Abstract
Classical (Pavlovian) conditioning procedures can be used to bias the appearance of physical stimuli. Under natural conditions this form of perceptual learning could cause perception to become more accurate by changing prior belief to be in accord with what is statistically likely. However, for learning to be of functional significance, it must last until similar stimuli are encountered again. Here, we used the apparent rotation direction of a revolving wire frame (Necker) cube to test whether a learned perceptual bias is long lasting. Apparent rotation direction was trained to have different bias at two different retinal locations by interleaving the presentation of ambiguous cubes with presentation of cubes that were disambiguated by disparity and occlusion cues. Four groups of eight subjects were subsequently tested either 1, 7, 14, or 28 days after initial training, respectively, using a counter-conditioning procedure. All four groups showed incomplete re-learning of the reversed contingency relationship during their second session. One group repeated the counter-conditioning and showed an increase in the reverse bias, showing that the first counter-conditioning session also had a long-lasting effect. The fact that the original learning was still evident four weeks after the initial training is consistent with the operation of a mechanism that ordinarily would improve the accuracy and efficiency of perception.
Keywords: perceptual learning, bistability, cue recruitment, structure from motion
1. Introduction
The role played by previous experience in determining how things look has been of interest for more than 300 years (Berkeley, 1709). Documenting a change in appearance caused by learning can be difficult, however, because observers may not accurately remember how a thing looked in the past. A strategy for overcoming this difficulty is to use perceptually bistable stimuli, because once the visual system itself makes the noisy dichotomous decision, the observer can effortlessly report the result of the visual system's decision (Backus, 2009; Backus, 2011; Pylyshyn, 1999).
A revolving wire-frame Necker cube is such a stimulus. It is perceived at stimulus onset to be rotating in one of exactly two directions, i.e., it is perceptually bistable. Furthermore, the apparent rotation direction of the cube can be conditioned to depend on retinal location, an effect that persists for at least 24 hours (Haijiang et al 2006; Backus and Haijiang, 2007; Harrison and Backus, 2010a). Specifically, if a cube presented above fixation on “training trials” is disambiguated by depth cues so that it appears to rotate one direction, while a cube presented below fixation on other training trials is disambiguated so that it appears to rotate the opposite direction, then ambiguous cubes on interleaved test trials will rapidly come to have the same apparent rotation direction as was trained at their respective locations. This training occurs mostly independently at each location (Harrison and Backus, 2010a), however, the difference in bias at the two locations, measured on test trials, is a useful measure of the learning because it is robust to their common initial bias. To control for initial bias that is different across locations but common across observers—a possible if unlikely situation—the contingency between location and rotation direction is counterbalanced across observers.
Short term priming effects, that may or may not be functionally important for vision, are sufficient to explain the learning that occurs within a single session (e.g. Brascamp et al., 2008). However, these same priming effects make it impossible to quantify the strength of any long term learning, because only one ambiguous trial at the start of the second session is independent; all the rest will be influenced by the previous trials in the second session (Pastukhov & Braun, 2008; Brascamp et al., 2008; Brascamp et al., 2009; Harrison & Backus, 2010; van Dam and Ernst, 2010).
Under these circumstances a useful strategy to quantify the learning is counter-conditioning: the strength of the initial learning can be assessed during the second session by measuring how resistant the system is to learning from training trial stimuli that rotate opposite to the training in the first session. The extent to which the perceptual outcomes for ambiguous cubes in the second session adopt the new location-rotation contingency (or alternatively, are perceived in accordance with the first session's contingency) is then a measure of the bias retained from the first session. This strategy of conditioning and then counter-conditioning has shown that learned biases last for many minutes (Backus, 2011) and even overnight (Haijiang et al., 2006). Here we ask whether learned biases last many days, as would presumably be the case if the learning is implemented by mechanisms that are useful for deciding the appearances of objects that are encountered repeatedly but not every day. A positive finding of persistent bias cannot prove a functional role, but failure to find it might argue against such a role.
2. Materials and methods
Most aspects of the materials and methods are as previously described (Harrison and Backus, 2010a). The methods were designed to ensure that subjects' responses reflect the visual appearance of the stimulus, rather than other factors such as a bias in post-perceptual cognitive decisions or motor choice, cognitive strategy, or fixation strategy (Haijiang, et al., 2006; Backus, 2009; Backus, 2011). For convenience we describe the most important of these design choices again, below.
