Abstract
Isoprenoids are one of the largest classes of natural products and all of them are constructed from two precursors, isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP). For decades, the mevalonic acid (MVA) pathway was proposed to be the only IPP and DMAPP biosynthetic pathway. This review summarizes the newly discovered IPP and DMAPP production pathways since late 1990s, their distribution among different kingdoms, and their roles in secondary metabolite production. These new IPP and DMAPP production pathways include the methylerythritol phosphate (MEP) pathway, a modified MVA pathway, and the 5-Methylthioadenosine shunt pathway. Relative to the studies on the MVA pathway, information on the MEP pathway regulation is limited and the mechanistic details of several of its novel transformations remain to be addressed. Current status on both MEP pathway regulation and mechanistic issues are also presented.
Keywords: isoprenoids, MVA, MEP, methylthioadenosine, regulation, biosynthesis
INTRODUCTION
Isoprenoids (also known as terpenoids or terpenes) are found in all domains of life (bacteria, archaea, and eukaryotes) and many have important biological functions [1]. For example, quinones act as part of the electron transport chain, sterols as eubacterial and eukaryotic cell membrane components, carotenoids as essential biopigments, dolichol in glycoprotein and bacterial cell wall biosynthesis, and linear prenyl diphosphates as protein prenylation units for intra-cellular protein targeting. In addition, isoprenoids are widely utilized in biomedical and commercial applications including pharmaceuticals, flavoring agents, fragrances, and nutrition products. In this paper, we review recent progress made in understanding the biosynthesis of the isoprenoid precursors, dimethylallyl diphosphate (DMAPP, 1) and isopentenyl diphosphate (IPP, 2).
BIOSYNTHESIS OF IPP AND DMAPP
The mevalonic acid (MVA) pathway
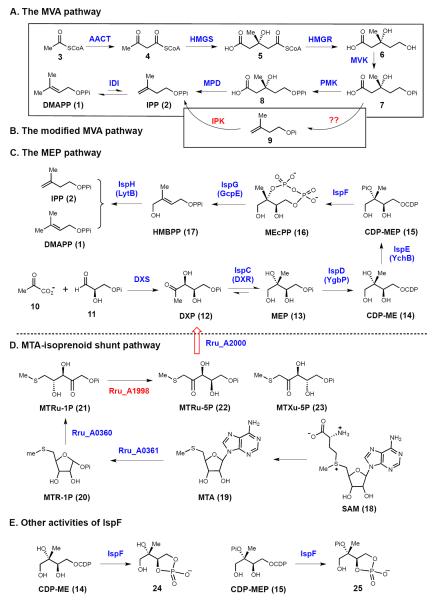
The mevalonic acid (MVA, 6) pathway of DMAPP (1) and IPP (2) biosynthesis (see Figure 1A) has been known for over half a century, and its discovery led to the awarding of the Nobel prize in physiology to Lynen and Bloch in 1964, and in chemistry to Cornforth in 1975 [2]. The pathway starts with the acetyl-CoA acetyltransferase (AACT) catalyzed condensation of two acetyl-CoA (3) molecules to form 4. This is followed by the hydroxylmethylglutaryl-CoA synthase (HMGS) catalyzed reaction to produce 3-hydroxy-3-methyl-glutaryl-CoA (5, HMG-CoA). The rate limiting step of the carbon flow through the MVA pathway is the reduction of 5 by HMG-CoA reductase (HMGR) with two equivalents of NADPH. The resulting MVA (6) is activated by two phosphorylation steps catalyzed by mevalonate kinase (MVK) and phosphomevalonate kinase (PMK). This is followed by an ATP-coupled decarboxylation of 8 catalyzed by mevalonate pyrophosphate decarboxylase (MPD) to yield IPP (2) as the product. Two structurally unrelated IPP:DMAPP isomerases (IDI-1 and IDI-2) are then responsible for the interconversion of IPP (2) and DMAPP (1) [3,4].
Figure 1.
Pathways for the production of isoprenoid precursors: (A) The mevalonic acid (MVA) pathway. (B) A modified MVA pathway in Methanocaldococcus jannaschii. (C) The methylerythritol phosphate (MEP) pathway. (D) A newly discovered isoprenoid shunt pathway related to S-adenosylmethioneine metabolism. Two new activities found for IspF are shown in E.
Recently, the existence of a modified mevalonate pathway (Figure 1B) was suggested based on the observation that genes encoding enzymes catalyzing the late steps of the MVA pathway are absent in some archaeal genomes. Grochowski et al. [5] identified an enzyme (isopentenyl phosphate kinase, IPK) from Methanocaldococcus jannaschii, which is capable of phosphorylating 9 to form IPP. It was thus proposed that 7 is decarboxylated to form 9 and then phosphorylated to produce IPP (2). However, the hypothesized decarboxylase (for 7 → 9) has not yet been identified. Hence, we cannot rule out the option of the existence of a classical MVA pathway in M. jannaschii, while its PMK and MPD have no or very low sequence similarities to the well-studied PMK and MPD enzymes.
