Abstract
Background Importance of the field
Therapeutic proteins and DNA constructs offer promise for the treatment of central nervous system disorders, yet significant biological barriers limit the ability of these molecules to reach the central nervous system from the bloodstream. Direct administrations to the cerebrospinal fluid (intrathecal administration) comprise an emerging field to facilitate the efficient delivery of these biological macromolecules to central nervous system tissues.
Areas covered in this review
Previous reports from 1990 to the present time describing the interactions and turnover of the cerebrospinal fluid within the intrathecal space, characterizations of the effects that therapeutic proteins and DNA have exhibited after intrathecal delivery via a lumbar route, and reports of emerging technologies to address the limitations of intrathecally administered macromolecules are reviewed.
What the reader will gain
This review provides an overview of the limitations that must be overcome for intrathecally administered biological macromolecules and the recent advances and promising approaches for surmounting these limitations.
Take home message
Emerging approaches that stabilize and sustain the delivery of intrathecally administered biological macromolecules may substantially enhance the clinical relevance of promising therapeutic proteins and DNA constructs for the treatment of various central nervous system disorders.
Keywords: Blood-brain barrier, Central nervous system, Intrathecal, Microparticle, PEGylation, PLGA
1. Introduction
There is a key need to facilitate the clinical relevance of therapeutic treatments for central nervous system disorders. Even after decades of aggressive research in the area, the number of people suffering from debilitating or fatal central nervous system diseases still far outnumbers those dying of all types of systemic cancer or heart disease, central nervous system disorders remain the world’s leading cause of disability, and necessitate more hospitalizations and prolonged care than almost all other diseases combined [1]. Debilitating central nervous system disorders include brain tumors [2], epilepsy [3], cerebrovascular diseases [4], neurodegenerative disorders including the widespread Parkinson’s [5] and Alzheimer’s diseases [6], multiple sclerosis or autoimmune encephalopathy [7], and chronic neuropathic pain [8]. Significant biological barriers that impede the delivery of drugs to the brain and spinal cord have dictated the physical properties of potential drug candidates for many of these disorders, and have consequently limited the number of clinically relevant treatment approaches for many of these conditions.
Biological macromolecules, including therapeutic proteins and DNA, are among the list of promising drug candidates that are particularly limited by the formidable obstacles of brain and spinal cord drug delivery. Currently promising macromolecules for central nervous system delivery include therapeutic neurotrophins [9, 10] and anti-inflammatory cytokines [11, 12] along with therapeutic plasmid DNA facilitating the endogenous production of neurotrophins [13, 14] and therapeutic cytokines [15, 16]. The development of improved delivery methods is required to render these macromolecule-based therapeutic approaches clinically relevant.
2.1 Intrathecal administration for delivery to the central nervous system
The blood-brain barrier, which has been extensively reviewed previously [1, 17], is the major obstacle for macromolecular delivery to the brain and spinal cord. Briefly, the blood-brain barrier is a membranous barrier consisting of blood capillaries that are structurally different from blood capillaries in other tissues, as capillaries of the brain and spinal cord lack the small pores that allow the rapid movement of solutes from the circulation into organs. These capillaries are lined with a layer of special endothelial cells that lack fenestrations and are sealed with tight junctions, similar to the barriers formed in the skin, bladder, colon and lung, which render the brain and spinal cord practically inaccessible to water-soluble compounds, such as polar molecules and small ions from the bloodstream. Passage of water-soluble compounds from the blood to the cerebrospinal fluid (CSF) surrounding the spinal cord is also limited by this same barrier, and can be referred to as the blood-CSF barrier. Overall, these tight barriers typically act to protect the brain and spinal cord from systemic microbial and viral infiltration, and often protect brain and spinal cord tissues from harmful toxins in the bloodstream, but have also significantly limited the currently available oral and parenteral therapeutic treatments for central nervous system disorders to water-insoluble compounds.
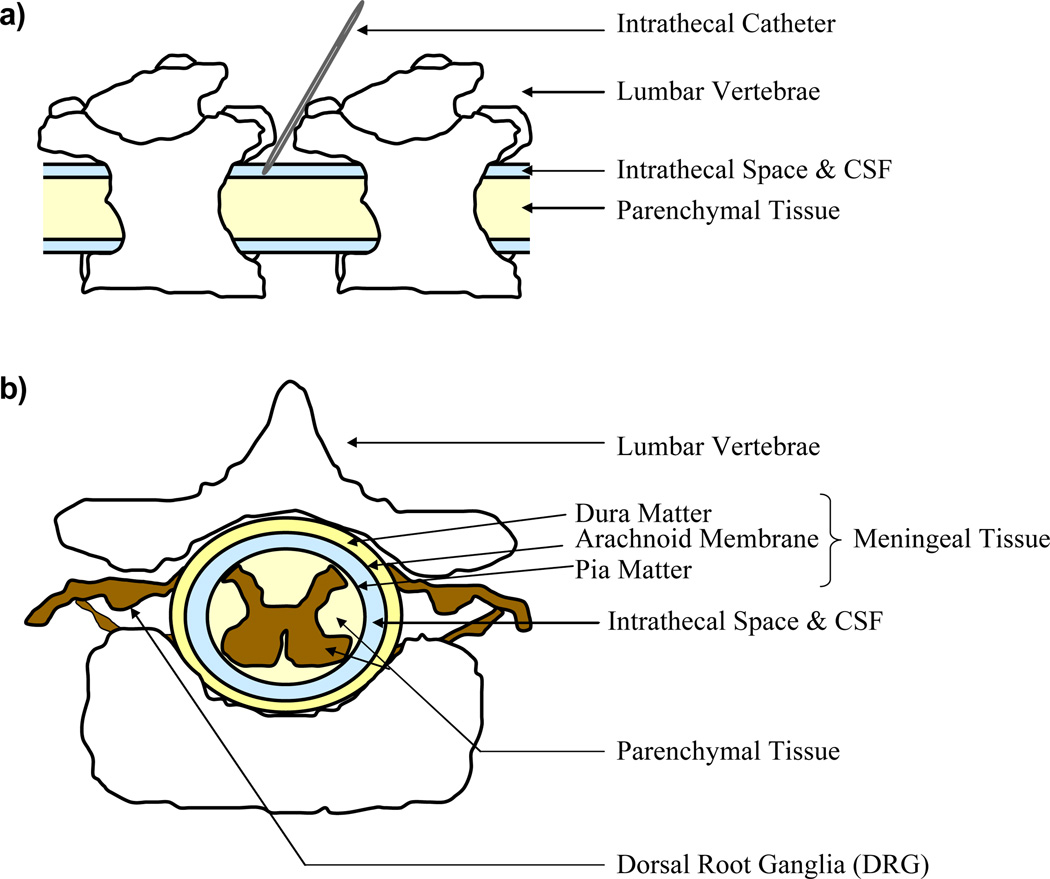
Novel strategies that enable water-soluble molecules to cross the blood-brain barrier have been developed and reviewed previously. These efforts include the use of receptor-mediated transport systems, peptidomimetic monoclonal antibodies and particulate drug carrier systems [17, 18]. While these efforts show some promise, the ability to deliver biological macromolecules directly to the CSF, which bathes the brain and spinal cord, is currently one of the most promising approaches that can surmount existing delivery barriers. The administration of a drug to the CSF surrounding the spinal cord is known as intrathecal administration, since the drug is delivered to the intrathecal space of the spinal cord (Figure 1a). The intrathecal space is bordered by the spinal cord pia matter on the inside and the arachnoid membrane on the outside, and as such it is also commonly referred to as the subarachnoid space (Figure 1b). Intrathecal delivery offers several advantages, beyond the obvious advantage of bypassing the blood-brain barrier, for the administration of biological macromolecules to the spinal cord. Many active drugs are more potent and safer when injected into the intrathecal space, and due to the increased proximity to the target tissue smaller dosages can be used which potentially minimizes systemic toxicity [19]. Additionally, some drugs encounter decreased enzymatic activity in the CSF relative to drugs in the plasma, and because the CSF exchanges molecules with the interstitial fluid of the brain and spinal cord parenchyma (interior tissue), delivery to the CSF can theoretically increase resultant parenchymal drug concentrations [1].