2.1 Subjects
Subjects were adults with normal or corrected-to-normal vision who were able to do the task correctly on training trials, recruited from the College of Optometry and from the New York City metropolitan area with advertisements at craigslist.com. We tested 4 groups of 8 subjects, with varying numbers of days between the first (conditioning) session and the second (counter-conditioning) session. Subjects returned for counter-conditioning on either the 2nd, 8th, 15th or 29th day. The group that received counter-conditioning on the 2nd day also received the same counter-conditioning on the 8th day, to evaluate the effect of elapsed time as compared to the effect of counter-conditioning per se as a factor in dissipating the bias.
2.2 Stimuli
On Session 1, all groups viewed 480 trials consisting of a 50:50 pseudorandom mixture of disambiguated and ambiguous cubes, presented by rear-projection, identical to those used previously (see Harrison and Backus, 2010a, for details). Disambiguated cubes contained binocular disparity and revolved around a central strip so as to provide an occlusion cue (Figure 1a). Ambiguous cubes were presented monocularly, and contained no other cues to depth (Figure 1b). Each transparent face of the cube contained 25 randomly placed dots, which stabilized the cube's appearance as a single rigid rotating body on ambiguous trials. All cubes were viewed through red-green glasses, and were presented using orthographic projection. Luminance in the red and green channels was balanced on training trials and cross-talk was minimized (Mulligan, 1986) to prevent the Pulfrich effect from determining apparent rotation direction on monocular test trials.
Figure 1.
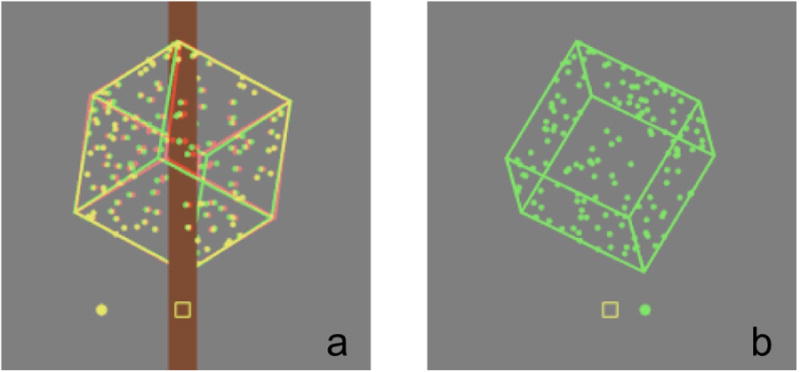
Cropped screen shots showing example stimuli: (a) cube disambiguated by (geometrically correct amplitude) binocular disparity and occlusion, and (b) ambiguous cube. Both cubes are depicted here at the “top” location, centered 12 degrees above the binocular fixation marker. Cube edges were of 20.0 cm length, hence subtended approximately 11.5 degrees of visual angle at the viewing distance of 1 m, when in the frontoparallel plane. Width and breadth of cube edges was 0.3 cm. Width of the central occlusion strip was 4.0 cm. Cubes rotated about a vertical axis at a rate of 45 degrees sec-1, and the comparison dot (shown to the left or right of fixation) had a speed of 15.7 cm sec-1, similar to the horizontal image speed of the nearest (and farthest) part of the cube.
2.3 Task
Subjects' task was to indicate whether the transit direction of a comparison dot, which completed horizontal paths through the fixation marker, was the same as the motion of the front (near part) or back (far part) of the cube. The comparison dot is shown to the left of the fixation square in Figure 1a. Subjects indicated “matches near” or “matches far” by pressing “2” or “8” on a numeric keypad. This task exploits a perceptual coupling (Hochberg and Peterson, 1987): on each trial, leftward or rightward transit direction was randomly chosen for the comparison dot, with equal probability, so the response mapping was randomly re-assigned on each trial. Thus subjects' responses were not correlated with the actual dependent variable of interest, namely apparent cube rotation, nor with the top vs. bottom position of the stimulus, nor with dot motion itself. This feature of the task design ensures that location-contingent motor bias cannot explain the data. The dot was presented at fixation depth on training trials and monocularly on test trials. The cube and comparison dot remained on the screen for a minimum of 1.5 seconds and the subject's response terminated the presentation.
2.4 Data analysis
From subjects' responses we calculated the fraction of ambiguous (test trial) cubes perceived as rotating the same as the disambiguated (training trial) cubes at the top location in Session 1 (Figures 2A-C and 3). These fractions were then transformed into z-scores, i.e. we used a probit (inverse-cumulative-normal) transformation (Backus, 2009, Dosher, Sperling & Wurst, 1986). For each subject, z-scores at the two locations were differenced to give “zDiff”, a measure of the difference in bias at the two (oppositely trained) locations. Thus, zDiff is twice the average of the acquired biases at the two locations (Figures 2D and 4).
