Abstract
Patients with sickle cell disease (SCD) often require transfusions to treat and prevent worsening anemia and other SCD complications. However, transfusions can trigger alloimmunization against transfused red blood cells (RBCs) with serious clinical sequelae. Risk factors for alloimmunization in SCD remain poorly understood. We recently reported altered regulatory T cell (Treg) and T helper (Th) responses with higher circulating Th1 (IFN-γ+) cytokines in chronically transfused SCD patients with alloantibodies as compared to those without alloantibodies. Since monocytes play a critical role in polarization of T cell subsets and participate in clearance of transfused RBCs, we tested the hypothesis that in response to RBC breakdown product, hemin, monocyte control of T cell polarization will differ between alloimmunized and non-alloimmunized SCD patients. Exogenous hemin induced Treg polarization in purified T-cell-monocyte cocultures from healthy volunteers through monocyte anti-inflammatory heme degrading enzyme HO-1. Importantly, hemin primarily through its effect on CD16+ monocytes induced an anti-inflammatory (higher Treg/lower Th1) polarization state in non-alloimmunized SCD group, whereas it had little effect in the alloimmunized group. Non-alloimmunized SCD CD16+ monocytes expressed higher basal levels of HO-1. Furthermore, IL-12, which contributed to a pro-inflammatory polarization state (low Treg/high Th1) in SCD, was dampened in hemin-treated stimulated monocytes from non-alloimmunized SCD patients, but not in alloimmunized group. These data suggest that unlike alloimmunized patients, non-alloimmunized SCD CD16+ monocytes in response to transfused RBC breakdown products promote an anti-inflammatory state that is less conductive to alloimmunization.
Keywords: Sickle cell disease, T cell polarization, Treg, Th1, hemin, HO-1, CD16+ monocytes, alloimmunization
Introduction
Sickle cell disease (SCD) results from a mutation in the β-globin gene which causes hemoglobin (Hb) S to polymerize when deoxygenated to form rigid polymers within RBCs. Hb S containing RBCs are deformed in sickle-prone conditions, resulting in chronic hemolytic anemia, shortened RBC survival in circulation, increased reticulocytosis, periodic painful vaso-occlusive crises, and end organ damage due to persistent tissue hypoxia.(1) Red blood cell (RBC) transfusions remain a very important modality of treatment for patients with SCD with the majority of patients receiving RBC transfusions in their lifetime.(2) Despite its benefits, RBC transfusion results in alloimmunization in approximately 20–50% of patients with SCD.(3) Alloantibodies can cause delayed hemolytic transfusion reactions which in SCD patients can trigger hyperhemolysis, a life-threatening poorly understood phenomena in which the transfused and the patient’s own RBCs are destroyed.(3) In addition, finding compatible units for patients with alloantibodies can be difficult and identifying the antibodies can be costly, time-consuming, causing transfusion delays. Even with provision of Rh-D, -C, and -E antigen-matched donor RBCs, patients continue to develop Rh antibodies, which may in part be due to genetic diversity of the RH locus in donors of African ancestry; many of these antibodies are considered clinically significant.(4) This highlights the need for better characterization of triggers of alloimmunization and identification of risk factors for alloimmunization in patients with SCD. Genetic as well as acquired patient-related factors are likely to influence the process of alloimmunization.(3) We recently reported reduced peripheral regulatory T cell (Treg) and B cell suppressive function and altered Th responses with higher circulating IFN-γ, but lower IL-10 levels in alloimmunized as compared to non-alloimmunized SCD patients.(5,6) These data are consistent with a model in which a generalized immune dysregulation exists in SCD alloimmunized patients with an imbalance between the regulatory (Tregs) and effector (Th) cells, possibly as a result of underlying inflammatory state,(7) that can potentially drive pathogenic responses against transfused RBCs. Studies that address how Treg/Th differentiation and expansion is controlled may improve our understanding of how SCD alloimmunization is triggered.
The monocyte/macrophage system is responsible for extravascular clearance of transfused RBCs.5 Following RBC transfusion, roughly 10% or more of donor RBCs are cleared from the circulation within 24 hours in healthy individuals.(8) Levels of hemin, a breakdown product of hemoglobin, are likely to build up in monocyte/macrophages following RBC transfusions. Heme oxygenase 1 (HO-1) is normally induced in response to heme, degrading it into iron, bilirubin and carbon monoxide, thereby reducing intracellular heme availability.(9,10) Several studies from mouse models indicate that hemin, probably through the anti-inflammatory activities of HO-1,(10) has potent immunoregulatory effect on both the innate(11) and adaptive immune response,(12) regulating the secretion of inflammatory as well as regulatory cytokines by monocytes.(13,14) In turn, monocytes can trigger and polarize Th responses(15,16) as well as both stimulate and suppress T-cell responses, depending on monocyte subset and their activation state.(16,17) Indeed, we recently showed in non-SCD setting that CD16+ monocyte subset, which constitute only about 5–10% of total monocytes in healthy individuals, controls Treg/Th proliferation,(18) inhibiting specific Treg subsets(19) while promoting Th1 expansion via IL-12.(18) The role of HO-1 in polarization of T cell responses in human disease setting has not been investigated. Monocytes in SCD are in an activated state,(20) but it remains to be determined whether they participate in modulating T cell responses in SCD alloimmunization. Since heme/HO-1 in mouse monocytes possess immunomodulatory activities,(21) we hypothesized that following transfusion of RBCs, the response of human monocytes to the breakdown products of hemoglobin will play a pivotal role in polarization of T cell immune response against transfused RBCs, and ultimately alloimmunization in human SCD.
Methods and Materials
Human samples
All the studies were approved by the Institutional Review Board of the New York Blood Center. Fresh leukopaks (n=14) containing leukocyte-enriched peripheral blood from healthy volunteer donors of the New York Blood Center were obtained without any identifiers. For SCD patients, blood was obtained solely from discard waste bags from SCD patients undergoing erythrocytapheresis procedures. Patients were selected randomly from a cohort of heavily transfused, infectious-disease free 15–34 year olds who were on a chronic transfusion protocol receiving leukoreduced blood matched for C, E and K at Children’s Hospital of Philadelphia on an outpatient basis. Patients with no history of antibody production were grouped as “non-alloimmunized” (n=9) and those with a history of having produced alloantibodies as “alloimmunized” (n=11). The apheresis waste bags stripped of all identifiers except the alloantibody state (“alloimmunized” or “negative”) were then sent to NYBC and analyzed within 18 hours of blood collection. Since patients on a chronic transfusion protocol can be transfused as regularly as every three weeks, all data presented in this study were derived from analysis of samples obtained within a three week period to ensure that each data point represents a different subject and not a duplicate.
Cell isolation and purification
PBMCs were separated by Ficoll (GE Healthcare, Port Washington, NY) density gradient centrifugation and subjected to cell subset purification by magnetic bead purification (all from Miltenyi Biotec, Auburn, CA). CD4+ T cells and total monocytes were purified using CD4+ T cell isolation kit and CD14 microbeads, respectively (purity>95% for both) following manufacturer’s instructions. For purification of CD16+ and CD16− monocyte subsets, the CD16+ monocyte isolation kit (Miltenyi Biotec) was used first to purify CD16+ monocytes by positive selection (purity>95%) and the negatively selected fraction was then incubated with CD14 microbeads to obtain the CD14+CD16− cell population (purity>95%) following manufacturer’s instructions.
T cell stimulation assays
Purified CD4+ T cells were stained with Carboxyfluoresceindiacetatesuccinimidyl ester (CFSE, Invitrogen, Grand Island, NY) and mixed (1.25×105 cells/ml) with autologous purified total monocytes at a ratio of 2:1 followed by stimulation with anti-CD3 antibody (1µg/ml, clone HIT3α, BD Biosciences, San Jose, CA) for 7 days in U-bottom 96 well plate as previously described.(19) Alternatively, purified CD14+CD16− cells and autologous CFSE-labeled CD4+ T cells (2:1 ratio) in the absence or presence of CD16+ monocytes (CD14+CD16− to CD16+ monocyte ratio of 2:1) were cultured for 7 days with anti-CD3 antibody. In the T cell stimulation experiments that were performed in the absence of monocytes, purified, CFSE stained CD4+ T cells (1.25×105 cells/ml) were cultured for 7 days in plates pre-coated with anti-CD3 antibody (clone HIT3α, 1µg/ml).
