Abstract
The successful demonstration that the selective estrogen receptor modulators (SERMs) tamoxifen and raloxifene reduce the risk of breast cancer has stimulated great interest in using drugs to prevent breast cancer in high-risk women. In addition, recent results from breast cancer treatment trials suggest that aromatase inhibitors may be even more effective at preventing breast cancer than are SERMs. However, while SERMs and aromatase inhibitors do prevent the development of many estrogen-receptor (ER)-positive breast cancers, these drugs do not prevent the development of ER-negative breast cancer. Thus, there is an urgent need to identify agents that can prevent ER-negative breast cancer. We have studied the cancer preventative activity of several classes of drugs for their ability to prevent ER-negative breast cancer in preclinical models. Results from these studies demonstrate that rexinoids (analogs of retinoids that bind and activate RXR receptors), tyrosine kinase inhibitors (such as EGFR inhibitors and dual kinase inhibitors that block EGFR and HER2/neu signaling), and cyclo-oxygenase 2 (COX-2) inhibitors all prevent ER-negative breast cancer in transgenic mice that develop ER-negative breast cancer. Other promising agents now under investigation include vitamin D and vitamin D analogs, drugs that activate PPAR-gamma nuclear receptors, and statins. Many of these agents are now being tested in early phase cancer prevention clinical trials to determine whether they will show activity in breast tissue and whether they are safe for use in high-risk women without breast cancer. The current status of these studies will be reviewed. It is anticipated that in the future, drugs that effectively prevent ER-negative breast cancer will be used in combination with hormonal agents such SERMs or aromatase inhibitors to prevent all forms of breast cancer.
Despite aggressive screening to detect early breast cancer and significant advances in treatment, breast cancer is still the most common cancer in women excluding skin cancer, and it remains the second leading cause of cancer death in women, exceeded only by lung cancer [1]. Recently, the incidence of breast cancer in the United States has declined. However, the decreased incidence was observed only in women aged 50 years or older and was more evident in estrogen receptor (ER)-positive than in ER-negative cancers. The incidence of ER-negative breast cancer, which has a poor prognosis and often occurs in premenopausal women, has not shown significant change. Therefore, there is an urgent need to prevent ER-negative breast cancer.
Primary prevention approaches of breast cancer can be categorized into prophylactic surgery, lifestyle changes, and chemoprevention. Prophylactic surgeries, which consist of bilateral oophorectomy and bilateral mastectomy, are highly invasive approaches that only apply to women with an extremely high risk of breast cancer, such as hereditary breast cancer. The invasive nature has limited their extensive clinical usage. Although lifestyle changes are considered as safe and natural processes, recent meta-analyses of clinical data failed to demonstrate consistent, strong, and statistically significant association between lifestyle changes and breast cancer incidence, except for regular alcohol consumption and weight gain [2, 3]. These interventions could reduce a women’s risk of breast cancer by 5%–10%. Given the limitation of prophylactic surgeries and the modest effect of lifestyle changes, recent breast cancer prevention studies have focused on preventative therapy, which has been shown to be effective in reducing the risk of breast cancer in randomized clinical trials.
13.1 SERMs and Aromatase Inhibitors
Chemoprevention was first defined by Michael Sporn as “prevention of cancer by the use of pharmacological agents (natural or synthetic) to inhibit or reverse the process of carcinogenesis” [4]. A critical issue in the development of chemopreventative agents is to understand the carcinogenesis process and identify targets that are essential for carcinogenesis. The study of estrogen signaling and the identification of ER ultimately led to the design of drugs targeting ER. Selective estrogen receptor modulators (SERMs), which exert selective agonist or antagonist effects on ER depending on different target tissues, represent the major group of compounds that block the ER signaling. Tamoxifen is a SERM that has estrogen antagonist effect to breast, but remains as an estrogen agonist in bone and the uterus. Tamoxifen was found to reduce the incidence of contralateral breast cancer by nearly 50% as a secondary endpoint in several adjuvant studies [5]. These observations suggested that giving tamoxifen to healthy high-risk women would produce equivalent results, and ultimately led to a series of cancer prevention trials using tamoxifen [6–9].
Cuzick et al. performed a metaanalysis of the four tamoxifen prevention trials [10]. The overall reduction in breast cancer incidence caused by tamoxifen was 38% (95% CI, 28–46, p<0.001). More importantly, tamoxifen reduced the risk of ER-positive breast cancer by 48%, but had no effect in reducing the risk of ER-negative breast cancer. Recent updated data based on extended follow-up shows similar results (Table 13.1) [6–9]. Based on the results of the National Surgical Adjuvant Breast and Bowel Project (NSABP) P1 trial, the United States Food and Drug Administration (FDA) approved tamoxifen for cancer risk reduction in women at high risk of breast cancer. However, tamoxifen caused increased risk of a variety of side effects including increased risk of endometrial cancer, venous thromboembolic events, hot flushes, and vaginal discharge. Concerns about these side effects have limited the use of tamoxifen for breast cancer prevention.
Table 13.1.
