Abstract
In contrast to the continuously growing number of methods that allow for the efficient α-functionalization of amines, few strategies exist that enable the direct functionalization of amines in the β-position. Outlined herein is a general redox-neutral strategy for amine β-functionalization and α,β-difunctionalization that utilizes in-situ-generated enamines. This concept is demonstrated in the context of preparing polycyclic N,O-acetals from simple 1-(aminomethyl)-β-naphthols and 2-(aminomethyl)-phenols.
Keywords: C–H functionalization, redox-neutral, enamines, azomethine ylides, heterocycles
While oxidative approaches continue to dominate the vibrant field of amine α-C–H bond functionalization,[1] redox-neutral methods continue to establish themselves as complementary strategies and viable alternatives.[2] Increasingly complex structures can be built efficiently from simple starting materials by replacing existing amine α-C–H bonds without requiring pre-functionalization or the use of external oxidants or reductants. Whereas many redox-neutral amine α-functionalizations involve hydride-shifts or sigmatropic H-transfer steps,[3] we have developed an alternative approach that involves azomethine ylides as reactive intermediates (Scheme 1).[4–6] In stark contrast to amine α-functionalization, methods that allow for the direct β-functionalization of amines remain rare and typically require precious metal catalysts.[7–10] Here we disclose a new strategy that allows for the simultaneous α,β-difunctionalization of amines via transient enamines.
Scheme 1.
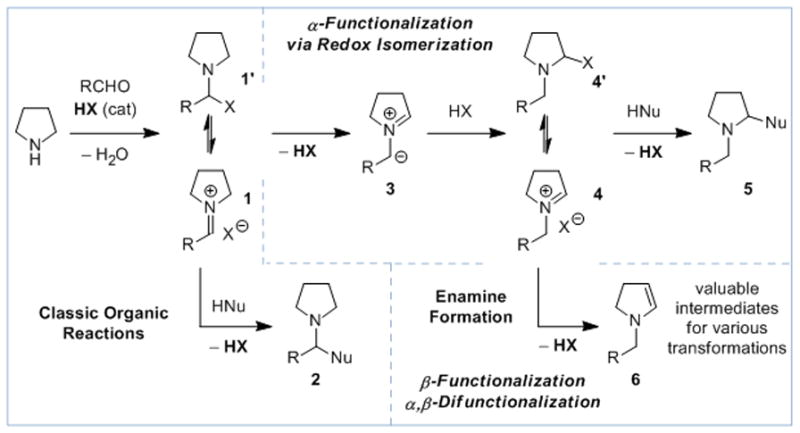
Concepts for the redox-neutral amine α-functionalization, β-functionalization and α,β-difunctionalization.
Classic organic transformations such as the Strecker and Mannich reactions involve the addition of a nucleophile to an in-situ-generated iminium ion 1 to result in the formation of products of type 2 (Scheme 1). We have developed a strategy that averts the traditional reaction pathway by incorporation of an isomerization step, allowing for amine α-functionalization and the generation of products with the general structure 5 (Scheme 1). These redox-transformations are thought to involve the intermediacy of azomethine ylides 3 and endocyclic iminium ions 4. Simple carboxylic acids have been found to catalyze a diverse set of amine α-C–H bond functionalizations including α-cyanation,[4f] α-phosphine oxide formation,[4j] and α-arylation.[4k] Copper carboxylates have been found to catalyze the α-alkynylation[4g] of amines. While considering new variants of these transformations we recognized an opportunity to fundamentally alter the course of these reactions. Specifically, we reasoned that under appropriate conditions, intermediates 4/4′ may provide access to endocyclic enamines 6 by simple loss of HX. Enamines 6 represent valuable precursors that could be utilized in a wide range of β-functionalization and α,β-difunctionalization reactions.