For the antibody blocking studies, anti-IL-12 p40/p70 antibody (clone C8.6, BD Biosciences) at a concentration of 2µg/ml as recommended by the manufacturer(19) or isotype matched controls (2µg/ml, R&D Systems, Minneapolis, MN) were added at the start of the CD4+ T cell-monocyte cocultures on day 0. Similarly, for hemin treatment studies, different concentrations of hemin (Frontier Scientific, Logan, UT) were added to the cocultures on day 0. For HO-1 blocking experiments, several concentrations of zinc protoporphyrin IX (ZnPPIX, Frontier Scientific, Logan, UT) together without or with hemin were first tested and based on optimal inhibition pattern, (22) concentration of ZnPPIX (2.5 µM) alone or with hemin (1.25µM) was chosen and added on day 0 to the cocultures.
Intracellular and surface expression analysis
For Treg subset analysis at day 7 of CD4+ T cell-monocyte cocultures, cells were harvested and intracellular expression of Foxp3 and helios in CD4+ cells was analyzed using anti-Foxp3 (clone PCH101; eBioscience, San Jose, CA) and anti-Helios (clone 22F6, Biolegend, Inc, San Diego, CA), respectively as previously described. Cytoplasmic IL-17 or IFN-γ expression in the CD4+ cells was also analyzed using anti-IL-17A (clone eBio64DEC17, eBioscience) and anti-IFN-γ (clone 4S.B3, eBioscience), respectively as previously described.(18) Percentage of Foxp3hi, IL-17+ and IFN-γ+ cells within divided (CFSElo) CD4+ T cells was used as the measurement for frequency of expanded Tregs, Th17 and Th1 cells, respectively. Similarly, the frequency of expanded Helios+ or Helios− regs was determined by measuring the percentage of Helios+Foxp3hi or Helios−Foxp3hi within CFSEloCD4+, respectively.
For HO-1 expression analysis, freshly isolated PBMCs were stained for 20 min with anti-CD3, anti-CD4 and anti-CD25 for T cell surface analysis or with anti-CD14 and anti-CD16 for monocyte subset analysis (all BD antibodies). After several washes, the cells were fixed and permeabilized (eBioscience), and incubated with an isotype control or anti-HO-1 antibody (clone 23/Heme Oxygenase 1, BD Biosciences) pre-labeled using the Zenon labeling Kit (Life Technologies, Grand Island, NY) without or with anti-Foxp3 antibody (eBioscience) for 30 min. To measure HO-1 expression after addition of hemin, PBMCs (5×105 cells/ml) were treated with different doses of hemin for 24 hours and different T and monocyte subsets were analyzed using the same staining pattern as above.
For intracellular IL-12 measurements, PBMCs that had been frozen in liquid nitrogen, were thawed and after several washes, were divided into 200µL aliquots (2.5×105/ml) and stimulated with IFN-γ (100ng/ml, R&D) in the absence or presence of hemin (1.25 µM) for 2 hours followed by addition of LPS (0.1ng/ml, from E. Coli 0111:B4, Sigma Aldrich) for 22 hours. Brefeldin A (1µl/ml, ebioscience) was added during the last five hours. The cells were then surface stained with PerCP conjugated anti-CD14 antibody (clone MφP9, BD Biosciences) followed by treatment with BD fixation/permeabilization solution (following manufacture’s instruction) and incubation with APC-conjugated isotype control (IgG1) or anti-IL-12 p40/p70 (clone C8.6, BD Biosciences). The samples were then analyzed by flow cytometry.
Statistical analysis
Data are expressed as mean values ±SEM. Statistical significance of differences between groups was determined by Mann-Whitney test, and statistical significance of differences of paired data was determined by paired t test. Statistical analyses were performed using PASW Statistics 18 software (IBM Inc, Armonk, NY).
Results
Hemin can regulate CD4+ T cell polarization
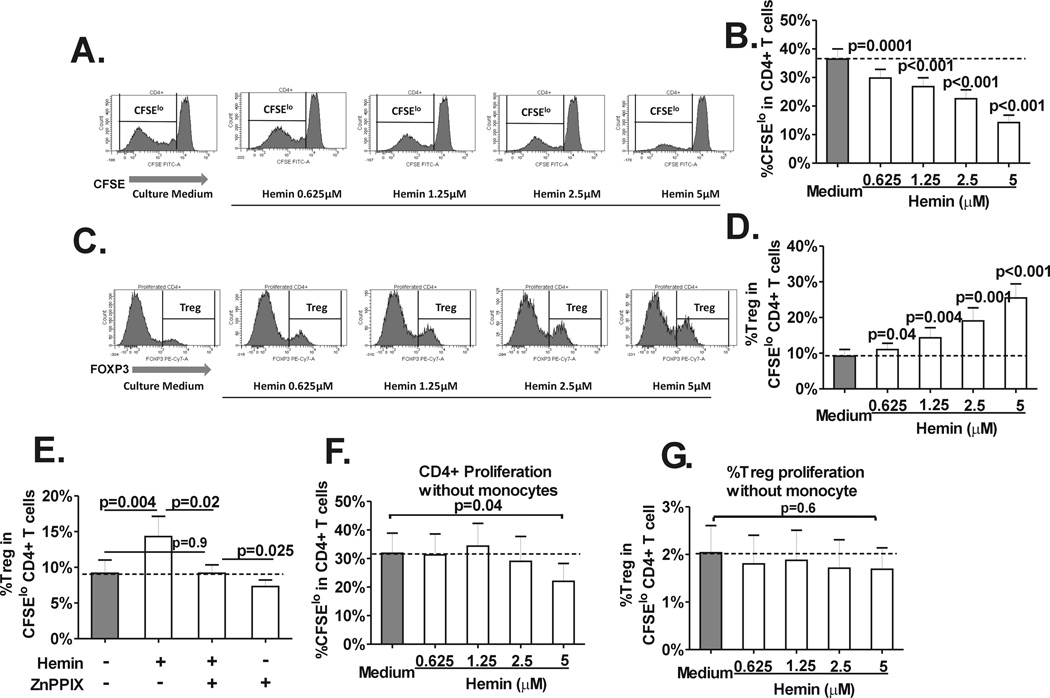
To investigate the effect of hemin on Treg polarization, purified peripheral CD4+ T cells (stained with CFSE) and autologous monocytes from healthy volunteer blood donors were cultured with various concentrations of hemin and stimulated with anti-CD3 antibody for 7 days. CD4+ proliferation (CFSElo, Fig. 1A) and frequency of Tregs (Foxp3hi) in divided (CFSElo) CD4+ T cells was measured as described previously (Fig. 1B).(18,19) Treatment with hemin resulted in a dose-dependent decrease in CD4+ T cell proliferation (Fig. 1A and B), consistent with previous reports.(23,24) Interestingly, however, within proliferating CD4+ T cells, the frequency of Tregs increased from 19% to 277% with increasing doses of hemin (Fig. 1C and D), indicating a polarizing effect of hemin on Treg cell population. Hemin is the substrate, activator and inducer of HO-1, an intracellular enzyme with complex immunoregulatory functions.(9) To test whether the Treg polarizing effect of hemin is mediated through hemin-HO-1 axis, we used the HO-1 activity blocker, ZnPPIX(9) in our CD4+T cell-monocyte assay. ZnPPIX reversed hemin-mediated Treg expansion to levels similar to untreated cultures (Fig. 1E), confirming that the polarization effect of hemin is mediated though HO-1. Monocytes were key in mediating CD4+ and Treg proliferation since in their absence, hemin inhibited CD4+ proliferation only at the highest concentration (Fig. 1F), and the baseline frequency of divided Tregs was much lower in the cultures without monocytes (1%) as compared to cultures with monocytes (Fig. 9%) and addition of hemin did not promote Treg proliferation and if anything inhibited its expansion (Fig. 1G).
Fig. 1. Hemin induces CD4+ Treg polarization.
Purified, CFSE-stained CD4+ T cells from normal healthy volunteers (n=14) were co-cultured with autologous purified total monocyte fraction in the absence (“Culture medium”) or presence of various concentrations of hemin (0 to 5µM) and stimulated with anti-CD3 antibody for 7 days. (A) Representative histograms of the staining pattern of CFSE in total CD4+ T cells after 7 days in cocultures showing the gating used to measure the extent of CD4+ T cell proliferation defined as the frequency of divided (CFSElo) population. (B) Dose-dependent inhibitory effect of hemin on the frequency of CD4+ T cell proliferation (CD4+CFSElo cells) in T cell-monocyte cocultures from healthy controls. P values were analyzed by paired t test comparing before (“medium”) and after addition of hemin. (C) Representative histograms of the staining pattern of T cell subset expressing Foxp3hi (Tregs) within the CD4+CFSElo population in cocultures untreated or treated with various concentrations of hemin (0 to 5µM) showing the gating used to analyze the frequency of Treg population that had undergone proliferation. (D) Dose dependent increase in the frequency of Tregs in divided CD4+ T cells in the same (C) cocultures. (E) Frequency of divided Tregs in cocultures without or with addition of hemin (1.25µM) and/or HO-1 inhibitor ZnPPIX (2.5 µM) at day 0. The dotted line marks the basal Treg proliferation (minus hemin or ZnPPIX). Frequency of (F) total CD4+ and (G) Treg proliferation in cocultures treated with different doses of hemin in the absence of monocytes are shown. Unlike cocultures with monocytes, Treg proliferation is not affected by hemin and total CD4+ proliferation is only inhibited at the highest hemin concentration (5 µM). All statistical analysis indicated by p values was performed by paired t test.