Breast cancer prevention trials using tamoxifen
| Trials | Population | Number randomized |
Therapy | Median follow-up |
IBC incidence |
|
|---|---|---|---|---|---|---|
| ER+ | ER− | |||||
| Royal Marsden [6] | Age 30–70 with family history of breast cancer | 2,471 | Tamoxifen 20 mg vs Placebo ×5–8 years | 13 years | 53 vs 86 | 24 vs 17 |
| NSABP-P1 [7] | Pre- or postmenopausal women age ≥35 with a >1.66% 5-year risk or with LCIS | 13,388 | Tamoxifen 20 mg vs Placebo ×5 years | 7 years | 70 vs 182 | 42 vs 56 |
| Italian [8] | Women aged 35–70 who had a total hysterectomy | 5,408 | Tamoxifen 20 mg vs Placebo ×5 years | 135 months | 40 vs 52a | 19 vs 21a |
| ISBS–1 [9] | Women aged 35–70 with increased risk of breast cancer | 7,154 | Tamoxifen 20 mg vs Placebo ×5 years | 96 months | 87 vs 132 | 35 vs 35 |
| Total | 28,421 | 250 vs 452 | 120 vs 129 | |||
Includes ductal carcinoma in situ (DCIS)
Raloxifene (Evista) is a second-generation SERM that has estrogen antagonist effects on the breast and uterus, but has estrogen agonist effects in bone and on lipid metabolism. It also has been shown to increase the risk of thromboembolic events. To directly compare the effectiveness and toxicity profile of raloxifene and tamoxifen, the NSABP launched the Study of Tamoxifen and Raloxifene (STAR) trial in postmenopausal women at high risk of breast cancer [11]. The results of the STAR trial demonstrated that raloxifene and tamoxifen were equivalent in preventing breast cancer, but that raloxifene had less toxicity. Because of these results, the FDA approved raloxifene to prevent breast cancer in postmenopausal women who are at high risk of breast cancer or who have osteoporosis. Raloxifene now joins tamoxifen as the second chemoprevention drug to be approved for breast cancer risk reduction. However, neither of these agents prevents the development of ER-negative breast cancer.
Aromatase inhibitors (AIs) offer an alternative approach to antagonize the estrogen signaling pathway by inhibiting the activity of aromatase, a rate-limiting enzyme catalyzing the last step in estrogen synthesis. Three third-generation AIs, anastrozole, letrozole, and exemestane, have shown superiority to tamoxifen in treating metastatic breast cancer, early stage breast cancer in the adjuvant setting, and in preventing the development of contralateral breast cancer in adjuvant studies [12]. These AIs are currently being tested in clinical trials in women with ductal carcinoma in situ (DCIS) breast cancer or in high-risk women without breast cancer to determine whether they can prevent the development of invasive breast cancer. However, in spite of the promising preventative effect of AIs, these agents are not expected to reduce the risk of ER-negative breast cancer. Thus, prevention of ER-negative breast cancer will rely on the development of novel chemopreventative agents that target nonestrogen signaling pathways.
13.2 Novel Agents for the Prevention of ER-Negative Breast Cancer
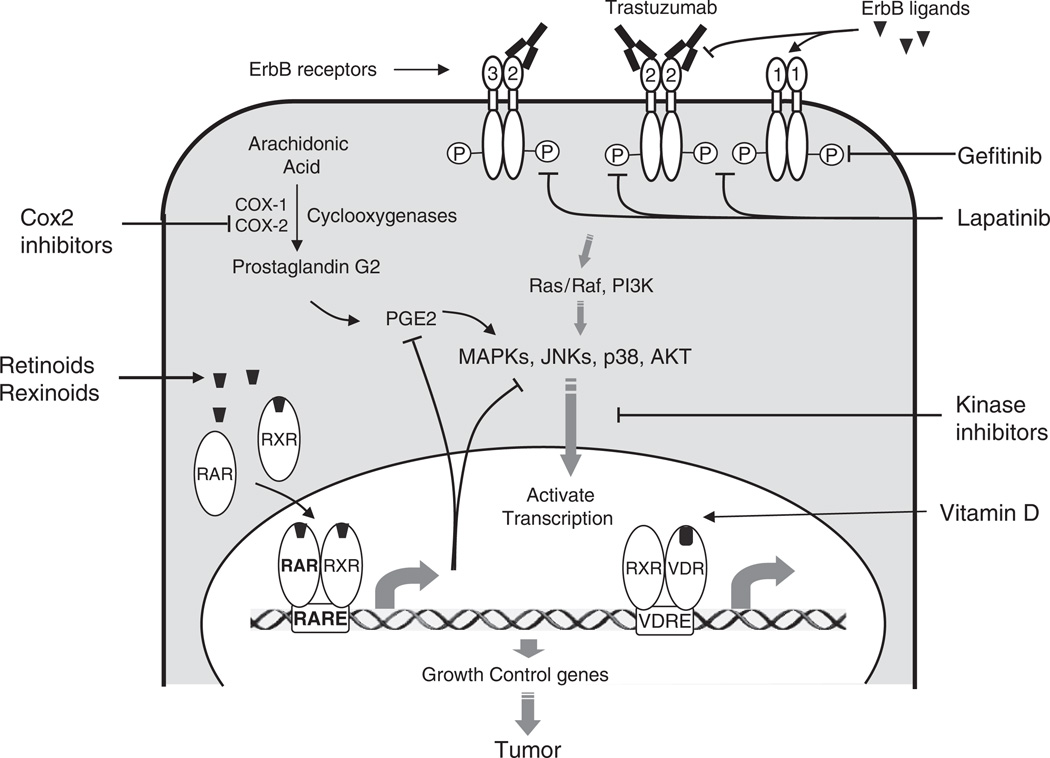
Mammary tumorigenesis is a complex process that involves aberrant regulation of multiple signaling pathways. To effectively prevent ER-negative breast cancer, identification of critical estrogen-independent signaling pathways will be necessary. Recently, molecular biology studies have revealed many signaling pathways that are involved in ER-negative mammary tumorigenesis. Targeting these pathways using pharmacological inhibitors represents a promising strategy for ER-negative breast cancer prevention. Figure 13.1 illustrates some of the estrogen-independent signaling pathways that are critical for breast cell growth. Novel agents targeting these nonendocrine pathways have shown cancer preventative effects in animal models. Representative agents include tyrosine kinase inhibitors against erbB receptors, COX-2 inhibitors, and ligands of nuclear receptor families such as retinoids and vitamin D-related compounds.
Fig. 13.1.
