Abstract
Transition-metal-catalyzed carbon–carbon and carbon–heteroatom bond formations are among the most heavily used types of reactions in both academic and industrial settings. As important as these are to the synthetic community, such cross-couplings come with a heavy price to our environment, and sustainability. E Factors are one measure of waste created, and organic solvents, by far, are the main contributors to the high values associated, in particular, with the pharmaceutical and fine-chemical companies which utilize these reactions. An alternative to organic solvents in which cross-couplings are run can be found in the form of micellar catalysis, wherein nanoparticles composed of newly introduced designer surfactants enable the same cross-couplings, albeit in water, with most taking place at room temperature. In the absence of an organic solvent as the reaction medium, organic waste and hence, E Factors, drop dramatically.
Keywords: cross-coupling, E Factors, green chemistry, micelles, water chemistry
1. Introduction
The chemistry enterprise continues to pay increasing attention to the environmental impact of its processes and products. While environmental issues have likely been under consideration for some time, well over a decade ago BASF created a metric based on eco-efficiency analyses, taking both economic and environmental measures into account.[1a] These included energy, materials, emissions, toxicity, hazards, and even land use.[2–5] Such an extensive analysis applies to individual processes, and while this approach is both cost- and time-intensive, it is also among the most accurate and informative. An alternative yardstick appearing at about the same time is the environmental waste or E Factor, which focuses entirely on one aspect, organic waste, of chemical processes as the measure of greenness.[6] Several related approaches to E Factors have since appeared, for example, the effective mass yield (EMY),[7] in recognition of the need to include front-end items such as starting materials and the investment associated with their production. Other considerations such as energy consumption and water usage form part of the overall picture.
Very recently, arguments have been advanced for a mass-based analysis, such as PMI (process mass intensity), as the metric of choice for the pharmaceutical arena.[8] PMI values are preferred over E Factors, which focus strictly on waste,[9] and as an alternative to a complete life cycle assessment (LCA)[10,11] which, in general, is too costly and time-consuming. Establishment of a tool for rapid calculation of PMIs, a tool which takes into account both the huge up-front and process entries, is an important step towards routine evaluation. Nonetheless, PMI calculations highlight the huge impact that both organic solvents and water have in calculating mass units associated with a process. Whether assessed up front in a PMI calculation, or at the back end as waste, organic solvents make up approximately 85% of organic waste.[12] Thus, it seems perfectly reasonable to continue to look towards E Factors as a guide to both the economics and extent of greenness of any individual process.
E Factors are defined as the amount of waste produced relative to the desired material, in kilograms [Eq. (1)].
 |
(1) |
According to calculations, fine-chemical companies and the pharmaceutical industry in particular are viewed as the major offenders, with E Factors typically in the 5–100 range.[13] These numbers can be misleading, however, as water used in the normal course of reaction workup is not taken into account, and can add greatly to the calculation. That the majority of organic waste produced is in the form of organic solvents[10] has led Sheldon and co-workers, in their monograph Green Chemistry and Catalysis,[13] to suggest that “…so many of the solvents that are favored by organic chemists have been blacklisted that the whole question of solvent use requires rethinking.” While the environmental implications may be clear, there are also economic incentives to get organic solvents out of organic reactions. Up-front costs associated with their purchase, and back-end expenses earmarked for their proper disposal, are just two of the major fiscal impacts. Others include measures that must be taken to deal with solvent toxicity and flammability, as well as worker safety issues which add to production costs.[9] Surely for synthetic organic chemists and those involved in catalysis in particular, there must be alternatives available which completely avoid organic solvents as reaction media. One approach focuses on modern micellar catalysis, which involves small amounts of environmentally benign designer surfactants,[14] dissolved in water only. This significantly reduces dependence on organic solvents and thereby, dramatically lowers associated E Factors.
To illustrate the potential offered by suitably engineered nanomicelles as nanoreactors, several palladium-catalyzed “name” reactions utilized by large pharmaceutical companies, as described in the literature,[15] have been selected and their E Factors calculated based on the total volume of organic solvent(s) employed divided by the amount of desired product isolated. To illustrate the impact of water on the calculation, E Factors were also calculated to include the associated aqueous workup, likewise leading to a waste stream, which may include organic materials (e.g., dissolved organic solvent). These same reactions were then run in an organic-solvent-free medium of water, which contained a mere 2–5 weight percent of a commercially available designer amphiphile, such as PTS[16] or TPGS-750-M[17,18] (Figure 1). E Factors for each run in water could then be calculated and compared directly with those based on couplings done in traditional organic media.
Figure 1.