The methylerythritol phosphate (MEP) pathway
In the 1990s and early 2000s, efforts by Rohmer, Arigoni, Lichtenthaler, and Seto etc. led to the discovery of a new isoprene biosynthetic pathway in both bacteria and the chloroplasts of green algae and higher plants [6–11]. This pathway begins with the condensation of pyruvate (10) and glyceraldehyde phosphate (11) to afford 1-deoxy-d-xylulose 5-phosphate (12, DXP) in a reaction catalyzed by the thiamin diphosphate-dependent enzyme 1-deoxy-d-xylulose 5-phosphate synthase (DXS, see Figure 1C) [12]. DXP is reductively isomerized by DXP reducto-isomerase (DXR, also known as IspC) to 2-C-methyl-d-erythritol 4-phosphate (13, MEP) [12], from which the MEP pathway receives its name. MEP is then activated by IspD to CDP-ME (14) prior to phosphorylation by IspE to produce 4-diphosphocytidyl-2-C-methyl-d-erythritol-2-phosphate (CDP-MEP, 15). Subsequent cyclization of CDP-MEP to 2-C-methyl-d-erythritol-2,4-cyclodiphosphate (MEcPP, 16) is catalyzed by IspF. The final two steps are catalyzed by two iron-sulfur containing enzymes, IspG and IspH. IspG is responsible for the ring-opening and reductive dehydration of MEcPP to produce 4-hydroxy-3-methylbutenyl 1-diphosphate (HMBPP, 17), whereas IspH catalyzes the reductive dehydration of HMBPP to yield both IPP (2) and DMAPP (1) (Figure 2C). Thus, in many organisms that utilize the MEP pathway, IDI is not essential for survival, though it may play a role in modulating the IPP/DMAPP ratio in the cell.
Two new activities were recently identified for IspF. Besides the physiological reaction (15 →16), E. coli and Plasmodium falciparum IspF can also catalyze the conversion of CDP-ME (14) to 2-C-methyl-d-erythritol 3,4-cyclophosphate (24) (Figure 2E) [13,14]. Similarly, the recombinant malaria IspF is capable of catalyzing the production of 2-phospho-2-C-methyl-d-erythritol 3,4-cyclophosphate (25) and MEcPP (16) at a ratio of 1:10 from CDP-MEP (15) (Figure 2E) [14]. However, the physiological relevance of these activities remains to be established.
Discovery of a MEP shunt pathway
5-Methylthioadenosine (MTA, 19) is a side-product of S-adenosylmethionine (SAM, 18)-dependent polyamine biosynthesis, and wild-type Rhodospirillum rubrum can grow using MTA as its sole source of sulfur [15]. It is therefore possible that an alternative MTA metabolic pathway is present R. rubrum and serves to compensate for the known absence of most canonical methionine salvage pathway genes in this organism. Recently, a ribulose-1,5-bisphosphate carboxylase like protein (RLP), Rru_A1998, was identified by Tabita and co-workers to be an enzyme involved in 5-methylthioadenosine (MTA, 19) metabolism in Rhodospirillum rubrum [16]. Later, Gerlt and co-workders demonstrated that MTA metabolism in R. rubrum is coupled to the MEP pathway by supplying DXP (12) [15]. In this shunt pathway, MTA is transformed to methylthio-d-ribulose-1-phosphate (MTRu-1P, 21) by early steps of a canonical methionine salvage pathway that R. rubrum is capable of (Figure 2D). MTRu-1P is then converted by Rru_A1998 to a 1:3 mixture of 1-methylthio-ribulose-5-phosphate (MTRu-5P, 22) and 1-methylthio-xylulose-5-phosphate (MTXu-5P, 23). A cupin family protein (Rru_A2000) is proposed to catalyze the subsequent C-S lysis reaction in the conversion of MTXu-5P to DXP, producing methanethiol as a co-product, which can be used as the sulfur source (Figure 2D). While the activity of Rru_A2000 has not yet been successfully reconstituted in vitro, phylogenetic analysis suggests that a number of organisms could make use of this metabolic shunt to produce IPP and DMAPP [15].
Distributions of MVA and MEP pathways among different kingdoms
The MEP pathway has been identified in eubacteria, green algae, and higher plants, whereas the MVA pathway is found in animals, plants (cytosol), fungi, and archaea [6]. Since the MEP pathway is absent in humans, enzymes involved in this pathway represent excellent targets for development of new broad-spectrum antibiotics and herbicides. This subject has been extensively discussed in many recent reviews [11,17–21].
Although bacteria are known to use the MEP pathway for the production of isoprenoids, there exist exceptions. Several actinobacteria possess genes for both the MEP and MVA pathways [22]. Indeed, feeding experiments using 13C-enriched precursors with Actinoplanes sp. A40644, Seto and co-workers demonstrated that menaquinones, which are primary metabolites, were derived from the MEP pathway, while the secondary metabolite, BE-406441, was assembled through the MVA pathway [23]. It was suggested that the MEP pathway operates at the early stage of fermentation when the production of primary metabolites is high, whereas the MVA pathway is switched on at a later stage when the production of secondary metabolites is more pronounced. This is consistent with the genes responsible for the MVA pathway being frequently localized to operons for secondary metabolite biosynthesis [24]. Such a genetic organization implies that additional MVA-derived isoprenoid biosynthetic gene clusters may be discovered in bacteria by screening for the highly conserved HMGR gene [24].
Interestingly, a recent study of the biosynthesis of furanonaphthoquinone (27, Figure 2), which is a polyketide-terpene hybrid, led to the identification of a mevalonate kinase gene in the furanonaphthoquinone biosynthetic operon, though all the remaining MVA pathway genes were absent [25]. Feeding studies using [13C]-labeled precursors, however, clearly indicated that the terpene portion of 27 (colored red) is derived from the MVA pathway and the quinone portion (26, 27, colored blue) is constructed from 3 by a polyketide synthase [25]. Therefore, the remaining genes of the MVA pathway must be expressed from other regions of the genome. Importantly, the MEP pathway can also serve as the source of the furanonaphthoquinone terpene fragment depending on the culture conditions. As the microbial genome sequencing information continues to expand, additional examples of overlap between the canonical MVA or MEP pathways are expected.