Figure 1.
Intrathecal Administration and the Intrathecal Space. (a) Schematic diagram of an intrathecal administration. (b) Schematic diagram of the spinal cord and the intrathecal space.
2.2 Historical Basis of Intrathecal Administration
Intrathecal administrations were primarily developed and have most commonly been used for the delivery of analgesics (pain medications), as other treatment avenues are ineffective and lead to unacceptable side effects for a large percentage of those suffering from acute and chronic pain [20, 21]. Even though the intrathecal administration technique must be performed by skilled medical personnel in aseptic conditions [19], current clinical practices in spinal pain intervention by intrathecal administration can be done on an outpatient basis, exhibit a good safety record with a low rate of complications, and have led to considerable improvements in the quality of life for the treatment populations [22, 23]. Although intrathecal delivery strategies were not commonly used until after the later 1980’s for the treatment of persistent pain [20], at a first glance it is somewhat surprising that more explorations to date have not been conducted with the delivery of therapeutic molecules for the treatment of additional central nervous system disorders beyond small molecule analgesics and chemotherapeutic agents.
2.3 Limitations for Macromolecule Delivery
The existing limitations for the intrathecal delivery of biological macromolecules may partially explain the lack of extensive development in this area. While the increased proximity of the CSF to the brain and spinal cord parenchyma is an advantage of intrathecal delivery, inconsistencies can arise between the interstitial fluid of parenchymal tissues and the CSF. In some cases, the concentration of a compound in the CSF can be used as a surrogate for assessing the concentration of a compound within the parenchymal tissue, but it has been shown that the CSF concentration is not necessarily an accurate predictor of unbound drug concentrations in the brain and spinal cord tissue [24]. Both the physical properties of a drug and the physiological state of the tissue, which can be altered by disorders such as epilepsy [25] and conditions such as hyperthermia [26], can increase the discrepancies that are observed between drug concentrations in the CSF and the interstitial fluid. This suggests that there may be a physiologically dependent and relatively uncharacterized CSF-brain barrier that consequently does not guarantee drug penetration into parenchymal tissues upon intrathecal administration, and may be an increasing limitation for biological macromolecules of increasing size and hydrophilicity [27–29].
Rapid clearance from the CSF is another significant limitation of intrathecally administered treatments. CSF in the brain is produced in choroid plexus, descends into the lateral, third and fourth ventricles along with the spinal cord and then ascends to the superior sagittal sinus where it is exposed to arachnoid granulations. These granulations are valve-like structures which allow CSF to pass into the lumen of the sinus when the CSF pressure exceeds venous pressure [30]. The CSF essentially passes through these granulations without filtration, which enables the passage of cellular components and macromolecules. CSF can also pass to the periphery through arachnoid villi and spinal nerve roots along the spinal cord, and CSF components that have passed into the interstitial fluid, if left unbound by parenchyma, can drain into lymphatic tissues in smaller quantities [31]. The main absorption of CSF through the central nervous system is ultimately to the blood. This occurs at multiple sites along the length of the brain and spinal cord by the above mechanisms, and is commonly referred to as bulk flow [32].
2.4 Implantable Infusion Pumps
To overcome the diffusive limitations and clearance of intrathecally administered molecules and promote more sustained and continuous drug concentrations in the spinal cord and its surrounding tissues, first generation approaches have utilized surgically implanted reservoir pumps connected to implanted intrathecal catheters. These surgically implanted pumps have the advantage of overcoming the need for repeated intrathecal injections and also can be designed to allow for the modification of infusion rates. These devices have exhibited many draw-backs, however, as they are expensive, invasive, exhibit complications due to the surgical procedure itself, lead to infection and inflammation and exhibit catheter dysfunctions over time [1, 19, 20]. A particular concern with observed catheter dysfunctions is the high incidence of intrathecal granuloma formations, which exhibit the potential for spinal cord compression and paralysis [20]. Finally, therapeutic agents must be maintained in an aqueous reservoir, which is not suitable for biological macromolecules that exhibit limited stability in solutions at physiological temperatures [33].
2.5 Single Administration Approaches
The examples provided previously require multiple and continuous administrations in order to attain a sustained therapeutic effect. Single administration strategies refer to administrations that can provide a sustained therapeutic effect without the need for implantable infusion pumps and repeated intrathecal administrations. In order to obtain sustained therapeutic effects from a single injection of biological macromolecules, including therapeutic proteins or DNA constructs, efforts must focus on prolonging the stability and residence of these drugs in the CSF, to the extent permitted by the limitations of bulk flow. Promoting the penetration of biological macromolecules into the parenchymal tissue, and prolonging their residence in the meningeal tissue (dura matter, arachnoid membrane and pia matter; Figure 1b) surrounding the intrathecal space and spinal cord is also needed in order to sustain the therapeutic effect of a single administration. Stabilization of biological macromolecules in the meningeal tissues surrounding the spinal cord is of particular interest for central nervous system disorders in which immune-central nervous system communication is hypothesized to play a key role. These meningeal tissues, which are comprised of highly immuno-competent cell types, have been shown to produce a range of cytokines involved in the development and maintenance of spinally-derived neuropathies [34], may play a key role in other central nervous system disorders [35], and are readily exposed to intrathecally administered macromolecules.