Novel target for the prevention of ER-negative breast cancer. Novel agents targeting nonendocrine pathways include retinoids, COX-2 inhibitors, EFGR/tyrosine kinase inhibitors, transcription factor inhibitors, and others
13.3 Retinoids
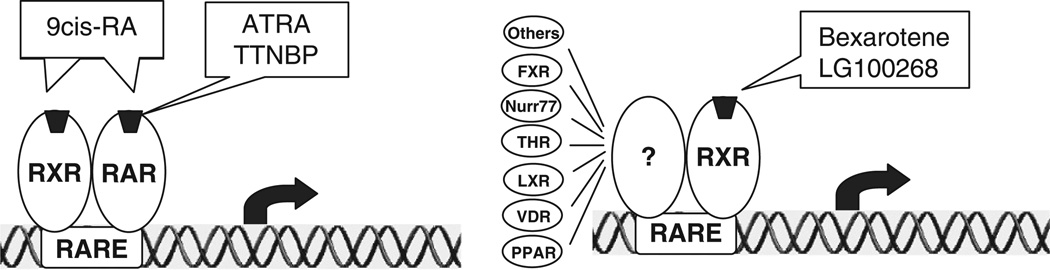
Retinoids are natural and synthetic derivatives of vitamin A (retinol) that have profound effects on development, metabolism, differentiation, and cell growth. Retinoids exert their activity primarily through binding to two types of nuclear receptors, retinoic acid receptors (RARα, -β, and -γ) and retinoid X receptors (RXRα, -β, and -γ). The ligand-bound receptors then form dimeric complexes which bind DNA at specific retinoid responsive elements and regulate the transcription of genes controlling cellular proliferation, differentiation, and apoptosis (Fig. 13.2). Accumulating epidemiological investigations, experimental studies using animal models, and clinical trials have provided strong evidence for the use of retinoids in cancer prevention.
Fig. 13.2.
Retinoids prevent cancer through different retinoid receptor pathways. Receptor-selective retinoids bind to either RAR or RXR. The ligand-bound receptors then form dimeric complexes that bind DNA at specific responsive elements and regulate the transcription of genes controlling cellular proliferation, differentiation, and apoptosis. RAR forms a dimer with RXR, while RXR is able to dimerize with many different partners
The cancer preventative activity of retinoids was first demonstrated by Waun Ki Hong in 1990, who showed that daily usage of isotretinoin (13-cis-retinoic acid) for 12 months prevented second primary tumors in patients with squamous-cell carcinoma of the head and neck [13]. Thereafter, naturally occurring retinoids 9cRA and retinyl acetate, and the synthetic retinoid fenretinide (N-4-hydroxyphenyl, 4HPR), have been reported to prevent breast cancer development in mice and rats exposed to chemical carcinogens dimethylbenz(a)anthracene (DMBA) and methylnitrosourea (MNU) [14, 15]. Fenretinide is one of the most extensively studied retinoids in cancer prevention due to its favorable toxicological profile in humans. A multicenter phase III chemoprevention trial using fenretinide to reduce the incidence of secondary breast cancer was conducted in Italy [16]. A total of 2,972 women with stage I breast cancer were randomized to receive 200 mg/day of fenretinide or no drug for 5 years. After a median follow-up of 97 months, fenretinide showed no effect on contralateral breast cancer occurrence and a nonsignificant 17% reduction in ipsilateral breast tumor reappearance. However, when menopausal status was considered, fenretinide significantly reduced the occurrences of both contralateral and ipsilateral breast cancer incidence in premenopausal women (HR=0.66, 95% CI=0.41–1.07; and HR=0.65.95% CI=0.46–0.92, respectively). In postmenopausal women, an opposite trend was observed in which fenretinide slightly increased the incidence of contralateral and ipsilateral breast cancer. Recently, an updated analysis after 14.6 years of follow-up of 1,739 women demonstrated similar results [17], showing the continuous protective effect of fenretinide in the premenopausal women even 10 years after cessation. Thus, these results suggest a beneficial effect of fenretinide only in premenopausal women. More importantly, fenretinide was observed to reduce second tumors in premenopausal women irrespective of the hormone receptor status of the primary cancer, suggesting that retinoids have a chemopreventative effect on ER-negative as well as ER-positive breast cancer.
Recently, RXR-selective retinoids, commonly referred as rexinoids, have been studied as cancer preventative agents. Rexinoids bind primarily to RXR, a multifunctional nuclear receptor that can form heterodimers with many different nuclear receptors including RAR, vitamin D receptor (VDR), peroxisome proliferator-activated receptor (PPAR), liver X receptor (LXR), and Nurr77. Preclinical studies have demonstrated that rexinoids maintain the chemopreventative effect of naturally occurring retinoids, but have greatly reduced toxicity. Wu et al. found that 9cRA, a retinoid that binds both RAR and RXR, significantly delayed the ER-negative tumor development in SV40 Tag mice and N-methyl-N-nitrosourea (MNU)-treated rats [15, 18]. However, 9cRA induced significant cutaneous toxicity including hair loss and skin erythema [18]. In contrast, the RXR-selective rexinoid bexarotene (LGD1069) demonstrated a similar cancer preventative effect in SV40 Tag mice with no observed toxicity [19]. The RAR-selective retinoid TTNPB was found to be highly toxic and minimally efficacious in suppressing mammary tumorigenesis in the same animal model [19]. Thus, these results suggested that the toxicity of retinoids is primarily mediated by the RAR signaling pathway, while the anticancer effect of retinoids is mediated by RXR-dependent pathways. Due to their favorable toxicity profile, rexinoids are particular attractive cancer preventative agents. In addition to its effect in SV40 Tag mice, bexarotene has also been shown to delay mammary tumor development in Mouse mammary tumor virus (MMTV)-ErbB2 transgenic mice and P53-null mice, two animal models that develop ER-negative breast cancers [20]. In the MMTV-ErbB2 transgenic mice, which exclusively form ER-negative mammary tumors, median time to tumor development was reduced from 230 days in the vehicle group vs 416 days in the bexarotene-treated mice. At the time when all the vehicle-treated mice have developed tumors, only 24% of the high-dose bexarotene-treated mice had tumors. Cutaneous toxicity was mild and only observed in the high-dose group after many months of treatment (an average of 205 days). These promising findings led to the development of a phase II clinical trial at Baylor College of Medicine to test the preventative efficacy of bexarotene in women at high risk of breast cancer. The results of this study were recently presented at the San Antonio Breast Cancer Symposium [21]. Bexarotene was found to reduce cyclin D1 RNA expression in breast cells from postmenopausal women (but not premenopausal women). A similar, but nonsignificant reduction in breast cell proliferation was seen in these post-menopausal women. These results demonstrate that bexarotene has a biological effect on breast cells in women at high risk of breast cancer.
