Abstract
Background
Chronic kidney disease (CKD) is associated with increased incidence of cardiac dysfunction. Recent animal studies have demonstrated that elevated levels of ceramides cause dilated lipotoxic cardiomyopathy. We hypothesized ceramides are increased in children with CKD and associated with abnormal cardiac structure and function.
Methods
Ceramide levels were determined in 93 children aged 1–16 years enrolled in the Chronic Kidney Disease in Children (CKiD) study and compared to levels from 24 healthy controls. Complete demographic, clinical, and laboratory information, and ceramide measurements were analyzed cross-sectionally. Echocardiography was performed to determine cardiac structure and function.
Results
Very long-chain C24:0 ceramides were the most abundant species in both control (56 %) and CKD subjects (55 %), followed by C24:1 (controls 19 %, CKD 23 %) and C22:0 (controls 19 %, CKD 13 %). Total serum ceramide levels were significantly higher in CKD children versus controls (p <0.001). Ceramide metabolites lactosylceramide, C24:0L, and C16:0L were significantly higher in CKD subjects than controls (p <0.001). The proportion of C24:0L was dramatically higher in CKD (59 %) versus control (17 %) subjects (p <0.001). In adjusted multivariate analyses, higher log10C24:0L and log10C16:0L were significant predictors of lower shortening fraction and mid-wall shortening.
Conclusions
Ceramide levels are increased in children with CKD. Our study identified lactosylceramides as an independent predictor of lower systolic function in these children.
Keywords: CKD, Ceramides, Sphingolipids, Lipids, Heart, Heart failure
Introduction
It is well known that chronic kidney disease (CKD) is associated with an increased incidence of cardiac dysfunction, even in patients with mild CKD [1]. Early cardiac dysfunction is frequently asymptomatic and therefore under-diagnosed, often resulting in the delay of therapeutic intervention. The pathogenesis of accelerated cardiomyopathy remains unknown in CKD.
Ceramides are sphingolipids that are synthesized from free fatty acid (FFA) precursors. In the blood, ceramides constitute a part of the circulating lipoprotein particles and are also present in blood cells and platelets [2]. Recent animal studies provided evidence that elevated ceramide levels cause dilated lipotoxic cardiomyopathy [3]. We postulate that abnormal sphingolipid homeostasis is present in CKD patients and may be involved in the mechanism of accelerated cardiac dysfunction in CKD. Ceramides have not been studied in patients with CKD.
The objectives of this pilot study were: (1) to determine and compare serum ceramide levels in children with CKD and healthy controls, and (2) to evaluate the association betweenthe serum ceramide levels and markers of cardiac structure and function in children with CKD. Data from the Chronic Kidney Disease in Children (CKiD) cohort, a prospective observational study of children with mild to moderate CKD, were utilized in the analysis.
Methods
Study population
The CKiD study is an observational cohort study of CKD in children being conducted at 49 pediatric nephrology centers in North America. The CKiD study protocol has been described elsewhere [4]. It has been reviewed and approved by the Institutional Review Boards of each participating center. Eligibility criteria for enrollment in CKiD include: age 1–16 years and estimated glomerular filtration rate (eGFR) as calculated by the Schwartz formula [5] of 30–90 ml/min/1.73 m2. In this pilot study, 93 subjects from Visit 4 (year 3 of the study) had their serum sphingolipid profile analyzed, 69 of whom also had complete echocardio-graphic data on cardiac structure and function, and other relevant demographic (age, gender, race, height, weight), clinical (blood pressure) and laboratory parameters (fasting lipids, insulin, iohexol GFR, protein/creatinine ratio, hemoglobin and serum albumin). Serum from 24 healthy children 9–17 years of age (median age (IQR) 13.5 (12.0, 15.5) years), including ten females and ten blacks, from the Cincinnati Genomic Control Cohort, was analyzed for sphingolipid profiles to serve as controls.
Cardiac structure and function
The specific procedure for echocardiographic determination of left ventricular mass (LVM) and definition of left ventricular hypertrophy (LVH) in the CKiD study has been described elsewhere [6]. Briefly, M-mode and Doppler ECHO are performed at individual participating centers, but reading and analyses of echocardiographic data are performed by the Cardiovascular Core Imaging Research Laboratory at Cincinnati Children’s Hospital Medical Center. To achieve standardization and uniformity of echocardiographic images across the centers, qualifying recordings were sent to each center and then certified at the Cardiovascular Core Imaging Research Laboratory. LVM was indexed (mass divided by height raised to a power of 2.7 [g/m2.7]) to account for body size. LVH was defined as LVM index≥the 95th percentile for normal children and adolescents [7]. Diastolic function was estimated by Doppler measurements of the maximal early (E wave) and late (A wave) diastolic flow velocities, E/A ratio, and by Tissue Doppler (E’/A’). LV systolic performance was assessed by calculation of shortening fraction (SF) and mid-wall shortening fraction (mwSF) to correct for overestimation of shortening fraction in hypertrophied ventricles [8, 9].