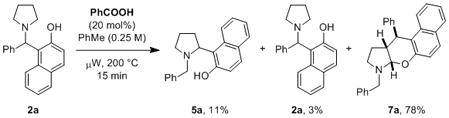
In the course of attempting to expand the scope of our recently reported amine α-arylation method,[4k] we discovered a unique opportunity to realize the first example of a redox-neutral amine α,β-difunctionalization. Specifically, we were curious to learn whether it would be possible to isomerize compound 2a to the regioisomeric product 5a. An experiment in which a solution of compound 2a was heated in toluene under microwave irradiation at 150 °C for 15 min in the presence of benzoic acid (20 mol%) did not result in any reaction, and starting material was recovered in essentially quantitative yield. However, upon increasing the temperature to 200 °C, the expected isomerization was indeed observed, giving 5a and 2a in an approximately 4:1 ratio albeit in low yields (eq 1). Interestingly, the main product of this reaction was the unusual N,O-acetal 7a which was isolated as a single diastereomer in 78% yield[11] (relative configuration established by X-ray crystallography).[12–15]
 |
(1) |
 |
(2) |
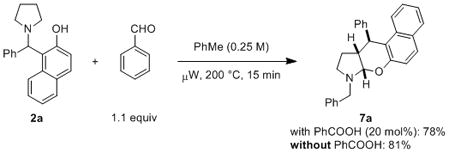
The formation of product 7a is consistent with the intermediacy of an endocyclic enamine corresponding to proposed compound 6 (Scheme 1). Considering that the formation of 7a from 2a requires the incorporation of one unit of benzaldehyde (or a benzaldehyde equivalent), we performed the reactions outlined in eq 2. Exposure of 2a to a small excess of benzaldehyde (1.1 equiv) yielded 7a in 78% yield for a reaction conducted in the presence of benzoic acid (20 mol%). Interestingly, the use of benzoic acid as a catalyst was not required. In fact, in the absence of any additive 7a was obtained in an improved yield of 81%.
With the goal to establish the course of this intriguing transformation, we performed the experiments shown in eqs 3 and 4 (Scheme 2). When 2a was allowed to react with 4-chlorobenzaldehyde, N,O-acetal 7b was isolated as the only product in 82% yield (eq 3). The complementary reaction of 2b and benzaldehyde afforded regioisomeric N,O-acetal 7c in 75% yield (eq 4).
Scheme 2.
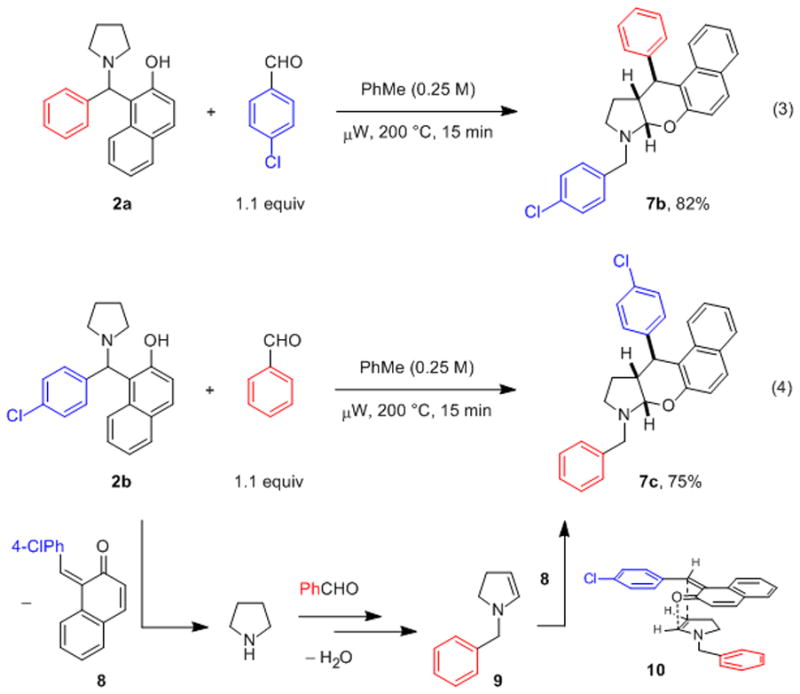
Experiments designed to determine the course of the annulation reaction and proposed mechanism.