Although bexarotene has promise as a drug to prevent breast cancer development, previous clinical trials using bexarotene to treat cutaneous T-cell lymphoma demonstrated that it has some adverse effects including hyperlipidemia, cutaneous toxicity, and rare mild hypothyroidism [22]. The toxicity of bexarotene may be attributed to its weak RAR-binding activity. Recently, a more RXR-specific rexinoid, LG100268, has been developed. This rexinoid has no detectable RAR-binding activity, and thus it is likely to be less toxic than bexarotene. Recently, we found that LG100268 is more effective than bexarotene in preventing the ER-negative mammary tumor development in MMTV-ErbB2 mice [23]. High-dose LG100268 treatment has almost totally prevented tumor development in MMTV-ErbB2 mice. Most importantly, no skin toxicity was observed in LG100268-treated mice. We also found that LG100268 significantly prevents the developments of premalignant lesions including hyperplasia and DCIS, suggesting that rexinoids might prevent both the initiation and progression of mammary tumorigenesis [23].
Although significant progress has been made toward understanding the RAR/RXR-mediated signaling pathway, the mechanism by which retinoids suppress carcinogenesis is still poorly understood. It is well accepted that retinoids exert their anticancer effects through altering the expression of genes regulating cell proliferation and apoptosis. These alterations are achieved through activation or repression of key signaling pathways including RAR/RXR, AP-1, mitogen-activated protein (MAP) kinases, and PI3/Akt pathways. Our preclinical data indicated that bexarotene and LG100268 prevent mammary tumorigenesis primarily through an antiproliferation effect. Rexinoids can either upregulate growth-inhibiting proteins such as RARβ, IGFBP-3, TGFβ, and DEC2, or downregulate growth-promoting proteins such as cyclin D1 and COX-2 [24]. All these changes lead to cell cycle blockade and/or induction of apoptosis. Considering the promiscuous nature of RXR protein, which can bind to a variety of nuclear receptors, the chemopreventative activity of rexinoids is due to regulation of a complex of multiple signaling pathways, rather than a single, specific mechanism.
13.4 Vitamin D Receptor
VDR is a nuclear receptor that modulates gene expression when activated by its ligand 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), a biologically active form of vitamin D. Activated VDR then forms a dimer with RXR, binds to VDR response element, and regulates the transcription of target genes (Fig. 13.2). Therefore, downstream signaling pathways of VDR may share some of the same pathways activated by rexinoids. 1,25(OH)2D3 has been shown to inhibit cell proliferation and promote apoptosis in breast cancers independent of ER status. Thus, vitamin D compounds that target VDRs are potential chemopreventative agents for ER-negative breast cancer. Consistent with these results, results from epidemiological studies suggest an inverse association exits between sunlight exposure (a major source of endogenous vitamin D production) and breast cancer incidence. Recent prospective cohort studies have analyzed the effect of vitamin D intake on breast cancer incidence regarding menopausal status. The four studies of vitamin D intake in postmenopausal women did not find significant correlation between vitamin D intake and breast cancer risk [25]. In contrast, the two prospective studies of premenopausal women demonstrated that vitamin D intake was associated with significant breast cancer risk reduction (35% reduction in the Women’s Health Study [26], and 34% reduction in women in the Nurse Health Study [27]).
In preclinical studies, 1,25(OH)D3 was shown to inhibit the growth of breast cancer cells in an ER-independent manner and reduce the risk of mammary tumors in animal models [28, 29]. However, the use of 1,25(OH)D3 for cancer prevention is hindered because of its hypercalcemic toxicity. To overcome this problem, several less calcemic vitamin D analogs have been synthesized and evaluated for their chemopreventative effects. Among them, 1alpha-hydroxy-24-ethylcholecalciferol (1alpha(OH)D5) and 22-oxa-1,25-(OH)2D3 (OCT) were found to inhibit the proliferation of both ER-positive and ER-negative breast cancer cells [30, 31]. Intratumor administration of OCT remarkably delayed the growth of human derived ER-negative breast cancer cell line (MX-1) implanted in athymic mice [29]. These results suggest that these analogs are promising agents to prevent the development of ER-negative breast cancers.
13.5 EGFR/Tyrosine Kinase Inhibitors
Estrogen, retinoids, and vitamin D regulate cell growth and differentiation through activation of nuclear receptors. Peptide growth factor receptors represent a different group of signaling molecules that are critical for the growth and differentiation of both normal and malignant tissues. Among these peptide growth factor receptors, the erbB family of type I tyrosine kinase receptors has been implicated in the development of breast cancer. ErbB receptors include epidermal growth factor receptor (EGFR; also termed HER-1 or ErbB1), ErbB2 (also termed HER-2 or neu), ErbB3 (HER-3), and ErbB4 (HER-4) (see Fig. 13.1). All four members have an extracellular ligand-binding domain, a single transmembrane domain, and an intracellular domain with tyrosine kinase activity. Ligand binding to the extracellular domain of ErbB receptors induces the auto- or heterodimerization of the ErbB family members and activates the intrinsic tyrosine kinase activity, resulting in phosphorylation of the specific tyrosine residue within the intracellular domain. Phosphorylated tyrosine residues then recruit effector proteins and activate downstream signal transduction cascades such as MAP kinase pathway, PI3K–AKT pathway, signal transducer and activator of transcription (STAT) pathway, and mammalian target of rapamycin (mTOR). Activation of these effectors leads to cell proliferation and increased survival ability, which promote breast cancer development independent of ER status. Thus, agents that block the erbB signaling pathways are promising agents to treat and prevent breast cancer.