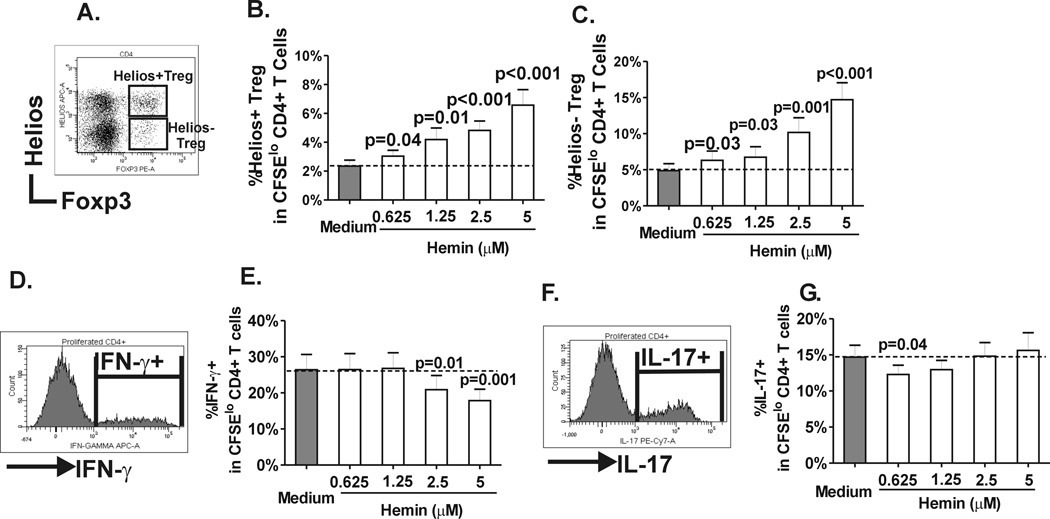
Analysis of proliferative Treg subsets based on expression of helios (Fig. 2A), which was originally described as a marker to distinguish naturally-occurring from peripherally induced Tregs,(25) indicated that expansion of helios+ and helios− Tregs at all doses of hemin tested were comparable (Fig. 2B and C), suggesting that the 2 subsets are equally responsive to hemin. In contrast, Th1 proliferation was inhibited by hemin only at higher concentrations (Fig. 2D and E) while Th17 expansion was inhibited albeit weakly at lower concentrations (Fig. 2F and G). Altogether, these data suggest that in addition to its previously described ability to inhibit CD4+ T cell proliferation, hemin can polarize CD4+ T cell subsets toward Tregs and to some extent dampen Th1 and Th17 development.
Fig. 2. Helios+/− Treg subset, Th1 and Th17 expansion in response to hemin.
(A) Representative dot plot showing helios and Foxp3 staining pattern in divided (CFSElo) CD4+ T cells in T cell-monocyte cocultures on day 7. Helios+ Tregs are defined by co-expression of helios in Foxp3hi population whereas Helios− Tregs lack helios expression. Frequency of (B) helios+ and (C) helios− Tregs that had undergone proliferation in the same cocultures from healthy control as in Fig. 1 before and after hemin treatment. (C) Representative histogram of IFN-γ expression in divided (CFSElo) CD4+ T cells on day 7 and (D) frequencies of IFN-γ + cells in divided CD4+ cells in the absence or presence of increasing concentrations of hemin in the same cocultures from healthy control as in Fig. 1. Similarly, (E) representative histogram of IL-17 expression in divided (CFSElo) CD4+ T cells on day 7 and (F) frequencies of IL-17+ cells in divided CD4+ cells without or with hemin in cocultures from Fig. 1. P values were analyzed by paired t test comparing before (“medium”) and after addition of hemin.
CD4+ T cell polarization by hemin in non-alloimmunized SCD patients
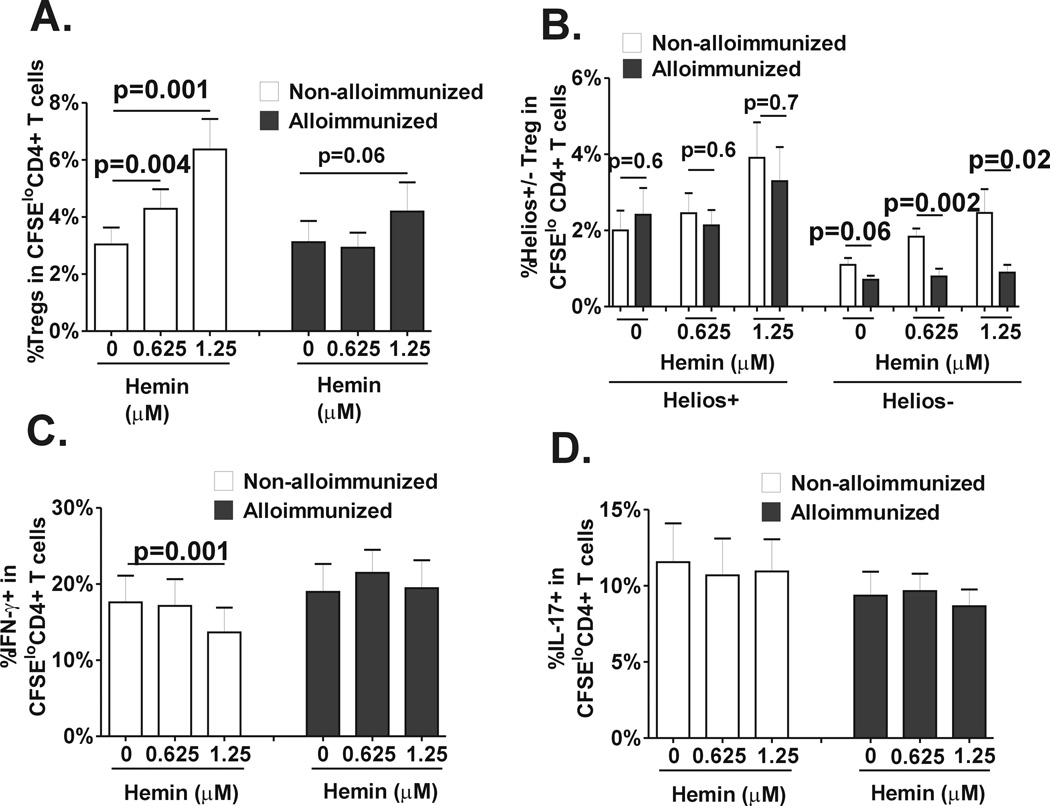
We next examined CD4+ T cell polarization in response to hemin in a cohort of regularly transfused patients with SCD comparing alloimmunized (black bars) and non-alloimmunized (white bars) proliferative responses. Basal Treg proliferative responses were comparable in alloimmunized and non-alloimmunized patients (Fig. 3A. comparing 0 hemin concentration). Interestingly, treatment with hemin increased Treg proliferation more significantly in non-alloimmunized as compared to alloimmunized SCD patients (Fig. 3A), indicating that hemin has a more potent Treg polarizing effect in non-alloimmunized as compared to alloimmunized SCD patients. Indeed, at 1.25µm hemin concentration, Treg proliferation doubled in the non-alloimmunized group (Fig. 3A, p=0.001), but the increase was less pronounced in alloimmunized patients (about 30%) (Fig. 3A, p=0.06). In the absence of hemin, helios+/− Treg subset proliferative responses was also comparable in alloimmunized and non-alloimmunized SCD groups (Fig. 3B). Hemin treatment increased helios+ Treg proliferative responses to the same extent in the 2 patient groups (Fig. 3B, p=0.7); in contrast helios− Treg expansion was increased only in the non-alloimmunized group (Fig. 3B), suggesting that hemin has a more profound effect on the expansion of Helios− Tregs in non-alloimmunized as compared to alloimmunized SCD patients. We also examined Th1 (Fig. 3C) and Th17 cell (Fig 3D) proliferative responses before and after hemin treatment. In the absence of hemin, basal Th1 proliferation was comparable in the 2 patient groups. Following hemin treatment, Th1 proliferation was inhibited only in non-alloimmunized SCD patients (p=0.001). Th17 proliferative responses were comparable in the 2 patients groups before hemin treatment and neither group was affected by hemin. Altogether, these data indicate that hemin induces a more robust proliferation of Treg, especially of helios− Treg subset, in non-alloimmunized SCD patients as compared to alloimmunized patients and that it inhibits Th1 polarization in non-alloimmunized SCD patients but not in alloimmunized group.