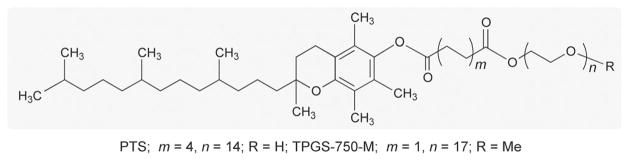
Structures for racemic vitamin E-based amphiphiles PTS and TPGS-750-M.
2. Comparisons: Micellar Catalysis in Water versus Organic Solvents
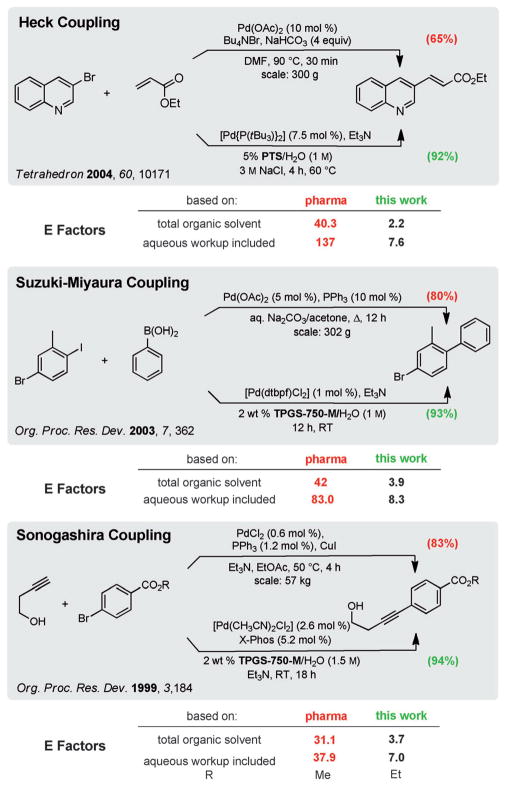
Among the array of well-known palladium-catalyzed cross-coupling reactions, the most commonly cited and patented in the previous decade, according to a review by Snieckus and Collacot,[19] are the Suzuki–Miyaura, Heck, and Sonogashira reactions. As the examples in Scheme 1 illustrate, E Factors for reactions associated with each type can be reduced dramatically, in some cases by greater than an order of magnitude. Since these were run in water on a far smaller, academic scale relative to those in the published studies, the corresponding drop in E Factor would likely be even more pronounced at larger scales where small losses are of little consequence. Moreover, in most cases, the hydrophobic effect[20] characteristic of aqueous micellar catalysis allows cross-coupling to take place at room temperature,[21] while in traditional organic media heating might well be needed to initiate and/or drive reactions to completion. With heating, especially in solvents such as DMF, usually comes the unavoidable appearance of varying amounts of side products along with potential complications associated with product purification and the resulting diminution in yields of the desired products. Reactions run in water at room temperature, by contrast, are typically very clean and the yields in comparison can be higher, as seen in each of these cases.
Scheme 1.
Representative comparison of E Factors for the three named reactions. DMF =N,N-dimethylformamide, dtbpf=1,1′-bis(di-tert-butyl-phosphino)ferrocene, X-Phos=2-dicyclohexylphosphanyl-2′,4′,6′-triiso-propylbiphenyl.
Running reactions that are water-based has the potential to greatly simplify set up, execution, and especially workup. A supply of an aqueous solution of the surfactant is degassed and stored on the shelf. At the time of use, the required volume can be transferred to the reaction vessel in air. Once the educts and catalyst have been added, high levels of conversion are achieved by efficient stirring, which is crucial. When the reaction is complete, no additional water need be added; that is, product extraction is a matter of adding a limited amount of a single organic solvent (e.g., EtOAc) to the reaction vessel with subsequent in-flask extraction and product removal. No additional waste-water stream is created and the reaction medium need not leave the reaction flask.
Representative procedure for a Heck coupling in water as shown in Scheme 1:
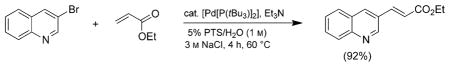
[Pd{P(tBu3)}2] (77 mg, 0.15 mmol) was added to an oven dried 5 mL microwave vial equipped with a PTFE stir bar (1×5 mm) and purged with Ar in a glove box. The vial was capped with a septum and removed from the box. 3-Bromoquinoline (416 mg, 271 μL, 2.00 mmol) was added through the septum by syringe followed by a 5 wt % PTS/H2O (3 M NaCl) solution (2.00 mL) and triethylamine (607 mg, 840 μL, 6.00 mmol). After stirring the solution for 1 min, ethyl acrylate (400 mg, 426 μL, 4.00 mmol) was added by syringe through the septum. The vial was placed in an oil bath at 60°C and vigorously stirred for 4 h and then cooled to room temperature. After extraction with EtOAc (4×250 μL) the resulting organic layers were combined and washed with brine (1×250 μL), passed over anhydrous MgSO4, and the solvent was removed by rotary evaporation to afford pure product as a white crystalline solid (417 mg, 92 %).