Figure 2.
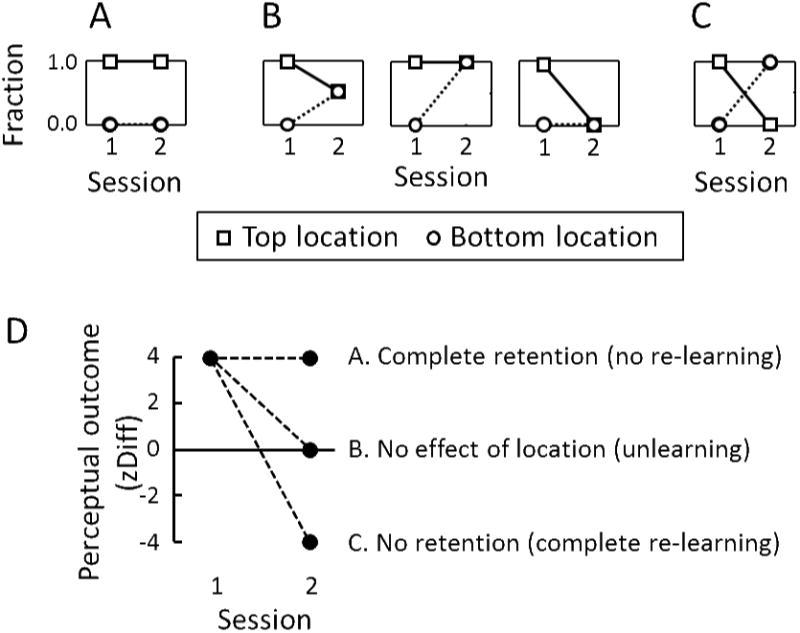
Predicted outcomes for ambiguous cubes in the first and second sessions, plotted as fractions (A, B, C) or plotted as z-score differences (D). A, B, C: The ordinate plots the fraction of test trials on which the cube appeared to rotate in the same direction as was trained at the top location in Session 1. Based on previous studies, near maximal effects are expected in Session 1, as shown by ordinates of 1.0 and 0.0 when cubes are presented at the top (squares) and bottom (circles) locations, respectively. Panel A shows the prediction if learning is retained from Session 1 and training in Session 2 has no effect. Panel B shows three possible predictions if Session 1 has a long-lasting effect, such that training in Session 2 is only partly effective. Shown is the special case in which Sessions 1 and 2 perfectly cancel each other. Panel C shows the prediction if Session 1 has no long-lasting effect, in which case responses in Session 2 will not reflect any previous learning from Session 1.
D. Predictions for the zDiff summary statistic. The ordinate plots perceptual outcomes as zDiff, which is the difference in z-score (z-transform of percent-seen-as-trained) for rthe two oppositely trained locations in Session 1. Near-maximal z-scores would be ±2, leading to an expected zDiff of 4 in Session 1. A zDiff of 0 indicates similar bias at both locations and a negative zDiff indicates biases that are opposite to the training in Session 1. The point with coordinates (2,4) therefore shows long-lasting learning from Session 1 and no re-learning during Session 2 (same as Panel A). The point at (2,0) shows unlearning of a long-lasting bias from Session 1 (same as B). The point at (2, −4) shows no effect of Session 1 on the bias measured in Session 2, as if Session 1 had not occurred (same as C).
Figure 3.
