Abstract
Dermal IL-17-producing γδT cells play a critical role in skin inflammation. However, their development and peripheral regulation have not been fully elucidated. Here we demonstrate that dermal γδT cells develop from the embryonic thymus and undergo homeostatic proliferation after birth with diversified TCR repertoire. Vγ6T cells are bona fide resident but precursors of dermal Vγ4T cells may require extrathymic environment for imprinting skin homing properties. Thymic Vγ6T cells are more competitive than Vγ4 for dermal γδT cell reconstitution and TCRδ−/− mice reconstituted with Vγ6 develop psoriasis-like inflammation after IMQ-application. Although both IL-23 and IL-1β promote Vγ4 and Vγ6 proliferation, Vγ4 are the main source of IL-17 production, which requires IL-1 signaling. Mice with deficiency of IL-1RI signaling have significantly decreased skin inflammation. These studies reveal a differential developmental requirement and peripheral regulation for dermal Vγ6 and Vγ4 γδT cells, implying a new mechanism that may be involved in skin inflammation.
Introduction
The skin has a unique composition of immune cells. In addition to adaptive αβT cells, many innate immune cells including dermal dendritic cells (DDCs) and γδT cells reside in the skin to establish a skin immune network that plays a critical role in host defense and tissue repair 1. In mice, Vγ5Vδ1T cells, named dendritic epidermal T cells (DETCs), uniquely reside in the epidermis during fetal development 2. These cells have been shown to recognize a putative antigen (Ag) expressed on the keratinocytes (KC) and are involved in the skin immunosurveillance 3. Recently, a new subset of γδT cells has been identified in the skin 4, 5, 6. In comparison to DETCs, this subset of γδT cells resides mainly in the dermis under the steady condition. They bear different Vγ usage and are the major IL-17 producers in the skin upon IL-23 or toll-like receptor (TLR)-7/8 agonist imiquimod (IMQ) stimulation 4, 7, 8. However, their development, trafficking, and peripheral regulation are not fully understood.
Previous studies have shown that DETCs are derived from early fetal thymic precursor cells 9. DETCs home to the skin between embryonic day 16 and 18 before birth. In addition, IL-17- producing γδT (γδT17) cells in the periphery such as lymph nodes (LN) also develop in the thymus after birth through a TGF-β-dependent mechanism 10. It appears that different subsets of γδT17 cells migrate from the thymus into the periphery in a functional wave manner 11. At the molecular level, a thymic epithelial cell determinant, Skint-1, plays a critical role in the development of IFN-γ-producing versus IL-17-producing γδT cells 12. Transcriptional factor Sox13 is essential for all IL-17-committed Vγ4 T cell development and function including dermal Vγ4 T cells 13, 14. Previous studies also identify scavenger receptor SCART2 is uniquely expressed in IL-17-producing γδT cells homing to the peripheral LN and dermis 15. Furthermore, studies have shown that γδT cells can traffick between LN and skin 13, 16, posing the question whether dermal γδT17 cells develop similarly as other peripheral γδT cells. Through bone marrow (BM) chimeras where BM cells were transplanted into lethally irradiated host mice, it showed that 90% of dermal γδT cells were from host origin whereas ~10% of dermal γδT cells were from donor BM 6, suggesting BM cells may contain precursor cells that give rise to dermal γδT cells. Although early studies from Gray EE et al suggested that dermal γδT cells could not be reconstituted by BM cells 5, their later studies showed that IL-17-producing Vγ4 T cells could be reconstituted by BM 13. However, a recent study demonstrated that IL-17-producing γδT cells develop before birth and maintain in adult mice as self-renewing cells 11, leaving the role of BM in the generation of dermal γδT cells uncertain. Furthermore, the detailed information for mature dermal γδT cell migration into skin is lacking. Previous studies have shown embryonic trafficking of DETCs to skin requires E/P selectin ligands and CCR4 17. CCR10 also plays a critical role in the migration and location of DETCs 18, 19. When and where dermal γδT cells develop and migrate into the skin are poorly understood.
Here we demonstrate that dermal γδT cells developed from fetal thymus and undergo homeostatic proliferation after birth, with diversified TCR repertoire. IL-17-producing Vγ6 T cells are bona fide resident in dermis and are reconstituted from fetal thymus while thymic Vγ4 T cells may require extrathymic environment for imprinting of their skin homing properties. Chemokine receptor CCR6 is critical for dermal Vγ4 but not for Vγ6 T cell migration. It appears that thymic Vγ6 T cells are more competitive than Vγ4 for dermal γδT cell reconstitution. In addition, Vγ6 T cells are pathogenic and can induce skin inflammation whereas Vγ4 T cells are preferentially expanded and are the major IL-17 producers in the IMQ model of psoriasis-like skin inflammation.. Although IL-23 and IL-1β are capable of driving dermal Vγ4 and Vγ6 T cell proliferation, IL-17 is mainly produced by Vγ4, for which IL-1 signaling is essential. Deficiency of IL-1R signaling pathway significantly decreases both IL-23 and IMQ induced skin inflammation. These results demonstrate the importance of IL-1β in the regulation of the proliferation and IL-17 production by different subsets of dermal γδT cells when interplaying with IL-23, implying a new mechanism that may be involved in skin inflammation.
Results
Dermal γδT cell development in mice before and after birth
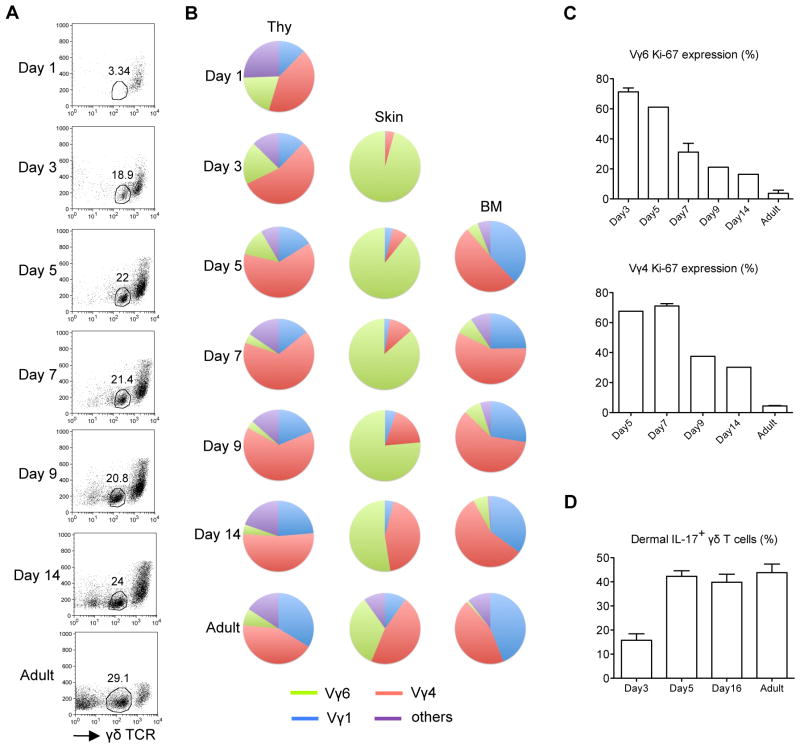
To investigate the development of dermal γδT cells, skin tissues from embryonic or neonatal pups were used to track dermal γδT cell appearance. γδTCRhi epidermal γδT cells were readily seen on E20 and day 1 (Supplementary Fig. 1a and Fig. 1a), consistent with a previous report 9. In contrast, γδTCRint dermal γδT cells were scarce on E20 or day 1. Dermal γδT cells became obvious on day 3 and had similar frequency as adult mice on day 14 (Fig. 1a). Next we examined the TCR repertoire of developing dermal γδT cells. TCR repertoire of dermal γδT cells on days 3–5 showed almost exclusive Vγ6 (Fig. 1b). Dermal Vγ4 became apparent on day 7. In adult mice, dermal γδT cells expressed TCRs mainly containing Vγ6 and Vγ4 (Fig. 1b). In contrast, TCR repertoire of thymic γδT cells on days 1–3 showed that Vγ1, Vγ4 and Vγ6 while Vγ6 was gradually decreased over the time (Fig. 1b). Side-by-side comparison studies indicated that Vγ1 and Vγ4 were the predominant γδT cells in the BM. Ki-67 staining suggested that both dermal Vγ6 and Vγ4 T cells had a significant more homeostatic proliferation at early days and then maintained lower proliferation rate in adult mice (Fig. 1c). In addition, dermal γδT cells were capable of producing IL-17 (Fig. 1d). We also examined γδT cells in the lung. Lung γδT cells were apparent on E20 and contained both Vγ6 and Vγ4. In contrast to dermal γδT cells, Vγ6 T cells were more than Vγ4 in the lung even after birth and in adult mice (Supplementary Fig. 1b). Taken together, these results suggest that dermal γδT cells migrate into the skin before birth and are further expanded after birth with more diversified TCR repertoire.
