Abstract
Background
Acute kidney injury is a serious complication of cardiac surgery for which there remains no specific therapy. Animal data and several observational studies suggest that statins prevent acute kidney injury, but the results are not conclusive, and many studies are retrospective in nature.
Methods
We conducted a multi-center prospective cohort study of 625 adult patients undergoing elective cardiac surgery. All patients were on statins and were grouped on whether statins were continued or held in the 24 hours prior to surgery. The primary outcome was acute kidney injury defined by a doubling of serum creatinine or dialysis. The secondary outcome was the peak level of several kidney injury biomarkers. Results were adjusted for demographic and clinical factors.
Results
Continuing (vs. holding) a statin prior to surgery was not associated with a lower risk of acute kidney injury defined by a doubling of serum creatinine or dialysis, [adjusted relative risk (RR) 1.09 (95% confidence interval (CI) 0.44, 2.70)]. However, continuing a statin was associated with a lower risk of elevation of the following AKI biomarkers: urine interleukin-18, urine neutrophil gelatinase-associated lipocalin, urine kidney injury molecule-1, and plasma neutrophil gelatinase-associated lipocalin [adjusted RR 0.34 (95% CI 0.18, 0.62), adjusted RR 0.41 (95% CI 0.22, 0.76), adjusted RR 0.37 (95% CI 0.20, 0.76), adjusted RR 0.62 (95% CI 0.39, 0.98), respectively].
Conclusions
Statins may prevent kidney injury after cardiac surgery as evidenced by lower levels of kidney injury biomarkers.
Keywords: CABG, kidney, renal failure
Introduction
Acute kidney injury (AKI) is a common complication of cardiac surgery associated with increased in-hospital mortality and resource utilization as well as poorer long-term survival and renal outcomes (1-3). Unfortunately, despite multiple trials of prevention and treatment strategies, current therapy for AKI remains limited to supportive measures (4). There is however increasing evidence to suggest that statins may be an effective preventative therapy. Statins possess anti-oxidant properties (5), improve endothelial function (6) and decrease inflammation (7-9). Several animal studies have shown that statins are reno-protective when given prior to an ischemia-reperfusion type injury (5-8). Unfortunately, the results in human studies are less consistent. Some observational studies have shown that statins decrease AKI and improve renal recovery when administered prior to or immediately following cardiac surgery (10-16), while others have not (17-19). Many prior studies are retrospective, with limited data on the details of perioperative statin administration. As well, prior studies have all relied on changes in serum creatinine, a marker of AKI known to be insensitive and non-specific (20-23). However, there is emerging data in support of AKI biomarkers, which may allow for a more accurate diagnosis of kidney injury in the cardiac surgery setting (24-26).
To investigate the issue further, we conducted a secondary analysis of a large prospective cohort study. Our goal was to contrast AKI outcomes among individuals who had a statin continued or held prior to surgery. We hypothesized that continuing a statin through cardiac surgery would associate with less AKI as defined by serum creatinine and lower levels of kidney injury biomarkers.
Material and Methods
Study Population
This cohort is fully described elsewhere (25, 26). In brief, we prospectively enrolled adults undergoing cardiac surgery (CABG or valve surgery) who were at high risk for AKI at 6 academic medical centers in North America between July 2007 and December 2009. Participants with multiple surgeries could only be enrolled once. All participants provided written informed consent and the study was approved by each institution's research ethics board. The reporting of this study follows guidelines set out in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (27).
Pre-operative Statin Use
All included patients were chronically taking a statin. Patients were grouped based on whether they were asked by their surgeon to continue or hold their statin in the 24 hours prior to surgery.
Outcome Definitions
The primary outcome was the development of AKI defined by the receipt of acute dialysis or a doubling in serum creatinine from the baseline outpatient pre-operative value during the entire hospital stay. In modern staging systems this reflects RIFLE stage “I” or Acute Kidney Injury Network (AKIN) stage 2 AKI (28). All pre-operative creatinine values were measured within two months prior to surgery. Pre- and post-operative serum creatinine levels were measured in the same clinical laboratory at each center. Serum creatinine values were recorded for every patient throughout the hospital stay. The secondary outcome was an elevation of kidney injury biomarkers. As fully described elsewhere, we carefully collected urine and plasma specimens pre-operatively and daily for up to five post-operative days (26).