Two strategies have been used to inhibit ErbB activity: the first involves blockade with monoclonal antibodies and the second the use of small molecule kinase inhibitors to inhibit ErbB activity. Monoclonal antibodies directly block the peptide binding at the extracellular domain. The monoclonal antibodies strategy has been particularly effective. Trastuzumab, a monoclonal antibody against HER-2 receptor, is highly effective in treating HER2-positive breast cancers. However, monoclonal antibody treatment may be difficult in women without breast cancer. Therefore, most chemopreventative studies have been conducted with small molecule tyrosine kinase inhibitors (TKI) due to their favorable oral bioavailability, potentially less toxicity, ability to inhibit truncated forms of EGFR and HER2 receptors (EGFR VIII and p95), and their ability to target multiple ErbB receptors.
Lenferink et al. found that blockade of the EGFR with tyrosine kinase inhibitor AG-1487 significantly delayed breast tumorigenesis in MMTV/neu+MMTV/TGF-alpha bigenic mice [32]. The delay was associated with inhibition of EGFR and neu signaling, reduction of cyclin-dependent kinase 2 (Cdk2) and MAPK activities, downregulation of cyclin D1, and an increase in the levels of the cell cycle inhibitor p27. Recently, our laboratory has demonstrated that gefitinib (ZD1839 or Iressa), an EGFR tyrosine kinase inhibitor, suppressed ER-negative mammary tumor formation in MMTV-ErbB2 transgenic mice [33]. Median time to tumor development was significantly delayed from 140 days of vehicle treatment to 220 days of high-dose gefitinib treatment. Moreover, we also demonstrated a strong growth-inhibitory effect of gefitinib in normal human mammary epithelial cells, which supports its role as a chemopreventative agent. We further observed that gefitinib prevented the development of pre-neoplastic diseases including hyperplasia, mammary intraepithelial neoplasia (MIN), and invasive breast cancer after 4 month of treatment, suggesting that gefitinib prevents cancer development at its early stages [33]. However, the rare side effects of gefitinib have limited its clinical use. In patients with lung cancer, gefitinib use was observed to be associated with interstitial lung disease (overall incidence at about 1%) [34]. Concerns about this potentially serious side effect caused the FDA to halt clinical cancer prevention trials using gefitinib.
Since erbB receptors can form heterodimers with other erbB proteins, blocking a single erbB receptor might induce the activity of other erbB heterodimers and result in drug resistance. Preclinical studies demonstrated that dual inhibition of ErbB-1 and ErbB-2 tyrosine kinases exerted greater biological effects in inhibiting cell proliferation and survival than inhibition of either receptor alone [35]. To obtain better anticancer activity, dual kinase inhibitors or pan-ErbB inhibitors, which target more than one erbB receptor, have been developed.
Lapatinib (GW572016, Tykerb) is a dual kinase receptor that targets both EGFR and ErbB2 receptors. It has been shown to inhibit tumor cell growth in vitro and in xenograft models for a variety of human tumors. Several clinical studies demonstrated that lapatinib was effective to treat ErbB1 and/or ErbB2 overexpressing metastatic breast cancers and trastuzumab-resistant breast cancers [36]. Recently, the FDA approved lapatinib to be used in combination with capecitabine (Xeloda) for patients with advanced or metastatic breast cancer whose tumors overexpress HER2 [and who have received prior therapy including an anthracycline, a taxane, and trastuzumab (Herceptin)]. Our group has studied the cancer preventative activity of lapatinib. The results from these studies showed that lapatinib significantly delayed breast cancer development in MMTV-ErbB2 transgenic mice (T.E. Strecker and P.H. Brown, in preparation). Like gefitinib, lapatinib also prevented the development of premalignant mammary lesions in these mice, suggesting that lapatinib inhibited both the initiation and progression of mammary carcinogenesis. The anticancer effect was associated with proliferation inhibition and apoptosis promotion, as well as reduced activation of downstream signaling effectors such as Erk1/2 and AKT.
Many other novel multitarget inhibitors have been developed. These include: HKI-272, BIBW-2992, and BMS-599626 targeting EGFR and ErbB2; CI-1033 targeting EGFR, ErbB2, and erbB4; and ZD6474 and AEE788 targeting EGFR, ErbB2, and VEGFR. All these agents are currently undergoing clinical trials for the treatment of solid tumors and breast cancers. Selection of appropriate candidate agents for prevention studies will depend heavily on the toxicity profiles of these agents.
13.6 COX-2 Inhibitors
Accumulating epidemiology data suggest that long-term usage of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with reduced risk of cancer from various tissues, especially the digestive tract. Recently, more data have linked NSAID usage to breast cancer. A number of observation studies demonstrated that NSAID usage was associated with a 20% reduction in risk of breast cancer [38], suggesting the chemopreventative potential of NSAIDs on breast cancer. The main target of NSAIDs is cyclooxygenase (COX), which consists of two isoforms, COX-1 and COX-2. COX enzymes catalyze the conversion of arachidonic acid to prostaglandin G2 (PGG2), which is further catalyzed by the peroxidase activity of COX to PGH2, a common precursor for all other prostanoids including PGI2, PGE2, PGF2, PGD2, and TXA2. COX-1 and COX-2 have similar catalytic activities but distinct expression patterns. COX-1 is constitutively expressed whereas COX-2 is expressed only under certain stimuli including growth factors, tumor promoters, and cytokines. Moreover, COX-2 is activated by many oncogenes including v-src, v-Ha-ras, and HER-2/neu. Aberrant expression of COX-2 is a marker of poor prognosis in human breast cancer and correlates with increased tumor size, negative ER status, HER-2 overexpression, and the presence of metastatic lesions. This correlation between COX-2 expression and breast cancer prognosis, as well as the results of several prevention studies that showed that NSAIDs prevent the development of breast cancer in rats and mice, indicates that COX-2 may be a useful target for breast cancer prevention. Celecoxib, a selective COX-2 inhibitor, has been shown to reduce the incidence and multiplicity of DMBA-induced mammary tumors in rat models by 68% and 86%, respectively [39]. Nimesulide, another selective COX-2 inhibitor, significantly reduced the incidence and multiplicity of PhIP- (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) and NMU-induced rat mammary tumors [40]. In MMTV-ErbB2 transgenic mice, which develop ER-negative cancers, celecoxib at 500 ppm delayed the onset of mammary tumor development and decreased the PGE2 level by 50%, suggesting that COX-2 inhibitors might be useful to prevent ER-negative breast cancer [41].
