high-sodium diet and/or aldosterone was much higher. The increase in oxidative stress is apparently higher in the combined high-sodium diet and aldosterone study compared with the high-sodium diet alone study, which then might be responsible for urine NO3/NO2 levels eventually falling in the P2Y2-R KO mice under these extremely high-stress conditions. While further studies are warranted to decipher how P2Y2-R is linked to suppression of oxidative stress induced by a high-sodium diet with/without aldosterone uncovered in our study, a recent report showed that 8-iso-PGF2α (8-isoprostane), increased Cl transport in the cortical thick ascending limb via protein kinase A-dependent mechanism, and this process was suppressed by blocking NKCC2. The report suggested that heightened NaCl retention caused by 8-iso-PGF2α, a product of oxidative stress, may contribute to the pathogenesis of hypertension (5). If established in further studies, one can predict that apart from its direct effect on ENaC, aldosterone may indirectly increase NaCl absorption in the cortical thick ascending limb by virtue of its ability to induce oxidative stress, especially in the setting of high salt intake.
Based on the information available in published literature and documented in this study, in Fig. 9 we schematically summarized the potential interactions between aldosterone and P2Y2-R in relation to other mediators, such as NO and oxidative stress, in regulating renal sodium excretion. It is increasingly becoming clear that an integrated approach, such as the one shown in Fig. 9, is needed to understand the complex nature of renal sodium handling in health and disease. The figure also shows the potential induction of COX-2 by the combined effect of aldosterone and a high-sodium diet in the absence of P2Y2-R.
Fig. 9.

Schematic representation of potential interactions between aldosterone and P2Y2-R nitric oxide (NO) and oxidative stress (OS) in relation to renal sodium excretion (top). The potential interactions depicted were based on the information available in published literature as well as the experimental evidence presented in this study. The bottom of the scheme depicts potential induction of COX-2 in P2Y2-R KO mice by the combined effect of aldosterone (Ald) and a high-sodium diet (HSD) based on the data presented in this study.
Finally, at the time of submission of this manuscript, Stockand and associates (40), using the P2Y2-R KO mice, reported that control of ENaC by purinergic signaling is necessary for aldosterone escape. A role for ENaC in aldosterone escape agrees with what we published previously (35) showing decreased abundance of the “70 kDa-band” of γ-ENaC, a band thought to be due to an activating cleavage of the major band in aldosterone escape in high-NaCl-fed vs. low-NaCl-fed aldosterone-infused rats. Our current study is also in agreement with the findings of Stockand et al. (40) in that we found relatively lower levels of the major band associated with β-ENaC in the P2Y2-R KO mice, which has also been reported as an aldosterone-like pattern (28). However, no significant difference was found for γ-ENaC. In addition, our study uncovers the potential role of important signaling mediators, such as NO and prostaglandins in determining these differences in the P2Y2-R KO mice. It also demonstrates, for the first time, the likelihood for the existence of oxidative stress in these mice in the absence of P2Y2-R, especially in the high-stress environment of high salt and/or aldosterone infusion.
GRANTS
This work was primarily supported by the Catalyst Grant from the University of Utah (to B. K. Kishore) and the resources and facilities at the VA Salt Lake City Health Care System; supplemented by funds from the Department of Veterans Affairs Merit Review Project (to B. K. Kishore), and the National Kidney Foundation of Utah and Idaho (to B. K. Kishore). Additional funding sources include an Established Investigator Award from the American Heart Association (to C. M. Ecelbarger).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Dr. Beverly Koller for generously supplying breeders of P2Y2-R null and wild-type mice, to Dr. Donald Kohan for helpful suggestions, and to Charlotte Hogan, R. Mayuri Garikepati, and Craig D. Kamerath for technical assistance.
Parts of this work have been presented in preliminary form at the 41st Annual Meeting of the American Society of Nephrology, November 2008, Philadelphia, PA, and appeared as printed abstracts in the proceedings of that meeting (25, 51).