Based on the outcome of these experiments, we propose the reaction mechanism shown in Scheme 2. Upon heating, 2b reversibly fragments into pyrrolidine and ortho-quinone methide 8. Hydrogen-bonding between the nitrogen atom and the naphthol hydroxyl group, as previously observed in X-ray crystal structures of related compounds,[16] is expected to facilitate this step. The released pyrrolidine is intercepted by benzaldehyde to eventually form enamine 9 via the sequence of events detailed in Scheme 1. The formation of N,O-acetal 7c from 8 and 9 is consistent with a diastereoselective hetero-Diels-Alder reaction (e.g., via 10) although a stepwise pathway cannot be ruled out.
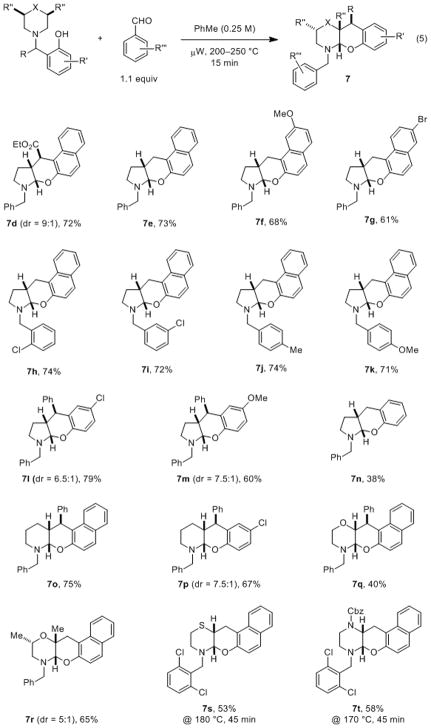
The scope of the N,O-acetal formation was examined next (Chart 1). Gratifyingly, a number of 1-(aminomethyl)-β-naphthols and 2-(aminomethyl)-phenols readily underwent the reaction with benzaldehyde and other aromatic aldehydes. The expected products were obtained in moderate to good yields following a reaction time of just 15 min. Good to excellent diastereoselectivities were observed in most instances. Notably, substrates incorporating piperidine, morpholine, thiomorpholine and piperazine moieties readily participated in the α,β-difunctionalization. This is despite the fact that these heterocycles typically represent challenging substrates for redox-neutral amine α-C–H bond functionalization.
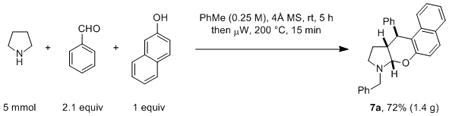
Given that 1-(aminomethyl)-β-naphthols such as 2a can be easily formed from the corresponding amine, β-naphthol and aldehyde,[17] we were curious to learn whether the amine N-alkylation/α,β-difunctionalization could be performed as a one-pot process simply by employing two equivalents of the aldehyde. In order to also evaluate the potential scalability of such a process, a test reaction was conducted on a 5 mmol scale (eq 6). Gratifyingly, treatment of a 1:2.1:1 mixture of pyrrolidine, benzaldehyde and β-naphthol with molecular sieves in toluene at room temperature for five hours, followed by exposure to microwave heating at 200 °C for 15 min, led to the isolation of 7a in 72% yield. Notably, the efficiency of this two-stage/one-pot approach is similar to that of the two-step procedure.
 |
(6) |
The product N,O-acetals can be transformed in a number of different ways (Scheme 3). For instance, treatment of compound 7e with lithium aluminum hydride in tetrahydrofuran resulted in C–O bond cleavage and the formation of β-substituted N-benzyl pyrrolidine 11 in 69% yield. In line with a previous report by Maulide et al. that nicely demonstrated the utility of N-aryl N,O-acetals in reactions with various nucleophiles,[3r] 7e also readily engaged Grignard reagents to form the α,β-disubstituted products 12 and 13 in excellent yields.
Scheme 3.