Kidney Injury Biomarker Measurements
We measured urine neutrophil gelatinase-associated lipocalin (NGAL) and urine interleukin-18 (IL-18) with the ARCHITECT® assay (Abbott Diagnostics, Abbott Park, IL). We measured urine creatinine by the modified Jaffe reaction. We performed the measurements in 2 batches, about 1 year apart. We measured plasma NGAL using the Triage NGAL immunoassay, in conjunction with the Triage Meter (Biosite Inc., San Diego, CA) in two batches 7 months apart. Urine NGAL, urine IL-18 and plasma NGAL concentrations are stable at -80°C for 2 years without any protease inhibitors with a variability of 2-8% (29, 30). Specifications and validation of the assays are fully described elsewhere (26). We measured urine kidney injury molecule-1 (KIM-1) and urine liver-fatty acid binding protein (L-FABP) using the Sekisui Diagnostics LLC assay. The detection range for KIM-1 is 0.056-60 ng/mL and for L-FABP, 0.057-1500ng/mL. The intra-assay coefficient of variation is <10% for both assays. Biomarkers were measured in duplicate and an average of the 2 values was used. All urine albumin assays were measured by immunoturbidimetry on a Siemens Dimension Plus with HM clinical analyzer, per manufacturer's instructions.
Personnel performing the biomarker measurements were blinded to each patient's clinical information. All biomarkers were measured from frozen aliquots that did not undergo any additional freeze-thaw cycles. Repeat measurement of randomly selected samples between the batches confirmed high correlation without any assay drift for all the assays measured in two batches.
Variable Definitions
We collected pre-operative characteristics, operative details and post-operative complications using definitions of the Society of Thoracic Surgeons (STS) (http://www.ctsnet.org/file/rptDataSpecifications252_1_ForVendorsPGS.pdf). We estimated pre-operative glomerular filtration rate (eGFR) using the CKD-EPI equation (31).
Analysis
We used a chi-square test or Fisher's exact test to compare dichotomous variables and a 2-sample t-test or Wilcoxon rank-sum test for continuous variables. We used a Poisson regression model to examine the association between continuing vs. holding a statin and dichotomous outcomes (32) and linear regression for continuous biomarker levels (log-transformed). For the dichotomous biomarker outcome, as in prior studies, an elevated biomarker level was defined as a level in the highest quintile (25, 26). The biomarker analyses were performed at 2 time points: 1. during the first 3 post-operative days; 2. within 6 hours after surgery. In multivariable analysis, we adjusted for 12 important covariates that predict AKI in the cardiac surgery setting (33): age (per year), gender, race, diabetes, type of surgery, pre-operative eGFR (<60, ≥60 mL/min/1.73 m2), congestive heart failure (CHF), cardiac catheterization, pre-operative urine albumin to creatinine ratio (ACR) (<10, 10-30, >30 mg/g), and pre-operative medication use (angiotensin converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB), diuretic, calcium channel blocker).
Results
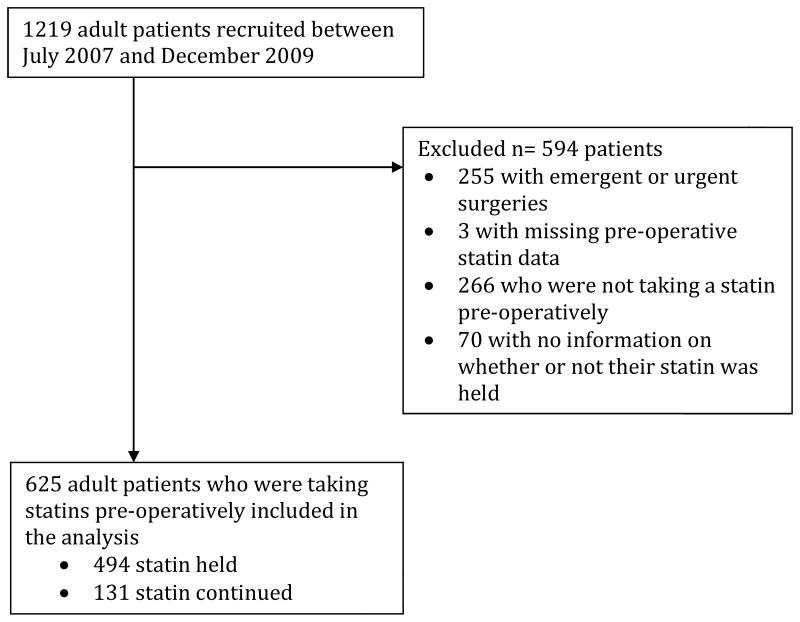
Patient selection is presented in Figure 1. Compared to those who had their statin held, patients who had their statin continued were more likely to have risk factors for AKI (Table 1). The decision to hold or continue a patient's statin was primarily surgeon preference (Supplementary Table 1).