2.6 Polymer-protein bio-conjugation
Polymer-protein bio-conjugation is rapidly becoming a common technique for the enhancement of protein stability in therapeutic applications that rely on oral and parenteral delivery, and may also be a useful approach for enhancing the stability, residence and tissue penetration of intrathecally administered proteins. Prior work with systemically administered proteins has in fact shown that covalently attaching a synthetic polymer to a protein significantly prolongs its half-life in the blood stream [36–38]. Polyethylene glycol (PEG) is the most common polymer used for the modification of proteins due to its excellent biocompatibility [39, 40] and approval by the United States Food and Drug Administration [41]. While the attachment of PEG to a protein (PEGylation) can sterically hinder the protein’s access to receptors and subsequent in vitro biological activity, PEGylated proteins typically have an increased systemic circulation time in the bloodstream [41–43] which compensates for marginal activity losses and often results in an overall in vivo therapeutic benefit [44, 45]. For intrathecal applications, however, where the impact of bulk flow significantly reduces protein residence times in the CSF relative to the bloodstream, minimizing the activity losses after PEGylation is of increasing importance. With a reduced time-scale for the onset of efficacy prior to protein clearance, it is necessary to identify PEGylation strategies that sufficiently preserve protein biological activities while increasing their stability and residence in the intrathecal space as much as possible.
PEGylation can also significantly enhance the diffusion of a molecule in tissues. Even though attaching PEG to a protein increases the overall size of a molecule, and larger molecules would theoretically exhibit a reduced ability to diffuse through tissue, PEGylation creates a hydration layer around a protein which increases its solubility [46, 47], reduces its non-specific electrostatic interactions [48], and shields it from receptor mediated uptake by surface tissues [40]. These effects therefore increase the potential for PEGylated proteins to penetrate and diffuse into tissue [49], including brain and spinal cord parenchyma. Prior work has indeed shown that PEGylated proteins exhibit enhanced diffusion in ex vivo brain tissue slices [48] and in vivo penetration into the spinal column and forebrain after prolonged exposure to continuous intrathecal infusions [50].
PEGylation has also been shown to decrease the immunogenicity of proteins and shield them from enzymatic degradation and antigenic determinants of the immune system in the bloodstream [46, 51]. These agents are less abundant in the CSF than in the bloodstream, but there is increasing evidence for the presence of serine proteases and antigenic determinants in the CSF [34, 52]. These effects can also impart additional protection to PEGylated proteins from proteases and antigenic determinants in brain and spinal cord parenchymal and meningeal tissues [53, 54]. Initial studies indicate that protein PEGylation exhibits significant potential for enhancing the CSF stability, spinal cord tissue distribution and therapeutic efficacy over time of intrathecally administered proteins. Intrathecally administered PEGylated brain-derived neurotrophic factor exhibits an enhanced stability in the CSF and penetration into spinal cord tissue relative to the unmodified protein [55], and it has more recently been shown that intrathecally administered PEGylated interleukin-10 exhibits an enhanced in vivo therapeutic efficacy over time for the treatment of neuropathic pain [56].
2.7 Polymer complexation
Lipids and cationic polymers have been shown to interact with negatively charged DNA in a self-assembly process [57] that results in the formation of liposomes or polyplexes, respectively [58]. The most widely used synthetic polymer for gene delivery by complexation is polyethylenimine (PEI) [59]. PEI complexation has been shown to reduce intrathecal gene delivery requirements by one-half to one-tenth [60–62]. PEI complexation with DNA has also been shown to increase its resultant gene expression in the central nervous system by 10 [63] to 40 [60] fold after an intrathecal administration. Additionally it has been shown that intrathecally administered PEI/DNA complexes can exhibit a significant migration to the dorsal root ganglia and significantly enhance the regeneration of transected rat sciatic nerves [64].
Although these delivery improvements are very promising, major disadvantages from the use of synthetic and natural polycations, including PEI, for complexation are toxicity [65, 66], lack of biodegradability and poor biocompatibility overall [67]. While these considerations have previously limited the broad applicability of this technology, more recent efforts have shown that potential toxicity can be reduced by covalently linking PEI to carrier molecules such as PEG [63] or cyclodextrin [68] and that the biodegradability of PEI can be increased by the incorporation of ester and caprolactone groups [69]. The further identification and development of polymers with improved biocompatibilities is therefore expected to increase the clinical utility of the polymer complexation approach for intrathecal gene delivery.
2.8 Polymer encapsulation
The encapsulation of therapeutic proteins and plasmid DNA within biodegradable polymer microparticles offers an approach that can promote therapeutic macromolecule release for prolonged periods of time with minimized toxicity concerns [70]. The term ‘microparticle’ specifically refers to a particle with a diameter of 1–1000 µm [71], but as intrathecal injections are typically conducted with an 18- to 22-gauge needle, microparticles less than 100 µm in diameter are preferred [19]. While there are variations in the nature of a microparticle, it is usually assumed that a microparticle formulation is a mixture of a polymer and a biological macromolecule that is released over time as the polymer degrades [71].
The most common polymers for biological macromolecule encapsulation and delivery are poly(lactic acid) (PLA) [72] and poly(lactic-co-glycolic acid) (PLGA) [72–75] due to their high biocompatibility and established approval by the United States Food and Drug Administration [71]. PLGA degrades into the natural products of lactic acid and glycolic acid that are eventually eliminated from the body without side effects [71, 76] and it has been shown that PLGA is biocompatible with and exhibits no evidence of toxicity to neural tissues [33]. Initial work has demonstrated that the encapsulation of intrathecally administered small molecule analgesics into PLGA microparticles can significantly prolong the potency of analgesia with few side effects for pain control [77]. Studies with the intrathecally administered anti-spasticity drug baclofen have also shown that PLGA encapsulation can enable its therapeutic presence in the CSF for greater than one month while reducing its toxicity relative to bolus dosages [78, 79].