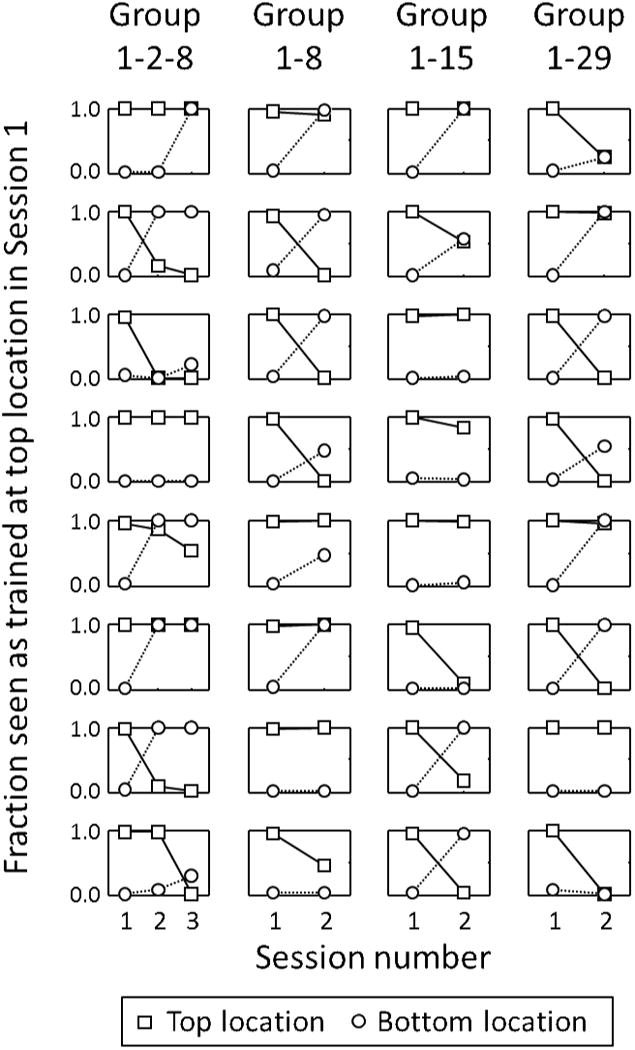
Individual subject data for the 32 subjects, plotted as fractions (as in Figure 2A-C). Each graph shows data for one of the eight subjects in one of the four groups. The ordinate is the fraction of test trials that appeared to rotate in the same direction as was trained at the top location on Session 1. Squares and circles plot data for test stimuli presented at the top and bottom locations—above and below fixation—respectively. Group 1-2-8 had sessions on Days 1, 2, and 8 of the experiment, and so on.
Figure 4.
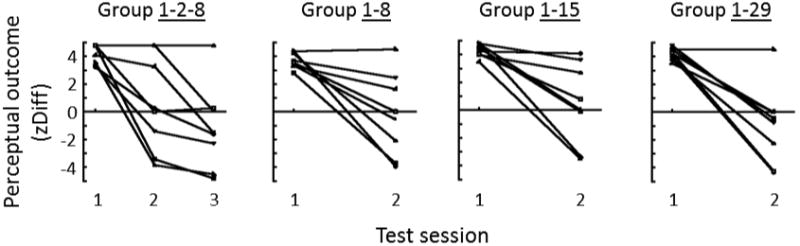
Perceptual outcomes for the four groups of eight subjects, plotted as z-score differences (same as Figure 2D).
2.5 Counter-contingency training
During Session 2 (on Day 2, 8, 15 or 29), all subjects once again viewed a 50:50 mixture of ambiguous and disambiguated cubes. Disambiguated cubes now had the opposite location-rotation contingency to that of Session 1. The percent of ambiguous cubes perceived according to the Session 1 contingency was used to calculate zDiff for Session 2 (see predictions, Figure 2D). If no long-term learningtoccurred on Session 1, then subjects should perceive ambiguous cubes in Session 2 according to the location-rotation contingency of Session 2 disambiguated stimuli, resulting in a negative zDiff for Session 2 with equal magnitude to the positive zDiff in Session 1. However, if Session 1 caused learning that persisted to Session 2, subjects' perception of ambiguous stimuli on Session 2 should reflect a residual bias in favor of the location-rotation contingency experienced on Session 1, resulting in a less negative (or perhaps even positive) zDiff on Session 2.
3. Results
Results are presented in Figures 3–5. Subjects in all four groups saw the same stimuli on Session 1 (with rotation direction being counter-balanced across subjects within groups) and so, as expected, there was no significant difference between groups in recruitment of the location-contingent bias on Session 1 (1-way ANOVA on zDiff: F(3,28) = 1.85, p = .16). Importantly, on the second session of testing in Group 1-29, the bias weaker than would be expected if the location-rotation contingency was recruited on Session 2 to the same extent as it was on Session 1 (Session 1 zDiff vs. additive inverse of Session 2 zDiff, two-tailed paired t-test: mean = 3.3, SE = 1.1, t(7) = 3.08, p = .02). The downward trend of the data in Figure 5 suggests that susceptibility to relearning may increase over time, but this trend was not statistically significant.
Figure 5.