REFERENCES
- 1. Audigé A, Yu ZR, Frey BM, Uehlinger DE, Frey FJ, Vogt B. Epithelial sodium channel (ENaC) subunit mRNA and protein expression in rats with puromycin aminonucleoside-induced nephritic syndrome. Clin Sci 104: 389– 395, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bailey MA, Imbert-Teboul M, Turner C, Marsy S, Srai K, Burnstock G, Unwin RJ. Axial distribution and characterization of basolateral P2Y receptors along the rat renal tubule. Kidney Int 58: 1893– 1901, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bianchi A, Moulin D, Sebillaud S, Koufany M, Galteau MM, Netter P, Terlain B, Jouzeau JY. Contrasting effects of peroxisome-proliferator-activated receptor (PPAR)γ agonists on membrane-associated prostaglandin E2 synthase-1 in IL-1β-stimulated rat chondrocytes : evidence for PPARγ-independent inhibition by 15-deoxy-Δ12,14 prostaglandin J2 Arthritis Res Ther 7: R1325– R1337, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nature Rev Nephrol 6: 261– 273, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Cabral PD, Silva GB, Baigorria ST, Juncos LA, Juncos LI, García NH. 8-Iso-prostaglandin-F2α stimulates chloride transport in thick ascending limbs: role of cAMP and protein kinase A. Am J Physiol Renal Physiol 299: F1396– F1400, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Churchill PC, Ellis VR. Purinergic P2y receptors stimulate renin secretion by rat renal cortical slices. J Pharmacol Exp Ther 266: 160– 163, 1993 [PubMed] [Google Scholar]
- 7. Cressman VL, Lazarowski E, Homolya L, Boucher RC, Koller BH, Grubb BR. Effect of loss of P2Y2 receptor gene expression on nucleotide regulation of murine epithelial Cl− transport. J Biol Chem 274: 26461– 26468, 1999 [DOI] [PubMed] [Google Scholar]
- 8. da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation 119: 871– 879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Djelidi S, Fay M, Cluzeaud F, Escoubet B, Eugene E, Capurro C, Bonvalet JP, Farman N, Blot-Chabaud M. Transcriptional regulation of sodium transport by vasopressin in renal cells. J Biol Chem 272: 32919– 32924, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol 279: F46– F53, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Ecelbarger CA, Kim GH, Wade JB, Knepper MA. Regulation of the abundance of renal sodium transporters and channels by vasopressin. Expt Nephrol 171: 227– 234, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Ecelbarger CA, Yu S, Lee AJ, Weinstein LS, Knepper MA. Decreased renal Na-K-2Cl cotransporter abundance in mice with heterozygous disruption of the Gsα gene. Am J Physiol Renal Physiol 277: F235– F244, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Farjah M, Roxas BP, Geenen DL, Danziger RS. Dietary salt regulates renal SGK1 abundance. Relevence to salt sensitivity in the Dahl rat. Hypertension 41: 874– 878, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Llama P, Ecelbarger CA, Ware JA, Andrews P, Lee AJ, Turner R, Nielsen S, Knepper MA. Cyclooxygenase inhibitors increase Na-K-2Cl cotransporter abundance in thick ascending limb of Henle's loop. Am J Physiol Renal Physiol 277: F219– F226, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Greenberg SS, Zhao X, Wang JF, Hua L, Ouyang J. cAMP and purinergic P2y receptors upregulate and enhance inducible NO synthase mRNA and protein in vivo. Am J Physiol Lung Cell Mol Physiol 273: L967– L979, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Homolya L, Watt WC, Lazarowski ER, Koller BH, Boucher RC. Nucleotide-regulated calcium signaling in lung fibroblasts and epithelial cells from normal and P2Y2 receptor (−/−) mice. J Biol Chem 274: 26454– 26460, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Jia Z, Zhang A, Zhang H, Dong Z, Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res 99: 1243– 1251, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Katada J, Meguro T, Saito H, Ohashi A, Anzai T, Ogawa S, Yoshikawa T. Persistent cardiac aldosterone synthesis in angiotensin II type 1A receptor-knockout mice after myocardial infarction. Circulation 111: 2157– 2164, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kim JS, Choi KC, Jeong MH, Kim SW, Oh Y, Lee J. Increased expression of sodium transporters in rats chronically inhibited of nitric oxide synthesis. J Korean Med Sci 21: 1– 4, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE. P2Y2 receptors and water transport in the kidney. Purinergic Signalling 5: 491– 499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knepper MA, Im GH, Masilamani S. Renal tubule sodium transporter abundance profiling in rat kidney. Response to aldosterone and variations in NaCl intake. Ann NY Acad Sci 986: 562– 569, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Kunzelmann K, Bachhuber T, Reeger R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. FASEB J 19: 142– 143, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284: F419– F432, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Listhrop R, Nelson RD, Ecelbarger CA, Kohan DE, Kishore BK. Genetic deletion of P2Y2 receptor (P2Y2-R) alters the protein abundances of proximal tubular and thick ascending limb sodium transporters (Abstract). FASEB J 21: 1328A, 2007 [Google Scholar]
- 25. Listhrop R, Zhang Y, Charlotte H, Kamerath CD, Ecelbarger CM, Kishore BK. Altered expression of renal sodium transporters/channels in P2Y2 receptor knockout mice fed high or low salt diet (Abstract). J Am Soc Nephrol 19: 352A, 2008 [Google Scholar]