Figure 1. Development of dermal γδT cells.
(A) Whole skin cell suspensions prepared from C57Bl/6 WT mice at different days (n=3–5) after birth were stained with CD3 and γδTCR and the frequency of γδTCRint dermal γδT cells was analyzed by flow cytometry. Flow plots gated on CD3+ cells are representative of three independent experiments with similar results. (B) Thymocytes (Thy), whole skin cells and BM cells from different days after birth were stained with CD3, γδTCR and Vγ (Vγ 1, 4, 6) mAbs. Percentages of Vγ1, Vγ4, and Vγ6 γδT cells were analyzed by flow cytometry and summarized in pie chart. (C) Percentages of Ki-67 expression on dermal Vγ4 and Vγ6 T cells were analyzed by flow cytometry. Cells were gated on CD3intγδTCRint Vγ4+ or Vγ6+ cells. Data are shown as mean ± SEM. (D) Whole skin cells were stimulated with PMA plus ionomycin for 5 hours and the percentage of IL-17-producing γδT cells gated on CD3intγδTCRint cells were analyzed by flow cytometry. Data are shown as mean ± SEM.
Fetal thymus is required for dermal γδT cell development
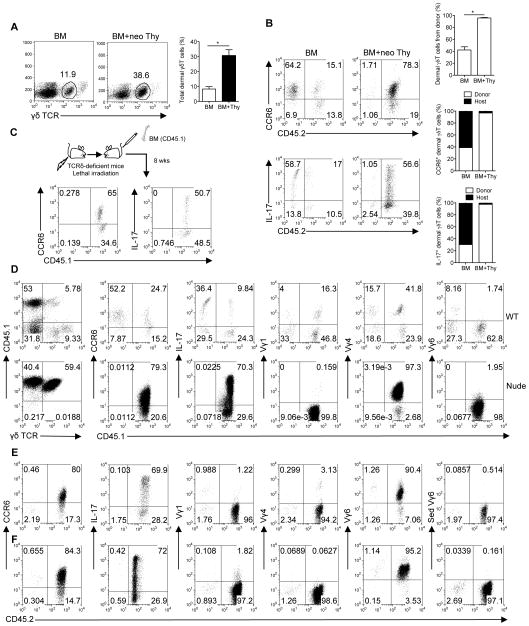
We next examined if development of dermal γδT cells was confined to the fetal thymus. To this end, we transplanted BM cells or BM cells plus neonatal thymocytes from wildtype (WT) C57Bl/6 mice (CD45.2) into CD45.1 congenic SJL mice. Mice transplanted with BM cells alone had significantly lower dermal γδT cells as compared to mice transplanted with BM plus neonatal thymocytes (Fig. 2a). In mice transplanted with BM alone, the majority of CCR6+ dermal γδT cells were host resident while approximately 15% were donor origin. Dermal γδT cells from both sources were capable of secreting IL-17 (Fig 2b). In contrast, mice transplanted with BM plus neonatal thymocytes, almost all dermal γδT cells were donor origin (Fig. 2b). To further confirm that BM cells contain precursors of dermal γδT cells, TCRδ-deficient mice were used as host to receive BM cells from SJL mice. Indeed, dermal γδT cells could be reconstituted from donor BM and were able to secrete IL-17 (Fig. 2c). To determine whether the thymus is required for dermal γδT cell development, we examined dermal γδT cells in athymic nude mice. As shown in Supplementary Fig. 2, dermal γδT cells were lacking in nude mouse skin. In addition, γδT cells in BM were also scarce. We then transplanted BM cells from CD45.1 SJL mice into CD45.2 nude or WT mice. Consistent with Fig. 2a, dermal γδT cells were reconstituted in both WT and nude mice and these cells were able to secrete IL-17 (Fig. 2d). Dermal γδT cells were significantly expanded in nude mice, compared to those in WT recipients (Fig. 2d). Further TCR usage analysis indicated that Vγ4 and Vγ1 were predominant in WT mice while nude mice reconstituted with WT BM had exclusively Vγ4. No Vγ6 was observed (Fig. 2d).
Figure 2. Fetal thymus is required for dermal γδT cell development and Vγ6 T cell reconstitution while dermal Vγ4 T cells originate mainly from BM.
BM cells or BM cells plus neonatal thymocytes (BM+neo Thy) from C57Bl/6 WT mice (CD45.2) were transplanted into lethally irradiated SJL mice (CD45.1, n=4–5). (A) Eight weeks after reconstitution, percentage of dermal γδ T cells gated on CD3+ cells from recipient mice was analyzed by flow cytometry and total frequency of dermal γδT cells was summarized. Data are shown as mean ± SEM. *p < 0.05 (unpaired Student’s t test). (B) Whole skin cells were stimulated with PMA plus ionomycin for 5 hours. CCR6+ and IL-17+CD3intγδTCRint cells were analyzed by flow cytometry. Flow plots were gated on CD3intγδTCRint cells. Percentages of dermal γδ T cells from donor are shown as mean ± SEM. *p < 0.05 (unpaired Student’s t test). Degree of chimerism of CCR6+ and IL-17-producing dermal γδ T cells are also shown as open bars (donor) and filled bars (host). Data are representative of at least three independent experiments with similar results. (C) BM cells from SJL mice (CD45.1) were transplanted into lethally irradiated TCRδ KO mice (CD45.2). After 8 weeks of reconstitution, CCR6+ and IL-17- producing dermal γδ T cells were analyzed by flow cytometry. Flow plots gated on CD3+γδTCR+ cells are representative of two independent experiments with similar results. (D) BM cells from SJL mice (CD45.1) were transplanted into lethally irradiated C57Bl/6 WT mice or Nude mice (CD45.2). After 8 weeks of reconstitution, percentage of dermal γδ T cells gated on CD3+ cells was detected by flow cytometry. Furthermore, CCR6 expression and Vγ usage (Vγ1, 4, 6) of dermal γδ T cells and intracellular IL-17 by dermal γδ T cells after PMA plus ionomycin stimulation were also analyzed by flow cytometry. Flow plots were gated on CD3intγδTCRint cells. (E, F) Thymocytes from C57Bl/6 WT neonatal (E) or E18 (F) pups plus BM cells from TCRδ KO mice were transplanted into lethally irradiated SJL mice. After 8 weeks of reconstitution, CCR6 expression and Vγ usage (Vγ1, 4, 6) of dermal γδT cells and intracellular IL-17 after PMA plus ionomycin stimulation were analyzed by flow cytometry. Flow plots were gated on CD3intγδTCRint cells.
Since dermal γδT cells consist both Vγ4 and Vγ6 TCR repertoire in adult mice, we reasoned whether dermal Vγ6 T cells were from fetal thymus. To exclude precursors of dermal γδT cells from BM, BM cells from TCRδ-deficient mice were mixed with neonatal thymocytes (Fig. 2e). Dermal γδT cells were almost all donor origin and predominately expressed Vγ6. Since previous study showed IL-17-producing γδT cells develop from embryonic thymus 11, we further used E18 embryonic thymocytes for reconstitution. Similar as neonatal thymocytes, dermal γδT cells from E18 thymocytes showed exclusively donor origin and were capable of producing IL-17 and predominantly expressed Vγ6 (Fig. 2f). These data suggest that IL-17-producing dermal γδT cells can be reconstituted from both fetal thymus and adult BM with different TCR repertoire and thymus is absolutely required for dermal γδT cell development.