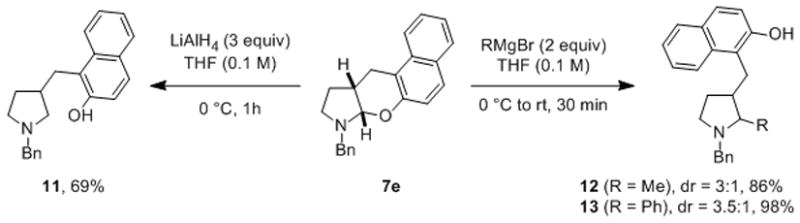
Product modification.
In conclusion, we have developed an unprecedented redox-neutral α,β-difunctionalization cascade reaction of simple cyclic amines. Polycyclic N,O-acetals are now available in just two steps from cheap commercial materials via an annulation of transient enamines. The method described herein is suited ideally to rapidly access new chemical space. We anticipate its widespread application and the emergence of related processes.
Experimental Section
General procedure for the redox-neutral α,β-difunctionalization of amines: A 10 mL microwave reaction tube was charged with a 10 × 8 mm SiC passive heating element, the 1-(aminomethyl)-β-naphthol or 2-(aminomethyl)-phenol (0.5 mmol, 1 equiv), toluene (2 mL) and the aldehyde (0.55 mmol, 1.1 equiv). The reaction tube was sealed with a Teflon-lined snap cap, and heated in a microwave reactor at 200 °C (200 W, 70–100 psi, for substrates containing a pyrrolidine moiety) or 250 °C (200W, 150–200 psi, for substrates containing piperidine/morpholine moieties) for 15 minutes. After cooling with compressed air flow, the solution was transferred to a 50 mL round-bottom flask. The solvent was then removed under reduced pressure and the residue was purified by silica gel chromatography.
Supplementary Material
Chart 1. Substrate scope for the α,β-difunctionalization/N,O-acetal formation.[a–c].
[a] Reactions were performed on a 0.5 mmol scale. [b] Substrates containing pyrrolidine and piperidine/morpholine moieties were heated at 200 and 250 °C, respectively. [c] Yields are combined yields of both diastereomers (if any), major diastereomer shown.
Footnotes
Financial support from the NIH–NIGMS (R01GM101389-01) is gratefully acknowledged. We thank Dr. Tom Emge for crystallographic analysis.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Selected reviews on amine α-functionalization: Murahashi SI. Angew Chem, Int Ed Engl. 1995;34:2443.Matyus P, Elias O, Tapolcsanyi P, Polonka-Balint A, Halasz-Dajka B. Synthesis. 2006:2625.Campos KR. Chem Soc Rev. 2007;36:1069. doi: 10.1039/b607547a.Murahashi SI, Zhang D. Chem Soc Rev. 2008;37:1490. doi: 10.1039/b706709g.Li CJ. Acc Chem Res. 2009;42:335. doi: 10.1021/ar800164n.Yoo WJ, Li CJ. Top Curr Chem. 2010;292:281. doi: 10.1007/128_2009_17.Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem Eur J. 2010;16:2654. doi: 10.1002/chem.200902374.Liu C, Zhang H, Shi W, Lei AW. Chem Rev. 2011;111:1780. doi: 10.1021/cr100379j.Yeung CS, Dong VM. Chem Rev. 2011;111:1215. doi: 10.1021/cr100280d.Sun CL, Li BJ, Shi ZJ. Chem Rev. 2011;111:1293. doi: 10.1021/cr100198w.Wendlandt AE, Suess AM, Stahl SS. Angew Chem Int Ed. 2011;50:11062. doi: 10.1002/anie.201103945.Jones KM, Klussmann M. Synlett. 2012;23:159.Zhang C, Tang CH, Jiao N. Chem Soc Rev. 2012;41:3464. doi: 10.1039/c2cs15323h.Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW. Chem Eur J. 2012;18:10092. doi: 10.1002/chem.201201539.Pan SC. Beilstein J Org Chem. 2012;8:1374. doi: 10.3762/bjoc.8.159.Platonova AY, Glukhareva TV, Zimovets OA, Morzherin YY. Chem Heterocycl Compd. 2013;49:357.Qin Y, Lv J, Luo S. Tetrahedron Lett. 2014;55:551.