Figure 1. Patient selection.
Table 1. Patient characteristics according to whether the statin was held or continued prior to surgery.
| Held (n=494) | Continued (n=131) | p value | |
|---|---|---|---|
| Age (years) | 71 (9) | 71 (11) | 0.3 |
| Male | 351 (71%) | 97 (74%) | 0.5 |
| White | 473 (96%) | 121 (92%) | 0.1 |
| Diabetes | 226 (46%) | 63 (48%) | 0.6 |
| Hypertension | 392 (79%) | 111 (85%) | 0.2 |
| Congestive heart failure | 63 (13%) | 47 (36%) | <.0001 |
| Elective procedure | 494 (100%) | 131 (100%) | 0.2 |
| Cardiac catheterization in the last 72 hours | 13 (3%) | 10 (8%) | 0.006 |
| Operative Characteristics | |||
| Incidence | |||
| first cardiovascular surgery | 437 (98%) | 114 (98%) | 1.0 |
| redo cardiovascular surgery | 8 (2%) | 2 (2%) | |
| Surgery | |||
| CABG and valve | 87 (18%) | 27 (21%) | 0.4 |
| CABG or Valve | 406 (82%) | 104 (79%) | |
| CPB | |||
| Combination | 16 (3%) | 1 (1%) | 0.02 |
| Full | 410 (83%) | 121 (92%) | |
| None | 68 (14%) | 9 (7%) | |
| Perfusion time (minutes) | 100.15 (56.42) | 114.02 (49.15) | 0.02 |
| Cross clamp time (minutes) | 66.19 (41.96) | 84.18 (41.89) | <0.0001 |
| Cardioplegia | 415 (84%) | 122 (93%) | 0.008 |
| Post-operative intra-aortic balloon pump | 7 (1%) | 6 (5%) | 0.02 |
| Renal function | |||
| Pre-operative serum creatinine (mg/dL)a | 1.05 (0.29) | 1.14 (0.34) | 0.007 |
| Pre-operative eGFR (mL/min per 1.73 m2) | 69.73 (18.43) | 65.39 (19.08) | 0.01 |
| Pre-operative eGFR | |||
| >60 (mL/min per 1.73 m2) | 349 (71%) | 80 (61%) | 0.2 |
| 45-60 (mL/min per 1.73 m2) | 100 (20%) | 32 (24%) | |
| 30-45 (mL/min per 1.73 m2) | 36 (7%) | 15 (11%) | |
| ≤30 (mL/min per 1.73 m2) | 9 (2%) | 4 (3%) | |
| Pre-operative ACEi or ARB | |||
| No | 136 (28%) | 48 (37%) | <0.0001 |
| Yes continued | 26 (5%) | 40 (31%) | |
| Yes held | 327 (67%) | 41 (32%) | |
| Pre-operative diuretic | |||
| No | 273 (56%) | 72 (55%) | <0.0001 |
| Yes continued | 4 (1%) | 46 (35%) | |
| Yes held | 212 (43%) | 13 (10%) | |
| Pre-operative calcium channel blocker | |||
| No | 330 (67%) | 99 (76%) | 0.001 |
| Yes continued | 82 (17%) | 26 (20%) | |
| Yes held | 81 (16%) | 5 (4%) | |
| Pre-operative urine albumin to creatinine ratio | |||
| <10 mg/g | 179 (36%) | 62 (47%) | 0.006 |
| 10-30 mg/g | 163 (33%) | 23 (18%) | |
| 31-300 mg/g | 126 (26%) | 39 (30%) | |
| >300 mg/g | 26 (5%) | 7 (5%) | |
| Pre-operative urine albumin to creatinine ratio (mg/g), median (IQR) | 17 (7, 42) | 14 (5, 58) | 0.7 |
Mean (SD) or n (%) presented unless otherwise indicated
IQR= interquartile range
eGFR, estimated glomerular filtration rate with CKD-EPI equation; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; CPB, cardiopulmonary bypass; CABG, coronary artery bypass graft
To convert serum creatinine values to umol/L, multiply by 88.4.