Fig. 3. Differences in Treg/Th polarization in response to hemin between alloimmunized and non-alloimmunized SCD patients.
Purified, CFSE-stained CD4+ T cells from regularly transfused non-alloimmunized (n=9, white bars) and alloimmunized (n=11, black bars) SCD patients were co-cultured with autologous purified total monocyte fraction in the absence or presence of 2 different concentrations of hemin (0.625µM and 1.25µM) and stimulated with anti-CD3 antibody for 7 days. Levels of (A) total Tregs, (B) helios+/− Treg subsets, (C) Th1 and (D) Th17 in CD4+ T population that had undergone proliferation were analyzed by flow cytometry. All statistical analysis comparing before and after hemin addition was performed using paired t test; comparison between alloimmunized and non-alloimmunized groups was performed using Mann-Whitney test.
Hemin regulation of Treg/Th polarization by CD16+ monocyte
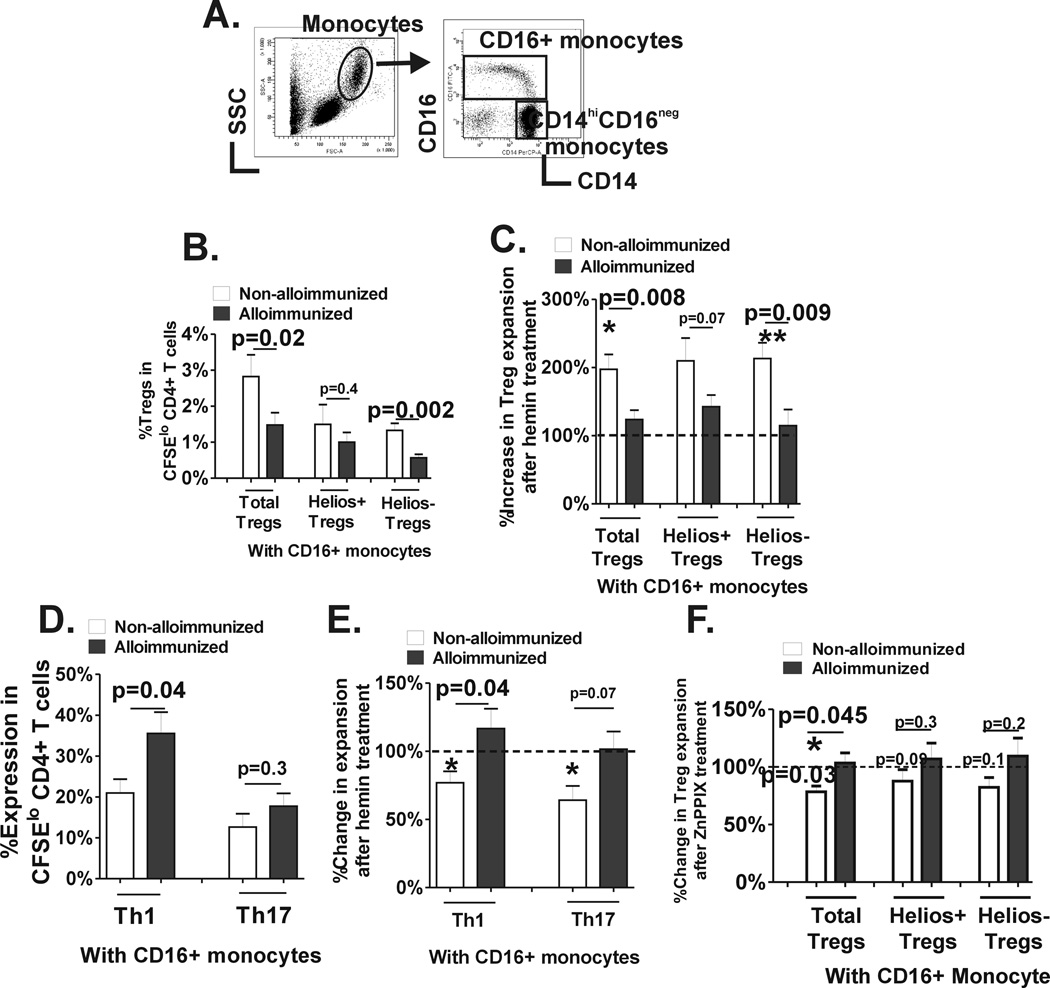
Monocytes can be divided into CD16+ and CD16− monocyte subsets based on CD16 surface expression (Fig. 4A) with disparate biologic and functional properties.(26) We have previously shown that CD16+ and CD16− monocyte subpopulations regulate polarization of distinct Treg/Th subsets in healthy controls.(18,19) To examine the role of CD16+/− monocyte subsets on T cell polarization, we performed our T cell proliferation assay system using purified CD16− monocytes cultured with autologous CD4+ cells (at 1:2 ratio) or together with purified autologous CD16+ monocyte subset (CD16− to CD16+ monocyte ratio of 2:1) in the absence or presence of hemin. Treg, Th1 and Th17 proliferative responses were comparable in alloimmunized and non-alloimmunized SCD groups in hemin treated or untreated cocultures T cell monocyte cocultures that lacked CD16+ monocytes (p>0.05 Supplemental Fig.1). In contrast, in the presence of CD16+ monocytes, basal Treg expansion, especially in Helios− Tregs, was lower in alloimmunized as compared to non-alloimmunized SCD patients (Fig. 4B). Moreover, addition of hemin resulted in doubling of total Treg and helios+/− Treg subset proliferation in non-alloimmunized SCD patients (p<0.05 as indicated by asterisk above white bars), but importantly it had no effect on Treg or Treg subset proliferation in the alloimmunized group (Fig. 4C). In the presence of CD16+ monocytes, basal Th1 proliferative responses was lower in non-alloimmunized as compared to alloimmunized patients (Fig. 4D, p=0.04), but Th17 proliferation was comparable in the 2 groups (Fig. 4D). Hemin further reduced Th1 and Th17 proliferation in the non-alloimmunized group in cocultures that included CD16+ monocytes (p<0.05 as indicated by asterisk above white bars), but had no effect in the alloimmunized group (Fig. 4E). Altogether, these data indicate that CD16+ monocytes from alloimmunized SCD patients have a stronger ability to inhibit Treg proliferation and promote Th1 expansion as compared to CD16+ monocytes from non-alloimmunized patients. Moreover, CD16+ subset is responsible for differential T cell polarization in response to exogenous hemin between alloimmunized vs non-alloimmunized SCD patients; hemin acting through CD16+ monocytes drives Treg/Th proliferation toward an anti-inflammatory state in non-alloimmunized patients whereas the same treatment has no effect in the alloimmunized group. To test whether differences in CD16+ monocyte-mediated Treg/Th proliferative responses was due to differential monocyte HO-1 activity in the 2 patient groups, ZnPPIX (2.5µM) was added at the start of the CD4+T cell-monocyte cocultures. In the presence of ZnPPIX, Treg expansion was not affected in cocultures without CD16+ monocytes in either of the 2 groups (Supplemental data). However, in cocultures that included CD16+ monocytes, Treg expansion was inhibited by ZnPPIX in non-alloimmunized (below 100%, paired t test p=0.027) but not in alloimmunized SCD patients (Fig, 4F), suggesting that basal CD16+ monocytes HO-1 activity with respect to Treg proliferative responses is indeed disparate in the 2 groups, being higher in the non-alloimmunized patients. Inhibition of HO-1 activity with ZnPPIX did not affect Th1 and Th17 proliferative responses in the presence or absence of CD16+ monocytes (data not shown), suggesting that Th1/Th17 as compared to Treg proliferative responses may be less sensitive to regulation by monocyte HO-1.
Fig. 4. CD16+ monocyte control of Treg/Th proliferation before and after hemin treatment.