- 2.Reviews on redox-economy and redox-neutral amine α-functionalization: Burns NZ, Baran PS, Hoffmann RW. Angew Chem Int Ed. 2009;48:2854. doi: 10.1002/anie.200806086.Newhouse T, Baran PS, Hoffmann RW. Chem Soc Rev. 2009;38:3010. doi: 10.1039/b821200g.Peng B, Maulide N. Chem Eur J. 2013;19:13274. doi: 10.1002/chem.201301522.
- 3.Selected recent examples of amine α-functionalization via H-shifts: Barluenga J, Fananas-Mastral M, Aznar F, Valdes C. Angew Chem Int Ed. 2008;47:6594. doi: 10.1002/anie.200802268.Polonka-Balint A, Saraceno C, Ludányi K, Bényei A, Matyus P. Synlett. 2008:2846.Zhang C, Murarka S, Seidel D. J Org Chem. 2009;74:419. doi: 10.1021/jo802325x.Murarka S, Zhang C, Konieczynska MD, Seidel D. Org Lett. 2009;11:129. doi: 10.1021/ol802519r.Mori K, Ohshima Y, Ehara K, Akiyama T. Chem Lett. 2009;38:524.Ruble JC, Hurd AR, Johnson TA, Sherry DA, Barbachyn MR, Toogood PL, Bundy GL, Graber DR, Kamilar GM. J Am Chem Soc. 2009;131:3991. doi: 10.1021/ja808014h.Cui L, Peng Y, Zhang L. J Am Chem Soc. 2009;131:8394. doi: 10.1021/ja903531g.Murarka S, Deb I, Zhang C, Seidel D. J Am Chem Soc. 2009;131:13226. doi: 10.1021/ja905213f.Dunkel P, Turos G, Benyei A, Ludanyi K, Matyus P. Tetrahedron. 2010;66:2331.Zhou G, Zhang J. Chem Commun. 2010;46:6593. doi: 10.1039/c0cc01946a.Kang YK, Kim SM, Kim DY. J Am Chem Soc. 2010;132:11847. doi: 10.1021/ja103786c.Cao WD, Liu XH, Wang WT, Lin LL, Feng XM. Org Lett. 2011;13:600. doi: 10.1021/ol1028282.Haibach MC, Deb I, De CK, Seidel D. J Am Chem Soc. 2011;133:2100. doi: 10.1021/ja110713k.Mori K, Ehara K, Kurihara K, Akiyama T. J Am Chem Soc. 2011;133:6166. doi: 10.1021/ja2014955.Barluenga J, Fananas-Mastral M, Fernandez A, Aznar F. Eur J Org Chem. 2011:1961.Zhou GH, Liu F, Zhang JL. Chem Eur J. 2011;17:3101. doi: 10.1002/chem.201100019.He YP, Du YL, Luo SW, Gong LZ. Tetrahedron Lett. 2011;52:7064.Jurberg ID, Peng B, Woestefeld E, Wasserloos M, Maulide N. Angew Chem, Int Ed. 2012;51:1950. doi: 10.1002/anie.201108639.Sugiishi T, Nakamura H. J Am Chem Soc. 2012;134:2504. doi: 10.1021/ja211092q.Wang Y, Chi Y, Zhang WX, Xi ZF. J Am Chem Soc. 2012;134:2926. doi: 10.1021/ja211486f.Han YY, Han WY, Hou X, Zhang XM, Yuan WC. Org Lett. 2012;14:4054. doi: 10.1021/ol301559k.Chen LJ, Zhang L, Lv J, Cheng JP, Luo SZ. Chem Eur J. 2012;18:8891. doi: 10.1002/chem.201201532.He YP, Wu H, Chen DF, Yu J, Gong LZ. Chem Eur J. 2013;19:5232. doi: 10.1002/chem.201300052.