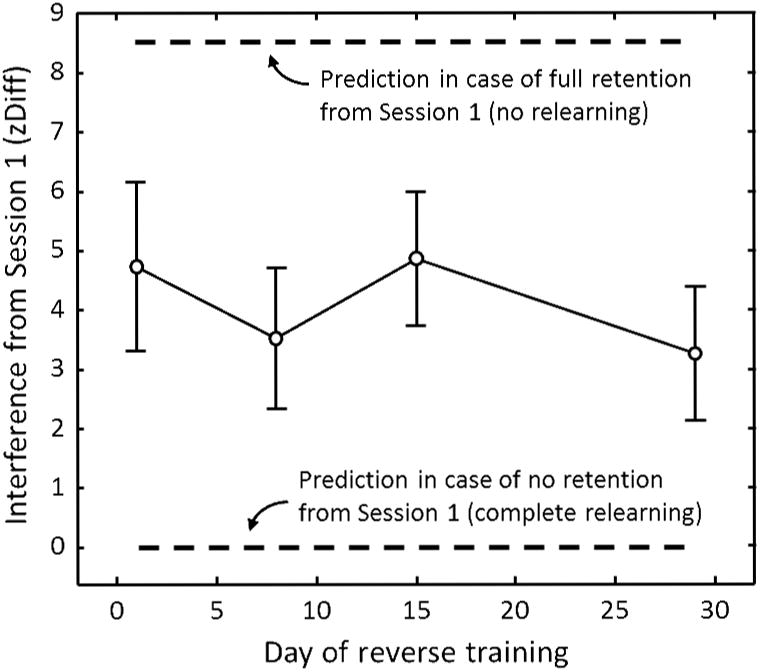
Interference from Session 1 on the bias measured in Session 2. The figure re-plots the data from Figure 4 as the zDiff from Session 1 minus the additive inverse of zDiff from Session 2, for each of the four groups. Bars show SE across observers within each group.
Individual differences are a highly salient feature of the data. The counter-conditioning on Session 2 was highly effective for several subjects in each group, yet it was completely ineffective for other individuals, as shown by the horizontal line segment(s) at the tops of the plots in Figure 4. There was no statistically significant difference across the four groups either in the bias on Session 2 (ANOVA on Session 2 zDiff: F(3,28) = .44, p = .73), or in the magnitude of difference between Session 1 and Session 2 bias (ANOVA on Session 1 zDiff – Session 2 zDiff: F(3,28) = .45, p = .72). Thus, any systematic effect of the length of delay between Session 1 and Session 2 was small compared to the individual differences.
Additionally, the difference in perceptual outcome on the 8th day between the 1-2-8 group, which had an intervening session of counter-conditioning, and the 1-8 group, which did not, was in the expected direction (it was more negative for the 1-2-8 group) but this difference was not statistically significant (two-sample t-test: difference in means = -1.0, t(14) = 0.67, p = .52). The 95% confidence interval for this difference was large, [-4.2, 2.3], meaning that we had little power to detect whether the additional session on Day 2 for the 1-2-8 group caused reverse learning to be greater on Day 8 than it would have been without Day 2. However, within the 1-2-8 group itself, a within-subjects (paired) comparison is possible. Additional (un)learning did occur from the 2nd day to the 8th day (Session 2 vs. Session 3, mean = 1.8, SE = 0.8, t(7) = 2.48, p = .04 two-tailed or .02 one-tailed). Furthermore, the bias from Session 1 was still not completely reversed (Session 1 vs. the additive inverse of Session 3, mean = 3.0, SE = 1.3, t(7) = 2.34, p < .05). Thus, additional counter-conditioning in Session 3 caused additional change in bias in the expected direction, but residual bias from Session 1 was still present. This cumulative effect of reverse-contingency training has been seen previously in shorter experiments that used similar stimuli (Backus, 2011).
The additional learning that occurred between Sessions 2 and 3 in the 1-2-8 group is important for another reason. Without this group, a possible interpretation of zDiff values close to 0 in Session 2 would be that learning decayed after Session 1, with no subsequent learning occurring in Session 2. That would still indicate a long term effect of Session 1, but not a long term retention of the bias from Session 1. However, since additional learning did occur in Session 3 that interpretation is untenable.
4. Discussion
Training a 3D rotation-direction bias at a specific retinal location prevented later counter-conditioning from being equally effective, even four weeks later. Counter-conditioning at any time during the four-week period had a short term effect within that same session that partially overcame the originally trained bias, but could not fully overcome it. In one group, counter-conditioning was repeated six days later in a third session, and the reversed bias became stronger, which showed that the second session also had a long-lasting effect. Yet even then, the bias during this third session was not fully reversed: it was not as strong as the originally trained bias observed during the first session.