These reactions in water are expected to be heterogeneous, as illustrated in Figure 2. Over time, and with good stirring, they may remain heterogeneous, although the contents tend to become more flocculent, powdery, or granular in nature. Color changes are typical, although these can vary widely depending upon the catalyst present in the medium. TLC analysis is easily performed either in the usual manner, or by an aliquot which has been treated in a vial with sub-milliliter quantities of non-chlorinated organic solvent. Once a reaction is judged to be complete, minimal amounts of an organic solvent are added to the flask, and after stirring, the layers separate for product isolation.
Figure 2.

Appearance of a Suzuki–Miyaura reaction mixture over time, and associated work up. 1: Reagents, 2: surfactant added, 3: after 1 minute, 4: reaction complete, 5: extraxtion solvent added, 6: extraction solvent mixing, 7: extraction solvent + aqueous surfactant solution.
2.1. Recycling of Aqueous Micellar Solutions
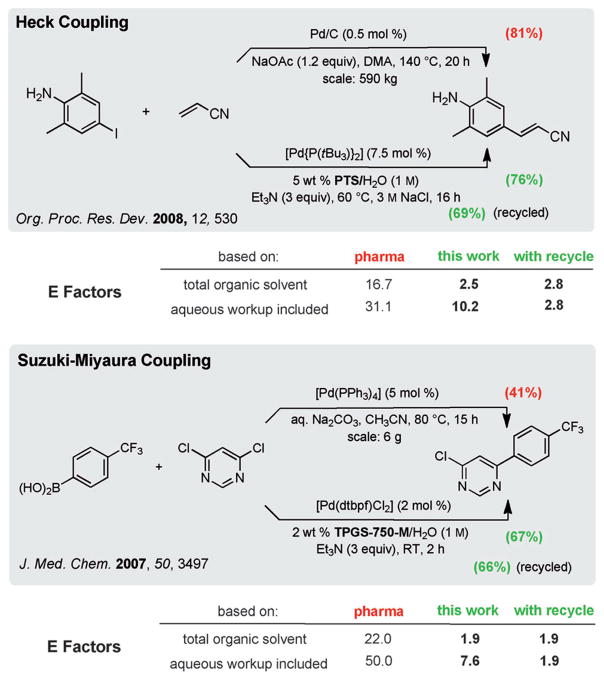
Yet another major advantage of modern micellar catalysis, which leads to further reductions in E Factors, is the facility with which these aqueous reaction mixtures can be recycled. As noted above, once a reaction is complete, in-flask extraction with a minimum amount of an organic solvent (e.g., EtOAc, ether, or any hydrocarbon) allows facile product isolation. Remaining in the aqueous phase, in the reaction vessel, is the surfactant which is ready for reuse. While the water invested remains at constant levels, it is likely to be eventually discarded as it becomes contaminated with water-soluble byproducts (e.g., salts) resulting from the couplings therein. Even here the aqueous waste is minimal, as many reactions in PTS or TPGS-750-M can be run at global concentrations in the 0.75 to 1.5 M range, which is uncommon in traditional palladium-catalyzed cross-couplings.[22] In addition, both surfactants, as diesters, are readily broken down into their component parts, each of which (i.e., vitamin E, a dicarboxylic acid, and PEG; PEG = polyethyleneglycol) is environmentally benign. Done at scale, it is likely that the organic solvent devoted to an in-flask extraction can be recovered to some extent, given that it is not contaminated by other solvents usually associated with both the reaction medium and extraction process which are oftentimes not the same. As shown in Scheme 2, even a single recycle of the medium can result in the further reduction in E Factors by well over 50% (10.2 → 2.8, and 7.6 → 1.9).[23]
Scheme 2.
Impact of a single recycling of the aqueous reaction mixture on E Factors. DMA =N,N-dimethylacetamide.
In several of these cross-couplings in water, which have been already reviewed,[14] recycling has been shown to be viable, and in one case (so far), as many as ten cycles have been documented.[24] Therefore, it is not unreasonable to anticipate that E Factors derived from both organic solvents and water usage can be reduced to less than unity.