Thymic Vγ4 requires extrathymic environment for skin homing
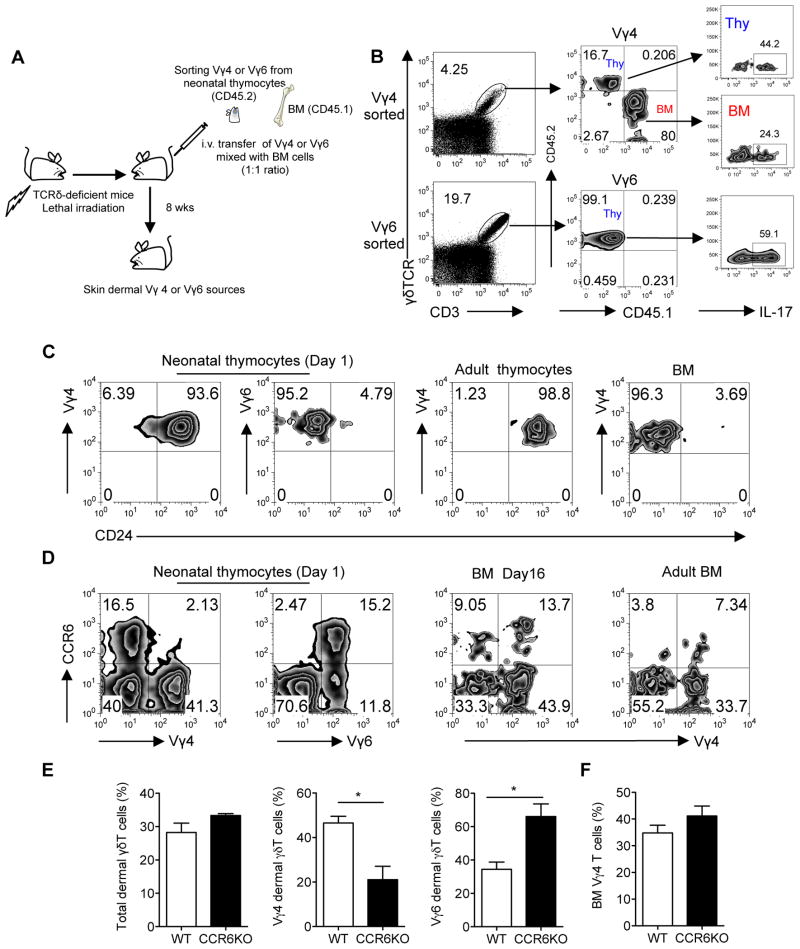
E18 and neonatal thymocytes contain both Vγ4 and Vγ6-expressing γδT cells with similar frequency (Fig. 1b). However, mice transplanted with E18 or fetal thymocytes had exclusive Vγ6 T cell reconstitution while majority of Vγ4 T cells were reconstituted from BM (Fig. 2). To further validate this, we sorted Vγ4 or Vγ6 T cells from neonatal thymocytes (CD45.2) and mixed with BM cells (CD45.1) as a source of BM Vγ4. TCRδ-deficient mice were used as recipient mice. The ratio of Vγ4 from both sources was approximately at 1:1 as well as Vγ6 to Vγ4 (Fig. 3a). As shown in Fig. 3b, mice transplanted with Vγ4 from neonatal thymus plus BM cells had dermal γδT cells expressing Vγ4, predominately from BM (>80%). In contrast, mice transplanted with neonatal Vγ6 plus BM cells had dermal γδT cells expressing Vγ6, exclusively from neonatal thymus (Fig. 3b). Both Vγ4 and Vγ6 T cells were capable of secreting IL-17 (Fig. 3b). These data suggest that Vγ4 and Vγ6 dermal γδT cells may have a differential developmental requirement.
Figure 3. Thymic Vγ4 T cells require extrathymic environment for imprinting of skin homing properties.
(A, B) Sorted Vγ4 or Vγ6 neonatal thymocytes from C57Bl/6 WT mice (CD45.2) mixed with BM cells from SJL mice (CD45.1) were transferred into lethally irradiated TCRδ KO mice (n=3–5). The ratio of Vγ4 from both sources was around 1:1 as well as Vγ6 to Vγ4 (based on Vγ4 frequency in BM). After 8 weeks of reconstitution, total dermal γδT cells (A) and the sources of Vγ4 or Vγ6 T cells as well as intracellular IL-17 stimulated with PMA plus ionomycin (B) were determined by flow cytometry. Flow plots were gated on CD3+ γδTCR+ cells. (C, D) CD24 (C) and CCR6 (D) expression by Vγ4 or Vγ6 cells on neonatal thymocytes, adult thymocytes, BM cells (Day16) and adult BM cells were determined by flow cytometry. Flow plot gated on CD3+ γδTCR+ cells are representative of three independent experiments with similar results. (E, F) Whole skin cells (E) or BM cells (F) from C57Bl/6 WT mice or CCR6KO mice (n=3) were stained with CD3, γδTCR, Vγ4 and Vγ6. Percentages of total dermal γδT cells as well as dermal Vγ4 and Vγ6 cells or BM Vγ4 cells were analyzed by flow cytometry. Data are shown as mean ± SEM and are representative of three independent experiments with similar results. *p < 0.05 (unpaired Student’s t test).
To gain insight into this difference, we examined maturation status of Vγ4 and Vγ6 T cells in thymus and BM. As shown in Fig. 3c, almost all Vγ6 T cells in neonatal thymus had mature phenotype (CD24lo). In contrast, most Vγ4 T cells were immature (CD24hi) in neonatal or adult thymus. This was consistent with CD44 CD62L staining patterns where the majority of Vγ6 T cells in E20 or neonatal stage were CD44hiCD62Llo while most Vγ4 T cells were CD44lo (Supplementary Fig. 3a). Strikingly, Vγ4 T cells from BM were almost all mature (CD24lo) (Fig. 3c), suggesting that Vγ4 T cells may require extrathymic environment for maturation. To rule out the possibility that Vγ4 T cells may have delayed maturation in the thymus as previously reported 13, we used thymocytes from different ages of mice ranging from day 2 to day 17 for dermal γδT reconstitution studies. Consistent with previous results (Fig. 1b), Vγ6 gradually decreased in the thymus after birth and were scarce on day 17 (Supplementary Fig. 4a). Mature Vγ4 T cells were indeed increased over time and peaked at day 5 and then decreased, which is consistent with a previous study 13. The ratios of matured Vγ4 versus Vγ6 were 1:5 at day 2, 1:1 at day 4, 4:1 at day 5. On day 17, almost no Vγ6 was found in the thymus (Supplementary Fig. 4a). After 8 weeks reconstitution, we examined γδT cell composition. Dermal γδT cells were fully reconstituted with thymocytes of days 2–5 mice (Supplementary Fig. 4b). Vγ6 T cells were predominant γδT cells in the skin although dermal Vγ4 T cells did increase using thymocytes of day 5 mice. However, this was not comparable with the ratio of matured Vγ4 versus Vγ6 in the thymus of day 5 mice (4:1). Both Vγ4 and Vγ6 were capable of producing IL-17 (Supplementary Fig. 4c). The unbalanced ratios of Vγ6 over Vγ4 were also shown in the lung but less striking in the peripheral blood, LN and spleen. Vγ4 and Vγ6 were almost at 1:1 ratio in the peripheral blood and LN using thymocytes from day 5 mice (Supplementary Fig. 4D). Dermal γδT cells were poorly reconstituted using thymocytes of day 17 mice, which predominantly constituted Vγ4. Taken together, these data suggest that thymic Vγ4 T cells may require an extrathymic environment to imprint their skin homing properties. It also suggests that thymic Vγ6 T cells may be more competitive than Vγ4 for dermal γδT cell reconstitution.
We also examined CCR6 expression on Vγ4 T cells since dermal γδT cells constitutively express CCR6 4. Vγ4 T cells from neonatal thymus had almost no CCR6 expression while Vγ6 T cells expressed an appreciable level of CCR6 (Fig. 3d and Supplementary Fig. 3b). In addition, 15% of Vγ4 T cells from BM expressed CCR6 (Fig. 3d), suggesting BM Vγ4 T cells may have gained skin homing receptor expression. Along this line, dermal Vγ4 T cells were significantly lower in CCR6-deficient mice but not in CCR10-deficient mice compared to those from WT mice (Fig. 3e and Supplementary Fig. 3c) although total dermal γδT cell frequency was not altered. In addition, the percentage of Vγ4 T cells in BM, LN or spleen was not significantly changed between WT and CCR6KO mice (Fig. 3f and supplementary Fig. 3d), suggesting that CCR6 is not essential for Vγ4 T cell trafficking from fetal thymus to BM, LN, or spleen but is critical for their homing to skin.