(A) Representative dot plot analysis of PBMCs based on forward and side scatter showing the gating strategy to identify the total monocyte population. Based on CD14 and CD16 expression pattern, CD16+ monocytes are further distinguished from CD14hiCD16− monocyte subset. Purified CFSE stained CD4+ T cells from non-alloimmunized (n=9, white bars) and alloimmunized (n=11, black bars) SCD patients were cocultured with autologous purified CD14+CD16− monocyte subset together with autologous purified CD16+ monocytes followed by stimulation with anti-CD3 for 7 days in the presence or absence of 1.25µM hemin. (B) Frequency of total and Helios helios +/− Treg subsets that had undergone proliferation in the absence of hemin are shown. (C) Fold change in proliferation of total and Helios helios+/− Treg subsets after addition of hemin was calculated (proliferation in the absence of hemin was set at 100%). The asterisks correspond to statistically significant differences in the proliferative responses before and after hemin treatment (paired t test). (D) Frequency IFN-γ+ and IL-17+ cells in divided CD4+ T cells from the same cocultures as in B are shown. (E) Fold change in Th1 and Th17 proliferation after addition of hemin was calculated (proliferation in the absence of hemin was set at 100%). The asterisks correspond to statistically significant differences in the proliferative responses before and after hemin treatment (paired t test). (F) Fold change in Treg proliferation after addition of ZnPPIX was calculated (proliferation in the absence of ZnPPIX was set at 100%). The p value indicated above the column was calculated by paired t test comparing before and after ZnPPIX treatment. All comparisons between alloimmunized and non-alloimmunized groups were performed using Mann-Whitney test.
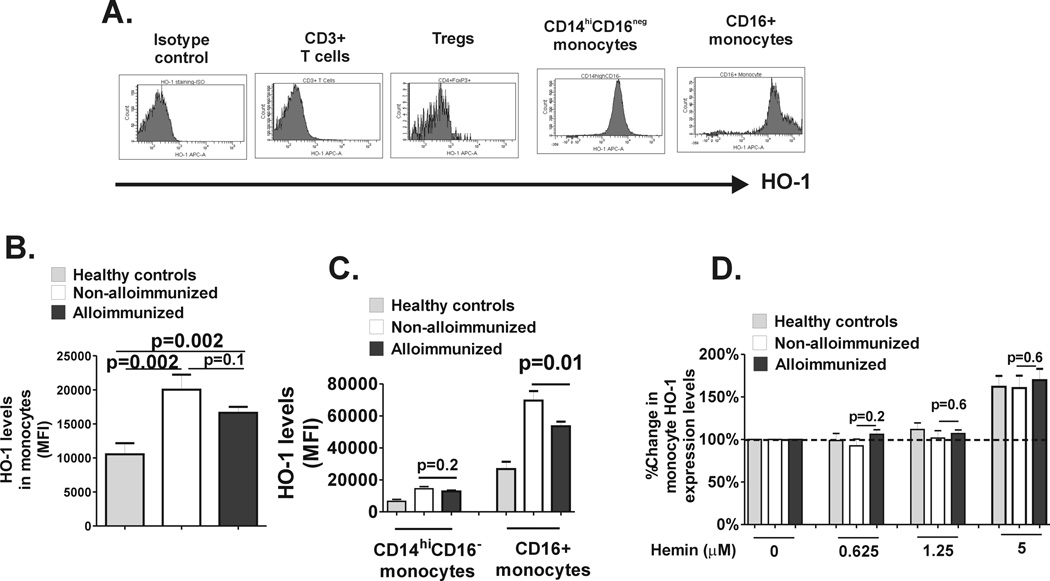
Finally, we examined the relative protein expression levels of HO-1 before and after hemin treatment. Analysis of PBMCs by flow cytometry indicated that in the absence of hemin monocytes expressed high levels of HO-1 with highest expression in CD16+ monocyte subset (Fig. 5A, consistent with a previous study in non-SCD setting.(21) Interestingly, we found extremely low levels of HO-1 in T cells and Tregs, although others have found HO-1 in T cells including Tregs (Fig. 5A).(27) These data suggest that HO-1 expressed in CD16+ monocytes rather than in Tregs may be responsible for the effects of hemin observed in our study, which is consistent with studies in a transgenic mouse system showing that HO-1 expressed in Tregs had little, if any, anti-inflammatory activity whereas when expressed in monocytes, it was highly immunosuppressive.(28) Monocyte HO-1 levels were higher in SCD patients than in healthy controls (Fig. 5B), as previously reported.(29–31) Importantly, non-alloimmunized SCD patients expressed significantly higher levels of HO-1 levels in CD16+ monocytes as compared to alloimmunized patients (Fig. 5C). Following 24 hour treatment with hemin, HO-1 levels in T cells/Tregs remained low (<2% of cells). Monocyte HO-1 levels were upregulated albeit only at the highest hemin concentrations used, although we did not detect any differences in upregulation of HO-1 between alloimmunized and non-alloimmunized patients (Fig. 3D). Altogether, these data indicate that basal levels of CD16+ monocyte HO-1, but not inducibility by hemin, differ between alloimmunized and non-alloimmunized SCD patients.
Fig. 5. HO-1 Expression levels of monocyte subset HO-1 levels.
Levels of intracellular HO-1 expression were measured in PBMCs and (A) representative histograms showing isotype control and HO-1 expression in T cells (CD3+) and Tregs (CD25+Foxp3hi) as well as in CD14hiCD16− and CD16+ monocytes are shown. (B) HO-1 expression in total monocyte fraction and (C) in CD14hiCD16− and CD16+ monocyte sunsets in PBMCs from healthy controls (grey bars) and non-alloimmunized (white bars) and alloimmunized (black bars) SCD patient as measured by relative mean fluorescent intensity (MFI) are shown. (D) PBMCs from healthy controls and SCD patients were cultured without (0) or with various concentrations of hemin for 24 hours and fold change in HO-1 expression relative to no added hemin (set at 100%) was calculated. Although at lower concentrations of hemin (0.625µM and 1.25µM), there was no change in HO-1 levels, at higher concentration (5µM), HO-1 expression level was induced (more than 100%). The differences in HO-1 inducibility between the groups were not different at any of the hemin concentrations tested. All comparisons between alloimmunized and non-alloimmunized groups were performed using Mann-Whitney test.
IL-12 mediated regulation of Treg/Th proliferation
We have previously shown that the Th1 polarizing cytokine IL-12 is involved in CD16+/− monocyte regulation of Treg polarization in healthy controls.(19) Specifically, CD16+ monocytes through secretion of IL-12 inhibit Treg proliferation, altering Helios+ Treg expansion. To test whether CD16+ monocyte-derived IL-12 also controls Treg/Th proliferation in SCD patients, we performed IL-12 neutralization studies in the CD4+ T cell-monocyte cocultures. Antibody blocking with anti-IL-12 resulted in expansion of total Tregs including Helios+/− Tregs and inhibition of Th1 expansion in cocultures that included CD16+ monocytes, regardless of alloimmunization state, confirming its role in SCD CD16+ monocyte mediated regulation of Treg/Th1 polarization (Fig. 6A). To test whether altered Treg/Th polarization in alloimmunized vs non-alloimmunized SCD patients may be due to differential IL-12 expression before and/or after hemin treatment, we measured IL-12 levels by ELISA in the T cell-monocyte cocultures. However, IL-12 was undetectable in the supernatants of many of the co-cultures, especially those that lacked CD16+ monocytes and the ones after hemin treatment. We therefore analyzed monocyte IL-12 expression in short-term LPS-stimulated PBMCs from alloimmunized and non-alloimmunized SCD patients before and after hemin treatment. We found a greater inhibition of IL-12 in non-alloimmunized monocytes as compared to alloimmunized group following hemin treatment (Fig. 6C), suggesting that hemin dampens IL-12 expression more effectively in non-alloimmunized patients.
Fig. 6. Role of IL-12 in CD16+ monocyte control of Treg/Th polarization and response to hemin.