Ceramide profiling
Seventeen ceramide species and their metabolites (C16:0, C16:0 dihydroceramide, C16:0 glucosylceramide, C16:0 lactosylceramide, C18:0, C18:0 dihydroceramide, C18:0 glucosylceramide, C18:1, C20:0, C22:0, C22:0 glucosylceramide, C24:0, C24:0 dihydroceramide, C24:0 lactosylceramide, C24:1, C24:1 dihydroceramide, C24:1 glucosylceramide) were quantified by HPLC-MS/MS at UT Southwestern Medical Center Mouse Metabolic Phenotyping Core. The instrument consisted of a Shimadzu Prominence HPLC system equipped with a CBM-20A controller, a DGUA3 degasser, three HPLC solvent delivery modules LC-ADXR, a CTO-20 AC column oven/chiller maintained at 30 °C, and a SIL-20ACTHTautosampler. The HPLC system is coupled to an API 5000 LC-MS/MS mass spectrometer (AB SCIEX, Framingham, MA) equipped with a Turbo V ion source operating the electrospray ionization (ESI) probe in positive mode. Quantitative analysis of sphingolipids was achieved using selective reaction monitoring scan mode. Chromatographic separations were obtained by reverse phase LC on a 2.1 (i.d.) × 150 mm Kinetex C8 (Phenomenex, Torrance, CA, USA) column under a complex gradient elution, using three different mobile phases: eluent A consisting of CH3OH/H2O/HCOOH, 58/41/1, v/v/v with 5 mM ammonium formate, eluent B consisting of CH3OH/HCOOH, 99/1, v/v with 5 mM ammonium formate, and eluent C consisting of CH3OH/CH2Cl2 35/65 with 5 mM ammonium formate [10, 11]. The concentration of each metabolite was determined according to calibration curves using the peak-area ratio of analyte vs. corresponding internal standard. Calibration curves were generated using serial dilutions of each target analyte. Sphingolipid standards and the Internal Standard Cocktail Mix II were purchased from Avanti Polar Lipids (Alabaster, AL, USA). C22:0 glucosylceramide was purchased from Matreya LLC (Pleasant Gap, PA, USA).
Sphingolipid analysis in healthy controls and CKiD subjects was performed at different time points and therefore in order to appropriately compare ceramide levels between healthy controls and CKD subjects, data were normalized to respective pooled normal human plasma control samples at the time of each sphingolipid analysis. This was accomplished by dividing the metabolite level in the sample by the average metabolite level in the pooled normal plasma control. Pooled normal human plasma was purchased from Innovative Research (Novi, MI, USA).
Statistical analysis
Ceramides and their metabolites were reported as medians with the interquartile range (IQR) as they were not normally distributed. Comparisons between categorical variables were performed using the Wilcoxon rank-sum test. For all continuous variables, Pearson production-moment correlation was used to determine association of the ceramides and their metabolites with SF, mwSF, and other factors. Multiple linear regression analysis was performed to determine predictors of SF and mwSF. In addition to ceramides, covariates included age, body mass index (BMI) z-score adjusted for age and gender, GFR, systolic blood pressure z-scores adjusted for age, gender and height (markers of afterload), left ventricular mass index (LVMI), presence of anemia and serum albumin (markers of preload). Serum ceramides were log-transformed (base 10) to adjust for the large range in values and nonnormal distribution. When GFR, as determined by directly measured plasma iohexol (assessment method has been published previously [5]) was unavailable, an estimated GFR value was used based on an internally derived estimating equation [12]. An additional regression analysis was performed to predict heart rate (HR) from ambulatory blood pressure monitoring data. A p value <0.05 indicated statistical significance. All analyses were performed using SAS statistical software (version 9.2 SAS Institute, Cary, NC, USA).
Results
In healthy controls, none of the levels of the 17 measured ceramides and their metabolites (C16–C24) were related to age; C16:0 levels were significantly higher in males and C18:1 levels were significantly higher in African American children. There was no significant difference in the levels of any other ceramides according to sex and race (data not shown).
The CKiD sub-cohort characteristics are shown in Table 1. There was no significant difference in demographic and clinical characteristics between study subjects and the rest of the CKiD cohort except that the ceramide sub-cohort subjects had a higher GFR and fewer were anemic. Four (5.6 %) of the subcohort had SF <25 % and 10 (13.5 %) had LVH. Two subjects were taking statins.
Table 1.