Outcomes
AKI Defined by Serum Creatinine
A total of 25/625 (4%) patients developed AKI defined by a doubling of serum creatinine or receipt of dialysis. This was 19/494 (4%) in the statin held group and 6/131 (5%) in the statin continued group [adjusted relative risk (RR) 1.09, 95% confidence interval (CI) (0.44, 2.70), statin held as the referent group] (Supplementary Table 2). AKI defined by smaller increases in serum creatinine was also no different between the statin held and continued groups (Supplementary Table 3).
Kidney Injury Biomarkers
The continuous biomarker levels for patients who had their statin continued vs. held are presented in Table 2. For all 5 biomarkers, patients who had their statin continued had significantly lower peak biomarker levels during the first 3 post-operative days compared to those who had their statin held. The effect sizes ranged from 6% lower for albumin to 59% lower for IL-18. The biomarkers analyzed dichotomously are presented in Table 3. Patients in the statin continued group were less likely to have a peak level of urine IL-18, urine NGAL, urine KIM-1, and plasma NGAL in the upper quintile during the first 3 post-operative days. Peak levels of L-FABP and urine albumin did not differ significantly between the statin continued and held groups. Unadjusted results were similar.
Table 2. Continuous peak post-operative biomarker levels according to whether the statin was held or continued prior to surgery.
| Held (n=494) | Continued (n=131) | Estimate (SE) P valuea | ||
|---|---|---|---|---|
| Unadjusted | Adjustedb | |||
| Urine IL-18 (pg/mL) | 103.5 (49.5, 197.7) | 42.9 (21.4, 83.1) | 0.75 (0.12) P<0.0001 | 0.67 (0.12) P<0.0001 |
| Urine NGAL (ng/mL) | 49.9 (24.6, 117.6) | 31.4 (13.7, 58.7) | 0.7 (0.14) P<0.0001 | 0.66 (0.15) P<0.0001 |
| Urine Kim-1 (ng/mL) | 9.3 (5.8, 16) | 5.9 (3.6, 10.5) | 0.49 (0.08) P<0.0001 | 0.42 (0.09) P<0.0001 |
| Plasma NGAL (ng/mL) | 209.9 (148.5, 288.7) | 168.6 (111.6, 252.3) | 0.19 (0.06) P=0.0005 | 0.24 (0.06) P<0.0001 |
| Urine L-FABP (ng/mL) | 38.7 (15.5, 130.6) | 26.8 (8.6, 86.8) | 0.43 (0.15) P=0.004 | 0.39 (0.16) P=0.01 |
| Urine Albumin (mg/L) | 54.9 (31.7, 97.8) | 51.4 (19.3, 93.9) | 0.28 (0.1) P=0.007 | 0.28 (0.11) P=0.009 |
Peak biomarker levels were defined as the highest biomarker value in the first 3 post-operative days
Median (25th percentile, 75th percentile) presented
Est (SE) is the estimate and standard error from the linear regression model on log-transformed biomarkers.