2.2. Sequential Reactions in Water at Room Temperature
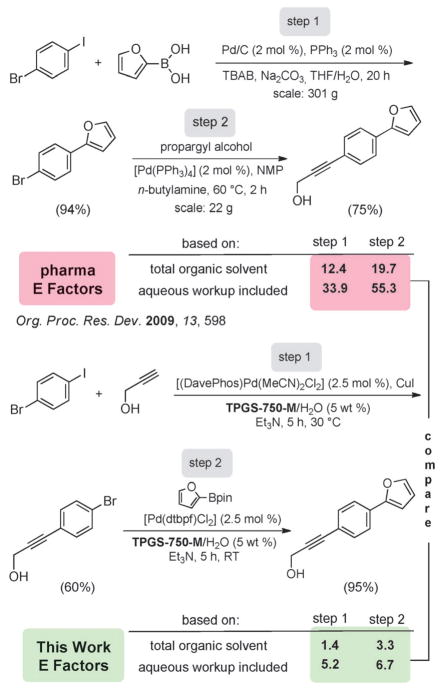
Sequential cross-couplings are also pursued in the pharma arena. One example, shown in Scheme 3, utilizes a dihaloarene that serves as a linchpin en route to the desired doubly substituted product. The initial Suzuki–Miyaura coupling relies on refluxing aqueous THF, while the second reaction, a Sonogashira coupling, is run in warm NMP (N-methyl-2-pyrrolidone). Both organic solvents are water-miscible and involve washing with copious amounts of water.[25] The E Factors based solely on organic solvent are 12.4 and 19.7 for steps one and two, respectively. Taking water from workup into account, the E Factors jump to almost 34 for step one, and over 55 for the second coupling. By contrast, each run (most efficiently pursued in the reverse order) under micellar catalysis at ≤30°C affords an E Factor which in the absence of water in the equation, is quite low (step 1: 1.4 and step 2: 3.3). Even with water as the global reaction medium, the values are significantly lower than those from reactions run initially in organic solvents (5.2 vs. 33.9; 6.7 vs. 55.3).
Scheme 3.
Comparison of E Factors from sequential cross-couplings. DavePhos=2-dicyclohexylphosphino-2′-(N,N-dimethylamino)biphenyl, TBAB =tetra-n-butylammonium bromide.
Another option to reduce E Factors associated with cross-couplings relies on reactions which are run sequentially in a single pot in water at room temperature; that is, in contrast to two reactions normally requiring two individual pots, two different organic solvents, and two separate workups. One example of such a sequence related to the case above involving a dihaloarene is shown in Scheme 4.[26] The initial Sonogashira coupling is followed, without workup, by introduction of naphthylboronic acid and additional catalyst. The overall E Factor for this two-step, one-pot process based on organic solvent invested is only 5.1. Even including the limited amounts of water present as the medium (reaction concentration is 2.0 M) raises the E Factor to only 7.5.
Scheme 4.
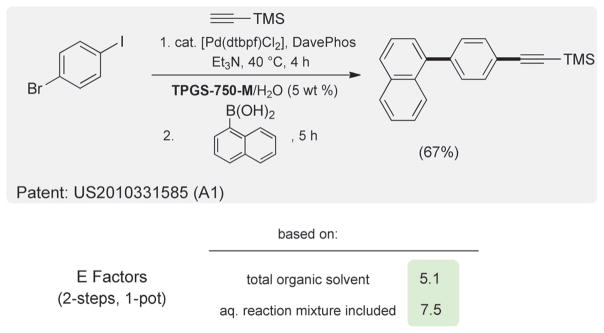
E Factors from a tandem two-step, one-pot sequence.
2.3. An Alternative Platform: PQS
Although these reactions document the enormous potential to minimize the extent to which organic solvents are involved in organic reactions, in each case the catalyst is lost from the aqueous phase during in-flask extraction. The loss of catalyst not only adds somewhat to the waste, but can be especially costly when precious metals are involved. To address this issue of catalyst loss the alternative water-soluble, nanomicelle-forming platform PQS[27] (Figure 3) was designed to provide an additional synthetic handle (i.e., the OH group) on the hydroquinone (derived from coenzyme Q10), a handle to which a catalyst could be covalently linked.
Figure 3.
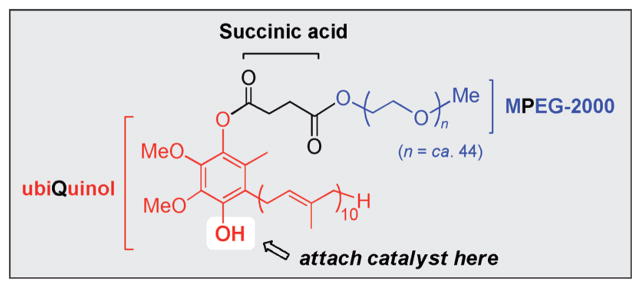
Structure and components of micelle-forming PQS.