Study characteristics. Data presented as median (IQR) or %
| Characteristic | Study sub-cohort n =93 |
CKiD cohort without sphingolipid profile n = 309 |
p |
|---|---|---|---|
| Age (years) | 12.8 (9.4–17.0) | 13.3 (10.1–16.7) | 0.959 |
| Male gender | 50 (53.8) | 196 (63.4) | 0.093 |
| White race | 65 (69.9) | 213 (68.9) | 0.860 |
| Height (m) | 149.1 (126.9–165.2) | 151.3 (133.7–164.0) | 0.526 |
| Tanner stage II through V | 54 (59.3) | 176 (60.3) | 0.874 |
| Birth weight = >2,500 g | 74 (83.1) | 234 (79.6) | 0.459 |
| Duration of chronic kidney disease (years) | 8.1 (6.0–12.3) | 9.7 (6.5–12.9) | 0.387 |
| Non-glomerular CKD etiology | 77 (82.8) | 255 (82.5) | 0.952 |
| Glomerular filtration rate (ml/min/1.73 m2) | 49.2 (38.3–64.2) | 42.5 (30.9–60.3) | 0.006 |
| Body mass index z-score | 51.2 (24.5–84.5) | 60.2 (33.8–87.5) | 0.185 |
| Body mass index =>85th percentile | 22 (24.2) | 81 (27.4) | 0.547 |
| Dyslipidemia | 39 (41.9) | 164 (53.1) | 0.060 |
| HDL cholesterol <40 mg/dl | 14 (15.1) | 79 (25.6) | 0.035 |
| HDL cholesterol (mg/dl) | 50.0 (43.0–60.0) | 48.0 (39.0–58.0) | 0.089 |
| Non-HDL cholesterol >160 mg/dl | 16 (17.2) | 47 (15.6) | 0.715 |
| Non-HDL cholesterol (mg/dl) | 118.0 (96.0–139.0) | 115.0 (97.0–140.0) | 0.738 |
| Triglycerides >130 mg/dl | 32 (34.4) | 107 (35.5) | 0.841 |
| Triglycerides (mg/dl) | 111.0 (73.0–149.0) | 105.0 (74.0–155.0) | 0.695 |
| Hypertension | 11 (12.1) | 40 (13.7) | 0.701 |
| Systolic blood pressure z-score | 48.8 (25.9–79.1) | 53.4 (29.5–79.8) | 0.610 |
| Systolic blood pressure >95th percentile | 9 (9.9) | 30 (10.2) | 0.923 |