Mice with predominate Vγ6 T cells develop skin inflammation
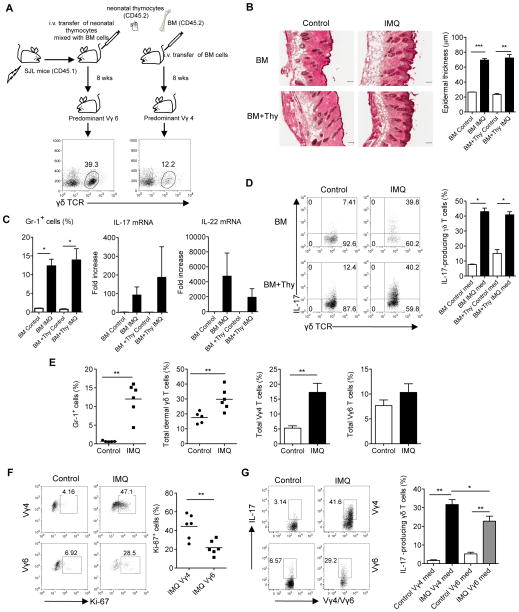
We have previously shown that dermal γδT cells are critical in skin inflammation such as psoriasis 4. Mice lacking Vγ4 IL-17T cells had significantly less epidermal thickening and neutrophil infiltration 13. To determine whether Vγ6 T cells also play a role in skin inflammation, we used mice reconstituted with neonatal thymocytes plus BM cells, which predominantly express Vγ6 T cells, for IMQ topical treatment. Mice reconstituted with BM alone containing mainly Vγ4 were used as control. Consistent with the previous experiment (Fig. 2a), mice reconstituted with neonatal thymocytes plus BM cells had 3-fold more dermal γδT cells than mice reconstituted with BM alone (Fig. 4a). Despite lower frequency of dermal γδT cells in BM alone reconstituted mice, histological analysis showed similar levels of epidermal thickening (acanthosis) in both groups (Fig. 4b). Daily application of IMQ to the back skin significantly increased neutrophil infiltration and mRNA levels of IL-17 and IL-22 in both groups (Fig. 4c). In addition, the frequencies of IL-17-producing dermal γδT cells were also similar (Fig. 4d). To specifically demonstrate whether Vγ6 T cells are able to induce skin inflammation, we sorted Vγ6 from neonatal thymocytes and co-transferred with BM cells from TCRδ−/− mice into TCRδ−/− mice to eliminate any resident dermal γδT cells or γδT cells from BM cells (Supplementary Fig. 5a). The reconstituted mice expressed exclusive Vγ6 and developed severe skin inflammation upon IMQ topical treatment. The epidermal thickness and neutrophil infiltration were significantly increased as compared to TCR δ−/− mice (Supplementary Fig. 5b, c). In addition, mRNA levels of IL-17 and IL-22 were also significantly increased (Supplementary Fig. 5d). Taken together, these findings suggest that dermal Vγ6 T cells are pathogenic and able to induce psoriasis-like dermatitis. However, Vγ4 T cells may be more prone to induce skin inflammation because reconstituted mice expressing Vγ4 develop similar magnitude of disease with significant fewer total dermal γδT cells.
Figure 4. Dermal Vγ6 T cells induce skin inflammation but dermal Vγ4 are preferentially expanded and are the major IL-17 producers.
(A) BM cells or BM cells plus neonatal thymocytes (BM+Thy) from C57Bl/6 WT mice (CD45.2) were transplanted into lethally irradiated SJL mice (CD45.1, n=5). After 8 weeks of reconstitution, frequency of dermal γδT cells was detected by flow cytometry. Mice receiving BM cells alone predominantly reconstituted Vγ4 while mice receiving BM plus neonatal thymocytes predominantly reconstituted Vγ6. (B) Reconstituted mice were treated daily for 5 days with IMQ or control cream (Control). Representative H&E-stained sections are shown and epidermal thickness were measured at day 5. Scale bar, 100 μm. Data are shown as mean ± SEM. **p < 0.01, ***p < 0.001 (unpaired Student’s t test). (C) Percentage of CD45+Gr-1+ cells after IMQ treatment was analyzed by flow cytometry. IL-17 and IL-22 mRNA levels were measured by qPCR. Data are shown as mean ± SEM. *p < 0.05 (unpaired Student’s t test). (D) Intracellular IL-17 production by skin dermal γδT cells from IMQ-treated or control mice was determined by flow cytometry (without stimulation). Flow plots were gated on CD3+ cells. Data are shown as mean ± SEM. *p < 0.05 (unpaired Student’s t test). (E) C57Bl/6 WT mice (n=5–6) were treated daily for 5 days with IMQ or control cream (Control). Percentages of CD45+Gr-1+ cells, total dermal γδT cells, dermal Vγ4 and Vγ6 cells were analyzed by flow cytometry. Data are shown as mean ± SEM and are representative of two independent experiments with similar results. **p < 0.01 (unpaired Student’s t test). (F, G) Ki-67 expression (F) and intracellular IL-17 production (G) by dermal Vγ4 and Vγ6 cells were determined by flow cytometry (without stimulation). Flow plots were gated on CD3intγδTCRint cells. Data are shown as mean ± SEM and are representative of two independent experiments with similar results. *p < 0.05, **p < 0.01 (unpaired Student’s t test).
Normal naïve mice have similar frequencies of dermal Vγ6 and Vγ4 T cells. We sought to determine whether dermal Vγ4 and Vγ6 T cells respond to IMQ differently in naïve mice. As expected, mice applied with IMQ cream developed massive neutrophil infiltration (Fig. 4e). Total dermal γδT cells were also increased. Among them, dermal Vγ4 T cells were preferentially expanded as compared to Vγ6 (Fig. 4e). Ki-67 staining indicated that Vγ4 had significantly more proliferation than Vγ6 (Fig. 4f). To assess IL-17 production by dermal γδ T cells ex vivo, skin tissues were digested and stained intracellularly for IL-17. Both Vγ4 and Vγ6 dermal T cells produced IL-17. However, Vγ4 T cells produced significantly more IL-17 than Vγ6 T cells (Fig. 4g). Thus, dermal Vγ4 T cells appear to play a more critical role compared to Vγ6 T cells in IMQ-induced psoriasis-like dermatitis.
IL-23 and IL-1β differently regulate dermal Vγ4 and Vγ6
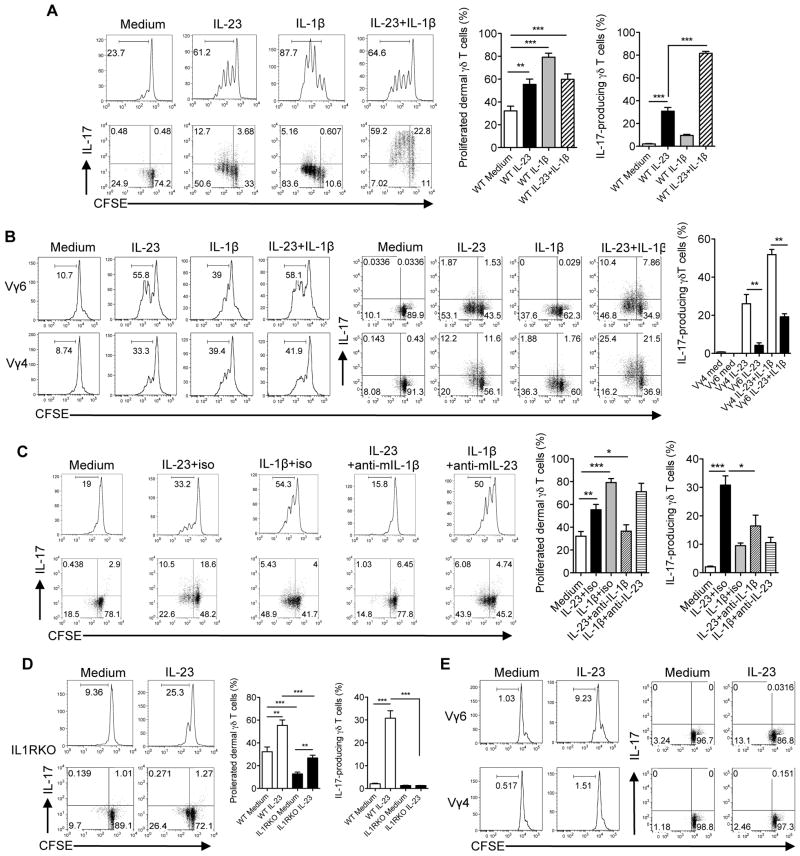
We reason that the differential responses to IMQ by dermal Vγ4 and Vγ6 T cells could be due to cytokine regulations since the IMQ-induced psoriasis-like dermatitis model is dependent of the IL-23/IL-17 pathway 20. To determine the role of IL-23 and IL-1β in the regulation of dermal γδ T cells, whole skin cells were labeled with CFSE and stimulated with IL-23, IL-1β, or IL-23 plus IL-1β. IL-23 alone or IL-1β alone was capable of driving dermal γδ T cell proliferation (Fig. 5a). Combination of IL-23 and IL-1β did not significantly increase dermal γδ T cell proliferation. In contrast, IL-17 production was largely induced upon IL-23 stimulation by both proliferated and un-proliferated dermal γδ T cells. IL-1β alone induced minimal IL-17 production. Combined IL-1β with IL-23 significantly enhanced IL-17 production. In addition, we found that either IL-23 or IL-1β was able to stimulate Vγ4 and Vγ6 proliferation. Strikingly, dermal Vγ4 T cells produced significantly more IL-17 than Vγ6 T cells upon IL-23 or IL-23 plus IL-1β stimulation (Fig. 5b).
Figure 5. IL-23 and IL-1β regulate dermal Vγ4 and Vγ6 γδ T cell proliferation and IL-17 production.
