Abstract
Purpose
Results from several studies suggest that there is value in evaluating the association between nonclinical characteristics of patients and quality of life (QoL), but few studies have focused on brain cancer. The primary goal of this feasibility study was to explore the relationship between clinical factors and nonclinical factors and QoL in brain cancer patients.
Methods
Participants in this cross-sectional study were drawn from two hospital sites. Eligible patients were 18–75 years old with a pathologically confirmed diagnosis of a brain cancer histology and stable disease after treatment. Data were obtained from medical chart review and a self-administered survey consisting of main study variables and two QoL standardized measures. Independent sample t test was used to determine differences between patient factors and QoL measures.
Results
The sample population was comprised of 26 patients with a median age at survey of 57.5 years (range 33–72). Quality of life was adversely associated with younger age, having underage children and living alone. Patients’ meaning of QoL differed by gender, however most patients viewed it as affecting multiple aspects of their lives.
Conclusions
Nonclinical characteristics were significantly associated with QoL more often than clinical characteristics. Identifying these factors may help improve the quality of care for these patients. This effort demonstrates the relevancy and feasibility of conducting a larger scale study to confirm or refute these findings.
Keywords: High-grade gliomas, Health-related quality of life, Sociodemographics, Perceptions/beliefs, Adults
Introduction
Patients with high-grade gliomas experience high morbidity and recurrence with a median survival ranging from just over a year for grade IV tumors [1] and 2–3 years for grade III tumors [2]. Although treatment advances for high-grade gliomas in recent years are promising [3], treatment generally has toxic effects with palliative results. While headaches, seizures, and nausea are common, other symptoms include personality changes, and neurological and cognitive deficits [4]; all which may negatively affect health-related quality of life (QoL). Quality of life is an important issue particularly for patients with an incurable disease [5] and an important outcome measurement in clinical practice but most efforts have been focused solely on clinical associations.
Results from several studies suggest that there is value in evaluating the association between QoL and nonclinical characteristics of patients, such as sociodemographics and perceptions/beliefs. Sociodemographics were shown to be better predictors of QoL than clinical characteristics in bladder and head/neck cancer patients, respectively [6, 7]. Patients’ perceptions/beliefs have been studied in non-brain tumor patients in relation to QoL [6, 8]. In brain cancer studies, the evaluation of sociodemographics in adults has been limited in scope and quantity [9, 10]; and there are no published reports that have evaluated the association between patients’ perceptions/beliefs and QoL. The current study explores whether there is an association between individual-level characteristics and QoL in brain cancer patients. The main goal of this study was to test the feasibility of assessing patients’ sociodemographics, clinical factors, and perceptions/beliefs as they relate to QoL in patients with high-grade gliomas using two QoL instruments.
Methods
Sample and procedures
For this cross-sectional study, sixty-nine eligible patients were identified from two participating brain tumor referral centers in the Chicago area upon institutional review board approval. Most eligible patients were approached in clinic; those that were not due in clinic for some time were approached by mail. Participating patients provided written consent to a detailed chart review, passive follow-up to ascertain their status, and chose to complete a paper-based survey or secure web-based survey via SurveyMonkey software (www.surveymonkey.com). They may have been undergoing chemotherapy, which was generally well-tolerated and did not hinder their participation, but had completed the remaining portion of their first course of treatment (surgery and/or radiation).
Eligibility criteria included having a pathologically confirmed diagnosis of a high-grade glioma with one of the following histologies: glioblastoma [International Classification of Diseases for Oncology version 3 (ICDO-3) histology codes 9440-9442], anaplastic astrocytoma (9401 and 9411), anaplastic oligodendroglioma (9451 and 9460), anaplastic ependymoma (9392), anaplastic ganglioglioma (9505/3), or anaplastic oligoastrocytoma/anaplastic mixed glioma (9382/3); diagnosis between July 2008 and March 2010; 18–75 years old, fluency in English or Spanish; and completion of surgery and/ or radiotherapy. Patients with speech or writing impairment were not excluded since the study design allowed for patient-assistance in completing the questionnaire. Patients with metastatic brain tumors were excluded as were patients that lacked the cognitive capacity to consent or complete the survey as determined by treating physicians using accepted methods.