Adjusted for age, gender, race, diabetes, type of surgery (CABG and valve vs. CABG or valve), pre-operative eGFR (<60, >=60), congestive heart failure, cardiac catheterization, pre-operative urine albumin to creatinine ratio (<10, 10-30, >30), pre-operative ACEi/ARB (yes/no), pre-operative diuretic (yes/no), pre-operative calcium channel blocker (yes/no)
Table 3. Association of continuing (vs holding) statins and AKI (defined by elevated peak post-operative biomarkersa).
| AKI (defined by elevated peak postoperative biomarkers) | Held (n=494) | Continued (n=131) | P value | |
|---|---|---|---|---|
| Urine IL-18 (pg/mL) | n (%) | 119 (24%) | 10 (8%) | <0.0001 |
| unadjusted RR (95% CI) | 1.0 (referent) | 0.32 (0.17,0.58) | ||
| adjustedb RR (95% CI) | 1.0 (referent) | 0.34 (0.18,0.62) | ||
| Urine NGAL (ng/mL) | n (%) | 103 (21%) | 11 (8%) | 0.001 |
| unadjusted RR (95% CI) | 1.0 (referent) | 0.4 (0.22,0.72) | ||
| adjustedb RR (95% CI) | 1.0 (referent) | 0.41 (0.22,0.76) | ||
| Urine KIM-1 (ng/mL) | n (%) | 125 (25%) | 12 (9%) | <0.0001 |
| unadjusted RR (95% CI) | 1.0 (referent) | 0.36 (0.21,0.63) | ||
| adjustedb RR (95% CI) | 1.0 (referent) | 0.37 (0.2,0.67) | ||
| Plasma NGAL (ng/mL) | n (%) | 99 (20%) | 20 (15%) | 0.2 |
| unadjusted RR (95% CI) | 1.0 (referent) | 0.76 (0.49,1.18) | ||
| adjustedb RR (95% CI) | 1.0 (referent) | 0.62 (0.39,0.98) | ||
| Urine L-FABP (ng/mL) | n (%) | 89 (18%) | 21 (16%) | 0.6 |
| unadjusted RR (95% CI) | 1.0 (referent) | 0.89 (0.57,1.37) | ||
| adjustedb RR (95% CI) | 1.0 (referent) | 0.93 (0.59,1.47) | ||
| Urine Albumin (mg/L) | n (%) | 95 (19%) | 24 (18%) | 0.8 |
| unadjusted RR (95% CI) | 1.0 (referent) | 0.95 (0.64,1.43) | ||
| adjustedb RR (95% CI) | 1.0 (referent) | 0.88 (0.58,1.33) | ||
Elevated peak biomarker levels were defined as the 5th quintile: (urine IL-18 > 201 pg/mL, urine NGAL > 156 ng/mL, urine KIM-1 > 15.8 ng/mL, plasma NGAL > 307 ng/mL, urine L-FABP > 199 ng/mL, urine Albumin > 121.6mg/L)
Peak biomarker levels were defined as the highest biomarker value in the first 3 post-operative days.
Adjusted for age, gender, race, diabetes, type of surgery (CABG and valve vs. CABG or valve), pre-operative eGFR (<60, >=60), congestive heart failure, cardiac catheterization, pre-operative urine albumin to creatinine ratio (<10, 10-30, >30), pre-operative ACEi/ARB, pre-operative diuretic (yes/no), pre-operative calcium channel blocker (yes/no)
Supplementary Analyses
The first post-operative biomarker levels are presented in Supplementary Tables 4 and 5. When analyzed as a continuous variable, urine IL-18, urine NGAL and plasma NGAL were significantly lower in the statin continued group compared to the statin held group at 0-6 hours post-operatively (Supplementary Table 4). When analyzed dichotomously (top quintile results), patients in the statin continued group were less likely to have elevated first post-operative biomarker levels of urine IL-18, urine NGAL and plasma NGAL. The first post-operative biomarker levels of urine KIM-1, urine L-FABP and urine albumin did not differ between the statin continued and held groups (Supplementary Table 5).
Comment
Our data suggest that statins may be an effective therapy for the prevention of kidney injury in the setting of cardiac surgery. The hypothesis warrants testing in definitive randomized controlled trials.
The predominant contributors to AKI in the cardiac surgery setting are thought to be ischemia and inflammation induced by cardiopulmonary bypass (3). It is therefore biologically plausible that statins, which possess anti-inflammatory properties and improve endothelial function could prevent cardiac surgery associated AKI (5-9). To our knowledge, our study is the first to examine the association of statins with kidney injury biomarkers.