| Diastolic blood pressure z-score | 53.7 (32.9–78.9) | 60.6 (38.4–81.2) | 0.120 |
| Diastolic blood pressure >95th percentile | 7 (7.7) | 24 (8.2) | 0.879 |
| Taking anti-hypertensive medication | 57 (61.3) | 205 (66.3) | 0.370 |
| Left ventricular hypertrophy | 10 (13.5) | 33 (12.7) | 0.852 |
| Left ventricular mass index | 30.3 (25.6–36.0) | 31.7 (26.7–36.6) | 0.275 |
| Shortening fraction (%) | 38.2 (33.1–42.5) | 38.2 (34.5–42.5) | 0.620 |
| Shortening fraction <25 | 4 (5.6) | 13 (5.0) | 0.867 |
| Hemoglobin (g/dl) | 12.9 (12.0–13.9) | 12.6 (11.4–13.6) | 0.054 |
| Anemia | 19 (21.3) | 110 (36.1) | 0.009 |
| Albumin (g/dl) | 4.4 (4.2–4.6) | 4.4 (4.1–4.6) | 0.386 |
HDL, high density lipoprotien; CKD, chronic kidney disease
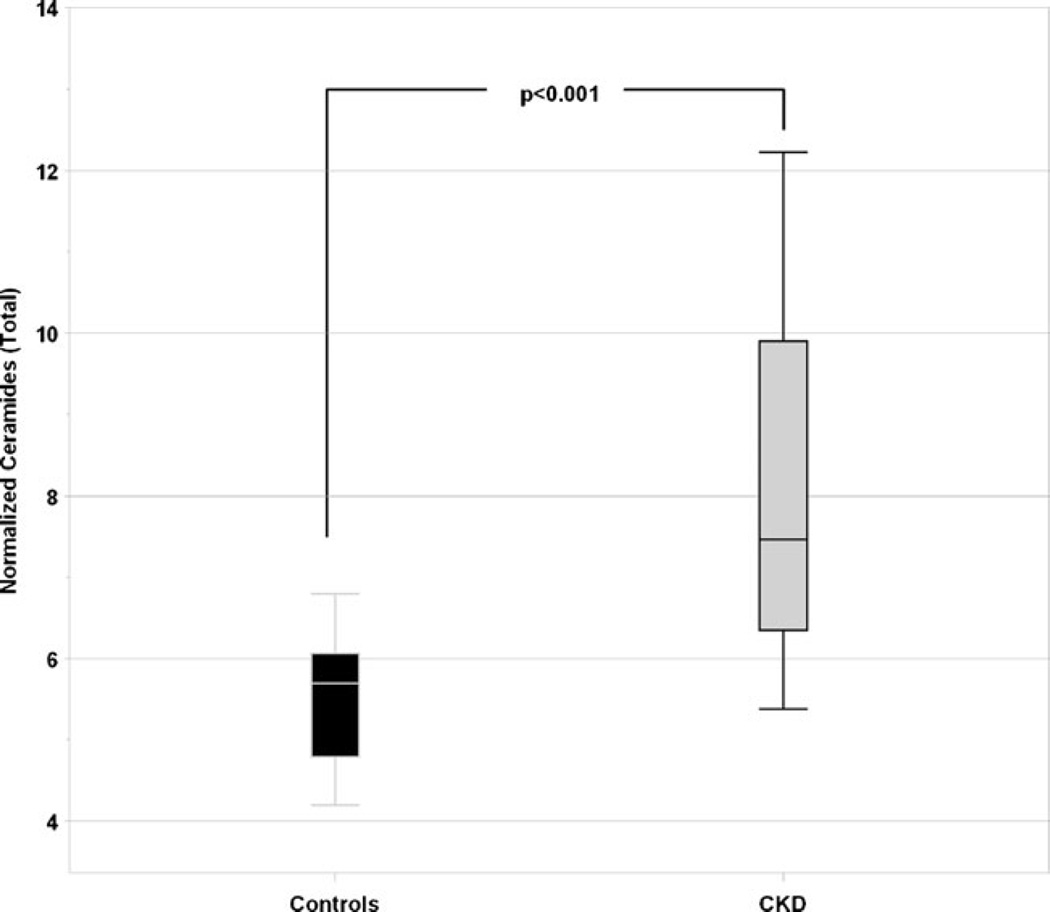
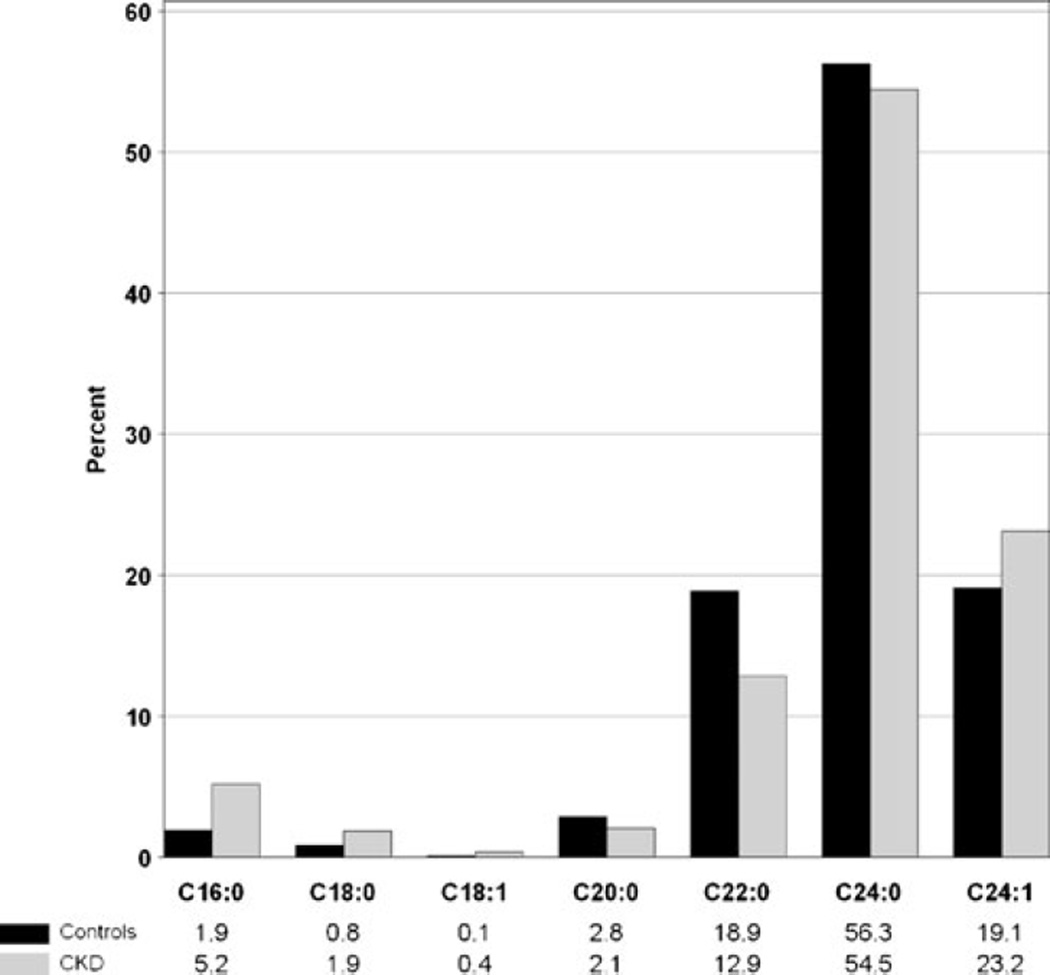
The serum level of total ceramides (C16:0, C18:0, C18:1, C20:0, C22:0, C24:0, C24:1) was significantly higher in the CKD children than healthy controls (median of 7.5 versus 5.7 units, p <0.001, normalized data to pooled human normal plasma), Fig. 1. Long-chain, C24:0 was the most abundant ceramide in both control (56 %) and CKD subjects (55 %), followed by C24:1 (controls 19 %, CKD 23 %) and C22:0 (controls 19 %, CKD 13 %), Fig. 2. The proportion of C16:0 was higher in CKD (5.2 %) than in controls (1.9 %).
Fig. 1.
Comparison of total ceramide levels in healthy children and children with chronic kidney disease (CKD). Data were normalized to respective pooled normal human plasma control samples at time of each sphingolipid analysis: metabolite level in the sample was divided by average metabolite level in pooled normal plasma control
Fig. 2.