4.1 Interpretation of the learning
The results admit explanation at two levels of analysis. First, what neural mechanism(s) were trained by the conditioning procedures? Brain area V5/MT is a good candidate for the primary site of these mechanisms (Harrison & Backus, 2010a) because many neurons in that area are jointly tuned for retinal location, motion direction, and binocular disparity; in macaque monkeys, microstimulating these neurons systematically biases behavioral 3D rotation judgments (Krug et al., 2013).
Second, does this instance of learning tells us anything important about how the visual system learns to see? In other words, does the learning reflect the operation of a functionally useful mechanism, one that would normally act to improve perception, and thus the organism's fitness or “achievement” (Brunswik, 1954)? While one instance of long term learning cannot decide this question, the long-lasting nature of the learning is at least consistent with learning how to disambiguate an otherwise ambiguous visual stimulus so as to be prepared the next time it occurs, in the service of fast and accurate perception (Harrison & Backus, 2010b).
A separate question is whether the current study provides support for the utility of cue recruitment (Haijiang et al, 2006) as a theoretical construct. Previous work showed that that the learned bias we have studied here depends on the stimulus's location within the retinocentric visual field, not its location in head-centric or exocentric coordinates (Harrison and Backus, 2010a). Formally, retinal location was a cue that our experiments successfully caused to be recruited, but it seems a peculiar sort of cue in the larger context of cue theory (Helmholtz, 1910). Like retinal location, canonical cues such as binocular disparity and retinal image size vary depending on the situation of the observer's sensory apparatus relative to the objects being perceived, and the observer can change their values by moving. Most cues, however, exhibit cue constancy: changes in the situation of the observer are corrected for during perception, so they do not cause dramatic changes in appearance. When the retinal location cue has been recruited, however, moving the eyes so as to put the cube at the other trained retinal location causes the apparent rotation direction of the cube to reverse.
Other visual cues besides retinal location that can be recruited by the visual system, for example: whether the object is moving up or down (Haijiang et al., 2006); binocular vertical disparity within the stimulus (Di Luca et al., 2010); the shape of the object (Harrison & Backus, 2012); illumination color (Kerrigan & Adams, 2013); and surface texture (Jain & Backus, 2013). Compared to retinal location, these other cues match more closely what perceptual scientists have in mind when they think of a cue, but it is not yet known whether the recruitment of these cues can be made to last, nor whether their recruitment generalizes to untrained retinal locations.
4.2 Comparison with other long-lasting perceptual effects
The McCollough effect is another learned contingent bias that can affect appearance for weeks (Jones & Holding, 1975). In one variant of this effect, the observer is exposed during the “adaptation phase” to vertical orange and black stripes that alternate in time with horizontal blue and black stripes. A test pattern consisting of vertical white and black stripes then look blue, while a pattern of horizontal white and black stripes looks orange (McCollough, 1965). Like cue recruitment, the McCollough effect can be interpreted as an instance of classical conditioning (Allan & Siegel, 1993).
However, the McCollough effect has important differences with the rotation-direction bias. The McCollough effect is a negative contingent aftereffect, not a positive one, and functionally, it is probably best understood as an internal recalibration that achieves normalization across responses in a population of sensory neurons, in order to eliminate bias, rather than the learning of a bias that reflects environmental statistics (as we suggest to be the case for the rotation bias).
This distinction becomes clearer when one considers how the biases are made visible. The McCollough effect is most visible when the sense data are well specified, and thus in need of calibration rather than disambiguation: the test stimulus in the McCollough effect physically specifies a color (white) which is corrected to appear orange or blue. In contrast, the rotation bias we studied here was most visible when sense data contained no other cues to specify rotation direction. A newly recruited cue becomes most useful when the sense data do not contain other disambiguating cues. Thus, the McCollough effect and the learned rotation bias are both long term contingent aftereffects, and both presumably reflect the operation of mechanisms that normally make perception more accurate—but for recalibration, or for disambiguation, respectively.
Tseng, Gobell, and Sperling (2004) also reported a long-lasting learned perceptual bias. Attention to one of two colors, reinforced using a search task, increased that color's participation weight in a seemingly unrelated dichotomous motion perception task. This color-contingent motion bias was still present one month later. The authors concluded that attention to the color gave a long-term boost to its salience in an early visual representation, and that feature-tracking motion mechanisms, that operate on salience maps, were affected in turn. This learning demonstrates the capacity of the adult visual system for long-lasting change, but it is probably not related to the location-contingent bias for 3D rotation that we have described here. One might speculate that leftward motion shown in crossed disparity (say) on training trials causes leftward motion to become more salient, and that this salience causes leftward motion to appear closer on test trials. At this point, however, an appeal to learned salience is not obviously necessary or helpful for explaining the learned rotation bias.