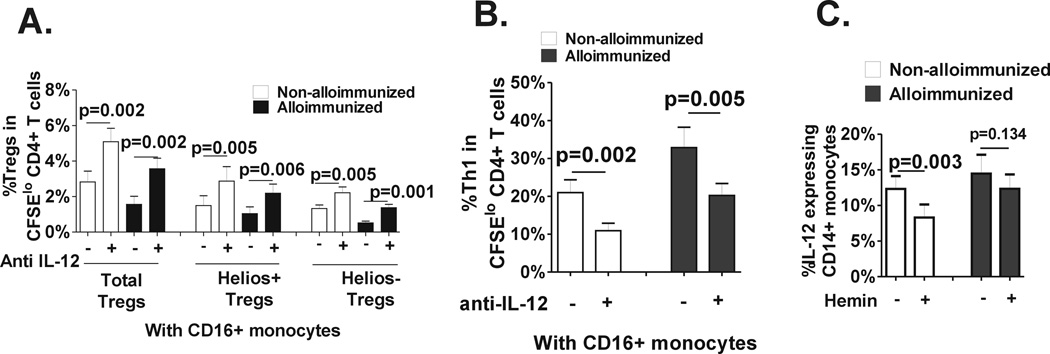
Isotype control (“minus”) or neutralizing anti-IL12 p40/p70 (“plus”) was added to the T cell-monocytes cocultures that included CD16+ monocytes from non-alloimmunized (white bars, n=8) and alloimmunized (black bars, n=8) SCD patients at day 0 and frequencies of (A) total Tregs and Helios+/− Treg subsets and (B) IFN-γ+ cells in divided CD4+T cells was measured on day 7. All statistical analysis comparing isotype vs anti-IL-12 was performed using paired t test. (C) Frozen and then thawed PBMCs from non-alloimmunized (white bars, n=8) and alloimmunized (black bars, n=7) SCD patients were stimulated with IFN-γ in the absence or presence of hemin (1.25 µM) for 2 hours followed by addition of LPS for another 22 hours. Frequency of IL-12 expressing monocytes in untreated and hemin treated samples are shown and the p values indicate paired t-test comparison analysis of untreated vs hemin-treated samples.
Discussion
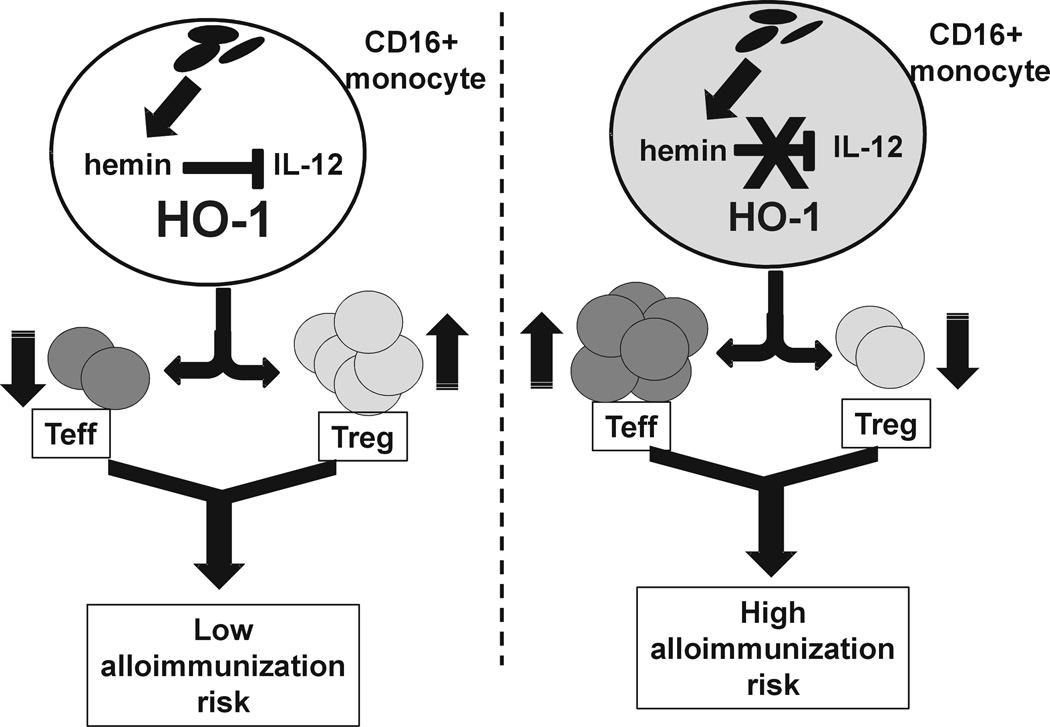
In the present study, we have found that hemin, a surrogate marker for transfused RBC breakdown products, can induce CD4+ T cell subset polarization mediated through monocyte HO-1, thereby establishing for the first time a role for HO-1 in T cell polarization in the human setting. Since aberrant T cells polarization, altered HO-1 expression(32–34) and/or monocyte activity(35–37) have been reported in various inflammatory diseases, it raises the possibility that the CD16+monocyte HO-1-T cell polarization axis may also play a pivotal role in the etiology and prognosis of such inflammatory diseases. HO-1 activity and levels can be regulated by multiple factors,(9) and therefore understanding the mechanisms of HO-1 mediated T cell polarization may not only provide insight into the etiology of diseases and but also may offer potential therapy targets for such diseases.(38,39) In this regard, the present study identified differences in Treg/Th proliferation in response to hemin between alloimmunized and non-alloimmunized transfused SCD patients. The difference was most notable under CD16+ monocyte-enriched conditions such that addition of hemin increased Treg expansion and inhibited Th1 proliferation in non-alloimmunized SCD patients but it had little effect on Treg/Th polarization in the alloimmunized group. Furthermore, higher baseline activity and levels of CD16+ monocyte HO-1, which is associated with anti-inflammatory response,(10) was detected in non-alloimmunized SCD group. At the hemin concentrations used in this study, IL-12, which we found to suppress CD16+ monocyte-mediated SCD Treg polarization, was more effectively inhibited in stimulated monocytes from non-alloimmunized patients. Multiple pro- and anti-inflammatory cytokines including TNF-α, IL-6 and IL-10, are also secreted by monocytes, can be regulated by HO-1,(13,40) and have been shown to affect Treg expansion.(19,41,42) As such, they can potentially affect Treg polarization in SCD alloimmunization. However, antibody blocking studies using anti- TNF-α, -IL-6 and -IL-10 did not reveal any difference in the Treg proliferative responses between non-alloimmunized and alloimmunized SCD patient groups (data not shown). We therefore believe while the other cytokines may also contribute, it is IL-12 which is the pivotal cytokine for alloimmunization in SCD patients. Based on all our data, we hypothesize that in the initial steps encountered following red blood cell transfusion, CD16+ monocytes from non-alloimmunized SCD patients induce polarization of Treg/Th responses toward a regulatory phenotype upon exposure to hemin possibly due to their higher baseline HO-1 levels/activity and their ability to suppress IL-12. As a result, an anti-inflammatory state is established that is less conducive to alloimmunization. In contrast, such an anti-inflammatory response fails to develop in alloimmunized SCD patients (Fig. 7). The model predicts that because of the inability of CD16+ monocytes to switch off their pro-inflammatory state in response to heme, alloimmunized patients are more likely to develop a strong immune response against allogeneic determinants on transfused RBCs, thus increasing the risk of further alloimmunization in this patient group. In support of this, it is estimated that once a patient makes an alloantibody his/her risk of alloimmunization increases by 20 fold.(43) Increasing evidence implicate a role for CD16+ monocytes in the control of Th polarization in autoimmune disease setting such as CD16+ mediated Th17 expansion in rheumatoid arthritis(16) and CD16+ driven Th1 polarization in immune thrombocytopenia.(18) Our data suggest that this monocyte population may also be involved in SCD alloimmunization. Clearly, longitudinal studies of SCD patients receiving RBC transfusions are needed to determine whether monocyte reactivity and Treg/Th polarization differ in patients who go on to develop alloantibodies as compared to those who don’t. Our cohort consisted of heavily transfused SCD patients. Since the cumulative number of transfused units appears to increase alloimmunization risk,(44) it remains to be determined whether differences in monocyte control of Treg/Th polarization would also be evident in less heavily transfused SCD patients or for that matter in transfused patient populations other than SCD patients.
Fig 7. Proposed mechanism of altered monocyte mediated Treg/Th polarization in SCD alloimmunization.
Levels of hemin, a breakdown product of hemoglobin, are likely to build up in monocyte/macrophages following RBC transfusions. If proinflammatory cytokines including IL-12 are at low levels and are maintained at low levels in response to hemin due to adequate HO-1 in CD16+ monocytes, Treg/Th polarization will be switched toward a regulatory response (higher Treg/lower Th1) that is less conducive to alloimmunization. However, if IL-12 levels in CD16+ monocytes are not optimally inhibited by hemin as a result of insufficient HO-1 activity/level, Tregs will not expand and Th1 proliferation will dominate, thereby increasing the risk of alloimmunization.