- 26. Manolopoulou J, Bielohuby M, Caton SJ, Gomez-Sanchez CE, Renner-Mueller I, Wolf E, Lichtenauer UD, Beuschlein F, Hoeflich A, Bidlingmaier M. A highly sensitive immunofluorometric assay for the measurement of aldosterone in small sample volumes: validation in mouse serum. J Endocrinol 196: 215– 224, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19– R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Masilamani S, Wang X, Kim GH, Brooks H, Nielsen J, Nielsen S, Nakamura K, Stokes JB, Knepper MA. Time course of renal Na-K-ATPase, NHE3, NKCC2, NCC, and ENaC abundance changes with dietary NaCl restriction. Am J Physiol Renal Physiol 283: F648– F657, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Mo J, Fisher MJ. Uridine nucleotide-induced stimulation of gluconeogenesis in isolated rat proximal tubules. Naunyn Schmiedebergs Arch Pharmacol 366: 151– 157, 2002 [DOI] [PubMed] [Google Scholar]
- 30. O'Mullane L, Cook DI, Dinudom A. Purinergic regulation of epithelial Na+ channel. Proc Australian Physiol Soc 40: 63– 70, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599– 36607, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prætorius HA, Leipiziger J. Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol 72: 377– 393, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Praticò D, Lawson JA, Rokach J, FitzGerald GA. The isoprostanes in biology and medicine. Trends Endocrinol Metab 12: 243– 247, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisted. FASEB J 22: 659– 661, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Riazi S, Khan O, Tiwari S, Hu X, Ecelbarger CA. Rosiglitazone regulates ENaC and Na-K-2Cl cotransporter (NKCC2) in the obese Zucker rat. Am J Nephrol 26: 245– 257, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Rieg T, Bundey RA, Chen Y, Deschenes G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717– 3726, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Schwiebert EM, Kishore BK. Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol 280: F945– F963, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Shirley DG, Bailey MA, Unwin RJ. In vivo stimulation of apical P2 receptors in collecting ducts: evidence for inhibition of sodium reabsorption. Am J Physiol Renal Physiol 288: F1243– F1248, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Song J, Hu X, Shi M, Knepper MA, Ecelbarger CA. Effects of dietary fat, NaCl, and fructose on renal sodium and water transporter abundances and systemic blood pressure. Am J Physiol Renal Physiol 287: F1204– F1212, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Stockand JD, Mironova E, Bugaj V, Rieg T, Insel PA, Vallon V, Peti-Peterdi J, Pochynyk O. Purinergic inhibition of ENaC produces aldosterone escape. J Am Soc Nephrol 21: 1903– 1911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stoos BA, Carretero OA, Garvin JL. Endothelial-derived nitric oxide inhibits sodium transport by affecting apical membrane channels in cultured collecting duct cells. J Am Soc Nephrol 4: 1855– 1860, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Sun R, Carlson NG, Hemmert AC, Kishore BK. P2Y2 receptor-mediated release of prostaglandin E2 by IMCD is altered in hydrated and dehydrated rats: relevance to AVP-independent regulation of IMCD function. Am J Physiol Renal Physiol 289: F585– F591, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Sun R, Miller RL, Hemmert AC, Zhang P, Shi H, Nelson RD, Kishore BK. Chronic dDAVP infusion in rats decreases the expression of P2Y2 receptor in inner medulla and P2Y2 receptor-mediated PGE2 by IMCD. Am J Physiol Renal Physiol 289: F768– F776, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Takeda M, Kobayashi M, Endou H. Establishment of a mouse clonal early proximal tubule cell line and outer medullary collecting duct cells expressing P2 purinoceptors. Biochem Mol Biol Int 44: 657– 664, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Tiwari S, Packer RK, Song J, Sugimura Y, Hu X, Verbalis JG, Ecelbarger CA. Increased renal α-ENaC and NCC abundance and elevated blood pressure are independent of hyperaldosteronism in vasopressin escape. Am J Physiol Renal Physiol 291: F49– F57, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10– F27, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Wang XY, Masilamani S, Nielsen J, Kwon TH, Brooks HL, Nielsen S, Knepper MA. The renal thiazide-sensitive Na-Cl cotransporter as mediator of the aldosterone-escape phenomenon. J Clin Invest 108: 215– 222, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Welch BD, Carlson NG, Shi H, Myatt L, Kishore BK. P2Y2 receptor-stimulated release of prostaglandin E2 by rat medullary collecting duct preparations. Am J Physiol Renal Physiol 285: F711– F721, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Wildman SSP, Kang ESK, King BF. EnaC, renal sodium excretion and extracellular ATP. Purnergic Signalling 5: 481– 489, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wildman SSP, Marks JM, Turner CM, Yew-Booth L, Peppiatt-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol 19: 731– 742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Y, Kohan DE, Kamerath CD, Kishore BK. Effect of aldosterone in a background of high sodium diet in P2Y2 receptor knockout mice (Abstract). J Am Soc Nephrol 19: 584A, 2008 [Google Scholar]
- 52. Zhang Y, Kohan DE, Nelson RD, Carlson NG, Kishore BK. Potential involvement of P2Y2 receptor in diuresis of postobstructive uropathy in rats. Am J Physiol Renal Physiol 298: F634– F642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang Y, Nelson RD, Carlson NG, Kamerath CD, Kohan DE, Kishore BK. Potential role of purinergic signaling in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 296: F1194– F1201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang Y, Sands JM, Kohan DE, Nelson RD, Martin CF, Carlson NG, Kamerath CD, Ge Y, Klein JD, Kishore BK. Potential role of purinergic signaling in urinary concentration in inner medulla: insights from P2Y2 receptor gene knockout mice. Am J Physiol Renal Physiol 295: F1715– F1724, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]