Figure 2.
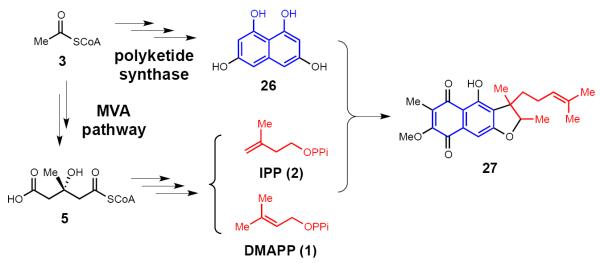
The involvement of the MVA pathway in the biosynthesis of furanonaphthoquinone (a secondary isoprenoid metabolite) in Streptoyces cinnamonensis DSM 1042.
MEP PATHWAY RELATED SIGNALING AND REGULATION
HMG-CoA reductase is the key regulatory step for IPP biosynthesis through the MVA pathway and has been extensively studied [26]. However, studies on the regulation of the MEP pathway and how this pathway interacts with other cellular processes are still at the rudimentary stage and some of the key discoveries are briefly summarized here.
DXR
Analysis of DXR from Francisella tularensis suggests that Ser177 (equivalent to Ser185 of E. coli DXR) is phosphorylated [27]. In view of the close proximity of Ser177 to the substrate binding site, phosphorylation of Ser177 could be related to DXR activity regulation.
IspF
There exists a central cavity in the crystal structure of IspF, which has been proposed to be the binding site for prenyl phosphate. This may permit regulation of IspF activity through a feedback mechanism [28–30].
IspG
Post-transcriptional regulation of other cellular processes might be achieved through IspG by engaging its iron-sulfur cluster and its substrate, MEcPP (16). The [4Fe-4S] cluster in IspG is labile and oxygen sensitive. Under some conditions (e.g., oxidative stress), IspG may become inactivated leading to the accumulation of MEcPP which may function as an anti-stressor [31]. Interestingly, study of histone-DNA interactions showed that MEcPP can modulate chromosome structures in Chlamydia trachomatis [32]. C. trachomatis is an intracellular pathogen with a biphasic developmental cycle composed of an infectious extracellular form called the elementary body (EB) and an intracellular replicative form known as the reticulate body (RB). The metabolically inert EB form has condensed nucleoid structure. But within a few hours after infection, the metabolically active RB form will develop and it has the dispersed chromatin. It was suggested that MEcPP can disrupt the histone-DNA interactions and is responsible for the transformation of C. trachomatis from the EB form to the RB form. In another report, MEcPP was found to reactivate the “nonculturable” form of Mycobacterium smegmatis by triggering the release of HupB, a histone-like protein, from nucleoids to regenerate a transcriptionally active dispersed state [33]. Clearly, IspG plays a pivotal role on the interplay of the MEP pathway with many other physiological processes.
IspH
IspH has been suggested to be part of a global regulation mechanism triggered by elevated levels of guanosine 3',5'-bispyrophosphate ((p)ppGpp) [34,35]. RelA protein is the enzyme involved in the biosynthesis of (p)ppGpp. Gustafson et al. proposed that IspH and RelA form a protein complex and the formation of such a complex restricts RelA's (p)ppGpp synthase activity [34]. Under certain conditions, disruption of the RelA-IspH complex would lead to release of RelA protein and increased production of (p)ppGpp. As a result, the elevated level of (p)ppGpp, a nutritional stress alarmone, would trigger a series of energy-consuming metabolic processes, including peptidoglycan synthesis and hydrolysis. However, the details of such an IspH-involved stringent response mechanism remain to be verified.
MECHANICTIC STUDIES ON IspG AND IspH
The MEP pathway exhibits a number of unusual transformations catalyzed by mechanistically unique enzymes. Studies on the mechanisms of these enzymes have become a major focus of research. In this section, we will describe the mechanistic models proposed for IspG and IspH and the most recent biochemical studies on these two enzymes.
2-C-methyl-d-erythritol-2,4-cyclodiphosphate reductase (IspG)
IspG is a [4Fe-4S] cluster-containing enzyme. It is a homodimer and the active site is located at the subunit interface of the two monomers [36,37]. Although progress had been made to improve the in vitro activity of IspG using methyl viologen as the reductant [38], the identity of the in vivo reducing system still needs to be established. All current IspG mechanistic models include the assumption that the IspG iron-sulfur cluster contributes to substrate activation and serves as an electron donor for substrate reduction [39]. However, a detailed understanding of the interactions between MEcPP (16) and the iron-sulfur cluster remains elusive. This is because the proposed binding site for MEcPP is separated by approximately 20 Å from the iron-sulfur cluster on the other IspG subunit, with which it is proposed to interact, and this would seem to preclude a direct interaction [36,37].