(A, B) Whole skin cell suspensions were labeled with CFSE and then stimulated with IL-23, IL- 1β or IL-23 plus IL-1β for 3 days. CFSE dilution and intracellular IL-17 production by dermal γδT cells (A) or dermal Vγ4 and Vγ6 γδ T cells (B) were determined by flow cytometry. Flow plots gated on CD3int γδTCRint cells are representative of at least three independent experiments with similar results. Data are shown as mean ± SEM (n=10). **p < 0.01, ***p < 0.001 (unpaired Student’s t test). (C) Whole skin cells suspensions were labeled with CFSE and then stimulated with IL-23, IL-1β, IL-23 plus anti-mouse IL-1β, IL-1β plus anti-mouse IL-23 or isotype control mAb in WT mice for 3 days. CFSE dilution and intracellular IL-17 production by dermal γδT cells were determined by flow cytometry. Flow plots gated on CD3int γδTCRint cells are representative of at least three independent experiments with similar results. Summarized data (n=8–9) are shown as mean± SEM. *p<0.05, **p < 0.01, ***p < 0.001 (unpaired Student’s t test). (D, E) Whole skin cells suspensions from IL-1RI KO mice labeled with CFSE were stimulated with IL- 23 for 3 days. CFSE dilution and intracellular IL-17 production by dermal γδ T cells (D) or dermal Vγ4 and Vγ6 γδ T cells (E) were determined by flow cytometry. Flow plots gated on CD3int γδTCRint cells are representative of at least three independent experiments with similar results. Data are shown as mean ± SEM (n=8–9). **p < 0.01, ***p < 0.001 (unpaired Student’s t test).
Whole skin cells contain endogenous IL-1β presumably produced by skin resident cells 21, 22. We next determined whether the proliferation of dermal γδT cells induced by IL-23 is dependent on IL-1β and conversely whether IL-1β-induced γδ T cell proliferation is dependent on IL-23. Using neutralizing mAbs against IL-23 and IL-1β and corresponding isotype control mAbs, we found that the proliferation and IL-17 production by dermal γδT cells in response to IL-23 stimulation were significantly decreased when blocking endogenous IL-1β (Fig. 5c). In contrast, IL-1β-induced dermal γδT cell proliferation and IL-17 production were not significantly influenced by IL-23 (Fig. 5c). Thus, IL-1β appears to be critical in both dermal γδT cell proliferation and IL-17 production induced by IL-23.
We next used IL-1RI KO mice to examine the regulatory role of IL-1β in dermal γδT cells. There was a significant lower basal level of proliferated dermal γδT cells in IL-1RI KO mice compared to WT mice (Fig. 5d). Dermal γδT cell proliferation was still observed upon IL-23 stimulation in IL-1RI KO mice although the overall level was significantly lower than WT mice (Fig. 5d). IL-17 production was completely abrogated in IL-1RI KO mice. Further dermal γδT cell subset studies indicated that dermal Vγ6 but not Vγ4 T cells had IL-23-induced proliferation independent of IL-1β signaling (Fig. 5e). However, IL-17 production by dermal Vγ4 and Vγ6 both required IL-1R signaling. Thus, IL-23-induced dermal Vγ6 T cell proliferation has both IL-1β-dependent and independent pathways. However, IL-1β signaling is essential for IL-23-induced dermal γδT cell IL-17 production.
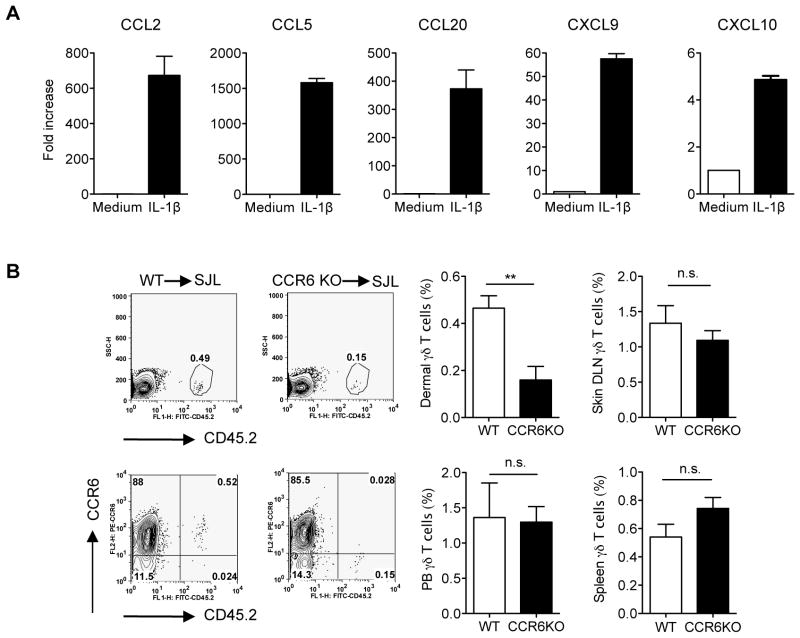
IL-1β stimulates keratinocytes for chemokine production
IL-1β has been identified as a key inflammatory cytokine in the pathogenesis of cutaneous inflammation including psoriasis 23. Elevated IL-1β levels were reported in psoriatic skin lesions 24. To further delineate the role of IL-1β on keratinocyte (KC) activation, we treated primary murine KC cells with IL-1β and found that mRNA levels of chemokines including CCL2, CCL5, CCL20, CXCL9, and CXCL10 were increased (Fig. 6a), suggesting that IL-1β may modulate dermal γδ T cell trafficking. CCL20-CCR6 axis has been implicated in dermal γδT cell trafficking and psoriasis pathogenesis 25, 26. To investigate whether γδT cells could migrate from peripheral lymphoid organs into dermis in addition to already established resident γδT cells, we sorted γδT cells from spleen and lymph nodes and then adoptively transferred them into SJL mice. As depicted in Fig. 6b, γδT cells migrated into dermis and expressed CCR6. γδT cells from CCR6-deficient mice had significantly lower frequency in dermis, suggesting CCR6 is critical in peripheral γδT cell trafficking into skin. In addition, no difference was observed in other anatomical sites (Fig. 6b).
Figure 6. CCR6 is essential for γδT cell trafficking from periphery to dermis.
(A) Primary murine KC were stimulated with IL-1β for 6 hours. CCL2, CCL5, CCL20, CXCL9, and CXCL10 mRNA levels were measured by qPCR. The figure shows fold changes normalized for β-MG mRNA versus medium alone. Data are shown as mean ± SEM. (B) Sorted γδT cells from spleen and lymph nodes from C57Bl/6 WT mice or CCR6KO mice were adoptively transferred into SJL mice (n=4). After 5–7 days, cells from skin, peripheral blood, lymph nodes and spleen were stained with CD3, γδ TCR and CCR6. Frequency of total γδT cells and CCR6+ γδT cells from donor (CD45.2) were determined by flow cytometry. Flow plots were gated on skin CD3intγδTCRint cells. Data are shown as mean ± SEM. **p < 0.01, n.s., not significant (unpaired Student’s t test).