AKI defined by serum creatinine did not differ between the statin continued and held groups; however, this could be due to a lack of statistical power. The number of AKI events defined by a doubling in serum creatinine or dialysis was small, which could be secondary to serum creatinine being an insensitive and non-specific marker for AKI (20-23). Haase et al. pooled the data from 10 studies (over 2,000 critically ill patients) and found that almost 20% of included patients had elevated levels of NGAL with a normal serum creatinine. These patients were labeled as likely having subclinical AKI (34). The majority of patients in our study would fall into this category. The label of subclinical AKI suggests that it is not a clinically meaningful outcome, but patients in the Haase et al. study were found to have a higher rate of adverse outcomes (34). As well, a recent study found that patients who had elevated urine NGAL or urine KIM-1 levels with a normal serum creatinine on admission to hospital had a higher risk of requiring dialysis or dying during their hospital stay (35). Another recent study found that elevated urinary NGAL levels are associated with higher one-year mortality (36). Taken together, these studies provide compelling evidence that elevated levels of kidney injury biomarkers are predictive of adverse short and long-term outcomes.
Strengths and Limitations
Our study has a number of strengths. It was conducted prospectively allowing for accurate data collection on pre-operative statin use, employed rigorous complete specimen collection, was performed under standardized conditions in consecutive patients undergoing cardiac surgery, and included multiple centers across the United States and Canada. As well, dividing patients into statin held and continued groups as opposed to starting a patient on a statin or not is likely most representative of how a statin AKI intervention trial could be conducted given that most patients presenting for cardiac surgery are already on a statin. Also, the decision to hold a statin is predominantly surgeon preference; therefore, an observational study comparing statin held vs. continued groups may be less prone to confounding by indication than the alternative design of pre-operative statin yes vs. no.
Our study is limited by a small number of renal events defined by serum creatinine or dialysis. Unfortunately, due to the sample size, we were also unable to account for center effects in our analysis, and we did not quantify or account for statin use after the surgery. We also did not account for dose or type of statin, which could impact the results, as there is evidence to suggest that higher potency statins are associated with less perioperative AKI compared to lower potency statins (37). As well, the allocation of pre-operative statin holding was non-random. Due to the observational nature of our study, the protective association seen between pre-operative statin administration and elevation of AKI biomarkers may not be causal. However, patients in the statin continued group had a greater number of risk factors for AKI, such as chronic kidney disease and cardiopulmonary bypass use. One might expect that residual confounding should, if anything, result in more AKI in the statin continued group.
Future Directions
A proposed reason for the failure of prior interventional trials is their reliance on serum creatinine and thus the inability to accurately diagnose AKI in its early stages. Also, trials that solely define AKI by a significant rise in serum creatinine or dialysis require thousands of patients for adequate statistical power. As a result, promising therapies may fail to undergo definitive testing if small, underpowered trials show no benefit of the intervention. As demonstrated in our proof of concept study, AKI biomarker(s) have a better signal to variability ratio and may decrease the required sample size to detect a signal of AKI. In this regard, both increases in serum creatinine and elevated biomarker levels are surrogate outcomes. Their utility therefore lies in identifying promising interventions worthy of definitive testing in large, expensive multi-centre randomized controlled trials with adequate statistical power to examine intervention effects on outcomes that matter most to patients and their health care providers.
Supplementary Material
Acknowledgments
The study was supported by the NIH grant RO1HL085757 (CRP) to fund the TRIBE-AKI Consortium to study novel biomarkers of acute kidney injury in cardiac surgery. SGC is supported by National Institutes of Health Grants K23DK080132, R01DK096549, and R01HL085757. CRP is also supported by NIH grant K24DK090203. SGC, AXG, and CRP are also members of the NIH-sponsored ASsess, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Consortium (U01DK082185).
The urine biomarker assays were donated by Abbott Diagnostics (IL-18 and NGAL) and Sekisui Diagnostics, LLC (KIM-1 and L-FABP).