Distribution of ceramides in healthy controls and children with chronic kidney disease (CKD). Significant differences were observed for all ceramide species (p <0.001) except C24:0 (p =0.11); Wilcoxon rank-sum test
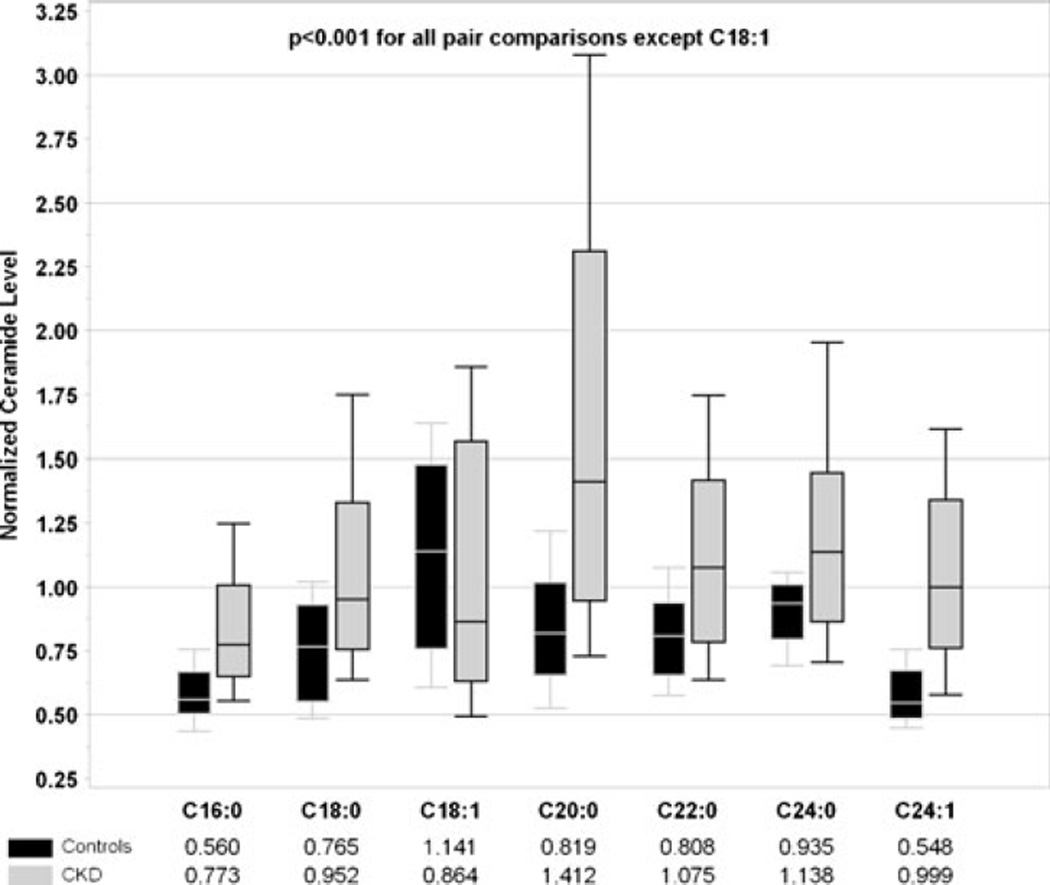
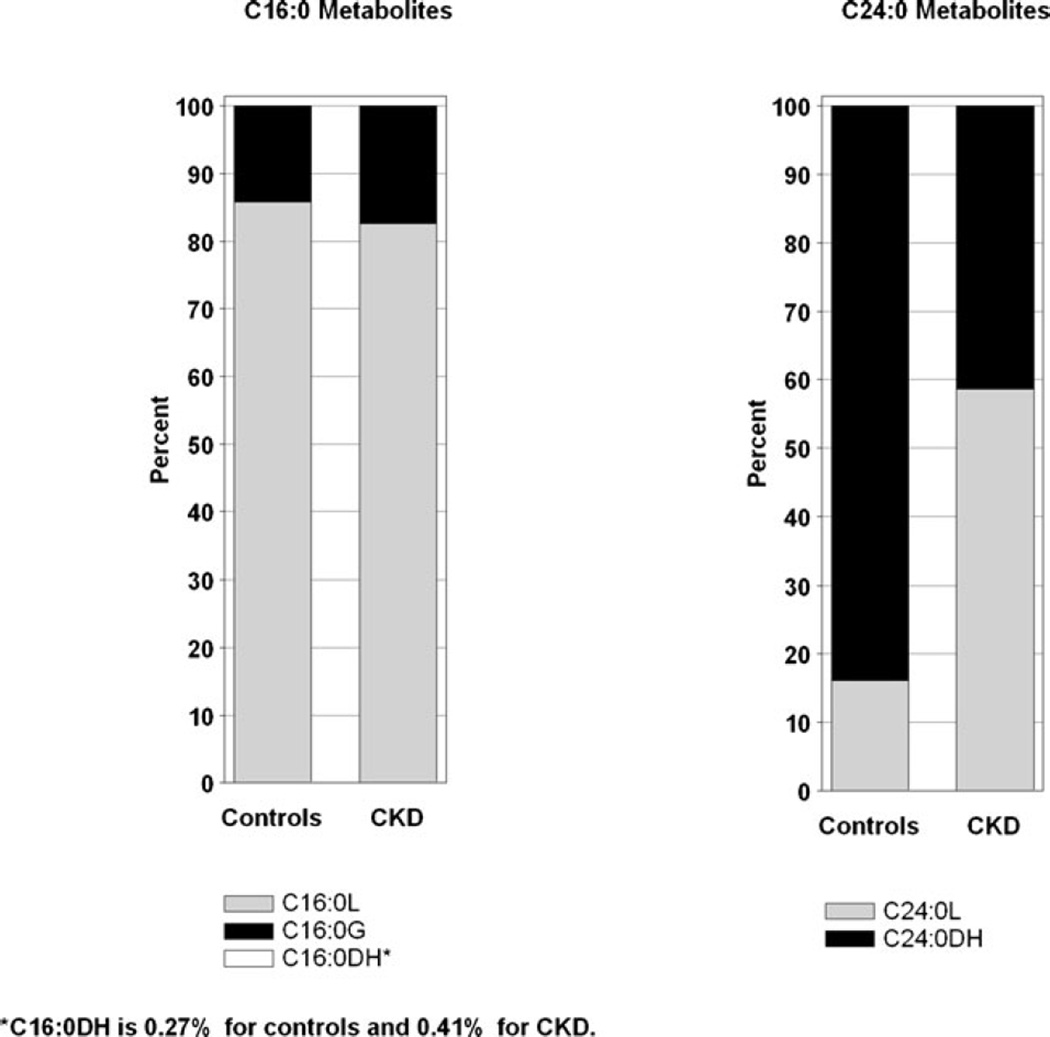
Comparison of individual ceramides is shown in Fig. 3. Children with CKD had significantly higher ceramide levels (all p <0.001, normalized data to pooled human normal plasma) except for C18:1 (p =ns). Serum levels of lactosylceramide (C16:0L and C24:0L) were significantly higher in CKD subjects (p <0.001, data not shown). Among C16:0 metabolites, C16:0L was the most abundant in both controls (85 %) and CKD subjects (82%). In contrast, the proportion of C24:0L was significantly higher (p <0.001) in CKD (59 %) versus control subjects (17 %), Fig. 4.