Compared to NSAIDs, COX-2 inhibitors have less gastrointestinal toxicity, which is believed to be due to COX-1 inhibition. This led to extensive clinical testing of the chemopreventative effect of selective COX-2 inhibitors. However, due to their selective inhibition of PGI2 synthesis, COX-2 inhibitors were found to increase the risk of thrombotic cardiovascular incidents. These rare but serious side effects have essentially halted the development of COX-2 inhibitors as cancer prevention agents. Therefore, researchers are searching for alternative strategies to antagonize the COX-2 pathway. Downstream activation of the COX-2 product, PGE2, is an important mediator for tumorigenesis. Blocking PGE2 activity through targeting prostanoid receptors (EP receptors) is thought to be a promising strategy to prevent cancer development [42, 43]. New agents targeting alternative COX-2 pathways are expected to retain the anticancer activity of COX-2 inhibitors, but may have reduced side effects. These new strategies will be the focus of future studies targeting COX-2 pathways.
13.7 Statins
Statins are 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (rate-limiting enzyme for mevalonate synthesis) inhibitors that are widely used in United States to lower the plasma cholesterol level and reduce mortality from cardiovascular disease. Recently, numerous observational and clinical studies indicate that statin usage may have potential beneficial effects on breast cancer risk. Lipophilic statins, which can permeate the cell membranes and affect cell and tumor growth in vitro and in vivo, have been found to be associated with a 50% reduction of breast cancers incidence in large observation studies [44]. In contrast, Bonovas et al. performed a meta-analysis of seven large randomized controlled trials (RCT) and nine observational trials (four cohort and five case-control) showing that there is no association between statin usage and breast cancer risk [45]. However, these divergent results have been criticized due to their multiple limiting factors including small numbers of cases, multiple statins, doses, and treatment durations, making any conclusions less than convincing. Thus, the cancer preventative potential of statins remains unclear.
Although the beneficial effects of statins on breast cancer development remain controversial, there is strong preclinical evidence suggesting that lipophilic statins can inhibit ER-negative breast tumor growth. Atorvastatin, lovastatin, simvastatin, and fluvastatin have been found to significantly inhibit the in vitro proliferation of both ER-positive and ER-negative breast cancer cell lines. Inhibition was between 10% and 90%, with greater efficacy observed in ER-negative cancer cells [46]. In addition, statins have been shown to reduce tumor growth in mouse models of ER-negative breast cancer [47, 48]. Laboratory investigations imply that the anticancer effects of statins may involve reducing levels of mevalonate and its downstream products such as isoprenoid intermediates that provide lipid attachment sites for activated Ras, Rac, and Rho family members. All these cytoplasmic signaling molecules affect important cancer pathways including apoptosis, proliferation, angiogenesis, and immune response, and ultimately lead to inhibition of tumor initiation and growth. Statins are currently being widely used to reduce hyperlipidemia and have been found to be relatively safe and well tolerated. It is possible that statins could promote health by reducing the risk of heart disease as well as cancer. However, the wide use of statins has made it very difficult to design randomized clinical trials to assess the breast cancer preventative effect of statins.
In addition to the agents summarized above, there is a growing list of molecularly targeted agents that block critical signaling pathways in cancer cells. Promising agents include PPAR ligands, imatinib mesylate (Gleevec), demethylating agents, histone deacetylase inhibitors, polyamine synthesis inhibitors, metalloprotease inhibitors, angiogenesis inhibitors, and triterpenoids. Future preclinical and clinical studies are needed to determine the efficacy of these agents in preventing ER-negative breast cancers.
13.8 Combination Chemoprevention
It is well accepted that carcinogenesis is a multistep process that involves the activation of complex signal transduction pathways. Breast cancer has many different subtypes that have different responses to specific anticancer agents. Therefore, many targeted agents are only effective in a specific subgroup of breast cancers. The ultimate aim of chemoprevention is to prevent all breast cancers. To achieve this goal, combination chemoprevention offers a promising approach.
Crosstalk between the ER pathway and the EGFR/ErbB2 pathway has been shown to contribute to tamoxifen resistance. Thus, coadministration of antiestrogens with EGFR or ErbB2 inhibitors may not only increase the efficacy of antiestrogens to prevent ER-positive cancer, but may also prevent the development of ER-negative cancer. In addition, preclinical studies have shown that combinations of SERMs with rexinoids effectively prevent breast cancer in transgenic mice when compared to either agent alone [49]. Results from Michael Sporn’s lab demonstrate that arzoxifene and rexinoid LG100268 together prevent the development of both ER-positive and ER-negative breast cancers in animal models [49, 50].
The combination of other chemopreventative agents that target nonendocrine signaling pathways represent novel approaches to prevent both ER-positive and ER-negative breast cancer. Promising combinations include PPAR-gamma ligands and rexinoids; EGFR inhibitors and COX-2 inhibitors; and rexinoid and COX-2 inhibitors (P. Brown, unpublished observation). Besides improved effectiveness, a potential advantage of combination chemoprevention is through decreasing the dose of each individual agent, which would likely decrease the incidence of adverse effects. Considering the complex nature of cancer and the safety requirement for preventative agents, combination chemoprevention is likely to offer the greatest efficacy with the least toxicity.