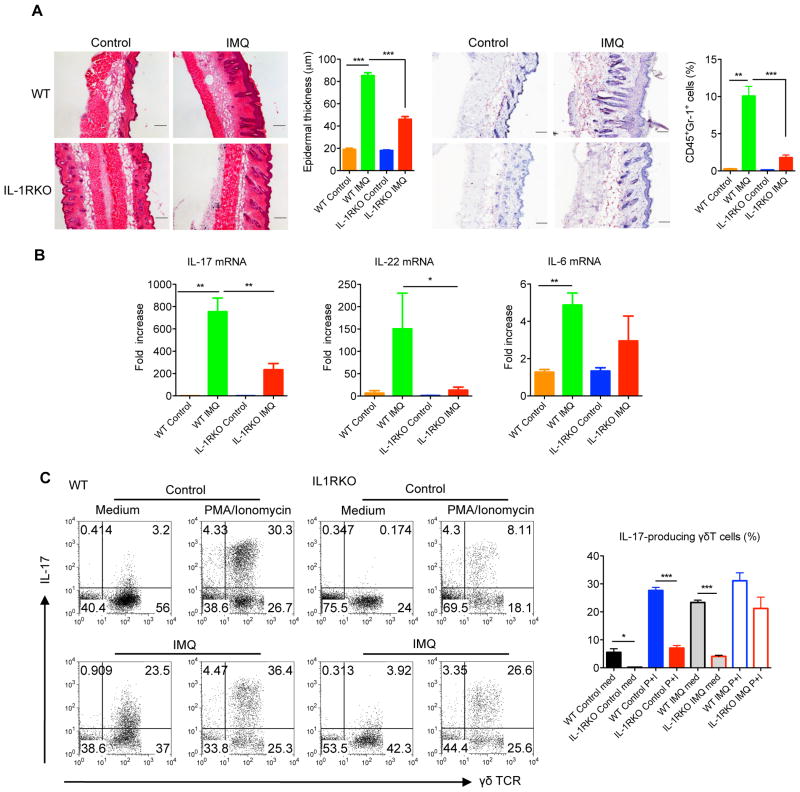
IL-1R signaling is critical in skin inflammation
We next examined whether IL-1β is involved in the skin inflammation such as psoriasis. As previously reported, both IL-23 dermal injection and IMQ induce human psoriasis-like skin inflammation and pathology 20, 27. The epidermal hyperplasia and neutrophil infiltration induced by IMQ or IL-23 were markedly decreased in IL-1RI KO mice compared to WT mice (Fig. 7a and Supplementary Fig. 6a). In addition, the mRNA levels of IL-17 and IL-22 were also significantly decreased in IL-1RI KO mice (Fig. 7b and Supplementary Fig. 6b). Further analysis of IL-17-producing dermal γδT cells from treated and untreated skin tissues indicated that the basal levels of IL-17-producing dermal γδT cells were significantly lower in IL-1R KO mice compared to those in WT mice (Fig. 7c and Supplementary Fig. 6c). Upon IL-23 or IMQ treatment, dermal γδT cells secreted large amounts of IL-17 in WT mice and IL-17 levels were significantly more in WT mice as compared to IL-1RI KO mice (Fig. 7c and Supplementary Fig. 6c). Taken together, these data suggest that IL-1RI expression is critical in both IL-23 and IMQ-induced skin inflammation and acanthosis.
Figure 7. IL-1R signaling is essential for IMQ-induced skin inflammation and acanthosis.
(A) C57Bl/6 WT and IL-1RI KO mice (n=6–8) were treated daily for 5 days with IMQ or control cream (Control). Representative H&E-stained sections and frozen sections stained with Gr-1 are shown. Gr-1 positive cells are brown. Skin tissues were also stained with CD45 and Gr-1 assessed by flow cytometry. Epidermal thickness and percentage of CD45+Gr-1+ cells were measured at day 5. Scale bar, 100 μm. Data are shown as mean ± SEM. **p < 0.01, ***P<0.001 (unpaired Student’s t test). (B) IL-17, IL-22 and IL-6 mRNA levels were measured by qPCR. The figure shows fold changes normalized for β-MG mRNA versus control skin from WT mice. Data are shown as mean ± SEM. *p < 0.05, **p< 0.01 (unpaired Student’s t test). (C) Intracellular IL- 17 production by dermal γδT cells with or without PMA plus ionomycin stimulation was determined by flow cytometry. Flow plots were gated on CD3+ cells. Data are shown as mean ± SEM. *p < 0.05, ***p<0.001 (unpaired Student’s t test).
Discussion
In this study, we examine dermal γδT cell development and find that these cells are scarce on E20 and neonatal pups but become obvious at day 3. They predominately express Vγ6 TCR, suggesting Vγ6 T cells are bona fide resident dermal γδT cells. After birth, dermal γδT cells undergo hemostatic proliferation with diversified TCR repertoire. Vγ4 T cells gradually increase over time in the dermis. This striking ontogeny profile may imply differential functions for dermal γδT cells with a distinct TCR repertoire. This is also in sharp contrast with lung γδT cells, which appear on E20 and maintain similar ratios of Vγ4 and Vγ6 in different ages of mice. Interestingly, conventional αβT cells occur in a significantly delayed fashion, suggesting that one of the primary contributions of dermal γδT cells is neonatal skin protection.
Previous studies suggest that DETCs or innate-like CD27−IL-17-producing cells cannot be generated from the BM 28. However, it is not definitive whether dermal γδT cells can be reconstituted by BM cells 5, 6, 11, 13. Our study clearly shows that dermal γδT cells can be reconstituted from the BM although thymus is absolutely required. This suggests that the precursors of dermal γδT cells in the BM are probably re-circulated from the thymus. The precursors of dermal γδT cells in the BM contain mainly Vγ4 and Vγ1 but not Vγ6. As shown in our study, Vγ6 T cells can be directly reconstituted from fetal thymus. It appears that this extra detour for thymic Vγ4 T cells is to gain skin homing properties. It is possible that transferred neonatal thymic Vγ4 T cells may require a longer time for skin seeding as compared to adult BM Vγ4. Using thymocytes from different ages of mice, we showed that matured Vγ4 T cells are significantly increased and are more than matured Vγ6 T cells in the thymus of day 5 mice. However, Vγ6 T cells are still the predominant dermal γδT cells in the reconstituted mice. This is different from γδT cell composition in the peripheral blood and LN, suggesting that thymic Vγ4 T cells need extrathymic environment for imprinting their skin homing properties, such as gaining an activated status or CCR6 expression that enables them home to the skin or sites of inflammation. It also suggests that thymic Vγ6 T cells may be more competitive than Vγ4 for dermal γδT cell reconstitution. Although it is unclear which factor(s) stimulates thymic egressed Vγ4 T cells for imprinting of their skin homing capability, it is conceivable that antigen (Ag) stimulation may be one of driving forces for their maturation and functional competency. Indeed, a recent study showed that γδT cells recognize a microbial encoded B cell Ag for activation and IL-17 production 29. These distinct developmental requirements between dermal Vγ4 and Vγ6 T cells are also supported by two recent studies showing their differential transcription factors for programming such as SOX4 and SOX13, respectively 13, 14. It is thus proposed that dermal γδT cells contain bona fide resident Vγ6 T cells, which are non-migratory, mature γδT cells with IL-17-producing ability and are the major force in the network of neonatal skin immunosurveillance. In contrast, dermal Vγ4 T cells, which require extrathymic environment for imprinting their skin homing properties and are probably migratory and the inducible IL-17-producing cells. Vγ4 T cells can be Ag-specific and produce large amounts of IL-17 in the presence of TCR engagement thus perpetuating the IL-17 response in inflammation 30.
Dermal Vγ4 and Vγ6 T cells also use differential chemokine receptors for their skin homing. Deficiency of CCR6 or CCR10 alone does not significantly alter dermal Vγ6 T cell skin migration. However, dermal Vγ4 T cells are significantly lower in CCR6-deficient mice but not in CCR10-deficient mice. CCR6 is critical for Vγ4 T cells in the BM homing to skin but not essential for Vγ4 thymic egress as frequencies of Vγ4 T cells in the BM of WT or CCR6 KO mice are similar. Although Vγ4 and Vγ6 dermal γδT cells have differential developmental requirement, it appears that both subsets are functional competent in terms of IL-17 production and induction of psoriasis-like skin inflammation. This is different from previous studies using Sox13-deficient mice or Sox-13 spontaneous mutant mice 13, 14. The discrepancy may arise from the overall frequency of dermal γδT cells. IL-17-producing Vγ4 T cells are selectively deficient in Sox13 mutated mice 13 and T-Sox4-deficient mice 14 leading to reduced skin inflammation. In our study, mice transplanted with fetal thymus or sorted Vγ6 from neonatal thymocytes have exclusively IL-17-producing Vγ6 T cells with the overall frequency similar as WT mice and develop severe skin inflammation upon IMQ treatment. Thus it appears that the different results could be in part due to the quantity of total IL-17-producing γδT cells in dermis. However, in naïve mice where both dermal Vγ4 and Vγ6 T cells exist, Vγ4 T cells are preferentially expanded and are the major IL-17 producers upon IMQ topical treatment, suggesting that a peripheral regulation program could be different for dermal Vγ4 and Vγ6 T cells.
Although γδT cells are considered as pre-committed functional competent cells, dermal γδT cells are subject to peripheral regulation. We found that IL-1β and IL-23 are both capable of stimulating Vγ4 and Vγ6 T cell proliferation. IL-23-stimulated dermal γδ T cell proliferation is largely driven by IL-1β signaling but also has a IL-1 independent pathway, mainly with Vγ6. It is possible that endogenous levels of IL-18 could effect in conjunction with IL-23 to stimulate minimal dermal γδ T cell proliferation in the absence of IL-1β since IL-18 is critical in human γδ T cell expansion and is independent of IL-1R signaling 31. We also found that dermal Vγ4 cells are the major IL-17 producer as compared to dermal Vγ6 upon IL-23 or IL23 plus IL-1β stimulation. This in vitro study is consistent with in vivo IMQ-induced psoriasis model in naïve mice where IL-17-producing Vγ4 T cells are significantly expanded. However, the molecular mechanism accounting for these differences between Vγ4 and Vγ6 is not presently known. Both dermal Vγ4 and Vγ6 T cells are CD44hiCD62LloCD24loCD27− and constitutively express CCR6 and RORγt (Supplementary Fig. 7). They do not express NK1.1 as most NK1.1+ γδT cells produce IFN-γ 32. IL-23R expression level is also low in both subsets, which may be related to their activation status 33. Previous studies also identify scavenger receptor SCART2 is uniquely expressed in IL-17-producing γδT cells 15. Nevertheless, we found that IL-1β is essential in both Vγ4 and Vγ6 T cell IL-17 production. Thus IL-1β is not only critical in regulating dermal γδT cell expansion under inflammatory conditions but also is essential for dermal γδT cell effector function.