Heme/hemin can act as the substrate for HO-1, thus activating its enzymatic activity as well as the ability to upregulate HO-1 expression, further increasing its activity.(9) HO-1 is reported to be upregulated in SCD,(29–31) which is consistent with our data of higher monocyte HO-1 levels in SCD patients as compared to healthy controls. In non-SCD setting, HO-1 is considered immunosuppressive as it was shown to inhibit T lymphocyte proliferation,(9) block maturation of dendritic cells and inhibit proinflammatory and allogeneic immune responses.(45,46) Indeed, the immunosuppressive effects of HO-1 are mediated through the anti-oxidative and anti-inflammatory activities of two of the heme breakdown products, namely carbon monoxide and biliverdin.(9) On the other hand, the third byproduct, free iron, is a pro-oxidant that has to be deactivated by ferritin.(9) A key finding of our study is that we found higher levels of HO-1 in CD16+ monocytes in non-alloimmunized SCD patients and a robust anti-inflammatory T cell polarization profile following stimulation with HO-1 inducer, hemin, as compared to alloimmunized group. Interestingly, the hemin concentrations used to elicit differences in monocyte control of T cell polarization did not induce upregulation of HO-1 expression at least at the protein level. This suggests that hemin may be acting as a substrate for HO-1 at these concentrations inducing a more effective anti-inflammatory response in these patients due to higher CD16+ monocyte HO-1 levels as compared to the alloimmunized group.
The molecular mechanism(s) responsible for lower basal CD16+ monocyte HO-1 levels/activity in our alloimmunized SCD patients is unknown. One possibility is that allosensitized group has higher baseline chronic intravascular hemolysis, a characteristic feature of SCD, which could account for lower HO-1 levels, although no differences in baseline chronic hemolysis between and non-allosensitized patients have previously been reported. In addition, our patient cohort was undergoing exchange transfusion as part of their regular transfusion procedure to maintain their HbS levels rather than because of acute need for transfusion, and it is therefore unlikely that the alloimmunized group had increased baseline hemolysis due to hemolytic transfusion reaction at the time of the study. Nevertheless, we were unable to examine any markers of hemolysis such as LDH, bilirubin, plasma hemoglobin levels which correlate with SCD HO-1 activity(47) since the patients were stripped of all identifiers with the exception of alloimmunization state. Genetic differences such as altered transcriptional control by microRNAs,(48) or promoter polymorphisms(49,50) may be responsible for lower CD16+ monocyte HO-1 levels and future studies will aim to explore these possibilities.
It remains to be determined which of the key HO-1 catalytic by-products are responsible for differences in Treg/Th polarization in alloimmunized vs non-alloimmunized SCD patients. Both CO(51) and bilirubin(52,53) have been shown to induce Treg expansion in mouse disease models, and their effects can be additive, indicating that both CO and bilirubin may be responsible for promoting HO-1-mediated Treg expansion. However, the direct effects of CO and/or bilirubin on Treg expansion in human disease setting or in culture conditions remain unexplored. Future studies focusing on the byproducts of HO-1 in SCD alloimmunization will help to dissect the mechanism of HO-1-mediated Treg expansion and may provide identify potential therapeutic targets for prevention of alloimmunization. Interestingly, iron chelation and treatment with anti-oxidants can modify expression and activation of HO-1.(54,55) This raises the possibility that transfusion-associated alloimmunization may be suppressed with peak induction of monocyte HO-1 levels through optimal use of iron chelation that can adequately lower iron levels in the monocyte/macrophage system and of anti-oxidants to reduce pro-oxidant state of monocytes.
In conclusion, we have shown that the immunoregulatory function of the innate immune cells in alloimmunized SCD patients may be altered such that in response to hemin, a surrogate for transfused RBC breakdown product, these cells are no longer able to induce Treg expansion or inhibit Th1 development, anti-inflammatory responses that are induced with the same treatment in non-alloimmunized patients. Although SCD patients before and after alloimmunization were not studied, we hypothesize that in the initial step of encountering the transfused RBCs, the activation state of the monocyte/macrophage system will be key to whether T cells are polarized toward regulatory or stimulatory type cells and ultimately impact the risk of alloimmunization.
Supplementary Material
Acknowledgments
This work was supported in part by National Heart, Lung, and Blood Institute grant HL096497-01 (K.Yazdanbakhsh) and the generous support from the Hugoton Foundation (K.Yazdanbakhsh).
Abbreviations
- SCD
Sickle cell disease
- Hb
hemoglobin
- Tregs
Regulatory T cells
- HO-1
heme oxygenase I
- RBC
red blood cell
- CFSE
Carboxyfluoresceindiacetatesuccinimidyl ester
- ZnPPIX
zinc protoporphyrin IX
References
- 1.Frenette PS, Atweh GF. Sickle cell disease: old discoveries, new concepts, and future promise. J. Clin. Invest. 2007;117:850–858. doi: 10.1172/JCI30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Josephson CD, Su LL, Hillyer KL, Hillyer CD. Transfusion in the patient with sickle cell disease: a critical review of the literature and transfusion guidelines. Transfus. Med. Rev. 2007;21:118–133. doi: 10.1016/j.tmrv.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Yazdanbakhsh K, Ware RE, Noizat-Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120:528–537. doi: 10.1182/blood-2011-11-327361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou ST, Jackson T, Vege S, Smith-Whitley K, Friedman DF, Westhoff CM. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122:1062–1071. doi: 10.1182/blood-2013-03-490623. [DOI] [PubMed] [Google Scholar]
- 5.Bao W, Zhong H, Li X, Lee MT, Schwartz J, Sheth S, Yazdanbakhsh K. Immune regulation in chronically transfused allo-antibody responder and nonresponder patients with sickle cell disease and beta-thalassemia major. Am. J. Hematol. 2011;86:1001–1006. doi: 10.1002/ajh.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao W, Zhong H, Manwani D, Vasovic L, Uehlinger J, Lee MT, Sheth S, Shi P, Yazdanbakhsh K. Regulatory B-cell compartment in transfused alloimmunized and non-alloimmunized patients with sickle cell disease. Am. J. Hematol. 2013;88:736–740. doi: 10.1002/ajh.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platt OS. Sickle cell anemia as an inflammatory disease. J. Clin. Invest. 2000;106:337–338. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumont LJ, Aubuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 9.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 10.Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat. Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 11.Piazza M, Damore G, Costa B, Gioannini TL, Weiss JP, Peri F. Hemin and a metabolic derivative coprohemin modulate the TLR4 pathway differently through different molecular targets. Innate. Immun. 2011;17:293–301. doi: 10.1177/1753425910369020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeda Y, Takeno M, Iwasaki M, Kobayashi H, Kirino Y, Ueda A, Nagahama K, Aoki I, Ishigatsubo Y. Chemical induction of HO-1 suppresses lupus nephritis by reducing local iNOS expression and synthesis of anti-dsDNA antibody. Clin. Exp. Immunol. 2004;138:237–244. doi: 10.1111/j.1365-2249.2004.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma JL, Yang PY, Rui YC, Lu L, Kang H, Zhang J. Hemin modulates cytokine expressions in macrophage-derived foam cells via heme oxygenase-1 induction. J. Pharmacol. Sci. 2007;103:261–266. doi: 10.1254/jphs.fp0060270. [DOI] [PubMed] [Google Scholar]
- 14.Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 2004;165:1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Ann. Rev. Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans HG, Gullick NJ, Kelly S, Pitzalis C, Lord GM, Kirkham BW, Taams LS. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc. Natl. Acad. Sci. USA. 2009;106:6232–6237. doi: 10.1073/pnas.0808144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De BP, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 18.Zhong H, Bao W, Li X, Miller A, Seery C, Haq N, Bussel J, Yazdanbakhsh K. CD16+ monocytes control T-cell subset development in immune thrombocytopenia. Blood. 2012;120:3326–3335. doi: 10.1182/blood-2012-06-434605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong H, Yazdanbakhsh K. Differential control of Helios(+/-) Treg development by monocyte subsets through disparate inflammatory cytokines. Blood. 2013;121:2494–2502. doi: 10.1182/blood-2012-11-469122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96:2451–2459. [PubMed] [Google Scholar]