2.9 Microparticle-mediated intrathecal protein delivery
The short (2–3 hr) half-life of most therapeutic proteins in the CSF necessitates multiple injections to obtain the desired therapeutic effects [80, 81]. Microparticle preparations can release protein products at a controlled rate in a sustained dosage form, and can further stabilize them from degradative enzymes and activity loss that occurs in an unprotected environment [70, 75]. Many applications for protein delivery have utilized microparticle encapsulation to improve the stability and delivery of therapeutic proteins in the bloodstream and peripheral tissue over time [70, 82, 83]. Prior work demonstrating the merits of microparticle-mediated intrathecal protein delivery is more limited in scope, but exhibits promise for increasing the clinical relevance of intrathecally administered protein formulations [33].
2.10 Microparticle-mediated intrathecal gene delivery
Due to the ability of therapeutic gene delivery to promote long-term therapeutic effects in vivo, there has been an increased degree of interest in the use of DNA delivery vectors in the central nervous system [84]. Due to concerns over the safety of viral-mediated DNA delivery systems [85], most efforts for gene delivery to the spinal cord have focused on the delivery of non-viral plasmid DNA (pDNA) which exhibits a good safety profile and ease of manufacturing [86]. Non-viral pDNA delivery, however, is often hampered by inefficient pDNA uptake and expression [85] and can require multiple high-dose intrathecal injections for enduring therapeutic efficacy in various central nervous system disorders [87].
High-dosages of pDNA, which are often necessary for successful in vivo gene therapy in the central nervous system [15, 80], are unfavorable from both a clinical and a process economics standpoint [88]. pDNA encapsulation with biodegradable PLGA microparticles can help meet the increasing need to induce human responses with lower and fewer doses of pDNA and additionally protects pDNA from nuclease degradation and rapid clearance [89]. Microparticle encapsulation can be used with large DNA plasmids, has simple preparations with flexibility in use and can offer cell-type specificity after chemical conjugation with a targeting ligand [90, 91]. Microparticle encapsulation also increases the persistence of pDNA released to the local environment which is critical for prolonged in vivo effects [92]. Overall, pDNA encapsulation within biodegradable microparticles can therefore enhance gene transfer by increasing the number of cells expressing the transgene, the extent of transgene expression, or by shielding the vector from clearance and the host’s immune response [33]. Even though there is only one reported use of the approach to date [62], improved pDNA delivery over time as a result of microparticle encapsulation offers potential for significantly enhancing and prolonging the ultimate expression of therapeutic proteins in the central nervous system after intrathecal administrations.
3. Conclusions
Biological macromolecules such as proteins and pDNA encounter significant obstacles after an intrathecal administration, yet they can still exhibit transient therapeutic effects. To overcome the clinical limitations of intrathecally administered biological macromolecules, advanced technologies including polymer conjugation, polymer complexation and polymer encapsulation of biological macromolecules have recently been explored. The findings of these foundational reports indicate that polymer-mediated delivery strategies may significantly enhance the clinical relevance of intrathecally administered biological macromolecules.
4. Expert Opinion
While the administration of therapeutic proteins and pDNA to the bloodstream is the easiest and most traditional route of administration, the obstacle presented by the blood-brain barrier renders this delivery route infeasible for many therapeutic proteins and pDNA constructs. Due a prior lack of more advanced administration and stabilization approaches, therapeutic treatments for many central nervous system disorders have been restricted to molecules that can easily traverse the blood-brain barrier. The number of potentially therapeutic molecules that have not been adequately explored due to this obstacle is unknown, but there is a continued and unacceptable cost to individuals and society as a whole due to a lack of adequate treatments for many central nervous system disorders. This therefore necessitates an adequate exploration of both small and large molecule therapeutic candidates, even if they are unable to cross the blood-brain barrier via systemic administration.
Efforts to develop delivery approaches that enable a therapeutic molecule to directly traverse the blood-brain barrier cannot be neglected and exhibit promise [17, 18]. The focus of this review, however, is on the potential of intrathecal delivery, which may ultimately be more widely applicable for a range of therapeutics when they are coupled with delivery vehicles that overcome the limitations inherent to the intrathecal space. Over the past two decades the emergence of a clinical infrastructure and trained personnel for intrathecal administration has made this route of delivery much more feasible [20], although the current clinical usage of the approach does not extend beyond the realm of small molecule analgesics. The inherent biological limitations of CSF flow and turnover in the intrathecal space currently require that intrathecally administered therapeutic proteins [80] and pDNA [87] occur with repeated and high dosages in order to promote a sustained therapeutic effect. Repeated and continuous intrathecal administration paradigms can lead to deleterious side-effects and patient non-compliance over time [93–95], hence significant improvements in the delivery of intrathecally administered macromolecules are still required to render these approaches clinically applicable.
Specific research directions for intrathecally administered macromolecules will likely focus on several different areas, as a single approach is unlikely to be optimal for every therapeutic candidate. The only approach that exhibits toxicity concerns is the use of positively charged polymers in a complexation approach. As concerns over vector toxicity and degradability therefore limit the use of liposomes and polyplexes, efforts with this technology will likely focus on the identification and development of materials that adequately address these concerns. Efforts with therapeutic protein PEGylation will likely focus on PEGylation approaches that minimize biological activity losses while stabilizing and sustaining the presence of therapeutic proteins in the intrathecal space as much as possible. Efforts with the encapsulation of therapeutic proteins and pDNA within degradable microparticles will likely focus on promoting the interaction of these vectors with targeted cell types, while sustaining therapeutic molecule release for as long as possible. Even though all of these delivery approaches still require development and extensive clinical testing before they are accepted for widespread clinical use, it is anticipated that the utilization of polymer-mediated delivery strategies will significantly expand the utility of intrathecally administered therapeutic treatments for a range of central nervous system disorders.
Abbreviations
- CSF
cerebrospinal fluid
- pDNA
plasmid DNA
- PEG
Polyethylene glycol
- PEI
polyethylenimine
- PLA
poly(lactic acid)
- PLGA
poly(lactic-co-glycolic acid)
Footnotes
The authors have no affiliations with any organizations that have a direct or indirect financial interest in the contents of this report.
Article Highlights
1. Introduction
Biological macromolecules…are particularly limited by the formidable obstacles of brain and spinal cord drug delivery. The development of improved delivery methods is required to render these macromolecule-based therapeutic approaches clinically relevant.
2.1 Intrathecal administration for delivery to the central nervous system
The ability to deliver biological macromolecules directly to the CSF, which bathes the brain and spinal cord [intrathecal administration], is currently one of the most promising approaches that can surmount existing delivery barriers.