IL-1β also stimulates KC to secrete many chemokines including CCR6 ligand CCL20. Since CCL20 expression is higher in skin inflammation such as psoriasis 34, this raises the possibility that IL-1β signaling may be involved in dermal γδT cell trafficking via chemokine secretion. We show that peripheral γδ T cells can be trafficked into the skin in a CCR6-dependent manner. Previous studies show that only CCR6+ γδT cells produce IL-17 32. CCR6+ γδT cells are also found in the cerebrospinal fluid in multiple sclerosis patients 35 and in other inflammatory conditions 36. However, CCR6 may be specific for Vγ4 T cell peripheral trafficking into skin because Vγ6 T cells are minimal in DLNs. This corroborates with a recent study showing two ways of Vγ4 dermal γδT cell migration from skin to draining LNs and back to inflamed skin 13. This may also be related to previous studies showing CCR6 KO mice have decreased skin inflammation in a murine psoriasis model 25, 37, 38. IL-1β has also been demonstrated to play an important role in the course of cutaneous inflammation such as psoriasis 39, 40. Psoriatic lesions are found to have increased IL-1β mRNA expression and caspase-1 activation, which is responsible for cleaving the inactive precursor of IL-1β into active form 41, 42. Indeed, mice with excessive IL-1β expression or the lack of IL-1 receptor (IL-1R) antagonist (Il1rn−/−) develop a psoriasis-like skin inflammation 43, 44, 45. In contrast, neutrophil infiltration and epidermal thickness are significantly decreased in IL-1RI deficient mice. This is consistent with the overall significantly decreased IL-17-producing γδT cells in the skin. In addition, mRNA levels of IL-17 and IL-22 in IL-1RI KO mice were significantly lower than WT mice. Although there is still controversy over the treatment of psoriasis patients with IL-1 receptor (IL-1R) antagonist, a promising therapeutic efficacy of targeting IL-1 pathway has been reported from a small clinical trial, indicating the importance of IL-1 signaling in the development of human psoriasis 46, 47.
Methods
Mice
C57Bl/6 WT, B6.SJLWT, TCRd−/−, CCR6−/−, and Il1r1−/− on C57Bl/6 background (female, 6–8 weeks old) were purchased from Jackson Laboratory. Nude mice (female, 6–8 weeks old) on C57Bl/6 background were purchased from Taconic. CCR10 KO mice were described previously 19. All animals were housed and treated in accordance with institutional guidelines and approved by the IACUC at the University of Louisville.
Tissue preparation and cell stimulation
Whole skin cells were prepared from mouse back skin 4. In brief, skin tissues were digested with a buffer containing collagenase IV, hyaluronidase, and DNase I. Skin cells were labeled with CFSE and stimulated with rIL- 23 (1ng/ml, eBioscience), rIL-1β (1ng/ml, eBioscience), or rIL-23 plus rIL-1β for 3 days. Cell proliferation and intracellular IL-17 were measured by flow cytometry. For blocking experiment, neutralizing mAbs for IL-1β (2μg/ml, eBioscience), IL-23 (12.5μg/ml, eBioscience), or matched isotype control mAbs were added. In addition, primary mouse keratinocytes (Cellntec) were stimulated with IL-1β for 6 hours and RNA were extracted for analysis of chemokine expression by real-time qPCR. For γδT cell development study, skin, thymus and BM were taken from pups before (E18 or E20) or after birth.
Flow cytometry analysis and intracellular cytokine staining
Fluorochrome-labeled mAbs including mouse γδTCR (GL3, 1:100), Vγ4 (UC3-10A6, 1:500), Vγ1 (2.11, 1:500), CD24 (M1/69, 1:500), CD27 (LG.3A10, 1:500), CD62L (MEL-14, 1:500), and IL-17A (TC11-18H10.1, 1:500, Biolegend), CD44 (IM7, 1:200), RORγt (AFKJS-9, 1:200), and Ki-67 (SolA15, 1:200, eBioscience), IL-23R (753317, 1:10) and CCR6 (140706, 1:50, R&D system) were used. Anti-mouse Vγ6 (17D1, 1:500) was kindly provided by Dr. Tigelaar (Department of Dermatology, Yale University). For intracellular cytokine staining, cells were first blocked with anti-CD16/32 and then stained with different cell surface Abs. Cells were then fixed, permeabilized and stained intracellularly for IL-17, RORγt or Ki-67. The relevant isotype control mAbs were also used. Samples were harvested with BD FACS Calibur or Canto (Becton Dickinson, San Jose, CA) and analyzed with FlowJo software (TreeStar).
BM chimeras
The BM chimeras were generated as previously reported 5. Briefly, recipient mice were lethally irradiated with 950 cGy and then were intravenously transferred with 5–10×106 thymocytes. After 24 hours, the recipient mice received 5–10×106 BM cells. In some experiments, the lethally irradiated WT, nude or TCRd−/− mice were transferred with BM cells alone. In addition, sorted Vγ4 or Vγ6 γδ T cells from C57Bl/6 neonatal thymocytes mixed with BM cells from SJL mice were transferred into irradiated TCRd−/− mice. In some experiments, sorted Vγ6 cells from neonatal thymocytes plus BM cells from TCRd−/− mice were transferred into irradiated TCRd−/− mice. Neonatal thymocytes used in all experiments were taken from pups born within 48 h. All chimeric mice were allowed to reconstitute for at least 8 weeks before use in experiments.
Peripheral γδT cells trafficking
γδ T cells were sorted from lymph nodes and spleens from C57Bl/6 or CCR6−/− mice and then intravenously transferred to SJL mice. After 5–7 days, recipient mice were sacrificed. Peripheral blood, skin, lymph nodes and spleen samples were collected for further analysis of the expression of γδT cells from donor by flow cytometry.
Establishment of psoriasis-like mouse models
IL-23-induced psoriasis-like mouse model was established as previously described 27. Briefly, IL-23 (1μg) or vehicle control was daily intradermally injected on the back skin of WT or Il1r1−/− mice for 4 days. For IMQ-induced psoriasis-like model 20, WT or Il1r1−/− mice were applied daily with IMQ cream (5%) (Aldara; 3M Pharmaceuticals) for 5 consecutive days. In some experiments, BM chimera mice were also applied with IMQ. Mice were sacrificed and the skin samples were embedded and froze in OCT for H&E and immunohistochemistry (IHC) staining. Skin samples were also excised in TRIzol (Invitrogen) for RNA extraction. Skin cell suspensions were stained for Gr-1 expression and IL-17 production.
Skin histology and IHC staining
Skin sections were stained with H&E and the epidermal thickness was determined by measuring the average interfollicular distance under the microscope in a blinded manner. For IHC staining, skin cryosections were fixed, blocked, and stained with rat-anti-mouse Gr-1 mAb (1:50) followed by goat-anti-rat IgG secondary Ab (1:200, Southern Biotech) 4. Slides were developed with 3-amino-9-ethylcarbazole (AEC) substrate solution (Vector Laboratories) and counterstained with hematoxylin. Images were acquired at x200 magnification using Aperio ScanScope digital scanners.
RNA extraction and real-time quantitative PCR
RNAs were isolated using a Qiagen RNeasy kit. After reverse transcription into cDNA, qPCR was performed on Bio-Rad MyiQ single color RT-PCR detection system using SYBR Green Supermix (Bio-Rad) and gene-specific primers were listed as follows: all chemokines (Real Time Primers, LLC, Elkins Park, PA); IL-17A (Mm_Il17a_SG, Qiagen); IL-22: 5′-ATA CAT CGT CAA CCG CAC CTT T-3′ (forward), 5′-AgC CGG ACA TCT GTG TTG TTA T-3′ (reverse); IL-6: 5′-GAG AAA AGA GTT CAA TGG C-3′ (forward), 5′-CCA GTT TGG TAG CAT CCA TCA T-3′ (reverse). We normalized gene expression level to β-2 microglobulin (β-MG) housekeeping gene and represented data as fold differences by the 2−ΔΔCt method, where ΔCt=Cttarget gene−Ctβ-MG and ΔΔCt=ΔCtinduced−ΔCtreference.