Fig. 3.
Comparison of individual ceramide levels in healthy children and children with chronic kidney disease (CKD). Data were normalized to respective pooled normal human plasma control samples at time of each sphingolipid analysis: metabolite level in the sample was divided by average metabolite level in pooled normal plasma control
Fig. 4.
Distribution of lactosylceramides C16:0L and C24:0L in healthy controls and children with chronic kidney disease (CKD)
In CKD children, there was no significant association between ceramides and demographic (age, gender, race, weight, height, BMI,), clinical (blood pressure) or laboratory parameters (hemoglobin, serum albumin, serum insulin, HOMA-IR, urine protein/creatinine ratio). Log10C16:0L (ρ=0.31, p =0.003) and log10C24:0L (ρ=0.20, p =0.05) levels were significantly correlated with LDL-cholesterol in univariate analysis. Log10C24:0L (ρ=−0.39, p <0.001) and log10 C16:0L (ρ=−0.35, p =0.003) were significantly associated with SF. Similar significant associations were seen for mwSF (Log10C24:0L ρ=−0.40, p <0.001, and Log10C16:0L ρ=−0.33, p =0.005). No significant association was found between any of the ceramides and markers of diastolic function (E/A ratio, E’/A’).
The results of multivariate analyses evaluating factors associated with SF are shown in Table 2. Log10C24:0L and log10C16:0L were independent significant predictors of SF and mwSF. Another set of multivariate analyses was performed to include heart rate (HR) from ambulatory blood pressure monitoring data (n =52). Log10C24:0L remained significantly associated with both SF (β=−12.6, p =0.03) and mwSF (β=−7.7, p =0.03), while HR was not a significant predictor. In contrast, adding HR resulted in log10C16:0L being non-significant predictor of SF (β=−5.5, p =0.35) and mwSF (β=−3.6, p =0.31).
Table 2.
Results from linear regression models predicting shortening fraction (SF) and mid-wall shortening fraction (mwSF) with covariates C24:0L and C16:0L
| Covariates | SF model | mwSF model | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | p | Estimate | SE | p | |
| Covariate C24:0L | ||||||
| Intercept | 95.44 | 14.99 | <0.001 | 49.15 | 8.58 | <0.001 |
| Log10 C24:0L | −11.7 | 2.53 | <0.001 | −5.24 | 1.45 | <0.001 |
| Age (years) | 0.11 | 0.15 | 0.46 | 0.01 | 0.09 | 0.89 |
| GFR (ml/min/1.73 m2) | 0.01 | 0.04 | 0.74 | 0.00 | 0.02 | 0.99 |
| BMI z-score | 0.81 | 0.61 | 0.19 | 0.43 | 0.35 | 0.23 |
| LVMI (g/m2.7) | −0.11 | 0.10 | 0.30 | −0.12 | 0.06 | 0.05 |
| Anemia | 4.26 | 1.92 | 0.03 | 1.67 | 1.10 | 0.13 |
| Serum albumin (mg/dl) | 0.25 | 1.84 | 0.89 | −0.11 | 1.05 | 0.92 |
| SBP z-score | 0.90 | 0.66 | 0.18 | 0.16 | 0.38 | 0.68 |
| Covariate C16:0L | ||||||
| Intercept | 99.94 | 19.66 | <0.001 | 50.74 | 10.95 | <0.001 |
| Log10 C16:0L | −8.67 | 2.54 | 0.001 | −3.81 | 1.42 | 0.009 |
| Age (years) | 0.15 | 0.16 | 0.38 | 0.02 | 0.09 | 0.80 |
| GFR (ml/min/1.73 m2) | 0.00 | 0.04 | 0.95 | −0.01 | 0.02 | 0.82 |
| BMI z-score | 0.76 | 0.66 | 0.25 | 0.41 | 0.37 | 0.27 |
| LVMI (g/m2.7) | −0.08 | 0.11 | 0.48 | −0.11 | 0.06 | 0.09 |
| Anemia | 2.44 | 2.00 | 0.23 | 0.87 | 1.12 | 0.44 |
| Serum albumin (mg/dl | −1.35 | 2.00 | 0.50 | −0.80 | 1.11 | 0.48 |
| SBP z-score | 0.75 | 0.71 | 0.29 | 0.09 | 0.40 | 0.82 |
GFR, glomerular filtration rate; BMI, body mass index; SBP, systolic blood pressure; LVMI, left ventricular mass index
Discussion
To our knowledge, this is the first study to describe elevated serum ceramides and their metabolite levels in CKD patients. The specific reasons for elevated ceramides in CKD patients are not clear. It is likely that the CKD-associated dyslipidemia is associated with an enhanced de novo biosynthesis as the major driving force for elevated ceramides, while chemokine-induced activation may also play a role through the activation of sphingomyelinase, though this is likely to play a less prominent role in the overall contributions [2]. Less likely, elevated ceramides in CKD could be due to decreased liver metabolism and clearance. Some ceramides contained in LDL cholesterol are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance [13]. In our study, we found significant association between ceramide levels and LDL cholesterol. However, the observational nature of the study did not allow us to draw any specific conclusions on the mechanism of these associations.