Catalysts that have been attached include the Grubbs-Hoveyda-1[24] and Grubbs-Hoveyda-2[28] derivatives (Figure 4; 1 and 2); each forms nanomicelles in water. These particles enable olefin cross- and ring-forming metathesis reactions to be run in water at room temperature, where in-flask recycling does not remove the valued ruthenium catalyst. Recycling is facile and takes place without changes in reaction outcome. Loss of ruthenium as determined by product analysis was found to be on the order of less than or equal to 2 ppm. Other PQS catalyst combinations include the PQS proline 3, use of which leads to organocatalysis in water at room temperature without loss of proline upon in-flask product extraction.[29]
Figure 4.
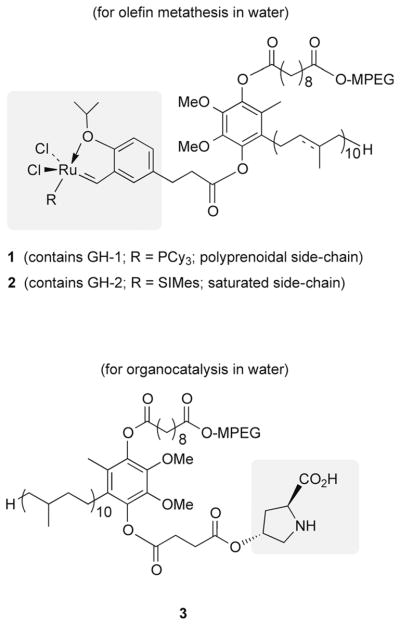
PQS-based covalently bound catalysts used for metathesis and organocatalysis.
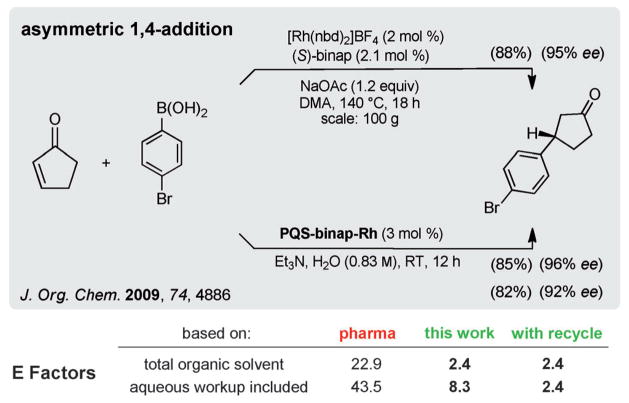
More recently, PQS-binap-Rh (4; Figure 5) has been prepared and utilized in rhodium-catalyzed asymmetric conjugate additions of boronic acids to enones, wherein rhodium is chelated by binap and remains in the aqueous layer.[30] From the standpoint of E Factors, a literature example involving such a 1,4-addition of a boronic acid is shown in Scheme 5.[31] Aside from the use of dimethylacetamide (DMA) and the input of external energy to achieve a reaction temperature of 140°C, the associated traditional organic-solvent-based E Factor is almost 23. By contrast, the identical reaction could be run in water at ambient temperature to afford the desired product in comparable yield and isomeric purity with an E Factor of 2.4.
Figure 5.
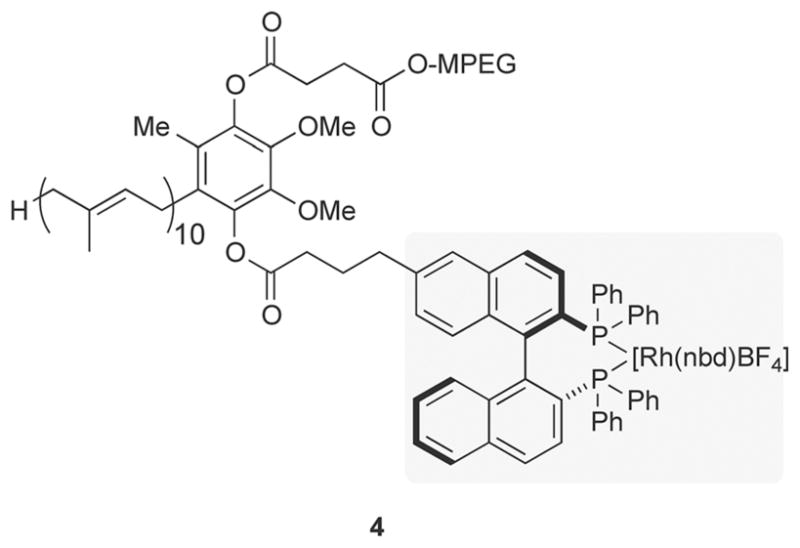
Structure of PQS-binap-RhI. binap=2,2′-bis(diphenylphosphanyl)-1,1′-binaphthyl, nbd =2,5-norbornadiene.