2.2 Historical Basis of Intrathecal Administration
Intrathecal administrations were primarily developed and have most commonly been used for the delivery of analgesics (pain medications)…
2.3 Limitations for Macromolecule Delivery
…there [is] a physiologically dependent and relatively uncharacterized CSF-brain barrier that [is] an increasing limitation for biological macromolecules of increasing size and hydrophilicity…Rapid clearance from the CSF is another significant limitation…
2.4 Implantable Infusion Pumps
… first generation approaches have utilized surgically implanted reservoir pumps connected to implanted intrathecal catheters. These surgically implanted pumps have the advantage of overcoming the need for repeated intrathecal injections…These devices have exhibited many draw-backs…
2.5 Single Administration Approaches
Single administration strategies refer to administrations that can provide a sustained therapeutic effect without the need for implantable infusion pumps and repeated intrathecal administrations.
2.6 Polymer-protein bio-conjugation
Initial studies indicate that protein PEGylation exhibits significant potential for enhancing the CSF stability, spinal cord tissue distribution and therapeutic efficacy over time of intrathecally administered proteins.
2.7 Polymer complexation
PEI complexation with DNA has also been shown to increase its resultant gene expression in the central nervous system…after an intrathecal administration…polymers with improved biocompatibilities [are] expected to increase the clinical utility of the polymer complexation approach for intrathecal gene delivery.
2.8 Polymer encapsulation
The encapsulation of therapeutic proteins and plasmid DNA within biodegradable polymer microparticles offers an approach that can promote therapeutic macromolecule release for prolonged periods of time.
2.9 Microparticle-mediated intrathecal protein delivery
Prior work demonstrating the merits of microparticle-mediated intrathecal protein delivery…exhibits promise for increasing the clinical relevance of intrathecally administered protein formulations.
2.10 Microparticle-mediated intrathecal gene delivery
…Improved pDNA delivery over time as a result of microparticle encapsulation offers potential for significantly enhancing and prolonging the ultimate expression of therapeutic proteins in the central nervous system after intrathecal administrations.
3. Conclusions
…Polymer-mediated delivery strategies may significantly enhance the clinical relevance of intrathecally administered biological macromolecules.
4. Expert Opinion
… A single approach is unlikely to be optimal for every therapeutic candidate…Even though all of these delivery approaches still require development and extensive clinical testing before they are accepted for widespread clinical use…polymer-mediated delivery strategies will significantly expand the utility of intrathecally administered therapeutic treatments…
References
- 1. Misra A, Ganesh S, Shahiwala A, et al. Drug delivery to the central nervous system: a review. Journal of Pharmacy and Pharmaceutical Sciences. 2003;6(2):252–273. **Provides an overview of drug targeting and delivery to the central nervous system
- 2.Martin-Villalba A, Okuducu AF, von Deimling A. The evolution of our understanding on glioma. Brain Pathology. 2008;18(3):455–463. doi: 10.1111/j.1750-3639.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prince DA, Parada I, Scalise K, et al. Epilepsy following cortical injury: Cellular and molecular mechanisms as targets for potential prophylaxis. Epilepsia. 2009;50:30–40. doi: 10.1111/j.1528-1167.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia How to move forward? Neurology. 2009;72(4):368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strecker K, Schwarz J. Parkinson's disease: emerging pharmacotherapy. Expert Opinion on Emerging Drugs. 2008;13(4):573–591. doi: 10.1517/14728210802596906. [DOI] [PubMed] [Google Scholar]
- 6.Biran Y, Masters CL, Barnham KJ, et al. Pharmacotherapeutic targets in Alzheimer's disease. Journal of Cellular and Molecular Medicine. 2009;13(1):61–86. doi: 10.1111/j.1582-4934.2008.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vernino S, Geschwind M, Boeve B. Autoimmune encephalopathies. Neurologist. 2007;13(3):140–147. doi: 10.1097/01.nrl.0000259483.70041.55. [DOI] [PubMed] [Google Scholar]
- 8.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nature Reviews Neuroscience. 2005;6(7):521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 9.Nagahara AH, Merrill DA, Coppola G, et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nat Med. 2009 doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawbarn D, Allen SJ. Neurotrophins and neurodegeneration. Neuropathology And Applied Neurobiology. 2003;29(3):211–230. doi: 10.1046/j.1365-2990.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 11.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117(4):1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 12.Walker D, Lue LF. Anti-inflammatory and immune therapy for Alzheimer's disease: Current status and future directions. Current Neuropharmacology. 2007;5(4):232–243. doi: 10.2174/157015907782793667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pettingill LN, Minter RL, Shepherd RK. Schwann cells genetically modified to express neurotrophins promote spiral ganglion neuron survival in vitro. Neuroscience. 2008;152(3):821–828. doi: 10.1016/j.neuroscience.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blesch A, Tuszynski MH. Transient growth factor delivery sustains regenerated axons after spinal cord injury. Journal Of Neuroscience. 2007;27(39):10535–10545. doi: 10.1523/JNEUROSCI.1903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao MZ, Gu JF, Wang JH, et al. Interleukin-2 gene therapy of chronic neuropathic pain. Neuroscience. 2002;112(2):409–416. doi: 10.1016/s0306-4522(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 16.Sloane E, Ledeboer A, Seibert W, et al. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis. Brain Behavior And Immunity. 2009;23(1):92–100. doi: 10.1016/j.bbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patel MM, Goyal BR, Bhadada SV, et al. Getting into the Brain Approaches to Enhance Brain Drug Delivery. Cns Drugs. 2009;23(1):35–58. doi: 10.2165/0023210-200923010-00003. **Provides an overview of novel strategies for enhancing drug delivery to the brain