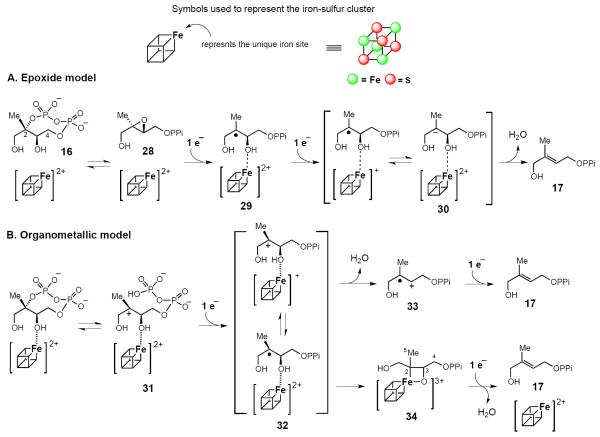
Rohdich et al. [40] proposed one of the first IspG mechanistic models, which involves the epoxide 2,3-epoxy-4-hydroxy-3-methyl-butenyl 1-diphosphate (28) as an intermediate. In this mechanism, the [4Fe-4S] cluster mediates the reductive deoxygenation of the epoxide 28 to HMBPP (17) via the radical intermediate 29 (Figure 3A). This mechanism is supported by the observation that IspG can indeed catalyze the conversion of 28 to HMBPP [41]. However, the rate of 28 → 17 conversion is only 10% of that of 16 →17 conversion. Thus, 28 may not be a true intermediate and the reductive dehydration of MEcPP and the reductive deoxygenation of 28 may be achieved through parallel pathways [42].
Figure 3.
Working models accounting for the IspG-catalyzed reaction. (A) The epoxide model. According to this model, the epoxide (28) serves as a key intermediate. Once 28 is formed, two sequential single electron reduction steps lead to the formation of HMBPP (17). (B) Cation and organometallic models. In this case, the first step is the formation of a carbocation intermediate (31) by the C-O cleavage. Subsequent reductive dehydroxylation can proceed via either the radical cation intermediate (33) or the organometallic intermediate (34).
Seemann and Kollas et al. instead suggested that IspG-catalysis might involve a carbocation intermediate (31, Figure 3B) [43,44]. The observation of a positional isotope exchange for the MEcPP C2 bridging oxygen provided evidence for a reversible cleavage at this position, which is consistent with the formation of a carbocation intermediate 31 [45]. Based on the characterization of paramagnetic species detected during IspG steady-state turnover, Oldfield and co-workers went on to suggest that IspG catalysis might also involve an organometallic intermediate (e.g., 34) downstream of the carbocation intermediate 31 [46]. A slightly different version has also been suggested by Duin, Hoffman and co-workders [47]. In these organometallic models, an Fe-C bond forms between the substrate and apical iron of the [4Fe-4S] cluster to yield the intermediate 34. Because the kinetic competence of this paramagnetic species has not yet been demonstrated, a mechanism involving the radical cation 33 should also be considered as a viable alternative.
4-Hydroxy-3-methyl-butenyl 1-diphosphate reductase (IspH)
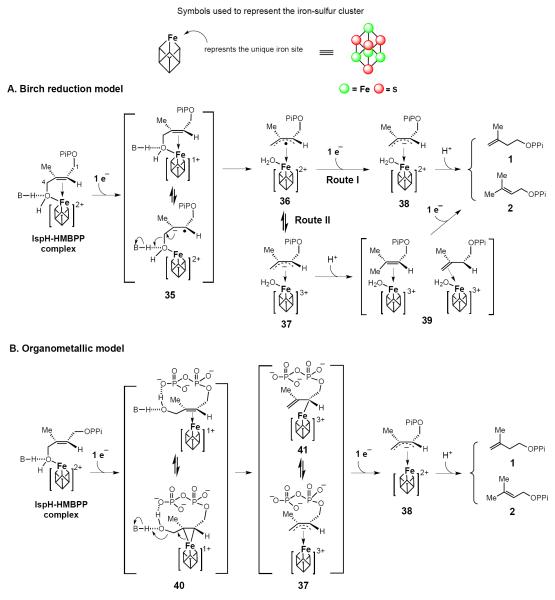
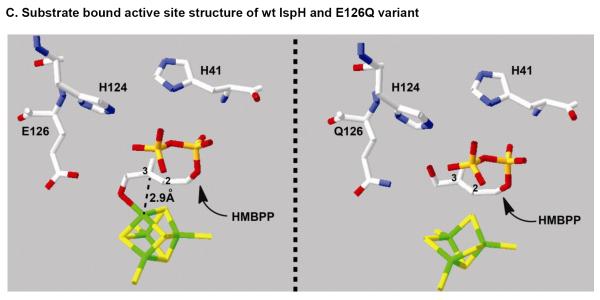
IspH is also an iron-sulfur cluster containing enzyme [48] and its iron-sulfur cluster contributes to both substrate activation and serves as an electron donor for substrate reduction [49]. One of two leading IspH mechanistic models is the Birch reduction model [40]. In this model, the reaction is initiated by the coordination of the HMBPP C4-OH to the [4Fe-4S]2+ apical iron site to yield an HMBPP-IspH complex (Figure 4). Reduction of HMBPP (17) by the reduced [4Fe-4S]+ cluster produces the radical anion intermediate 35 (see Figure 4A), which eliminates hydroxide at C4 to form an allyl radical-[4Fe-4S]2+ intermediate (36). Subsequent one-electron reduction may proceed via one of two different routes involving formation of 38 (route I) or 39 (route II) as intermediates. As revealed by a 1.7-Å resolution structure of the IspH-HMBPP complex, HMBPP indeed coordinates to the apical iron site of the [4Fe-4S] cluster using its C4-OH group [49]. Furthermore, studies using a substrate analog indicate that the HMBPP C4-OH plays the dominant role in positioning the substrate in IspH active site [50]. The HMBPP olefinic carbons are also positioned adjacent to apical iron site at a distance of 2.8–3.0 Å (Figure 4C, left panel). Thus, the proposed one electron transfer from the iron-sulfur cluster to HMBPP should be feasible.