The granting agencies, Abbott Diagnostics and Sekisui Diagnostics, Inc., did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Abbreviations
- AKI
Acute kidney injury
- AKIN
Acute Kidney Injury Network
- ACR
Albumin to creatinine ration
- ACEi
Angiotensin converting enzyme inhibitor
- ARB
Angiotensin receptor blocker
- CCB
Calcium channel blocker
- CI
Confidence interval
- CHF
Congestive heart failure
- eGFR
Estimated glomerular filtration rate
- IL-18
Interleukin-18
- KIM-1
Kidney injury molecule-1
- L-FABP
Liver-fatty acid binding protein
- NGAL
Neutrophil gelatinase-associated lipocalin
- RR
Relative risk
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bahar I, Akgul A, Ozatik MA, et al. Acute renal failure following open heart surgery: Risk factors and prognosis. Perfusion. 2005;20(6):317–322. doi: 10.1191/0267659105pf829oa. [DOI] [PubMed] [Google Scholar]
- 2.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (aki) following cardiac surgery. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23(6):1970–1974. doi: 10.1093/ndt/gfm908. [DOI] [PubMed] [Google Scholar]
- 3.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clinical journal of the American Society of Nephrology : CJASN. 2006;1(1):19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 4.Park M, Coca SG, Nigwekar SU, Garg AX, Garwood S, Parikh CR. Prevention and treatment of acute kidney injury in patients undergoing cardiac surgery: A systematic review. Am J Nephrol. 31(5):408–418. doi: 10.1159/000296277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gueler F, Park JK, Rong S, et al. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. Am J Pathol. 2007;170(4):1192–1199. doi: 10.2353/ajpath.2007.060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joyce M, Kelly C, Winter D, Chen G, Leahy A, Bouchier-Hayes D. Pravastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, attenuates renal injury in an experimental model of ischemia-reperfusion. J Surg Res. 2001;101(1):79–84. doi: 10.1006/jsre.2001.6256. [DOI] [PubMed] [Google Scholar]
- 7.Sharyo S, Yokota-Ikeda N, Mori M, et al. Pravastatin improves renal ischemia-reperfusion injury by inhibiting the mevalonate pathway. Kidney Int. 2008;74(5):577–584. doi: 10.1038/ki.2008.210. [DOI] [PubMed] [Google Scholar]
- 8.Patel NS, Chatterjee PK, Di Paola R, et al. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther. 2005;312(3):1170–1178. doi: 10.1124/jpet.104.078659. [DOI] [PubMed] [Google Scholar]
- 9.Dereli Y, Ege E, Kurban S, Narin C, Sarigul A, Yeniterzi M. Pre-operative atorvastatin therapy to decrease the systemic inflammatory response after coronary artery bypass grafting. The Journal of international medical research. 2008;36(6):1248–1254. doi: 10.1177/147323000803600611. [DOI] [PubMed] [Google Scholar]
- 10.Virani SS, Nambi V, Polsani VR, et al. Preoperative statin therapy decreases risk of postoperative renal insufficiency. Cardiovasc Ther. 28(2):80–86. doi: 10.1111/j.1755-5922.2009.00124.x. [DOI] [PubMed] [Google Scholar]
- 11.Tabata M, Khalpey Z, Pirundini PA, Byrne ML, Cohn LH, Rawn JD. Renoprotective effect of preoperative statins in coronary artery bypass grafting. Am J Cardiol. 2007;100(3):442–444. doi: 10.1016/j.amjcard.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 12.Presta P, Rubino AS, Lucisano G, et al. Preoperative statins improve recovery of renal function but not by an anti-inflammatory effect: Observational study in 69 elderly patients undergoing cardiac surgery. International urology and nephrology. 2011;43(2):601–609. doi: 10.1007/s11255-011-9956-3. [DOI] [PubMed] [Google Scholar]
- 13.Huffmyer JL, Mauermann WJ, Thiele RH, Ma JZ, Nemergut EC. Preoperative statin administration is associated with lower mortality and decreased need for postoperative hemodialysis in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2009;23(4):468–473. doi: 10.1053/j.jvca.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Clark LL, Ikonomidis JS, Crawford FA, Jr, et al. Preoperative statin treatment is associated with reduced postoperative mortality and morbidity in patients undergoing cardiac surgery: An 8-year retrospective cohort study. J Thorac Cardiovasc Surg. 2006;131(3):679–685. doi: 10.1016/j.jtcvs.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Billings FTt, Pretorius M, Siew ED, Yu C, Brown NJ. Early postoperative statin therapy is associated with a lower incidence of acute kidney injury after cardiac surgery. J Cardiothorac Vasc Anesth. 2010;24(6):913–920. doi: 10.1053/j.jvca.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brunelli SM, Waikar SS, Bateman BT, et al. Preoperative statin use and postoperative acute kidney injury. Am J Med. 2012;125(12):1195–1204. doi: 10.1016/j.amjmed.2012.06.021. e1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mithani S, Kuskowski M, Slinin Y, Ishani A, McFalls E, Adabag S. Dose-dependent effect of statins on the incidence of acute kidney injury after cardiac surgery. Ann Thorac Surg. 2011;91(2):520–525. doi: 10.1016/j.athoracsur.2010.10.061. [DOI] [PubMed] [Google Scholar]