13.9 Conclusion
Clinical cancer prevention studies have demonstrated that SERMs reduce the incidence of breast cancer and that chemoprevention is clinically feasible. Current chemoprevention studies are now testing the ability of AIs to prevent breast cancer. However, while SERMs are, and AIs may be, effective agents to prevent ER-positive breast cancer, they have no effect in reducing the incidence of ER-negative breast cancers. Through a better understanding of the estrogen-independent pathways that lead to mammary tumorigenesis, a growing number of chemopreventative agents have emerged that prevent ER-negative breast cancers in preclinical models. Rexinoids, COX-2 inhibitors, and EGFR tyrosine kinase inhibitors are the most promising agents that have been shown to prevent ER-negative tumorigenesis. Despite the promising effect of these novel agents, issues of safety and toxicity still hamper progress in the field. Clinically observed toxicity has adversely affected several ongoing chemoprevention trials including those of celecoxib and gefitinib. While many of these drugs are tolerated by cancer patients, the severity and frequency of side effects becomes a major concern when considering chronic preventative therapy in healthy women. Thus, future clinical studies of chemoprevention will depend heavily on the balance between efficacy and tolerability. With breast cancer risk assessment, it becomes critical to select the high-risk women who will be benefit most from chemoprevention. More recently, preclinical studies have shown that combination chemoprevention is a promising strategy that will greatly enhance the efficacy of cancer preventative effect. Thus, to ultimately prevent all forms of breast cancers, it will be necessary to combine safe and effective drugs targeting the ER as well as drugs inhibiting critical estrogen-independent pathways.
Acknowledgement
This work was supported by NCI/NIH grant RO1 CA10121.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Monninkhof EM, Elias SG, Vlems FA, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 3.Michels KB, Mohllajee AP, Roset-Bahmanyar E, Beehler GP, Moysich KB. Diet and breast cancer: a review of the prospective observational studies. Cancer. 2007;109:2712–2749. doi: 10.1002/cncr.22654. [DOI] [PubMed] [Google Scholar]
- 4.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids) Fed Proc. 1976;35:1332–1338. [PubMed] [Google Scholar]
- 5.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451–1467. [No authors listed] [PubMed] [Google Scholar]
- 6.Powles TJ, Ashley S, Tidy A, Smith IE, Dowsett M. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 8.Veronesi U, Maisonneuve P, Rotmensz N, et al. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99:727–737. doi: 10.1093/jnci/djk154. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer—96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 10.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 11.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Brown PH. Translational approaches for the prevention of estrogen receptor-negative breast cancer. Eur J Cancer Prev. 2007;16:203–215. doi: 10.1097/CEJ.0b013e328011ed98. [DOI] [PubMed] [Google Scholar]
- 13.Hong WK, Lippman SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323:795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 14.Moon RC, Thompson HJ, Becci PJ, et al. N-(4-Hydroxyphenyl)retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res. 1979;39:1339–1346. [PubMed] [Google Scholar]
- 15.Anzano MA, Byers SW, Smith JM, et al. Prevention of breast cancer in the rat with 9-cis-retinoic acid as a single agent and in combination with tamoxifen. Cancer Res. 1994;54:4614–4617. [PubMed] [Google Scholar]
- 16.Veronesi U, De Palo G, Marubini E, et al. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst. 1999;91:1847–1856. doi: 10.1093/jnci/91.21.1847. [DOI] [PubMed] [Google Scholar]
- 17.Veronesi U, Mariani L, Decensi A, et al. Fifteen-year results of a randomized phase III trial of fenretinide to prevent second breast cancer. Ann Oncol. 2006;17:1065–1071. doi: 10.1093/annonc/mdl047. [DOI] [PubMed] [Google Scholar]
- 18.Wu K, Kim HT, Rodriquez JL, et al. 9-cis-Retinoic acid suppresses mammary tumorigenesis in C3(1)-simian virus 40 T antigen-transgenic mice. Clin Cancer Res. 2000;6:3696–3704. [PubMed] [Google Scholar]
- 19.Wu K, Kim HT, Rodriquez JL, et al. Suppression of mammary tumorigenesis in transgenic mice by the RXR-selective retinoid, LGD1069. Cancer Epidemiol Biomarkers Prev. 2002;11:467–474. [PubMed] [Google Scholar]
- 20.Wu K, Zhang Y, Xu XC, et al. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptornegative mammary tumors in transgenic mice. Cancer Res. 2002;62:6376–6380. [PubMed] [Google Scholar]
- 21.Brown P, Arun B, Miller A, et al. Prevention of breast cancer using rexinoids: results of a phase II biomarker modulation trial using bexarotene in women at high risk of breast caner. 30th Annual San Antonio Breast Cancer Symposium; San Antonio. 2007. p. S181. [Google Scholar]
- 22.Querfeld C, Rosen ST, Guitart J, et al. Comparison of selective retinoic acid receptor- and retinoic X receptor-mediated efficacy, tolerance, and survival in cutaneous T-cell lymphoma. J Am Acad Dermatol. 2004;51:25–32. doi: 10.1016/j.jaad.2003.11.058. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Zhang Y, Hill J, et al. The rexinoid LG100268 prevents the development of preinvasive and invasive estrogen receptor negative tumors in MMTV-erbB2 mice. Clin Cancer Res. 2007;13:6224–6231. doi: 10.1158/1078-0432.CCR-06-2681. [DOI] [PubMed] [Google Scholar]
- 24.Kim HT, Kong G, Denardo D, et al. Identification of biomarkers modulated by the rexinoid LGD1069 (bexarotene) in human breast cells using oligonucleotide arrays. Cancer Res. 2006;66:12009–12018. doi: 10.1158/0008-5472.CAN-05-2515. [DOI] [PubMed] [Google Scholar]