4.3 Individual differences in initial bias and susceptibility to conditioning
Most of the learning in these experiments presumably occurred during ambiguous test trials, as was the case in studies of shorter duration (Harrison & Backus, 2010; van Dam & Ernst, 2010). This fact makes it impossible to measure the initial bias at the two locations for each subject before the start of training, because the act of measuring the bias causes it to increase (note that the counter-conditioning design of the current experiment is robust to this effect). Thus, while we can be sure that the training has a long-lasting effect, we cannot determine how much of the observed inter-subject variability is due to inter-subject differences in the strength of initial bias. Initial bias, if present, could have been of two kinds: an overall bias affecting both locations, that should not have affected our results, and a differential bias between the two locations that agreed with Session 1 training for some subjects and not for others, that could have affected the results of individual subjects. Nevertheless, the fact that intersubject differences were so much larger in Session 2 than in Session 1 suggests to us that individual differences in initial bias were probably not a significant factor. More likely, some individuals change their biases more readily than others. Visual attention affects the strength of the acquired bias (Backus & Fuller, 2010), so inter-subject differences in attention may also have played a role.
Inter-subject differences in susceptibility to counter-conditioning could be measured by using several reversals of contingency, not just the one reversal we used here. A short term experiment with four subjects, in which contingency was reversed five times in a single session, did show inter-subject differences in susceptibility to counter-conditioning (Figure 6.6 in Backus, 2011). That study (with N = 1 subject) also showed a marked decrease in susceptibility over the course of 8 sessions. Individual differences in the cumulative effect of sustained counter-conditioning are also evident in single-session data (Figure 6.5 in Backus, 2011).
The optimal learning strategy is not in fact obvious for a perceptual system that is trained with cues that first have one meaning and then have the opposite meaning (see Backus, 2011 for discussion). Should the system track the meaning of the cue across the change, in order to track changes in the environment? Or should it assume that the environment is stable, in which case what is learned early can be assumed to be true in the future? The persistence of Session 1 bias into Session 2 shows that observers did not forget what they learned in Session 1; operationally their perceptual systems acted as though what was learned early was worth remembering.
5. Conclusion
Learning must persist to be useful. The location-specific bias for 3D rotation, established in less than one hour, persists for at least 4 weeks. This fact suggests that the bias could reflect the operation of perceptual learning mechanisms that normally act to improve perception by making visual appearances more accurate, or more efficiently constructed, or both, across weeks or longer.
Supplementary Material
Highlights (for review).
Perceptual biases can be trained, specific to retinal location
These biases last for many weeks
These biases may reflect useful learning
Acknowledgments
This research was supported by grants NSF BCS-0810944, NIH R01 EY 013988, and HFSP RPG 3/2006. We thank Martha Lain and Hunter McFadden for help with subject recruitment and data collection, and two anonymous reviewers for suggestions that improved the manuscript
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah J. Harrison, Email: sarah.harrison@rhul.ac.uk.
Benjamin T. Backus, Email: bbackus@sunyopt.edu.
References
- Allan LG, Siegel S. McCollough effects as conditioned responses: reply to Dodwell and Humphrey. Psychol Rev. 1993;100:342–6. doi: 10.1037/0033-295x.100.2.342. [DOI] [PubMed] [Google Scholar]
- Backus BT, Fuller S. Attention mediates learned perceptual bias for bistable stimuli (abstract) Journal of Vision. 2010;10:1106. [Google Scholar]
- Backus BT, Haijiang Q. Competition between newly recruited and preexisting visual cues during the construction of visual appearance. Vision Research. 2007;47:919–924. doi: 10.1016/j.visres.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus BT. The Mixture of Bernoulli Experts: a theory to quantify reliance on cues in dichotomous perceptual decisions. Journal of Vision. 2009;9(1):6, 1–19. doi: 10.1167/9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus BT. Recruitment of new visual cues for perceptual appearance. In: Trommershauser, Kording, Landy, editors. Sensory Cue Integration. Chapter 6. Oxford University Press; Oxford, U.K.: 2011. [Google Scholar]