- 18.Liakopoulos OJ, Choi YH, Haldenwang PL, et al. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: A meta-analysis of over 30,000 patients. Eur Heart J. 2008;29(12):1548–1559. doi: 10.1093/eurheartj/ehn198. [DOI] [PubMed] [Google Scholar]
- 19.Argalious M, Xu M, Sun Z, Smedira N, Koch CG. Preoperative statin therapy is not associated with a reduced incidence of postoperative acute kidney injury after cardiac surgery. Anesth Analg. 111(2):324–330. doi: 10.1213/ANE.0b013e3181d8a078. [DOI] [PubMed] [Google Scholar]
- 20.Devarajan P. Emerging urinary biomarkers in the diagnosis of acute kidney injury. Expert Opin Med Diagn. 2008;2(4):387–398. doi: 10.1517/17530059.2.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doi K, Yuen PS, Eisner C, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. Journal of the American Society of Nephrology : JASN. 2009;20(6):1217–1221. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. Journal of the American Society of Nephrology : JASN. 22(5):810–820. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- 23.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. Journal of the American Society of Nephrology : JASN. 2009;20(3):672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Che M, Xue S, et al. Urinary l-fabp and its combination with urinary ngal in early diagnosis of acute kidney injury after cardiac surgery in adult patients. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2012 doi: 10.3109/1354750X.2012.740687. [DOI] [PubMed] [Google Scholar]
- 25.Molnar AO, Parikh CR, Sint K, et al. Association of postoperative proteinuria with aki after cardiac surgery among patients at high risk. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(11):1749–1760. doi: 10.2215/CJN.13421211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. Journal of the American Society of Nephrology : JASN. 22(9):1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Von EE, Altman DG, Egger M, Popcock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening of the reporting of observational studies in epidemiology (strobe) statement: Guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (adqi) group. Crit Care. 2004;8(4):R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grenier FC, Ali S, Syed H, et al. Evaluation of the architect urine ngal assay: Assay performance, specimen handling requirements and biological variability. Clinical biochemistry. 2010;43(6):615–620. doi: 10.1016/j.clinbiochem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(5):873–882. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou G. A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 33.Mehta RH, Grab JD, O'Brien SM, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114(21):2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. quiz 2208. [DOI] [PubMed] [Google Scholar]
- 34.Haase M, Bellomo R, Haase-Fielitz A. Neutrophil gelatinase-associated lipocalin: A superior biomarker for detection of subclinical acute kidney injury and poor prognosis. Biomarkers in medicine. 2011;5(4):415–417. doi: 10.2217/bmm.11.49. [DOI] [PubMed] [Google Scholar]
- 35.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: A multicenter prospective cohort study. Journal of the American College of Cardiology. 2012;59(3):246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ralib AM, Pickering JW, Shaw GM, et al. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. Journal of the American Society of Nephrology : JASN. 2012;23(2):322–333. doi: 10.1681/ASN.2011040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molnar AO, Coca SG, Devereaux PJ, et al. Statin use associates with a lower incidence of acute kidney injury after major elective surgery. Journal of the American Society of Nephrology : JASN. 2011;22(5):939–946. doi: 10.1681/ASN.2010050442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.