Figure 4.
Working models accounting for the IspH-catalyzed reaction. (A) Birch reduction model. In this model, the iron-sulfur cluster has two functions: (1) mediating two sequential one-electron reduction, and (2) serving as the Lewis acid to facilitate C4-dehydroxylation. (B) Organometallic model. This model has two unique features: (1) the HMBPP C4-OH group rotates away from the [4Fe-4S] cluster to form an internal H-bond (40), and (2) an iron-sulfur cluster mediated two-electron reductive dehydroxylation step (40 → 41). (C) Substrate bound active site structure of wt and E126Q variant IspH.
Recently, a different binding conformation was observed in the structure of the HMBPP-IspH/E126Q mutant. In this case the HMBPP C4-OH is not coordinated to the [4Fe-4S] cluster, but instead rotates to the other side of the HMBPP double bond to form an internal H-bond with the β-phosphate group (see 40) [51]. ENDOR spectroscopic analysis of this complex also implicates a similar structure [52]. These observations led to a second mechanistic model, the organometallic model (Figure 4B and 4C, right panel). Following formation of the initial enzyme-substrate complex, this model predicts the olefin moiety of the substrate to interact with the [4Fe-4S] cluster to form a π complex or the η2-alkenyl/metallacycle intermediate 40. In doing so, the free HMBPP C4-OH rotates to the other side of the molecule to form a H-bond with the β-phosphate group (40) and facilitate the subsequent dehydration to 41 (Figure 4B). Results from recent feeding studies using isotopically labeled precursors indicate that such a rotation of the HMBPP C4-OH is very likely during the IspH catalysis [53].
The Birch reduction and organometallic models are similar in many aspects and share several common intermediates (species 37, 38). However, the proposed rotation of the HMBPP C4-OH and formation of a Fe-C bond involving the olefinic moiety and the [4Fe-4S] cluster are the major points of departure between these two models. In the Birch model, reductive dehydration proceeds in a stepwise one-electron/one-electron transfer manner involving a substrate-based radical intermediate (36). By contrast, the reductive C-O bond cleavage step (40 → 41) is a two-electron transfer process with concomitant oxidation of [4Fe-4S]+ to [4Fe-4S]3+ in the organometallic model. Clearly, trapping and characterization of kinetically competent intermediates are essential to distinguish whether IspH catalysis involves stepwise one-electron (the Birch reduction model) or one-step two-electron chemistries (the organometallic model).
SUMMARY
The review provides a brief account of the recent advances in our understanding of the biosynthesis of isoprenoid precursors. While the MVA pathway has been studied for decades, research on the MEP pathway is still at an early stage due to its relatively more recent discovery in the late 1990s. Many questions regarding the interplay between the MVA and MEP pathways, the regulation and control of these pathways, the intracellular distribution of the biosynthetic enzymes involved, and the catalytic mechanisms of the enzymes in the MEP pathway, remain unanswered. These questions are fascinating, albeit challenging, and are being actively pursued by many research laboratories around the world. It is expected that many new and interesting discoveries will result from these efforts with important implications for pharmaceutical and biomedical research.
Isoprenoids are widely utilized in biomedical and commercial applications
Recent progress in understanding the biosynthesis of DMAPP and IPP is reviewed
While the MVA pathway has been studied for decades, research on the MEP pathway is at an early stage
Many questions regarding MEP pathways remain unanswered
ACKNOWLEGEMENTS
We thank the grant supports of our work from the National Institutes of Health (GM040541 to H.-w.L., and GM093903 to P.L.) and Welch Foundation (F-1511 to H.-w.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Breitmaier E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones. WILEY-VCH, Weinheim; Germany: 2006. p. ix. [Google Scholar]
- 2.Bochar DA, Friesen JA, Stauffacher CV, Rodwell VW. Biosynthesis of mevalonic acid from acetyl-CoA. In: Cane DE, editor. Comprehensive Natural Product Chemistry. Pergamon; Oxford: 1999. pp. 15–44. [Google Scholar]
- 3.Agranoff BW, Eggerer H, Henning U, Lynen F. Isopentenyl pyrophosphate isomerase. J Am Chem Soc. 1959;81:1254–1255. [PubMed] [Google Scholar]
- 4.Kaneda K, Kuzuyama T, Takagi M, Hayakawa Y, Seto H. An unusual isopentenyl diphosphate isomerase found in the mevalonate pathway gene cluster from Streptomyces sp strain CL190. Proc Natl Acad Sci USA. 2001;98:932–937. doi: 10.1073/pnas.020472198. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Discovery of type II Isopentenyl pyrophosphate isomerase.
- 5.Grochowski LL, Xu H, White RH. Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate. J Bacteriol. 2006;188:3192–3198. doi: 10.1128/JB.188.9.3192-3198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Initial evidence leading to the suggestion of the presence of a modified MVA pathway.