- 4.a) Zhang C, De CK, Mal R, Seidel D. J Am Chem Soc. 2008;130:416. doi: 10.1021/ja077473r. [DOI] [PubMed] [Google Scholar]; b) Deb I, Seidel D. Tetrahedron Lett. 2010;51:2945. [Google Scholar]; c) Zhang C, Das D, Seidel D. Chem Sci. 2011;2:233. [Google Scholar]; d) Deb I, Das D, Seidel D. Org Lett. 2011;13:812. doi: 10.1021/ol1031359. [DOI] [PubMed] [Google Scholar]; e) Zhang C, De CK, Seidel D. Org Synth. 2012;89:274. [Google Scholar]; f) Ma L, Chen W, Seidel D. J Am Chem Soc. 2012;134:15305. doi: 10.1021/ja308009g. [DOI] [PubMed] [Google Scholar]; g) Das D, Sun AX, Seidel D. Angew Chem Int Ed. 2013;52:3765. doi: 10.1002/anie.201300021. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Dieckmann A, Richers MT, Platonova AY, Zhang C, Seidel D, Houk KN. J Org Chem. 2013;78:4132. doi: 10.1021/jo400483h. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Richers MT, Deb I, Platonova AY, Zhang C, Seidel D. Synthesis. 2013;45:1730. [PMC free article] [PubMed] [Google Scholar]; j) Das D, Seidel D. Org Lett. 2013;15:4358. doi: 10.1021/ol401858k. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Chen W, Wilde RG, Seidel D. Org Lett. 2014;16:730. doi: 10.1021/ol403431u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Examples of related amine redox transformations: Oda M, Fukuchi Y, Ito S, Thanh NC, Kuroda S. Tetrahedron Lett. 2007;48:9159.Zheng L, Yang F, Dang Q, Bai X. Org Lett. 2008;10:889. doi: 10.1021/ol703049j.Pahadi NK, Paley M, Jana R, Waetzig SR, Tunge JA. J Am Chem Soc. 2009;131:16626. doi: 10.1021/ja907357g.Mao H, Xu R, Wan J, Jiang Z, Sun C, Pan Y. Chem Eur J. 2010;16:13352. doi: 10.1002/chem.201001896.Ghavtadze N, Narayan R, Wibbeling B, Wuerthwein EU. J Org Chem. 2011;76:5185. doi: 10.1021/jo200896y.Xue XS, Yu A, Cai Y, Cheng JP. Org Lett. 2011;13:6054. doi: 10.1021/ol2025247.Zheng QH, Meng W, Jiang GJ, Yu ZX. Org Lett. 2013;15:5928. doi: 10.1021/ol402517e.Lin W, Cao T, Fan W, Han Y, Kuang J, Luo H, Miao B, Tang X, Yu Q, Yuan W, Zhang J, Zhu C, Ma S. Angew Chem Int Ed. 2014;53:277. doi: 10.1002/anie.201308699.Haldar S, Mahato S, Jana CK. Asian J Org Chem. 2014;3:44.
- 6.Selected reviews on azomethine ylides: Padwa A, Pearson WH. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. Vol. 59. Wiley; Chichester, U. K: 2002. Najera C, Sansano JM. Curr Org Chem. 2003;7:1105.Coldham I, Hufton R. Chem Rev. 2005;105:2765. doi: 10.1021/cr040004c.Pandey G, Banerjee P, Gadre SR. Chem Rev. 2006;106:4484. doi: 10.1021/cr050011g.Pinho e Melo TMVD. Eur J Org Chem. 2006:2873.Najera C, Sansano JM. Top Heterocycl Chem. 2008;12:117.Nyerges M, Toth J, Groundwater PW. Synlett. 2008:1269.Adrio J, Carretero JC. Chem Commun. 2011;47:6784. doi: 10.1039/c1cc10779h.