- Brascamp JW, Knapen TH, Kanai R, Noest AJ, van Ee R, van den Berg AV. Multi-timescale perceptual history resolves visual ambiguity. Plos One. 2008;3:e1497, 1–8. doi: 10.1371/journal.pone.0001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brascamp JW, Pearson J, Blake R, van den Berg AV. Intermittent ambiguous stimuli: Implicit memory causes periodic perceptual alternations. Journal of Vision. 2009;9(3):3, 1–23. doi: 10.1167/9.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswik E. Organismic Achievement and Environmental Probability. Psychological Review. 1943;50:255–272. [Google Scholar]
- Brunswik E. Perception and the representative design of psychological experiments. University of California Press; Berkeley, CA: 1956. pp. 92pp. 96pp. 123–131. [Google Scholar]
- Di Luca M, Ernst MO, Backus BT. Learning to use an invisible visual signal for perception. Current Biology. 2010;20:1860–1863. doi: 10.1016/j.cub.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA, Sperling G, Wurst SA. Tradeoffs between stereopsis and proximity luminance covariance as determinants of perceived 3D structure. Vision Research. 1986;26:973–990. doi: 10.1016/0042-6989(86)90154-9. [DOI] [PubMed] [Google Scholar]
- Haijiang Q, Saunders JA, Stone RW, Backus BT. Demonstration of cue recruitment: Change in visual appearance by means of Pavlovian conditioning. Proc Natl Acad Sci U S A. 2006;103:483–488. doi: 10.1073/pnas.0506728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SJ, Backus BT. Disambiguating Necker cube rotation using a location cue: What types of spatial location signal can the visual system learn? Journal of Vision. 2010a;10(6):23, 1–15. doi: 10.1167/10.6.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SJ, Backus BT. Uninformative visual experience establishes long term perceptual bias. Vision Research. 2010b;50:1905–1911. doi: 10.1016/j.visres.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SJ, Backus BT. Associative learning of shape as a cue to appearance: a new demonstration of cue recruitment. Journal of Vision. 2012;12(3):15, 1–17. doi: 10.1167/12.3.15. [DOI] [PubMed] [Google Scholar]
- Hebb DO. Organization of Behavior. Wiley; New York: 1949. [DOI] [PubMed] [Google Scholar]
- Hochberg J, Peterson MA. Piecemeal organization and cognitive components in object perception: perceptually coupled responses to moving objects. J Exp Psychol Gen. 1987;116:370–80. doi: 10.1037//0096-3445.116.4.370. [DOI] [PubMed] [Google Scholar]
- Helmholtz H von. Southall translation (1925) III. Optical Society of America; New York: 1910. Treatise on Physiological Optics. [Google Scholar]
- Jain A, Backus BT. Generalization of cue recruitment to non-moving stimuli: location and surface-texture contingent biases for 3-D shape perception. Vision Research. 2013;82:13–21. doi: 10.1016/j.visres.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PD, Holding DH. Extremely long-term persistence of the McCollough effect. J Exp Psychol Hum Percept Perform. 1975;1:323–7. doi: 10.1037//0096-1523.1.4.323. [DOI] [PubMed] [Google Scholar]
- Kerrigan IS, Adams WJ. Learning different light prior distributions for different contexts. Cognition. 2013;127:99–104. doi: 10.1016/j.cognition.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Krug K, Cicmil N, Parker AJ, Cumming BG. A Causal Role for V5/MT Neurons Coding Motion-Disparity Conjunctions in Resolving Perceptual Ambiguity. Current Biology. 2013;23:1454–1459. doi: 10.1016/j.cub.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollough C. Colour adaptation of edge detectors in the human visual system. Science. 1965;149:1115–1116. doi: 10.1126/science.149.3688.1115. [DOI] [PubMed] [Google Scholar]
- Mulligan JB. Optimizing stereo separation in color television anaglyphs. Perception. 1986;15:27–36. doi: 10.1068/p150027. [DOI] [PubMed] [Google Scholar]
- Pastukhov A, Braun J. A short-term memory of multi-stable perception. Journal of Vision. 2008;8(13):7, 1–14. doi: 10.1167/8.13.7. [DOI] [PubMed] [Google Scholar]
- Pylyshyn Z. Is vision continuous with cognition? The case for cognitive impenetrability of visual perception. Behav Brain Sci. 1999;22:341–65. doi: 10.1017/s0140525x99002022. with discussion pp. 366-423. [DOI] [PubMed] [Google Scholar]
- Tseng CH, Gobell JL, Sperling G. Long-lasting sensitization to a given colour after visual search. Nature. 2004;428:657–660. doi: 10.1038/nature02443. [DOI] [PubMed] [Google Scholar]
- van Dam LCJ, Ernst MO. Preexposure disrupts learning of location-contingent perceptual biases for ambiguous stimuli. Journal of Vision. 2010;10(8):15, 1–17. doi: 10.1167/10.8.15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