Independent variables
Independent variables were obtained by both medical chart review and survey completion. The following sociodemographic and clinical variables were dichotomized for analytic purposes: adult children (yes vs. no), underage children (yes vs. no), employment status (employed vs. other; retired vs. other); income (<$100,000 vs. ≥$100,000); surgical resection (gross/total vs. other); primary site (frontal/temporal vs. other); and postneurodiagnostic results (evidence of tumor vs. other). Postoperative length of hospital stay was defined as (date of surgical discharge−date of surgical treatment) and time from diagnosis to survey as (date of survey completion−date of diagnosis). Three types of therapy were considered; physical, occupational, and speech. Investigator-generated perception/ belief variables were dichotomized as follows: ease of travel to/from medical visits as (“very easy/easy” vs. “sometimes easy, sometimes difficult”, “difficult, very difficult”); perception of income as (“I can save/I get enough for my needs” vs. “I am just able to make ends meet”, “I don't get enough for my needs”, and “I am in desperate need”); and treatment expectation (be cured vs. some cancer left and/or symptoms, no expectations/no effect).
Outcome measures
The Functional Assessment of Cancer Therapy for brain tumor patients version 4 (FACT-Br) is a 50-item instrument, comprised of 27 core items and a 23-item brain subscale that assesses QoL in brain tumor patients [11]. The Ferrans and Powers Quality of Life Index Cancer version III (FPQLI-C) is a validated 66-item instrument that is separated into two 33-item sections; one measures QoL of oncology patients in terms of importance and the other in terms of satisfaction with life [12]. These instruments were chosen because both are self-administered, multidimensional, and have been validated in English and Spanish for use with brain tumor [11, 13] and cancer [12, 14] patients, respectively. Both measures gather subjective information but from different perspectives. The FACT-Br is composed of a combination of status and evaluation questions which ask for information on the status of a particular aspect of life whereas FPQLI-C is comprised completely of evaluation questions which go a step further by asking the subjects to evaluate the status [15]. Additionally, because they were conceptualized differently each instrument may identify factors associated with different aspects of QoL [10], which is valuable given little research has been conducted regarding nonclinical characteristics. For both instruments, a higher score indicates better QoL.
Statistical analysis
Descriptive statistics were computed for all patient characteristics and for overall outcomes and subscales. Reliability was evaluated with Cronbach's alpha coefficient for each QoL scale. An acceptable Cronbach's alpha (≥.70) provides stronger support for the results yielded from using the instruments [16], particularly when they are administered to patient populations that differ from the one used to develop the QoL instrument. Pearson correlation analysis was performed to identify significant relationships between measures of FACT-Br and FPQLI-C. Two-sided independent sample t test was used to compare mean scores between patient factors and QoL measures with the significance level of p <0.10 for this exploratory analysis. Quantitative analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Content analysis was used to analyze the qualitative responses to the question: “What does quality of life mean to you?” Theme groupings were created based on the subscale names from the FACT-Br and FPQLI-C. Common subscale names were combined to create a single theme category (i.e., emotional well-being (FACT-Br) and spiritual/psychological (FPQLI-C)=emotional/psychological theme) because the analysis was a textual account and did not involve scoring the subscales. Patients’ responses were converted into quantitative measures by tallying responses within theme groupings. In some instances, an individual response could be tallied in multiple theme groupings. Responses were stratified by gender. Qualitative analyses were performed using computerized tables.
Results
Sample characteristics
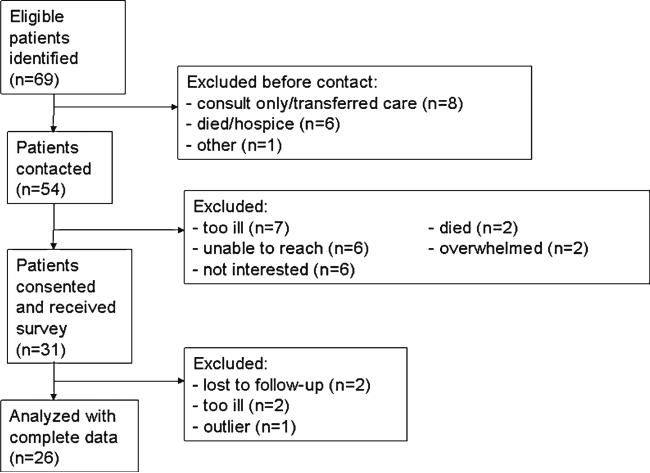
Thirty-one of the 54 eligible patients contacted, enrolled in the study for an overall response rate of 57.4 %. The analytic sample included 26 patients as outlined in Fig. 1. Six participants were deceased at the time of follow-up, which was a minimum of 6 months post-diagnosis. Characteristics of decedents included median age of 58 years (range=43–68 years), median time since diagnosis to death of 11.5 months (range= 8–19 months), equal gender distribution, and a diagnosis of glioblastoma (data not shown).