- 7.For Ru/Ir catalyzed β-functionalization of amines with alcohols or aldehydes, see: Sundararaju B, Tang Z, Achard M, Sharma GVM, Toupet L, Bruneau C. Adv Synth Catal. 2010;352:3141.Sundararaju B, Achard M, Sharma GVM, Bruneau C. J Am Chem Soc. 2011;133:10340. doi: 10.1021/ja203875d.Boudiar T, Sahli Z, Sundararaju B, Achard M, Kabouche Z, Doucet H, Bruneau C. J Org Chem. 2012;77:3674. doi: 10.1021/jo300237s.Yuan KD, Jiang F, Sahli Z, Achard M, Roisnel T, Bruneau C. Angew Chem Int Ed. 2012;51:8876. doi: 10.1002/anie.201204582.Sahli Z, Sundararaju B, Achard M, Bruneau C. Green Chem. 2013;15:775.
- 8.Examples of oxidative amine β-functionalization and α,β-difunctionalization: Xia XF, Shu XZ, Ji KG, Yang YF, Shaukat A, Liu XY, Liang YM. J Org Chem. 2010;75:2893. doi: 10.1021/jo100133z.Xia XF, Shu XZ, Ji KG, Shaukat A, Liu XY, Liang YM. J Org Chem. 2011;76:342. doi: 10.1021/jo102219z.Takasu N, Oisaki K, Kanai M. Org Lett. 2013;15:1918. doi: 10.1021/ol400568u.
- 9.For a Pd-catalyzed β-functionalization of amines via organozinc intermediates, see: Millet A, Larini P, Clot E, Baudoin O. Chem Sci. 2013;4:2241.
- 10.Examples of intramolecular β-arylation of protected amines via a Pd-catalyzed metalation–deprotonation pathway: Watanabe T, Oishi S, Fujii N, Ohno H. Org Lett. 2008;10:1759. doi: 10.1021/ol800425z.Saget T, Lemouzy SJ, Cramer N. Angew Chem Int Ed. 2012;51:2238. doi: 10.1002/anie.201108511.Larionov E, Nakanishi M, Katayev D, Besnard C, Kundig EP. Chem Sci. 2013;4:1995.
- 11.The yield of 7a is based on the fact that two equivalents of starting material are needed to form one equivalent of product.
- 12.Examples of oxidative N,O-acetal formation: Kienzle F. Tetrahedron Lett. 1983;24:2213.Pandey G, Gadre SR. ARKIVOC. 2003:45.Okimoto M, Yoshida T, Hoshi M, Hattori K, Komata M, Tomozawa K. Heterocycles. 2006;68:2563.Kumaraswamy G, Murthy AN, Pitchaiah A. J Org Chem. 2010;75:3916. doi: 10.1021/jo1005813.Xuan J, Feng ZJ, Duan SW, Xiao WJ. RSC Adv. 2012;2:4065.Mathis CL, Gist BM, Frederickson CK, Midkiff KM, Marvin CC. Tetrahedron Lett. 2013;54:2101.Deb ML, Dey SS, Bento I, Barros MT, Maycock CD. Angew Chem, Int Ed. 2013;52:9791. doi: 10.1002/anie.201304654.Mahato S, Haldar S, Jana CK. Chem Commun. 2014;50:332. doi: 10.1039/c3cc46191b.
- 13.For an alternate redox-neutral approach to N,O-acetals, see: Shaaban S, Peng B, Maulide N. Synlett. 2013;24:1722.
- 14.A decarboxylative approach to N,O-acetals: Cohen N, Blount JF, Lopresti RJ, Trullinger DP. J Org Chem. 1979;44:4005.
- 15.For alternate approaches to related compounds, see: Troxler F. Helv Chim Acta. 1973;56:374.Winkler JD, Haddad N, Ogilvie RJ. Tetrahedron Lett. 1989;30:5703.Pathak TP, Sigman MS. Org Lett. 2011;13:2774. doi: 10.1021/ol200913r.
- 16.Mukhopadhyay C, Rana S, Butcher RJ. Synth Commun. 2012;42:3077. [Google Scholar]
- 17.See, for example: Haslinger E, Wolschann P. Monatsh Chem. 1980;111:563.Periasamy M, Reddy MN, Anwar S. Tetrahedron: Asymmetry. 2004;15:1809.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.