- 18. Tosi G, Costantino L, Ruozi B, et al. Polymeric nanoparticles for the drug delivery to the central nervous system. Expert Opinion on Drug Delivery. 2008;5(2):155–174. doi: 10.1517/17425247.5.2.155. *Review of particulate drug carriers for central nervous system delivery
- 19. Lagarce F, Benoit JP. Sustained release formulations for spinal drug delivery. Journal Of Drug Delivery Science And Technology. 2004;14(5):331–343. **Review of strategies to enhance drug residence time after spinal delivery
- 20. Smith HS, Deer TR, Staats PS, et al. Intrathecal Drug Delivery. Pain Physician. 2008;(11):S89–S104. Opiod Special Issue. *Provides an overview of intrathecal analgesic development from a clinical perspective
- 21.Goss JR, Goins WF, Glorioso JC. Gene therapy applications for the treatment of neuropathic pain. Expert Rev. Neurotherapeutics. 2007;7(5):487–506. doi: 10.1586/14737175.7.5.487. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher RM. Intrathecal drug delivery for chronic back pain: Better science for clinical innovation. Pain Medicine. 2004;5(1):1–3. doi: 10.1111/j.1526-4637.2004.04014.x. [DOI] [PubMed] [Google Scholar]
- 23.Rosenblum LY, Johnson RC, Schmahai TJ. Preclinical safety evaluation of recombinant human interleukin-10. Regulatory Toxicology and Pharmacology. 2002;35(1):56–71. doi: 10.1006/rtph.2001.1504. [DOI] [PubMed] [Google Scholar]
- 24.Lin JH. CSF as a surrogate for assessing CNS exposure: An industrial perspective. Current Drug Metabolism. 2008;9(1):46–59. doi: 10.2174/138920008783331077. [DOI] [PubMed] [Google Scholar]
- 25.Rambeck B, Jurgens UH, May TW, et al. Comparison of brain extracellular fluid, brain tissue, cerebrospinal fluid, and serum concentrations of antiepileptic drugs measured intraoperatively in patients with intractable epilepsy. Epilepsia. 2006;47(4):681–694. doi: 10.1111/j.1528-1167.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 26.Sharma HS, Johanson CE. Neurobiology of Hyperthermia. Amsterdam: Elsevier Science Bv; 2007. Blood-cerebrospinal fluid barrier in hyperthermia; pp. 459–478. [DOI] [PubMed] [Google Scholar]
- 27.Krewson CE, Klarman ML, Saltzman WM. Distribution of nerve growth-factor following direct delivery to brain interstitium. Brain Research. 1995;680(1–2):196–206. doi: 10.1016/0006-8993(95)00261-n. [DOI] [PubMed] [Google Scholar]
- 28.Shen DD, Artru AA, Adkison KK. Principles and applicability of CSF sampling for the assessment of CNS drug delivery and pharmacodynamics. Advanced Drug Delivery Reviews. 2004;56(12):1825–1857. doi: 10.1016/j.addr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Newcomb R, Abbruscato TJ, Singh T, et al. Bioavailability of Ziconotide in brain: influx from blood, stability, and diffusion. Peptides. 2000;21(4):491–501. doi: 10.1016/s0196-9781(00)00175-3. [DOI] [PubMed] [Google Scholar]
- 30.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4 ed. New York: McGraw-Hill; 2000. [Google Scholar]
- 31.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochemistry International. 2004;45(4):545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 32. Greitz D, Hannerz J. A proposed model of cerebrospinal fluid circulation: Observations with radionuclide cisternography. American Journal of Neuroradiology. 1996;17(3):431–438. *Provides a useful understanding of CSF flow
- 33. Whittlesey KJ, Shea LD. Delivery systems for small molecule drugs, proteins, and DNA: the neuroscience/biomaterial interface. Experimental Neurology. 2004;190(1):1–16. doi: 10.1016/j.expneurol.2004.06.020. **Provides an overview of sustained release systems for large molecule delivery to neural tissues
- 34.Wieseler-Frank J, Jekich BM, Mahoney JH, et al. A novel immune-to-CNS communication pathway: Cells of the meninges surrounding the spinal cord CSF space produce proinflammatory cytokines in response to an inflammatory stimulus. Brain Behavior And Immunity. 2007;21(5):711–718. doi: 10.1016/j.bbi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Mercier F, Hatton GI. Immunocytochemical basis for a meningeo-glial network. Journal of Comparative Neurology. 2000;420(4):445–465. doi: 10.1002/(sici)1096-9861(20000515)420:4<445::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 36.Tsutsami Y, Tsunoda S-I, Kamada H, et al. PEGylation of Interleukin-6 Effectively Increases Its Thrombopoietic Potency. Thrombosis and Haemostasis. 1997;77(1):168–173. [PubMed] [Google Scholar]
- 37.Basu A, Yang K, Wang ML, et al. Structure-function engineering of interferon-beta-1b for improving stability, solubility, potency, immunogenicity, and pharmacokinetic properties by site-selective mono-PEGylation. Bioconjugate Chemistry. 2006;17(3):618–630. doi: 10.1021/bc050322y. [DOI] [PubMed] [Google Scholar]
- 38.Ramon J, Saez V, Baez R, et al. PEGylated interferon-alpha 2b: A branched 40K polyethylene glycol derivative. Pharmaceutical Research. 2005;22(8):1374–1386. doi: 10.1007/s11095-005-5278-4. [DOI] [PubMed] [Google Scholar]
- 39.Bailon P, Berthold W. Polyethylene glycol-conjugated pharmaceutical proteins. Pharmaceutical Science & Technology Today. 1998;1(8):352–356. [Google Scholar]
- 40.Roberts MJ, Bentley MD, Harris JM. Chemistry for peptide and protein PEGylation. Advanced Drug Delivery Reviews. 2002;54(4):459–476. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 41.Veronese FM, Pasut G. PEGylation, successful approach to drug delivery. Drug Discovery Today. 2005;10(21–24):1451–1458. doi: 10.1016/S1359-6446(05)03575-0. [DOI] [PubMed] [Google Scholar]
- 42.Harris MJ, Martin NE, Modi M. Pegylation: a novel process for modifying pharmacokinetics. Clinical pharmacokinetics. 2001;40(7):539–551. doi: 10.2165/00003088-200140070-00005. [DOI] [PubMed] [Google Scholar]
- 43.Koumenis IL, Shahrokh Z, Leong S, et al. Modulating pharmacokinetics of an anti-interleukin-8 F(ab ')(2) by amine-specific PEGylation with preserved bioactivity. International Journal Of Pharmaceutics. 2000;198(1):83–95. doi: 10.1016/s0378-5173(99)00458-5. [DOI] [PubMed] [Google Scholar]
- 44.Clark R, Olson K, Fuh G, et al. Long-acting growth hormones produced by conjugation with polyethylene glycol. Journal Of Biological Chemistry. 1996;271(36):21969–21977. doi: 10.1074/jbc.271.36.21969. [DOI] [PubMed] [Google Scholar]