It is unlikely that decreased kidney clearance is the cause of elevated ceramides, since ceramides are part of large circulating lipoprotein particles and are also present in blood cells and platelets. A previous CKiD analysis showed that lower GFR was associated with higher triglycerides, lower HDL cholesterol, and higher non-HDL cholesterol, even though these associations were not directly caused by decreased kidney clearance [14]. In contrast, current analysis showed no significant relationship between GFR and any of the studied ceramides.
As in healthy controls, the long-chain C24:0 and C24:1 ceramides were the most abundant species in children with CKD, comprising more than 75%of all ceramides. In contrast, the composition of some of the metabolites was different in CKD children. The levels of one of the metabolites, lactosylceramide, C24:0Lwas especially high, more than three times greater than in controls. Importantly, lactosylceramide C24:0L was an independent predictor of SF and mwSF after adjusting for other preload- (e.g., anemia, BMI, HR) and afterload- (e.g., blood pressure) related factors affecting systolic function in these children with mild-to-moderate CKD. This observation raises questions about the potential role of sphingolipid metabolism in the development of accelerated cardiac dysfunction in CKD patients.
In advanced CKD, maladaptive cardiac hypertrophy that develops secondary to prolonged and proportionally increased pressure (afterload) and volume (preload) overload with impaired renal function is a proposed mechanism for the development of congestive heart failure [15]. This in turn results in excessive cardiac myocyte work relative to the supply of oxygen. As a consequence, myocyte death and fibrosis develops, with chamber dilatation and systolic dysfunction. This model, however, cannot fully explain the high prevalence of accelerated cardiac dysfunction in early CKD [1].
Our results, showing an association between higher C24:0L and C16:0L and lower systolic function, suggest that a condition known as cardiac lipotoxicity might be involved in the pathogenesis of abnormal cardiac function in CKD. Cardiac lipotoxicity leading to dilated cardiomyopathy has been described in congenitally obese Zucker diabetic fatty (ZDF) rats [16]. In this animal model, increased myocardial ceramide accumulation and its association with decreased LV contractility was identified. The authors proposed that elevated ceramides lead to increased apoptosis and subsequent myocyte death. Recent studies from the Goldberg lab have confirmed a causal relationship between elevated ceramideand dilated lipotoxic cardiomyopathy. In their initial study, these authors showed that mice with cardiomyocyte overexpression of a glycosylphosphatidylinositol (GPI) membrane-anchored form of lipoprotein lipase (LpLGPI) developed cardiomyopathy with accumulation of ceramide in the heart [17]. In a follow-up study, de novo ceramide biosynthesis was inhibited pharmacologically by myriocin and genetically by a heterozygous deletion of LCB1, a subunit of serine palmitoyltransferase (SPT). Inhibition of SPT, a rate-limiting enzyme in ceramide biosynthesis, reduced fatty acid levels, increased glucose oxidation in isolated perfused LpLGPI hearts, improved systolic function, and prolonged survival rates [3].
Lipid accumulation in the heart of morbidly obese and diabetic adults with heart failure was shown in a study by Sharma et al. [18]. The authors studied hearts from 27 consecutive patients who underwent heart transplantation due to nonischemic heart failure: nearly 30 % had high intramyocardial lipid deposition. This is especially relevant to CKD patients, a population with a high prevalence of obesity, metabolic syndrome, and diabetes. Sphingolipid levels were not measured in that study.
Previous data have implicated glucosylceramides, but not lactosylceramides, in metabolic diseases. Inhibiting glucosylceramide synthesis can significantly improve insulin sensitivity and glucose homeostasis [19]. However, we found that unlike the vastmajority of other ceramide species, steadystate plasma glucosylceramide concentrations decreased in type 2 diabetic children [20]. Even though the cellular function of C24:0L and its specific involvement in cardiomyopathy processes have not yet been elucidated, recent studies indicate that lactosylceramide can induce critical phenotypes such as cell proliferation, migration, adhesion, angiogenesis, and apoptosis [21, 22], the same mechanisms involved in the final pathways of the development of heart failure [23].
There are limitations of this pilot study. It is relatively small and cannot be generalized, especially in the presence of a relatively small control group. The observational design does not allow for the examination of a causal relationship between ceramides and cardiac function. Mechanistic studies utilizing animal models are now needed to prove the concept of cardiac lipotoxicity in CKD as it is not known how circulating ceramides relate to ceramide populations in tissues (e.g., heart). However, in light of our results in this pilot study, larger confirmatory studies are needed to further evaluate ceramides as a possible biomarker or a cause of abnormal cardiac function in children and adults with CKD.
Acknowledgments
Data were collected by the Chronic Kidney Disease in Children prospective cohort study (CKiD) with clinical coordinating centers at Children’s Mercy Hospital and the University of Missouri-Kansas City (Bradley Warady MD, PI) and University of Pennsylvania (Susan Furth MD, PhD, PI) and data coordinating center at the Johns Hopkins Bloomberg School of Public Health (Alvaro Munoz, PhD, PI).
Data were partially presented in abstract form at Kidney Week 2012 in Denver, CO.