- 6.Eisenreich W, Bacher A, Arigoni D, Rohdich F. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell Mol Life Sci. 2004;61:1401–1426. doi: 10.1007/s00018-004-3381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohmer M. Diversity in isoprene unit biosynthesis: The methylerythritol phosphate pathway in bacteria and plastids. Pure Appl Chem. 2007;79:739–751. [Google Scholar]
- 8.Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep. 1999;16:565–574. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- 9.Gabrielsen M, Rohdich F, Eisenreich W, Gräwert T, Hecht S, Bacher A, Hunter WN. Biosynthesis of isoprenoids: a bifunctional IspDF enzyme from Campylobacter jejuni. Eur J Biochem. 2004;271:3028–3035. doi: 10.1111/j.1432-1033.2004.04234.x. [DOI] [PubMed] [Google Scholar]
- 10.Meyer O, Grosdemange-Billiard C, Tritsch D, Rohmer M. Synthesis and activity of two trifluorinated analogues of 1-deoxy-D-xylulose 5-phosphate. Tetrahedron Lett. 2007;48:711–714. [Google Scholar]
- 11.Rohmer M. Methylerythritol phosphate pathway. In: Liu H-w, Mander L., editors. Comprehensive Natural Products II — Chemistry and Biology. Elsevier Science ltd.; Oxford: 2010. pp. 517–556. [Google Scholar]
- 12.Kuzuyama T, Takahashi S, Watanabe H, Seto H. Direct formation of 2-C-methyl-D-erythritol 4-phosphate from 1-deoxy-D-xylulose 5-phosphate by 1-deoxy-D-xylulose 5-phosphate reductoisomerase, a new enzyme in the non-mevalonate pathway to isopentenyl diphosphate. Tetrahedron Lett. 1998;39:4509–4512. [Google Scholar]
- 13.Herz S, Wungsintaweekul J, Schuhr CA, Hecht S, Luttgen H, Sagner S, Fellermeier M, Eisenreich W, Zenk MH, Bacher A, Rohdich F. Biosynthesis of terpenoids: YgbB protein converts 4-diphosphocytidyl-2C-methyl-D-erythritol 2-phosphate to 2C-methyl-D-erythritol 2,4-cyclodiphosphate. Proc Natl Acad Sci USA. 2000;97:2486–2490. doi: 10.1073/pnas.040554697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohdich F, Eisenreich W, Wungsintaweekul J, Hecht S, Schuhr CA, Bacher A. Biosynthesis of terpenoids. 2C-Methyl-D-erythritol 2,4-cyclodiphosphate synthase (IspF) from Plasmodium falciparum. Eur J Biochem. 2001;268:3190–3197. doi: 10.1046/j.1432-1327.2001.02204.x. [DOI] [PubMed] [Google Scholar]; • The discovery of new IspF chemistries.
- 15.Erb TJ, Evans BS, Cho K, Warlick BP, Sriram J, Wood BM, Imker HJ, Sweedler JV, Tabita FR, Gerlt JA. A RubisCO-like protein links SAM metabolism with isoprenoid biosynthesis. Nat Chem Biol. 2012;8:926–932. doi: 10.1038/nchembio.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The discovery of a MEP shunt pathway.
- 16.Singh J, Tabita FR. Roles of RubisCO and the RubisCO-Like Protein in 5-Methylthioadenosine Metabolism in the Nonsulfur Purple Bacterium Rhodospirillum rubrum. J Bacteriol. 2010;192:1324–1331. doi: 10.1128/JB.01442-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hale I, O'Neill PM, Berry NG, Odom A, Sharma R. The MEP pathway and the development of inhibitors as potential anti-infective agents. MedChemComm. 2012;3:418–433. [Google Scholar]
- 18.Obiol-Pardo C, Rubio-Martinez J, Imperial S. The methylerythritol phosphate (MEP) pathway for isoprenoid biosynthesis as a target for the development of new drugs against tuberculosis. Curr Med Chem. 2011;18:1325–1338. doi: 10.2174/092986711795029582. [DOI] [PubMed] [Google Scholar]
- 19.Wiemer AJ, Hsiao CH, Wiemer DF. Isoprenoid metabolism as a therapeutic target in gram-negative pathogens. Curr Top Med Chem. 2010;10:1858–1871. doi: 10.2174/156802610793176602. [DOI] [PubMed] [Google Scholar]
- 20.Gräwert T, Groll M, Rohdich F, Bacher A, Eisenreich W. Biochemistry of the non-mevalonate isoprenoid pathway. Cell Mol Life Sci. 2011;68:3797–3814. doi: 10.1007/s00018-011-0753-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiesner J, Jomaa H. Isoprenoid biosynthesis of the apicoplast as drug target. Curr Drug Targets. 2007;8:3–13. doi: 10.2174/138945007779315551. [DOI] [PubMed] [Google Scholar]
- 22.Kuzuyama T, Seto H. Diversity of the biosynthesis of the isoprene units. Nat Prod Rep. 2003;20:171–183. doi: 10.1039/b109860h. [DOI] [PubMed] [Google Scholar]
- 23.Seto H, Orihara N, Furihata K. Studies on the biosynthesis of terpenoids produced by actinomycetes. Part 4. Formation of BE-40644 by the mevalonate and nonmevalonate pathways. Tetrahedron Lett. 1998;39:9497–9500. [Google Scholar]; • The involvement of the MVA pathway in actinomycetes terpene biosynthesis.