- 45.Francis GE, Fisher D, Delgado C, et al. PEGylation of cytokines and other therapeutic proteins and peptides: the importance of biological optimisation of coupling techniques. International Journal of Hematology. 1998;68:1–18. doi: 10.1016/s0925-5710(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 46.Molineux G. Pegylation: Engineering Improved Biopharmaceuticals for Oncology. Pharmacotherapy. 2003;23(8 Pt 2):3S–8S. doi: 10.1592/phco.23.9.3s.32886. [DOI] [PubMed] [Google Scholar]
- 47.Dhalluin C, Ross A, Leuthold LA, et al. Structural and biophysical characterization of the 40 kDa PEG-interferon-alpha(2a) and its individual positional isomers. Bioconjugate Chemistry. 2005;16(3):504–517. doi: 10.1021/bc049781+. [DOI] [PubMed] [Google Scholar]
- 48. Stroh M, Zipfel WR, Williams RM, et al. Multiphoton microscopy guides neurotrophin modification with poly(ethylene glycol) to enhance interstitial diffusion. Nature Materials. 2004;3(7):489–494. doi: 10.1038/nmat1159. *Provides an understanding of large molecule diffusion in neural tissues before and after PEGylation
- 49.Dang W, Colvin MO, Brem H, et al. Covalent coupling of methotrexate to dextran enhances the penetration of cytotoxicity into a tissue-like matrix. Cancer Research. 1994;54:1729–1735. [PubMed] [Google Scholar]
- 50. Ankeny DP, McTigue DM, Guan Z, et al. Pegylated brain-derived neurotrophic factor shows improved distribution into the spinal cord and stimulates locomotor activity and morphological changes after injury. Experimental Neurology. 2001;170(1):85–100. doi: 10.1006/exnr.2001.7699. *Provides an understanding of large molecule diffusion and residence in the CSF and tissue before and after PEGylation
- 51.Greenwald RB, Yang K, Zhao H, et al. Controlled release of proteins from their poly(ethylene glycol) conjugates: drug delivery systems employing 1,6-elimination. Bioconjugate Chemistry. 2003;14(2):395–403. doi: 10.1021/bc025652m. [DOI] [PubMed] [Google Scholar]
- 52.Scarisbrick IA, Towner MD, Isackson PJ. Nervous system-specific expression of a novel serine protease: Regulation in the adult rat spinal cord by excitotoxic injury. Journal Of Neuroscience. 1997;17(21):8156–8168. doi: 10.1523/JNEUROSCI.17-21-08156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polfliet MMJ, Goede PH, van Kesteren-Hendrikx EML, et al. A method for the selective depletion of perivascular and meningeal macrophages in the central nervous system. Journal Of Neuroimmunology. 2001;116(2):188–195. doi: 10.1016/s0165-5728(01)00282-x. [DOI] [PubMed] [Google Scholar]
- 54.McMenamin PG, Wealthall RJ, Deverall M, et al. Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell And Tissue Research. 2003;313(3):259–269. doi: 10.1007/s00441-003-0779-0. [DOI] [PubMed] [Google Scholar]
- 55.Soderquist RG, Milligan ED, Sloane EM, et al. PEGylation of brain-derived neurotrophic factor for preserved biological activity and enhanced spinal cord distribution. Journal of Biomedical Materials Research Part A. 2009;91(3):719–729. doi: 10.1002/jbm.a.32254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soderquist RG, Milligan ED, Harrison JA, et al. PEGylation of interleukin-10 for the mitigation of enhanced pain states. Journal of Biomedical Materials Research Part A. 2009 doi: 10.1002/jbm.a.32611. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radler JO, Koltover I, Salditt T, et al. Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science. 1997;275(5301):810–814. doi: 10.1126/science.275.5301.810. [DOI] [PubMed] [Google Scholar]
- 58.Park JH, Lee S, Kim JH, et al. Polymeric nanomedicine for cancer therapy. Progress in Polymer Science. 2008;33(1):113–137. [Google Scholar]
- 59.Boussif O, Lezoualch F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo - polyethylenimine. Proceedings Of The National Academy Of Sciences Of The United States Of America. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi L, Tang GP, Gao SJ, et al. Repeated intrathecal administration of plasmid DNA complexed with polyethylene glycol-grafted polyethylenimine led to prolonged transgene expression in the spinal cord. Gene Therapy. 2003;10(14):1179–1188. doi: 10.1038/sj.gt.3301970. [DOI] [PubMed] [Google Scholar]
- 61.Meuli-Simmen C, Liu Y, Yeo TT, et al. Gene expression along the cerebral-spinal axis after regional gene delivery. Human Gene Therapy. 1999;10(16):2689–2700. doi: 10.1089/10430349950016735. [DOI] [PubMed] [Google Scholar]
- 62.Milligan ED, Soderquist RG, Malone SM, et al. Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron Glia Biology. 2006;2(4):293–308. doi: 10.1017/S1740925X07000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang GP, Zeng JM, Gao SJ, et al. Polyethylene glycol modified polyethylenimine for improved CNS gene transfer: effects of PEGylation extent. Biomaterials. 2003;24(13):2351–2362. doi: 10.1016/s0142-9612(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Wang CY, Zeng JM, et al. Gene transfer to dorsal root ganglia by intrathecal injection: Effects on regeneration of peripheral nerves. Molecular Therapy. 2005;12(2):314–320. doi: 10.1016/j.ymthe.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 65.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22(5):471–480. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 66.Fischer D, Li YX, Ahlemeyer B, et al. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24(7):1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 67.Tiera MJ, Winnik FM, Fernandes JC. Synthetic and natural polycations for gene therapy: State of the art and new perspectives. Current Gene Therapy. 2006;6(1):59–71. doi: 10.2174/156652306775515510. [DOI] [PubMed] [Google Scholar]
- 68.Tang GP, Guo HY, Alexis F, et al. Low molecular weight polyethylenimines linked by beta-cyclodextrin for gene transfer into the nervous system. Journal Of Gene Medicine. 2006;8(6):736–744. doi: 10.1002/jgm.874. [DOI] [PubMed] [Google Scholar]
- 69.Arote R, Kim TH, Kim YK, et al. A biodegradable poly(ester amine) based on polycaprolactone and polyethylenimine as a gene carrier. Biomaterials. 2007;28(4):735–744. doi: 10.1016/j.biomaterials.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 70. Edlund U, Albertsson AC. Degradable polymer microspheres for controlled drug delivery. Degradable Aliphatic Polyesters. 2002:67–112. *Good overview of polymer encapsulation technology