Statistical analysis
All quantitative data are shown as mean± s.e.m unless otherwise indicated. All samples were compared using two-tailed, unpaired Student’s T test. A P value less than 0.05 was considered significant. Statistical analysis was performed with GraphPad Prism software.
Supplementary Material
Acknowledgments
This work was supported by the NIH and the National Psoriasis Foundation. The authors declare no competing financial interests.
Footnotes
Author contributions:
Y.H.C. and F.X. designed and performed the experiments and wrote the manuscript; C.F., J.Y., C.L.D., Y.F.M., M.L. participated experiments, H.G.Z., J.Z., N.X. participated experimental design; J.Y. designed the experiments and wrote the manuscript.
References
- 1.Di Meglio P, Perera GK, Nestle FO. The multitasking organ: recent insights into skin immune function. Immunity. 2011;35:857–869. doi: 10.1016/j.immuni.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Payer E, Elbe A, Stingl G. Epidermal T lymphocytes--ontogeny, features and function. Springer seminars in immunopathology. 1992;13:315–331. doi: 10.1007/BF00200531. [DOI] [PubMed] [Google Scholar]
- 3.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 4.Cai Y, et al. Pivotal Role of Dermal IL-17-Producing gammadelta T Cells in Skin Inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumaria N, et al. Cutaneous immunosurveillance by self-renewing dermal {gamma}{delta} T cells. J Exp Med. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantelyushin S, et al. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122:2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becher B, Pantelyushin S. Hiding under the skin: Interleukin-17-producing gammadelta T cells go under the skin? Nat Med. 2012;18:1748–1750. doi: 10.1038/nm.3016. [DOI] [PubMed] [Google Scholar]
- 9.Havran WL, Allison JP. Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature. 1990;344:68–70. doi: 10.1038/344068a0. [DOI] [PubMed] [Google Scholar]
- 10.Do JS, et al. Cutting edge: spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J Immunol. 2010;184:1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas JD, et al. Development of Interleukin-17-Producing gammadelta T Cells Is Restricted to a Functional Embryonic Wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon-gamma-secreting versus interleukin-17-secreting gammadelta T cells. Immunity. 2011;35:59–68. doi: 10.1016/j.immuni.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Gray EE, et al. Deficiency in IL-17-committed V4 gammadelta T cells in a spontaneous Sox13-mutant CD45.1 congenic mouse substrain provides protection from dermatitis. Nat Immunol. 2013 doi: 10.1038/ni.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malhotra N, et al. A network of high-mobility group box transcription factors programs innate interleukin-17 production. Immunity. 2013;38:681–693. doi: 10.1016/j.immuni.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kisielow J, Kopf M, Karjalainen K. SCART scavenger receptors identify a novel subset of adult gammadelta T cells. J Immunol. 2008;181:1710–1716. doi: 10.4049/jimmunol.181.3.1710. [DOI] [PubMed] [Google Scholar]
- 16.Gatzka M, et al. Reduction of CD18 promotes expansion of inflammatory gammadelta T cells collaborating with CD4+ T cells in chronic murine psoriasiform dermatitis. J Immunol. 2013;191:5477–5488. doi: 10.4049/jimmunol.1300976. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Campbell JJ, Kupper TS. Embryonic trafficking of gammadelta T cells to skin is dependent on E/P selectin ligands and CCR4. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7443–7448. doi: 10.1073/pnas.0912943107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin Y, et al. Cutting edge: Intrinsic programming of thymic gammadeltaT cells for specific peripheral tissue localization. J Immunol. 2010;185:7156–7160. doi: 10.4049/jimmunol.1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin Y, Xia M, Sun A, Saylor CM, Xiong N. CCR10 is important for the development of skin-specific gammadeltaT cells by regulating their migration and location. J Immunol. 2010;185:5723–5731. doi: 10.4049/jimmunol.1001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Fits L, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 21.Kupper TS, et al. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1 alpha and beta mRNA. Keratinocyte epidermal cell-derived thymocyte-activating factor is identical to interleukin 1. The Journal of experimental medicine. 1986;164:2095–2100. doi: 10.1084/jem.164.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushima H, Ogawa Y, Miyazaki T, Tanaka H, Nishibu A, Takashima A. Intravital imaging of IL-1beta production in skin. Journal of Investigative Dermatology. 2010;130:1571–1580. doi: 10.1038/jid.2010.11. [DOI] [PubMed] [Google Scholar]
- 23.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 24.Dombrowski Y, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Science translational medicine. 2011;3:82ra38. doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedrick MN, et al. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J Clin Invest. 2009;119:2317–2329. doi: 10.1172/JCI37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mabuchi T, et al. CCR6 is required for epidermal trafficking of gammadelta-T cells in an IL-23-induced model of psoriasiform dermatitis. J Invest Dermatol. 2013;133:164–171. doi: 10.1038/jid.2012.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan JR, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng X, et al. gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity. 2012;37:524–534. doi: 10.1016/j.immuni.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chien YH, Zeng X, Prinz I. The natural and the inducible: interleukin (IL)-17-producing gammadelta T cells. Trends in immunology. 2013;34:151–154. doi: 10.1016/j.it.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsuda J, et al. Involvement of CD56brightCD11c+ cells in IL-18-mediated expansion of human gammadelta T cells. J Immunol. 2011;186:2003–2012. doi: 10.4049/jimmunol.1001919. [DOI] [PubMed] [Google Scholar]
- 32.Haas JD, et al. CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur J Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 33.Liang D, et al. IL-23 receptor expression on gammadelta T cells correlates with their enhancing or suppressive effects on autoreactive T cells in experimental autoimmune uveitis. J Immunol. 2013;191:1118–1125. doi: 10.4049/jimmunol.1300626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homey B, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621–6632. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- 35.Schirmer L, Rothhammer V, Hemmer B, Korn T. Enriched CD161high CCR6+ gammadelta T cells in the cerebrospinal fluid of patients with multiple sclerosis. JAMA neurology. 2013;70:345–351. doi: 10.1001/2013.jamaneurol.409. [DOI] [PubMed] [Google Scholar]
- 36.Serre K, Silva-Santos B. Molecular Mechanisms of Differentiation of Murine Pro-Inflammatory gammadelta T Cell Subsets. Frontiers in immunology. 2013;4:431. doi: 10.3389/fimmu.2013.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mabuchi T, et al. CCR6 Is Required for Epidermal Trafficking of gammadelta-T Cells in an IL-23-Induced Model of Psoriasiform Dermatitis. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hedrick MN, Lonsdorf AS, Hwang ST, Farber JM. CCR6 as a possible therapeutic target in psoriasis. Expert opinion on therapeutic targets. 2010;14:911–922. doi: 10.1517/14728222.2010.504716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nestle FO, Kaplan DH, Barker J. Psoriasis. The New England journal of medicine. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 40.Renne J, Schäfer V, Werfel T, Wittmann M. Interleukin-1 from epithelial cells fosters T cell-dependent skin inflammation. The British journal of dermatology. 2010;162:1198–1205. doi: 10.1111/j.1365-2133.2010.09662.x. [DOI] [PubMed] [Google Scholar]
- 41.Dombrowski Y, et al. Cytosolic DNA Triggers Inflammasome Activation in Keratinocytes in Psoriatic Lesions. Science translational medicine. 2011;3:82ra38. doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johansen C, Moeller K, Kragballe K, Iversen L. The activity of caspase-1 is increased in lesional psoriatic epidermis. Journal of Investigative Dermatology. 2007;127:2857–2864. doi: 10.1038/sj.jid.5700922. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd J, Little MC, Nicklin MJH. Psoriasis-like cutaneous inflammation in mice lacking interleukin-1 receptor antagonist. The Journal of investigative dermatology. 2004;122:665–669. doi: 10.1111/j.0022-202X.2004.22305.x. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima A, et al. TNF, but not IL-6 and IL-17, is crucial for the development of T cell-independent psoriasis-like dermatitis in Il1rn−/− mice. Journal of immunology (Baltimore, Md: 1950) 2010;185:1887–1893. doi: 10.4049/jimmunol.1001227. [DOI] [PubMed] [Google Scholar]
- 45.Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viguier M, Guigue P, Pagès C, Smahi A, Bachelez H. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Annals of internal medicine. 2010;153:66–67. doi: 10.7326/0003-4819-153-1-201007060-00030. [DOI] [PubMed] [Google Scholar]
- 47.González-López MA, Martínez-Taboada VM, González-Vela MC, Fernández-Llaca H, Val-Bernal JF. New-onset psoriasis following treatment with the interleukin-1 receptor antagonist anakinra. The British journal of dermatology. 2008;158:1146–1148. doi: 10.1111/j.1365-2133.2008.08470.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.