Scheme 5.
Comparison of E Factors using PQS-binap-Rh in water versus organic solvent.
Using a water workup to remove the DMA leads to an E Factor of over 43; with water as the reaction medium under micellar conditions, the E Factor drops to 8.3. Once the initial reaction in aqueous nanoparticles has led to the targeted 1,4-adduct, re-introduction of only additional enone and coupling partner (i.e., no additional catalyst) produced the desired ketone. The overall E Factor, therefore, when no additional water has been added in the workup, and based on this single recycle, drops to 2.4. Tellingly, the measured analytical loss of this valued transition metal, as found within the product ketone in a previous study,[30] was less than 10 ppb.
In addition to the absence of organic solvents and the recycling of the aqueous medium and valuable catalyst, especially for larger scale reactions, these ambient-temperature reactions based on the PQS platform offer many additional advantages: 1) eliminating the need for handling/cleaning of the reaction vessel after each reaction, 2) facile reaction monitoring of room temperature reactions, and 3) potential increased throughput, as time is not lost with heated or cooled reaction vessels which must be returned to ambient temperatures.
3. Summary and Outlook
This review takes aim at E Factors, and the implications for industrial processes which generate huge amounts of organic waste derived mainly from usage of organic solvents as reaction media. Several of the most commonly employed palladium-catalyzed cross-couplings, as described by several pharmaceutical companies, have been selected for comparison purposes to illustrate alternative chemistry which may provide a pathway to significantly reduce E Factors to levels comparable to those associated with other chemical industries (e.g., bulk chemicals). The key to lowering E Factors in these reactions is the application of environmentally benign surfactants which spontaneously self-assemble in water into nanomicelles of a particularly engineered shape and size such that they maximize reaction efficiency. Cross-coupling and related reactions run within these lipophilic cores, especially at ambient temperatures, are typically clean and thus, can oftentimes be higher yielding than those run in organic media. Opportunities for recycling of these aqueous reaction mixtures serve to further reduce dependence on organic solvents. New industrial processes, therefore, that begin green are very likely to remain green for the lifetime of a given process; that could be years, thus translating into a potential economic and environmental windfall for each green step in a synthesis. Notwithstanding the potential for these enormous savings, unknown challenges may well arise with scale up of such micellar-enabled chemistry. Put another way, what is the downside to this chemistry? When asked of an industrial colleague, the respononse was: “I don’t see any.” And so as the library of such processes grows, so should the prospects for sustainability. After all, many organic solvents are derived from the world’s petroleum reserves. Going forward, one can imagine that many alternative amphiphiles and/or procedures will be devised in an effort to lower E Factors even further. We challenge the chemical community to do better!
Acknowledgments
Financial support for this work provided by the NIH (R01GM 86485) and (in part) the NSF (CHE 0948479) is warmly acknowledged with thanks. Catalysts generously provided by Johnson Matthey and Materia are most appreciated. We warmly thank Wyatt Laboratories in Santa Barbara, CA, for their assistance with DLS measurements on TPGS-750-M. Comments on the manuscript received from Drs. Lawrence Hamann (Novartis) and Thomas Colacot (Johnson Matthey) were especially valuable. We also thank Dr. Volker Berl (Mycell Technologies) for early discussions on TPGS-750-M, and Dr. John Tucker (Amgen) for suggesting that this study be done.
Biographies
 Bruce Lipshutz has been at UCSB since 1979, where he is Professor of Chemistry. His program in synthesis focuses in large measure on green chemistry, with the specific goal of getting organic solvents out of organic reactions. Towards this goal, designer surfactants have been introduced to enable transition-metal-catalyzed cross-couplings to be carried out in water at room temperature.
Bruce Lipshutz has been at UCSB since 1979, where he is Professor of Chemistry. His program in synthesis focuses in large measure on green chemistry, with the specific goal of getting organic solvents out of organic reactions. Towards this goal, designer surfactants have been introduced to enable transition-metal-catalyzed cross-couplings to be carried out in water at room temperature.