Fig. 1.
Flowchart of recruitment
Patient characteristics are summarized in Table 1. While 23 patients were still undergoing chemotherapy at time of survey, the remaining patients either had surgery only (n =1) or had completed all treatment (n =2). At least two-thirds of patients (65.4–73.8 %) reported completing each of the four survey sections without assistance (data not shown). Patients preferred the paper-based survey (65.4 %) and the median time interval from diagnosis to survey was 7.6 months. No significant differences between participants and nonparticipants, paper and web-based surveys or recruitment were observed for gender, age at diagnosis, race/ethnicity, facility, histology, or education (data not shown).
Table 1.
Patient characteristics: sociodemographics, clinical, and perception/beliefs
| Characteristics | # of patients (%) |
|---|---|
| Sociodemographics | |
| Median age at survey | 57.5 years (SD 10.8) |
| Gender | |
| Male | 13 (50.0) |
| Female | 13 (50.0) |
| Race/ethnicity | |
| Non-hispanic white | 23 (88.5) |
| Other | 3 (11.5) |
| Education | |
| Completed college | 14 (53.9) |
| Not college graduate | 12 (46.2) |
| Handedness | |
| Right | 24 (92.3) |
| Right/ambidextrous | 2 (7.7) |
| Living arrangement | |
| Alone | 4(15.4) |
| With others | 22(84.6) |
| Marital status | |
| Married with a partner | 21(80.8) |
| Single/widowed | 5(19.2) |
| Children | |
| Yes | 24 (92.3) |
| No | 2 (7.7) |
| Lifetime occupation | |
| White collar | 20 (80.0) |
| Blue collar | 5 (20.0) |
| Employment status | |
| Employed | 13 (50.0) |
| Retired | 5 (19.2) |
| Unemployed/other | 8 (30.8) |
| Health insurance | |
| Private | 17 (65.4) |
| Medicare/COBRA/None | 9 (34.6) |
| Income, annual household | |
| <$50,000 | 5 (21.7) |
| $50,000–99,999 | 6 (26.1) |
| ≥$100,000 | 12 (52.2) |
| Clinical | |
| Type of surgical resection | |
| Biopsy | 4 (15.4) |
| Subtotal | 10 (38.5) |
| Gross/total | 12 (46.2) |
| Laterality | |
| Left | 14 (53.9) |
| Right | 9 (34.6) |
| Bilateral/midline | 3 (11.5) |
| Primary site | |
| Frontal | 11 (42.3) |
| Temporal | 10 (38.5) |
| Parietal/ventricle, NOS/spinal cord/brain, NOS | 5 (19.2) |
| Histology | |
| Glioblastoma | 21 (80.8) |
| Anaplastic astrocytoma/oligodendroglioma/oliastrocytoma/mixed glioma | 5 (19.2) |
| Tumor size (mm) | |
| ≤36 | 18 (69.2) |
| 37+ | 8 (30.8) |
| Length hospital stay | |
| 0–3days | 9 (34.6) |
| 4+ days | 17 (65.4) |
| Disposition discharge | |
| Home, independent | 12 (70.6) |
| Home, dependent/rehabilitation center | 5 (29.4) |
| Unknown | 9 (34.6) |
| Postneurodiagnostic results | |
| Evidence of tumor | 14 (56.0) |
| No evidence | 7 (28.0) |
| Postneurodiagnostic test, biopsy or results unknown | 4 (16.0) |
| Comorbid condition | |
| None | 8 (30.8) |
| 1 | 6 (23.1) |
| 2 | 7 (26.9) |
| 3 or more | 5 (19.2) |
| Chemotherapy ended by survey completion | |
| Yes | 2 (8.0) |
| No | 23 (92.0) |
| Perceptions/beliefs | |
| Ease of travel to/from doctor visits/treatment | |
| Very easy/easy | 11 (42.3) |
| Sometimes easy, sometimes difficult/difficult | 15 (57.7) |
| Perception of income | |
| I can save | 5 (19.2) |