- 24.Kuzuyama T, Takahashi S, Dairi T, Seto H. Detection of the mevalonate pathway in streptomyces species using the 3-hydroxy-3-methylglutaryl coenzyme A reductase gene. J Antibiot. 2002;55:919–923. doi: 10.7164/antibiotics.55.919. [DOI] [PubMed] [Google Scholar]
- 25.Bringmann G, Haagen Y, Gulder TAM, Gulder T, Heide L. Biosynthesis of the isoprenoid moieties of furanonaphthoquinone I and endophenazine A in Streptomyces cinnamonensis DSM 1042. J Org Chem. 2007;72:4198–4204. doi: 10.1021/jo0703404. [DOI] [PubMed] [Google Scholar]; • Discovery of an example where the MVA pathway is involved in the biosynthesis of a secondary metabolite, yet, the biosynthtic operon does not have the complete MVA pathway genes.
- 26.Brown MS, Goldstein JL. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980;21:505–517. [PubMed] [Google Scholar]
- 27.Jawaid S, Seidle H, Zhou WD, Abdirahman H, Abadeer M, Hix JH, van Hoek ML, Couch RD. Kinetic Characterization and Phosphoregulation of the Francisella tularensis 1-Deoxy-D-Xylulose 5-Phosphate Reductoisomerase (MEP Synthase) PLoS One. 2009;4:e8288. doi: 10.1371/journal.pone.0008288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richard SB, Ferrer JL, Bowman ME, Lillo AM, Tetzlaff CN, Cane DE, Noel JP. Structure and mechanism of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase - An enzyme in the mevalonate-independent isoprenoid biosynthetic pathway. J Biol Chem. 2002;277:8667–8672. doi: 10.1074/jbc.C100739200. [DOI] [PubMed] [Google Scholar]
- 29.Ni SS, Robinson H, Marsing GC, Bussiere DE, Kennedy MA. Structure of 2C-methyl-D-erythritol-2,4-cyclodiphosphate synthase from Shewanella oneidensis at 1.6 angstrom: identification of farnesyl pyrophosphate trapped in a hydrophobic cavity. Acta Crystallogr D. 2004;60:1949–1957. doi: 10.1107/S0907444904021791. [DOI] [PubMed] [Google Scholar]
- 30.Kemp LE, Bond CS, Hunter WN. Structure of 2C-methyl-D-erythritol 2,4-cyclodiphosphate synthase: An essential enzyme for isoprenoid biosynthesis and target for antimicrobial drug development. Proc Natl Acad Sci USA. 2002;99:6591–6596. doi: 10.1073/pnas.102679799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostrovsky D, Diomina G, Lysak E, Matveeva E, Ogrel O, Trutko S. Effect of oxidative stress on the biosynthesis of 2-C-methyl-D-erythritol-2,4-cyclopyrophosphate and isoprenoids by several bacterial strains. Arch Microbiol. 1998;171:69–72. doi: 10.1007/s002030050680. [DOI] [PubMed] [Google Scholar]
- 32.Grieshaber NA, Fischer ER, Mead DJ, Dooley CA, Hackstadt T. Chlamydial histone-DNA interactions are disrupted by a metabolite in the methylerythritol phosphate pathway of isoprenoid biosynthesis. Proc Natl Acad Sci USA. 2004;101:7451–7456. doi: 10.1073/pnas.0400754101. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A report suggesting the link between MEcPP and histone-DNA interactions.
- 33.Goncharenko AV, Ershov YV, Salina EG, Wiesner J, Vostroknutova GN, Sandanov AA, Kaprelyants AS, Ostrovsky DN. The role of 2-C-methylerythritol-2,4-cyclopyrophosphate in the resuscitation of the “nonculturable” forms of Mycobacterium smegmatis. Microbiology. 2007;76:147–152. [PubMed] [Google Scholar]; • A report suggesting the link between MEcPP and the resuscitation of nonculturable form of Mycobacterium.
- 34.Gustafson CE, Kaul S, Ishiguro EE. Identification of the Escherichia-Coli Lytb Gene, Which Is Involved in Penicillin Tolerance and Control of the Stringent Response. J Bacteriol. 1993;175:1203–1205. doi: 10.1128/jb.175.4.1203-1205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A report suggesting the link between IspH and stringent responses.
- 35.Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 36.Lee M, Grawert T, Quitterer F, Rohdich F, Eppinger J, Eisenreich W, Bacher A, Groll M. Biosynthesis of Isoprenoids: Crystal Structure of the [4Fe-4S] Cluster Protein IspG. J Mol Biol. 2010;404:600–610. doi: 10.1016/j.jmb.2010.09.050. [DOI] [PubMed] [Google Scholar]; • One of the first two IspG structural paper.
- 37.Rekittke I, Nonaka T, Wiesner J, Demmer U, Warkentin E, Jomaa H, Ermler U. Structure of the E-1-hydroxy-2-methyl-but-2-enyl-4-diphosphate synthase (GcpE) from Thermus thermophilus. FEBS Lett. 2011;585:447–451. doi: 10.1016/j.febslet.2010.12.012. [DOI] [PubMed] [Google Scholar]; • One of the first two IspG structural paper.