- 25.Cui Y, Rohan TE. Vitamin D, calcium, and breast cancer risk: a review. Cancer Epidemiol Biomarkers Prev. 2006;15:1427–1437. doi: 10.1158/1055-9965.EPI-06-0075. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;167:1050–1059. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- 27.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin d and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–1311. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 28.Colston KW, Perks CM, Xie SP, Holly JM. Growth inhibition of both MCF-7 and Hs578T human breast cancer cell lines by vitamin D analogues is associated with increased expression of insulin-like growth factor binding protein-3. J Mol Endocrinol. 1998;20:157–162. doi: 10.1677/jme.0.0200157. [DOI] [PubMed] [Google Scholar]
- 29.Abe J, Nakano T, Nishii Y, Matsumoto T, Ogata EIkeda K. A novel vitamin D3 analog, 22-oxa-1,25-dihydroxyvitamin D3, inhibits the growth of human breast cancer in vitro and in vivo without causing hypercalcemia. Endocrinology. 1991;129:832–837. doi: 10.1210/endo-129-2-832. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto H, Iino Y, Koibuchi Y, et al. Antitumor effect of 22-oxacalcitriol on estrogen receptor-negative MDA-MB-231 tumors in athymic mice. Oncol Rep. 1999;6:349–352. [PubMed] [Google Scholar]
- 31.Hussain EA, Mehta RR, Ray R, Das Gupta TK, Mehta RG. Efficacy and mechanism of action of 1alpha-hydroxy-24-ethyl-cholecalciferol (1alpha[OH]D5) in breast cancer prevention and therapy. Recent Results Cancer Res. 2003;164:393–411. doi: 10.1007/978-3-642-55580-0_29. [DOI] [PubMed] [Google Scholar]
- 32.Lenferink AE, Simpson JF, Shawver LK, Coffey RJ, Forbes JT, Arteaga CL. Blockade of the epidermal growth factor receptor tyrosine kinase suppresses tumorigenesis in MMTV/Neu+MMTV/TGF-alpha bigenic mice. Proc Natl Acad Sci U S A. 2000;97:9609–9614. doi: 10.1073/pnas.160564197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu C, Speers C, Zhang Y, et al. Effect of epidermal growth factor receptor inhibitor on development of estrogen receptor-negative mammary tumors. J Natl Cancer Inst. 2003;95:1825–1833. doi: 10.1093/jnci/djg117. [DOI] [PubMed] [Google Scholar]
- 34.Nagaria NC, Cogswell J, Choe JK, Kasimis B. Side effects and good effects from new chemotherapeutic agents. Case 1. Gefitinib-induced interstitial fibrosis. J Clin Oncol. 2005;23:2423–2424. doi: 10.1200/JCO.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 35.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61:8887–8895. [PubMed] [Google Scholar]
- 36.Bilancia D, Rosati G, Dinota A, Germano D, Romano R, Manzione L. Lapatinib in breast cancer. Ann Oncol. 2007;18(Suppl 6):vi26–vi30. doi: 10.1093/annonc/mdm220. [DOI] [PubMed] [Google Scholar]
- 37.Reference deleted in proof
- 38.Khuder SA, Mutgi AB. Breast cancer and NSAID use: a meta-analysis. Br J Cancer. 2001;84:1188–1192. doi: 10.1054/bjoc.2000.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60:2101–2103. [PubMed] [Google Scholar]
- 40.Nakatsugi S, Ohta T, Kawamori T, et al. Chemoprevention by nimesulide, a selective cyclooxygenase-2 inhibitor, of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary gland carcinogenesis in rats. Jpn J Cancer Res. 2000;91:886–892. doi: 10.1111/j.1349-7006.2000.tb01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howe LR, Subbaramaiah K, Patel J, et al. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 2002;62:5405–5407. [PubMed] [Google Scholar]
- 42.Thorat MA, Morimiya A, Mehrotra S, Konger R, Badve SS. Prostanoid receptor EP1 expression in breast cancer. Mod Pathol. 2008;21:15–21. doi: 10.1038/modpathol.3800970. [DOI] [PubMed] [Google Scholar]
- 43.Ma X, Kundu N, Rifat S, Walser T, Fulton AM. Prostaglandin E receptor EP4 antagonism inhibits breast cancer metastasis. Cancer Res. 2006;66:2923–2927. doi: 10.1158/0008-5472.CAN-05-4348. [DOI] [PubMed] [Google Scholar]
- 44.Kumar AS, Campbell M, Benz CC, Esserman LJ. A call for clinical trials: lipophilic statins may prove effective in treatment and prevention of particular breast cancer subtypes. J Clin Oncol. 2006;24:2127–2128. doi: 10.1200/JCO.2005.04.9882. [DOI] [PubMed] [Google Scholar]
- 45.Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. 2005;23:8606–8612. doi: 10.1200/JCO.2005.02.7045. [DOI] [PubMed] [Google Scholar]
- 46.Mueck AO, Seeger H, Wallwiener D. Effect of statins combined with estradiol on the proliferation of human receptor-positive and receptor-negative breast cancer cells. Menopause. 2003;10:332–336. doi: 10.1097/01.GME.0000055485.06076.00. [DOI] [PubMed] [Google Scholar]
- 47.Shibata MA, Ito Y, Morimoto J, Otsuki Y. Lovastatin inhibits tumor growth and lung metastasis in mouse mammary carcinoma model: a p53-independent mitochondrial-mediated apoptotic mechanism. Carcinogenesis. 2004;25:1887–1898. doi: 10.1093/carcin/bgh201. [DOI] [PubMed] [Google Scholar]
- 48.Campbell MJ, Esserman LJ, Zhou Y, et al. Breast cancer growth prevention by statins. Cancer Res. 2006;66:8707–8714. doi: 10.1158/0008-5472.CAN-05-4061. [DOI] [PubMed] [Google Scholar]
- 49.Liby K, Rendi M, Suh N, et al. The combination of the rexinoid, LG100268, and a selective estrogen receptor modulator, either arzoxifene or acolbifene, synergizes in the prevention and treatment of mammary tumors in an estrogen receptor-negative model of breast cancer. Clin Cancer Res. 2006;12:5902–5909. doi: 10.1158/1078-0432.CCR-06-1119. [DOI] [PubMed] [Google Scholar]
- 50.Suh N, Lamph WW, Glasebrook AL, et al. Prevention and treatment of experimental breast cancer with the combination of a new selective estrogen receptor modulator, arzoxifene, and a new rexinoid, LG 100268. Clin Cancer Res. 2002;8:3270–3275. [PubMed] [Google Scholar]