| I get enough for my needs | 9 (34.6) |
| I am just able to make ends meet | 6 (23.1) |
| I don't get enough for my needs | 4 (15.4) |
| I am in desperate need | 2 (7.7) |
| Out-of pocket expense | |
| Very manageable | 7 (26.9) |
| Somewhat manageable | 13 (50.0) |
| Barely manageable | 2 (7.7) |
| Not at all manageable | 4 (15.4) |
| Treatment expectation | |
| Be cured of my cancer | 11 (42.3) |
| Some cancer left/symptoms left | 10 (38.5) |
| No expectations/no effect | 5 (19.2) |
| Prognosis explained | |
| Very well | 14 (53.9) |
| Somewhat | 10 (38.5) |
| Not very well | 2 (7.7) |
| Current health | |
| Same/better | 8 (32.0) |
| Worse | 17 (68.0) |
| Any therapy (physical, occupational, speech) | |
| Yes | 10 (38.5) |
| No | 16 (61.5) |
| Diagnosis to survey | |
| 3–7 months | 15 (57.7) |
| >7 months | 11 (42.3) |
| Radiation completion to survey | |
| 1–4 months | 14 (56.0) |
| ≥5 months | 11 (44.0) |
| Survey administration | |
| Paper-based | 17 (65.4) |
| Web-based | 9 (34.6) |
Correlation of the FACT-BR and FPQLI-C
Table 2 demonstrates the relationship between the FACT-Br and FPQLI-C domains. The overall scale for the FACT-Br had a moderately high correlation with the overall scale for the FPQLI-C (r =0.74, p <0.01), suggesting that the instruments measure similar QoL aspects overall. However, the correlation coefficients between the individual subscales varied widely, ranging from r =−0.12 to 0.59. Correlation coefficients >0.42 were significant (p <0.05) and coefficients ≥0.52 were very significant (p <0.01). With few exceptions, the socioeconomic and family subscales of the FPQLI-C had relatively low correlations with the overall and subscale of the FACT-Br. Similarly, the social/family subscale of the FACT-Br correlates moderately low with the score of the FPQLI-C (r =0.27, p =0.20). Instances of low correlation suggest that each QoL instrument has distinct domains that measure aspects of QoL not found in the other.
Table 2.
Pearson correlation matrix for subscale and total scores of the FACT-Br and FPQLI-C for 26 brain cancer patients
| Ferrans and Powers quality of life index—cancer |
|||||
|---|---|---|---|---|---|
| Functional assessment of cancer therapy—brain | Health and functioning | Socioeconomic | Psychological/spiritual | Family | Total score |
| Physical well-being | 0.58a | 0.03 | 0.52a | –0.12 | 0.45b |
| Social/family well-being | 0.12 | 0.25 | 0.26 | 0.42b | 0.27 |
| Emotional well-being | 0.51b | 0.11 | 0.58a | 0.25 | 0.51b |
| Functional well-being | 0.59a | 0.23 | 0.50b | 0.06 | 0.52a |
| Brain cancer subscale | 0.49b | 0.46b | 0.29 | 0.11 | 0.45b |
| Total score | 0.79a | 0.42b | 0.69a | 0.21 | 0.74a |
Twenty-four patients completed the FACT-Br and all 26 patients completed the FPQLI-C
p < 0.01
p < 0.05
Quality of life score statistics
The descriptive statistics for the FACT-Br and FPQLI-C scales are summarized in Tables 3 and 4. Mean scores varied by survey type but were not statistically significant for either QoL instrument (data not shown). The Cronbach's alpha coefficients varied by survey type but each scale demonstrated good internal consistency.
Table 3.