- 38.Xiao Y, Zahariou G, Sanakis Y, Liu P. IspG enzyme activity in the deoxyxylulose phosphate pathway: Roles of the iron-sulfur cluster. Biochemistry. 2009;48:10483–10485. doi: 10.1021/bi901519q. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L, Chang W-c, Xiao Y, Liu H-w, Liu P. Methylerythritol phosphate pathway of isoprenoid biosynthesis. Annu Rev Biochem. 2013;82 doi: 10.1146/annurev-biochem-052010-100934. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohdich F, Zepeck F, Adam P, Hecht S, Kaiser J, Laupitz R, Gräwert T, Amslinger S, Eisenreich W, Bacher A, Arigoni D. The deoxyxylulose phosphate pathway of isoprenoid biosynthesis: studies on the mechanisms of the reactions catalyzed by IspG and IspH protein. Proc Natl Acad Sci USA. 2003;100:1586–1591. doi: 10.1073/pnas.0337742100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nyland RL, II, Xiao Y, Liu P, Freel Meyers CL. IspG converts an epoxide substrate analogue to (E)-4-hydroxy-3-methylbut-2-enyl diphosphate: implications for IspG catalysis in isoprenoid biosynthesis. J Am Chem Soc. 2009;131:17734–17735. doi: 10.1021/ja907470n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao Y, Nyland RL, II, Meyers CL, Liu P. Methylerythritol cyclodiphosphate (MEcPP) in deoxyxylulose phosphate pathway: synthesis from an epoxide and mechanisms. Chem Commun. 2010;46:7220–7222. doi: 10.1039/c0cc02594a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kollas AK, Duin EC, Eberl M, Altincicek B, Hintz M, Reichenberg A, Henschker D, Henne A, Steinbrecher I, Ostrovsky DN, Hedderich R, et al. Functional characterization of GcpE, an essential enzyme of the non-mevalonate pathway of isoprenoid biosynthesis. FEBS Lett. 2002;532:432–436. doi: 10.1016/s0014-5793(02)03725-0. [DOI] [PubMed] [Google Scholar]; • IspG cation mechanistic model.
- 44.Seemann M, Bui BTS, Wolff M, Tritsch D, Campos N, Boronat A, Marquet A, Rohmer M. Isoprenoid biosynthesis through the methylerythritol phosphate pathway: The (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE) is a [4Fe-4S] protein. Angew Chem Int Ed. 2002;41:4337–4339. doi: 10.1002/1521-3773(20021115)41:22<4337::AID-ANIE4337>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]; • IspG cation mechanistic model.
- 45.Xiao Y, Rooker D, You Q, Meyers CLF, Liu P. IspG-Catalyzed Positional Isotopic Exchange in Methylerythritol Cyclodiphosphate of the Deoxyxylulose Phosphate Pathway: Mechanistic Implications. ChemBiochem. 2011;12:527–530. doi: 10.1002/cbic.201000716. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Biochemical evidence supporting a reversible cleavage of the MEcPP C2 position C-O bond, which provide evidences supporting the feasibility of the formation of a cation intermediate.
- 46.Wang W, Li J, Wang K, Huang C, Zhang Y, Oldfield E. Organometallic mechanism of action and inhibition of the 4Fe-4S isoprenoid biosynthesis protein GcpE (IspG) Proc Natl Acad Sci USA. 2010;107:11189–11193. doi: 10.1073/pnas.1000264107. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The initial organometallic model.
- 47.Xu W, Lees NS, Adedeji D, Wiesner J, Jomaa H, Hoffman BM, Duin EC. Paramagnetic intermediates of (E)-4-hydroxy-3-methylbut-2-enyl diphosphate synthase (GcpE/IspG) under steady-state and pre-steady-state conditions. J Am Chem Soc. 2010;132:14509–14520. doi: 10.1021/ja101764w. [DOI] [PubMed] [Google Scholar]; • Modification of the organometallic model based on more detailed analysis of the spectroscopic data.
- 48.Xiao Y, Chu L, Sanakis Y, Liu P. Revisiting the IspH catalytic system in the deoxyxylulose phosphate pathway: achieving high activity. J Am Chem Soc. 2009;131:9931–9933. doi: 10.1021/ja903778d. [DOI] [PubMed] [Google Scholar]; • Discovering the relationship between reduction potential and the IspH activities and improving the IspH activity by ~ 100 fold.
- 49.Gräwert T, Span I, Eisenreich W, Rohdich F, Eppinger J, Bacher A, Groll M. Probing the reaction mechanism of IspH protein by x-ray structure analysis. Proc Natl Acad Sci USA. 2010;107:1077–1081. doi: 10.1073/pnas.0913045107. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First report of IspH-HMBPP complex structure.
- 50.Chang W-c, Xiao Y, Liu H-w, Liu P. Mechanistic studies of an IspH-catalyzed reaction: Implications for substrate binding and protonation in the biosynthesis of isoprenoids. Angew Chem Int Ed. 2011;50:12304–12307. doi: 10.1002/anie.201104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Span I, Grawert T, Bacher A, Eisenreich W, Groll M. Crystal structures of mutant IspH proteins reveal a rotation of the substrate's hydroxymethyl group during catalysis. J Mol Biol. 2012;416:1–9. doi: 10.1016/j.jmb.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 52.Wang WX, Wang K, Liu YL, No JH, Li JK, Nilges MJ, Oldfield E. Bioorganometallic mechanism of action, and inhibition, of IspH. Proc Natl Acad Sci USA. 2010;107:4522–4527. doi: 10.1073/pnas.0911087107. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Characterization of a paramagnetic species and the proposal of the organometallic mechanism model.
- 53.Citron CA, Brock NL, Rabe P, Dickschat JS. The stereochemical course and mechanism of the IspH reaction. Angew Chem Int Ed. 2012;51:4053–4057. doi: 10.1002/anie.201201110. [DOI] [PubMed] [Google Scholar]; • Feeding studies suggest that one of the steps in the MEP pathway from MEP to IPP involves a stereochemistry change at the C4 position.