- 21.Mizuno K, Toma T, Tsukiji H, Okamoto H, Yamazaki H, Ohta K, Ohta K, Kasahara Y, Koizumi S, Yachie A. Selective expansion of CD16highCCR2-subpopulation of circulating monocytes with preferential production of haem oxygenase (HO)-1 in response to acute inflammation. Clin. Exp. Immunol. 2005;142:461–470. doi: 10.1111/j.1365-2249.2005.02932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong RJ, Vreman HJ, Schulz S, Kalish FS, Pierce NW, Stevenson DK. In vitro inhibition of heme oxygenase isoenzymes by metalloporphyrins. J. Perinatol. 2011;31(Suppl 1):S35–S41. doi: 10.1038/jp.2010.173. [DOI] [PubMed] [Google Scholar]
- 23.Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, Chung KR, Chung HT. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J. Immunol. 2004;172:4744–4751. doi: 10.4049/jimmunol.172.8.4744. [DOI] [PubMed] [Google Scholar]
- 24.Song R, Mahidhara RS, Zhou Z, Hoffman RA, Seol DW, Flavell RA, Billiar TR, Otterbein LE, Choi AM. Carbon monoxide inhibits T lymphocyte proliferation via caspase-dependent pathway. J. Immunol. 2004;172:1220–1226. doi: 10.4049/jimmunol.172.2.1220. [DOI] [PubMed] [Google Scholar]
- 25.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Ann. Rev. Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 27.Choi BM, Pae HO, Jeong YR, Kim YM, Chung HT. Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression. Biochem. Biophys. Res. Commun. 2005;327:1066–1071. doi: 10.1016/j.bbrc.2004.12.106. [DOI] [PubMed] [Google Scholar]
- 28.George JF, Braun A, Brusko TM, Joseph R, Bolisetty S, Wasserfall CH, Atkinson MA, Agarwal A, Kapturczak MH. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am. J. Pathol. 2008;173:154–160. doi: 10.2353/ajpath.2008.070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nath KA, Grande JP, Haggard JJ, Croatt AJ, Katusic ZS, Solovey A, Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am. J. Pathol. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jison ML, Munson PJ, Barb JJ, Suffredini AF, Talwar S, Logun C, Raghavachari N, Beigel JH, Shelhamer JH, Danner RL, Gladwin MT. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104:270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanaro C, Franco-Penteado CF, Albuqueque DM, Saad ST, Conran N, Costa FF. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. J. Leukoc. Biol. 2009;85:235–242. doi: 10.1189/jlb.0708445. [DOI] [PubMed] [Google Scholar]
- 32.Haines DD, Lekli I, Teissier P, Bak I, Tosaki A. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol (Oxf) 2012;204:487–501. doi: 10.1111/j.1748-1716.2011.02387.x. [DOI] [PubMed] [Google Scholar]
- 33.Deshane J, Wright M, Agarwal A. Heme oxygenase-1 expression in disease states. Acta Biochim. Pol. 2005;52:273–284. [PubMed] [Google Scholar]
- 34.Fagone P, Patti F, Mangano K, Mammana S, Coco M, Touil-Boukoffa C, Chikovani T, Di MR, Nicoletti F. Heme oxygenase-1 expression in peripheral blood mononuclear cells correlates with disease activity in multiple sclerosis. J. Neuroimmunol. 2013;261:82–86. doi: 10.1016/j.jneuroim.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol. Res. 2012;53:41–57. doi: 10.1007/s12026-012-8297-3. [DOI] [PubMed] [Google Scholar]
- 36.Zawada AM, Rogacev KS, Schirmer SH, Sester M, Bohm M, Fliser D, Heine GH. Monocyte heterogeneity in human cardiovascular disease. Immunobiology. 2012;217:1273–1284. doi: 10.1016/j.imbio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Byrne JC, Ni GJ, Lazzari E, Mahony R, Smith S, Stacey K, Wynne C, Jefferies CA. Genetics of SLE: functional relevance for monocytes/macrophages in disease. Clin. Dev. Immunol. 2012;2012:582352. doi: 10.1155/2012/582352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbagallo I, Galvano F, Frigiola A, Cappello F, Riccioni G, Murabito P, D'Orazio N, Torella M, Gazzolo D, Li VG. Potential therapeutic effects of natural heme oxygenase-1 inducers in cardiovascular diseases. Antioxid. Redox. Signal. 2013;18:507–521. doi: 10.1089/ars.2011.4360. [DOI] [PubMed] [Google Scholar]
- 39.Ryter SW, Choi AM. Heme oxygenase-1/carbon monoxide: novel therapeutic strategies in critical care medicine. Curr. Drug Targets. 2010;11:1485–1494. doi: 10.2174/1389450111009011485. [DOI] [PubMed] [Google Scholar]
- 40.Roach JP, Moore EE, Partrick DA, Damle SS, Silliman CC, McIntyre RC, Jr, Banerjee A. Heme oxygenase-1 induction in macrophages by a hemoglobin-based oxygen carrier reduces endotoxin-stimulated cytokine secretion. Shock. 2009;31:251–257. doi: 10.1097/SHK.0b013e3181834115. [DOI] [PubMed] [Google Scholar]
- 41.Fujimoto M, Nakano M, Terabe F, Kawahata H, Ohkawara T, Han Y, Ripley B, Serada S, Nishikawa T, Kimura A, Nomura S, Kishimoto T, Naka T. The influence of excessive IL-6 production in vivo on the development and function of Foxp3+ regulatory T cells. J. Immunol. 2011;186:32–40. doi: 10.4049/jimmunol.0903314. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Bruning JC, Muller W, Rudensky AY. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schonewille H, Van de Watering LM, Brand A. Additional red blood cell alloantibodies after blood transfusions in a nonhematologic alloimmunized patient cohort: is it time to take precautionary measures? Transfusion. 2006;46:630–635. doi: 10.1111/j.1537-2995.2006.00764.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosse WF, Gallagher D, Kinney TR, Castro O, Dosik H, Moohr J, Wang W, Levy PS Cooperative Study of Sickle Cell Disease. Transfusion and alloimmunization in sickle cell disease. Blood. 1990;76:1431–1437. [PubMed] [Google Scholar]
- 45.Chauveau C, Remy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, Tesson L, Brion R, Beriou G, Gregoire M, Josien R, Cuturi MC, Anegon I. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 46.Remy S, Blancou P, Tesson L, Tardif V, Brion R, Royer PJ, Motterlini R, Foresti R, Painchaut M, Pogu S, Gregoire M, Bach JM, Anegon I, Chauveau C. Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J. Immunol. 2009;182:1877–1884. doi: 10.4049/jimmunol.0802436. [DOI] [PubMed] [Google Scholar]
- 47.Bains SK, Foresti R, Howard J, Atwal S, Green CJ, Motterlini R. Human sickle cell blood modulates endothelial heme oxygenase activity: effects on vascular adhesion and reactivity. Arterioscler. Thromb. Vasc. Biol. 2010;30:305–312. doi: 10.1161/ATVBAHA.109.196360. [DOI] [PubMed] [Google Scholar]
- 48.Beckman JD, Chen C, Nguyen J, Thayanithy V, Subramanian S, Steer CJ, Vercellotti GM. Regulation of heme oxygenase-1 protein expression by miR-377 in combination with miR-217. J. Biol. Chem. 2011;286:3194–3202. doi: 10.1074/jbc.M110.148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bean CJ, Boulet SL, Ellingsen D, Pyle ME, Barron-Casella EA, Casella JF, Payne AB, Driggers J, Trau HA, Yang G, Jones K, Ofori-Acquah SF, Hooper WC, DeBaun MR. Heme oxygenase-1 gene promoter polymorphism is associated with reduced incidence of acute chest syndrome among children with sickle cell disease. Blood. 2012;120:3822–3828. doi: 10.1182/blood-2011-06-361642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia-Santos D, Chies JA. HO-1 polymorphism as a genetic determinant behind the malaria resistance afforded by haemolytic disorders. Med. Hypotheses. 2010;74:807–813. doi: 10.1016/j.mehy.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 51.Nikolic I, Saksida T, Mangano K, Vujicic M, Stojanovic I, Nicoletti F, Stosic-Grujicic S. Pharmacological application of carbon monoxide ameliorates islet-directed autoimmunity in mice via anti-inflammatory and anti-apoptotic effects. Diabetologia. 2014 doi: 10.1007/s00125-014-3170-7. [DOI] [PubMed] [Google Scholar]
- 52.Rocuts F, Zhang X, Yan J, Yue Y, Thomas M, Bach FH, Czismadia E, Wang H. Bilirubin promotes de novo generation of T regulatory cells. Cell Transplant. 2010;19:443–451. doi: 10.3727/096368909X484680. [DOI] [PubMed] [Google Scholar]
- 53.Lee SS, Gao W, Mazzola S, Thomas MN, Csizmadia E, Otterbein LE, Bach FH, Wang H. Heme oxygenase-1, carbon monoxide, and bilirubin induce tolerance in recipients toward islet allografts by modulating T regulatory cells. FASEB J. 2007;21:3450–3457. doi: 10.1096/fj.07-8472com. [DOI] [PubMed] [Google Scholar]
- 54.Kruger AL, Peterson SJ, Schwartzman ML, Fusco H, McClung JA, Weiss M, Shenouda S, Goodman AI, Goligorsky MS, Kappas A, Abraham NG. Up-regulation of heme oxygenase provides vascular protection in an animal model of diabetes through its antioxidant and antiapoptotic effects. J. Pharmacol. Exp. Ther. 2006;319:1144–1152. doi: 10.1124/jpet.106.107482. [DOI] [PubMed] [Google Scholar]
- 55.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.