 Nicholas Isley received his B.S. from Western Washington University (2010). After being exposed to undergraduate research, he decided to obtain a Ph.D. at UCSB. In 2012, he received an NSF Graduate Research Fellowship Honorable Mention Award, and the Robert H. DeWolfe Graduate Teaching Fellowship in Organic Chemistry at UCSB.
Nicholas Isley received his B.S. from Western Washington University (2010). After being exposed to undergraduate research, he decided to obtain a Ph.D. at UCSB. In 2012, he received an NSF Graduate Research Fellowship Honorable Mention Award, and the Robert H. DeWolfe Graduate Teaching Fellowship in Organic Chemistry at UCSB.
 James Fennewald received his B.S. from Evergreen State College (2008) and his M.S. from the University of Oregon (2010). After working at a start-up pharma company, he decided to pursue his Ph.D. studies and joined the Lipshutz group at the University of California, Santa Barbara.
James Fennewald received his B.S. from Evergreen State College (2008) and his M.S. from the University of Oregon (2010). After working at a start-up pharma company, he decided to pursue his Ph.D. studies and joined the Lipshutz group at the University of California, Santa Barbara.
 Eric Slack received his B.S. from California State University at Fullerton in 2010. He is currently in his third year of graduate study working in both areas of green chemistry and total synthesis in the group of Prof. Bruce Lipshutz at UCSB.
Eric Slack received his B.S. from California State University at Fullerton in 2010. He is currently in his third year of graduate study working in both areas of green chemistry and total synthesis in the group of Prof. Bruce Lipshutz at UCSB.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201302020.
Contributor Information
Bruce H. Lipshutz, Email: lipshutz@chem.ucsb.edu, Dept. of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, CA 93106 (USA), Homepage: http://web.chem.ucsb.edu/~lipshutzgroup/
Nicholas A. Isley, Dept. of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, CA 93106 (USA), Homepage: http://web.chem.ucsb.edu/~lipshutzgroup/
James C. Fennewald, Dept. of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, CA 93106 (USA), Homepage: http://web.chem.ucsb.edu/~lipshutzgroup/
Eric D. Slack, Dept. of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, CA 93106 (USA), Homepage: http://web.chem.ucsb.edu/~lipshutzgroup/
References
- 1.For BASF-related, see: Landsiedel R, Saling P. Int J Life Cycle Assess. 2002;7:261.For an alternative discussion, see: Van Aken K, Strekowski L, Patiny L. Beilstein J Org Chem. 2006;2:3. doi: 10.1186/1860-5397-2-3.
- 2.Feuerherd K-H. Macromol Symp. 2003;201:253. [Google Scholar]
- 3.Shonnard DR, Kicherer A, Saling P. Environ Sci Technol. 2003;37:5340. doi: 10.1021/es034462z. [DOI] [PubMed] [Google Scholar]
- 4.Wall-Markowski CA, Kicherer A, Saling P. Environ Prog. 2004;23:329. [Google Scholar]
- 5.Saling P. Appl Microbiol Biotechnol. 2005;68:1. doi: 10.1007/s00253-005-1951-0. [DOI] [PubMed] [Google Scholar]
- 6.Sheldon RA. Green Chem. 2007;9:1273. [Google Scholar]
- 7.Constable DJC, Curzons AD, Cunningham VL. Green Chem. 2002;4:521. [Google Scholar]
- 8.Hudlicky T, Frey DA, Koroniak L, Claeboe CD, Brammer LE. Green Chem. 1999;1:57. doi: 10.1021/jo990382v. [DOI] [PubMed] [Google Scholar]
- 9.Jiménez-GonzQlez C, Ponder CS, Broxterman QB, Manley JB. Org Process Res Dev. 2011;15:912. [Google Scholar]; Jiménez-GonzQlez C, Ollech C, Pyrz W, Hughes D, Broxterman QB, Bhathela N. Org Proc Res Dev. 2013;17:239. [Google Scholar]
- 10.National Service Center for Environmental Publications (NSCEP); National Risk Management Research Laboratory (NRMRL) Life Cycle Assessment, Principles and Practice, EPA/600/R-06/060. U.S. Environmental Protection Agency; Cincinnati, OH: May, 2006. [Google Scholar]
- 11.Wenzel H, Hauschild M, Alting L. Methodology, Tools and Case Studies in Product Development, Environmental Assessment of Products. Vol. 1. Chapman and Hall; New York: 1997. [Google Scholar]