- 71. Birnbaum DT, Brannon-Peppas L. Microparticle drug delivery systems. In: Brown DM, editor. Drug Delivery Systems in Cancer Therapy. Totowa, NJ: Humana Press Inc.; 2003. pp. 117–135. *Overview of polymer encapsulation technology with a balanced in vitro and in vivo perspective
- 72.Kumar M, Bakowsky U, Lehr CM. Preparation and characterization of cationic PLGA nanospheres as DNA carriers. Biomaterials. 2004;25(10):1771–1777. doi: 10.1016/j.biomaterials.2003.08.069. [DOI] [PubMed] [Google Scholar]
- 73.Barman SP, Lunsford L, Chambers P, et al. Two methods for quantifying DNA extracted from poly(lactide-co-glycolide) microspheres. Journal Of Controlled Release. 2000;69(3):337–344. doi: 10.1016/s0168-3659(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 74.Tinsley-Bown AM, Fretwell R, Dowsett AB, et al. Formulation of poly(D,L-lactic-co-glycolic acid) microparticles for rapid plasmid DNA delivery. Journal Of Controlled Release. 2000;66(2–3):229–241. doi: 10.1016/s0168-3659(99)00275-8. [DOI] [PubMed] [Google Scholar]
- 75.Cohen S, Yoshioka T, Lucarelli M, et al. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharmaceutical Research. 1991;8(6):713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y, Deng XM. Influences of preparation conditions on particle size and DNA-loading efficiency for poly(DL-lactic acid-polyethylene glycol) microspheres entrapping free DNA. Journal Of Controlled Release. 2002;83(1):147–155. doi: 10.1016/s0168-3659(02)00176-1. [DOI] [PubMed] [Google Scholar]
- 77.Sendil D, Bonney IM, Carr DB, et al. Antinociceptive effects of hydromorphone, bupivacaine and biphalin released from PLGA polymer after intrathecal implantation in rats. Biomaterials. 2003;24(11):1969–1976. doi: 10.1016/s0142-9612(02)00567-7. [DOI] [PubMed] [Google Scholar]
- 78.Lagarce F, Renaud P, Faisant N, et al. Baclofen-loaded microspheres: preparation and efficacy testing in a new rabbit model. European Journal Of Pharmaceutics And Biopharmaceutics. 2005;59(3):449–459. doi: 10.1016/j.ejpb.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 79.Lagarce F, Faisant N, Desfontis JC, et al. Baclofen-loaded microspheres in gel suspensions for intrathecal drug delivery: In vitro and in vivo evaluation. European Journal Of Pharmaceutics And Biopharmaceutics. 2005;61(3):171–180. doi: 10.1016/j.ejpb.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Milligan ED, Langer SJ, Sloane EM, et al. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. European Journal Of Neuroscience. 2005;21(8):2136–2148. doi: 10.1111/j.1460-9568.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- 81.Berg SL, Balis FM, McCully CL, et al. Pharmacokinetics Of Peg-L-Asparaginase And Plasma And Cerebrospinal-Fluid L-Asparagine Concentrations In The Rhesus-Monkey. Cancer Chemotherapy And Pharmacology. 1993;32(4):310–314. doi: 10.1007/BF00686177. [DOI] [PubMed] [Google Scholar]
- 82.Hinds KD, Campbell KM, Holland KM, et al. PEGylated insulin in PLGA microparticles. In vivo and in vitro analysis. Journal Of Controlled Release. 2005;104(3):447–460. doi: 10.1016/j.jconrel.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 83. Gander B, Meinel L, Walter E, et al. Polymers as a platform for drug delivery: Reviewing our current portfolio on poly(lactide-co-glycolide) (PLGA) microspheres. Chimia. 2001;55(3):212–217. *Good overview of polymer encapsulation technology for DNA
- 84.Wang CY, Wang S. Adeno-associated virus inverted terminal repeats improve neuronal transgene expression mediated by baculoviral vectors in rat brain. Human Gene Therapy. 2005;16(10):1219–1226. doi: 10.1089/hum.2005.16.1219. [DOI] [PubMed] [Google Scholar]
- 85.Kaneda Y, Tabata Y. Non-viral vectors for cancer therapy. Cancer Science. 2006;97(5):348–354. doi: 10.1111/j.1349-7006.2006.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anderson WF. Human gene therapy. Nature. 1998;392(6679):25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 87.Milligan ED, Sloane EM, Langer SJ, et al. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006;126(1–3):294–308. doi: 10.1016/j.pain.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 88.Singh M, Briones M, Ott G, et al. Cationic microparticles: A potent delivery system for DNA vaccines. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2000;97(2):811–816. doi: 10.1073/pnas.97.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang DQ, Robinson DR, Kwon GS, et al. Encapsulation of plasmid DNA in biodegradable poly(D,L-lactic-co-glycolic acid) microspheres as a novel approach for immunogene delivery. Journal Of Controlled Release. 1999;57(1):9–18. doi: 10.1016/s0168-3659(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 90.Li Y, Wang J, Lee CGL, et al. CNS gene transfer mediated by a novel controlled release system based on DNA complexes of degradable polycation PPE-EA: a comparison with polyethylenimine/DNA complexes. Gene Therapy. 2004;11(1):109–114. doi: 10.1038/sj.gt.3302135. [DOI] [PubMed] [Google Scholar]
- 91.Luo D, Woodrow-Mumford K, Belcheva N, et al. Controlled DNA delivery systems. Pharmaceutical Research. 1999;16(8):1300–1308. doi: 10.1023/a:1014870102295. [DOI] [PubMed] [Google Scholar]
- 92.Lunsford L, McKeever U, Eckstein V, et al. Tissue distribution and persistence in mice of plasmid DNA encapsulated in a PLGA-based microsphere delivery vehicle. Journal Of Drug Targeting. 2000;8(1):39–50. doi: 10.3109/10611860009009208. [DOI] [PubMed] [Google Scholar]
- 93.Ward AB, Kadies M. The management of pain in spasticity. Disability and Rehabilitation. 2002;24(8):443–453. doi: 10.1080/09638280110108878. [DOI] [PubMed] [Google Scholar]
- 94.Plassat R, Verbe BP, Menei P, et al. Treatment of spasticity with intrathecal baclofen administration: long-term follow-up, review of 40 patients. Spinal Cord. 2004;42(12):686–693. doi: 10.1038/sj.sc.3101647. [DOI] [PubMed] [Google Scholar]
- 95.Delhaas EM, Beersen N, Redekop WK, et al. Long-term outcomes of continuous intrathecal baclofen infusion for treatment of spasticity: A prospective multicenter follow-up study. Neuromodulation. 2008;11(3):227–236. doi: 10.1111/j.1525-1403.2008.00170.x. [DOI] [PubMed] [Google Scholar]