Sources of funding This study was funded by the research grant DK076957 from the National Institute of Diabetes and Digestive and Kidney Diseases (M.M.M). The CKiD prospective cohort study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute of Child Health and Human Development; the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01-DK-082194, and U01-DK66116).
Footnotes
Statement of competing financial interests No conflicts are reported.
Statement of non-financial competing interestsNo conflicts are reported.
Authors’ contribution
M.M. and P.H. made substantial contributions to the conception and design of this study and data interpretation and were involved in drafting the manuscript or revising it critically for important intellectual content as well as giving final approval of the version to be published. R.G. and L.F. were involved in analysis and interpretation of the data. S.F. and B.W. were involved in drafting the manuscript or revising it critically for important intellectual content, and gave final approval of the version to be published.
Contributor Information
Mark Mitsnefes, Email: mark.mitsnefes@cchmc.org, Division of Nephrology and Hypertension, Cincinnati Children’s, Hospital Medical Center, MLC: 7022, 3333 Burnet Avenue, Cincinnati, OH 45229-3039, USA.
Philipp E. Scherer, The University of Texas Southwestern Medical Center, Dallas, TX, USA
Lisa Aronson Friedman, Johns Hopkins University School of Public Health, Baltimore, MD, USA.
Ruth Gordillo, The University of Texas Southwestern Medical Center, Dallas, TX, USA.
Susan Furth, University of Pennsylvania, Philadelphia, PA, USA.
Bradley A Warady, Children’s Mercy Hospital, Kansas City, MO, USA.
References
- 1.Shlipak MG, Lash JP, Yang W, Teal V, Keane M, Cappola T, Keller C, Jamerson K, Kusek J, Delafontaine P, He J, Miller ER, 3rd, Schreiber M, Go AS Investigators CRIC. Symptoms characteristic of heart failure among CKD patients without diagnosed heart failure. J Card Fail. 2011;17:17–23. doi: 10.1016/j.cardfail.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammad SM. Blood sphingolipids in homeostasis and pathobiology. Adv Exp Med Biol. 2011;721:57–66. doi: 10.1007/978-1-4614-0650-1_4. [DOI] [PubMed] [Google Scholar]
- 3.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, Kimball T, Furth S, Warady B CKiD Study Group. Masked hypertension and associated left ventricular hypertrophy in children with CKD. J Am Soc Nephrol. 2010;21:137–144. doi: 10.1681/ASN.2009060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22:709–714. doi: 10.1016/j.echo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu G, Zile MR, Blaustein AS, Gaash WH. Left ventricular chamber filling and midwall fiber lengthening in patients with left ventricular hypertrophy: overestimation of fiber velocities by conventional midwall measurements. Circulation. 1985;71:266–272. doi: 10.1161/01.cir.71.2.266. [DOI] [PubMed] [Google Scholar]
- 9.De Simone G, Devereux RB, Koren MJ, Menash GA, Casale PN, Laragh JH. Midwall left ventricular mechanics. An independent predictor of cardiovascular risk in arterial hypertension. Circulation. 1996;93:259–265. doi: 10.1161/01.cir.93.2.259. [DOI] [PubMed] [Google Scholar]
- 10.Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, Klein RL, Hannun YA, Bielawski J, Bielawska A. Blood sphingolipidomic in healthy humans: impact of sample collection methodology. J Lipid Res. 2010;51:3074–3087. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaner RL, Allegood JC, Park H, Wang E, Kelly S, Haynes CA, Sullards MC, Merrill AH., Jr Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res. 2009;50:1692–1707. doi: 10.1194/jlr.D800051-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka V, Warady BA, Furth SL, Muñoz A. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boon J, Hoy AJ, Stark R, Brown RD, Meex RC, Henstridge DC, Schenk S, Meikle PJ, Horowitz JF, Kingwell BA, Bruce CR, Watt MJ. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2013;62:401–410. doi: 10.2337/db12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saland JM, Pierce CB, Mitsnefes MM, Flynn JT, Goebel J, Kupferman JC, Warady BA, Furth SL. Dyslipidemia in children with chronic kidney disease. Kidney Int. 2010;78:1154–1163. doi: 10.1038/ki.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 16.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, Seo T, Hu Y, Lutz EP, Merkel M, Bensadoun A, Homma S, Goldberg IJ. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 19.Zhao H, Przybylska M, Wu IH, Zhang J, Siegel C, Komarnitsky S, Yew NS, Cheng SH. Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes. 2007;56:1210–1218. doi: 10.2337/db06-0719. [DOI] [PubMed] [Google Scholar]
- 20.Lopez X, Goldfine AB, Holland WL, Gordillo R, Scherer PE. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J Pediatr Endocrinol Metab. 2013;24:1–4. doi: 10.1515/jpem-2012-0407. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee S, Pandey A. The Yin and Yang of lactosylceramide metabolism: implications in cell function. Biochim Biophys Acta. 2008;1780:370–382. doi: 10.1016/j.bbagen.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee S, Alsaeedi N. Lactosylceramide synthase as a therapeutic target to mitigate multiple human diseases in animal models. Adv Exp Med Biol. 2012;749:153–169. doi: 10.1007/978-1-4614-3381-1_11. [DOI] [PubMed] [Google Scholar]
- 23.Seta Y, Shan K, Bozkurt B, Oral H, Mann DL. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail. 1996;2:243–249. doi: 10.1016/s1071-9164(96)80047-9. [DOI] [PubMed] [Google Scholar]