- 12.Dunn P, Henderson R, Mergelsberg I, Wells A. Collaboration to Deliver a Solvent Selection Guide for the Pharmaceutical Industry Moving Towards Greener Solvents for Pharmaceutical Manufacturing—An Industry Perspective. College Park, Maryland: ACS Green Chemistry Institute Pharmaceutical; Jun 23–25, 2009. http://acs.confex.com/acs/green09/recordingredirect.cgi/id/510. [Google Scholar]
- 13.Sheldon RA, Arends IWCE, Hanefeld U. Green Chemistry and Catalysis. Wiley-VCH; Weinheim: 2007. [Google Scholar]
- 14.Lipshutz BH, Ghorai S. Aldrichimica Acta. 2012;45:3. [PMC free article] [PubMed] [Google Scholar]; Lipshutz BH, Ghorai S. Aldrichimica Acta. 2008;41:59. [PMC free article] [PubMed] [Google Scholar]
- 15.Magano J, Dunetz JR. Chem Rev. 2011;111:2177. doi: 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]
- 16.Lipshutz BH, Ghorai S, Leong WWY, Taft BJ. J Org Chem. 2011;76:5061. doi: 10.1021/jo200746y. and references therein. PTS: Aldrich catalogue number 698717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipshutz BH, Ghorai S, Abela AR, Moser R, Nishikata T, Duplais C, Krasovskiy A. J Org Chem. 2011;76:4379. doi: 10.1021/jo101974u. See Aldrich catalog numbers 733857 and 763918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Both PTS and TPGS-750-M are unsymmetrical diesters, and as such they are subject to hydrolysis. Each surfactant is composed of components which themselves are environmentally benign and generally recognized as safe (GRAS): vitamin E, sebacic or succinic acids, and PEG. Hence, their eventual treatment as part of an aqueous waste stream is likely to be of little environmental consequence.
- 19.Carin CC, Seechurn J, Kitching MO, Colacot TJ, Snieckus V. Angew Chem. 2012;124:5150. [Google Scholar]; Angew Chem Int Ed. 2012;51:5062. [Google Scholar]
- 20.Lindström UM, Andersson F. Angew Chem. 2006;118:562. doi: 10.1002/anie.200502882. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:548. [Google Scholar]
- 21.Lipshutz BH. Applying the Hydrophobic Effect to Transition Metal-Catalyzed Couplings in Water at Room Temperature. In: Magano J, Dunetz JR, editors. Transition Metal-Catalyzed Couplings in Process Chemistry: Case Studies from the Pharmaceutical Industry. Wiley-VCH; Weinheim: in press. [Google Scholar]
- 22.Negishi E, Gagneur S. In: Handbook of Organopalladium Chemistry for Organic Synthesis. Negishi E, editor. Vol. 1. Wiley; New York: 2002. p. 2. [Google Scholar]
- 23.The Suzuki–Miyaura coupling in Scheme 2 was run using the same catalyst, [Pd(PPh3)4], and base, Na2CO3, but under micellar conditions, by replacing CH3CN with TPGS-750-M/H2O. After 2 h the desired product was formed in less than 7% yield (by GC), illustrating the crucial role played by the ligands, and that ligands known to work well in organic media are not necessarily the best choice under micellar conditions.
- 24.Lipshutz BH, Ghorai S. Org Lett. 2009;11:705. doi: 10.1021/ol8027829. [DOI] [PubMed] [Google Scholar]
- 25.Houpis IN, Shilds D, Nettekoven U, Schnyder A, Bappert E, Weerts K, Canters M, Vermuelen W. Org Process Res Dev. 2009;13:598. [Google Scholar]
- 26.Masahiro K, Kazuki N, Yoriyuki T, Mistunori I, Toshihiro I, Toshinari O. Phenanthrene Derivative, and Material for Organic EL Element. 331,585 (A1) US Patent Application. 2010 Dec 30;
- 27.Moser R, Ghorai S, Lipshutz BH. J Org Chem. 2012;77:3143. doi: 10.1021/jo202564b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipshutz BH, Ghorai S. Tetrahedron. 2010;66:1057. [Google Scholar]
- 29.Lipshutz BH, Ghorai S. Org Lett. 2012;14:422. doi: 10.1021/ol203242r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipshutz BH, Isley NA, Moser R, Ghorai S, Leuser H, Taft BR. Adv Synth Catal. 2012;354:3175. doi: 10.1002/adsc.201200160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace GA, Gordon TD, Hayes ME, Konopacki DB, Fix-Stenzel SR, Zhang X, Grongsaard P, Cusak KP, Schaffter LM, Henry RF, Stoffel RH. J Org Chem. 2009;74:4886. doi: 10.1021/jo900376b. [DOI] [PubMed] [Google Scholar]