Study-based statistics and mean scores for FACT-Br and patient factors
| Mean (SD) | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Totala | Physical | Social/family | Emotional | Functional | Brain |
| FACT-Br | ||||||
| Score rangeb | 90–151 | 3–26 | 13–27 | 6–24 | 6–23 | 34–69 |
| Overall Mean (SD) | 122.0 (16.6) | 19.0 (5.2) | 21.1 (3.6) | 16.3 (4.9) | 14.4 (4.3) | 51.2 (9.4) |
| Cronbach's alpha | 0.70 | 0.77 | 0.82 | 0.80 | 0.78 | 0.79 |
| Patient | ||||||
| Age at survey | ||||||
| 33–49 years | 109.9 (16.5)c | 15.1 (7.3)c | 19.9 (4.2) | 13.0 (4.7)c | 11.0 (4.3)c | 50.9 (9.0) |
| >50 years | 126.9 (14.3) | 20.4 (3.1) | 21.4 (3.5) | 17.2 (4.7) | 15.3 (3.8) | 51.4 (9.6) |
| Gender | ||||||
| Male | 122.9 (12.9) | 19.8 (3.4) | 20.5 (3.3) | 16.5 (4.3) | 14.2 (3.3) | 52.1 (9.0) |
| Female | 120.8 (20.7) | 18.0 (6.5) | 21.5 (4.2) | 15.6 (5.7) | 14.1 (5.2) | 50.3 (9.8) |
| Race/ethnicity | ||||||
| White | 122.0 (16.9) | 19.6 (4.0)c | 20.9 (3.5) | 15.9 (5.2) | 14.1 (4.3) | 50.7 (9.4) |
| Other | 122.0 (17.7) | 14.0 (10.1) | 21.3 (5.5) | 17.0 (3.0) | 14.7 (4.9) | 55.0 (8.0) |
| Education | ||||||
| Completed college | 123.1 (18.0) | 18.7 (6.6) | 21.1 (4.2) | 15.3 (4.7) | 13.5 (5.2) | 54.3 (7.2)c |
| Not college grad | 120.7 (15.5) | 19.1 (3.0) | 20.8 (3.2) | 16.8 (5.3) | 14.8 (2.9) | 47.9 (10.3) |
| Handedness | ||||||
| Right | 122.8 (17.1) | 19.0 (5.3) | 21.2 (3.6) | 15.8 (5.1) | 14.2 (4.5) | 51.8 (9.4) |
| Other | 113.0 (5.7) | 18.0 (0.0) | 18.6 (5.1) | 18.5 (0.7) | 13.0 (0.) | 44.9 (2.7) |
| Living arrangement | ||||||
| With others | 122.1 (17.3) | 18.9 (5.4) | 21.2 (3.8) | 16.5 (5.1) | 14.2 (4.4) | 51.3 (9.8) |
| Alone | 121.1 (2.7) | 19.2 (1.8) | 19.3 (2.7) | 12.8 (2.3) | 13.5 (4.3) | 50.8 (4.3) |
| Marital status | ||||||
| Married/with a partner | 122.6 (17.6) | 18.9 (5.6) | 21.1 (3.9) | 16.4 (5.2) | 14.2 (4.5) | 52.0 (9.4) |
| Single/widowed | 117.7 (6.2) | 18.9 (1.5) | 20.2 (2.9) | 14.1 (3.2) | 14.0 (3.9) | 47.1 (8.2) |
| Children (minors) | ||||||
| Yes | 118.4 (17.5) | 17.3 (8.2) | 20.3 (3.9) | 13.2 (4.2)c | 12.5 (5.6) | 55.1 (5.6) |
| No | 123.8 (16.3) | 19.7 (2.8) | 21.3 (3.6) | 17.4 (4.8) | 14.8 (3.5) | 49.4 (10.1) |
| Children (adults) | ||||||
| Yes | 124.7 (15.8) | 20.3 (3.3)c | 21.3 (3.5) | 16.6 (4.5) | 15.3 (3.8)c | 49.9 (10.0) |
| No | 115.5 (19.4) | 14.2 (7.1) | 21.2 (3.5) | 14.0 (6.4) | 11.2 (4.4) | 55.0 (6.6) |
| Lifetime occupation | ||||||
| White collar | 119.6 (16.4) | 18.7 (5.5) | 21.5 (3.8) | 15.9 (5.0) | 14.1 (4.6) | 49.5 (9.2)c |
| Blue collar | 134.0 (12.6) | 20.5 (3.1) | 19.5 (2.7) | 18.3 (4.6) | 15.5 (2.3) | 59.5 (5.9) |
| Employment status | ||||||
| Employed | 118.6 (16.2) | 19.8 (4.0) | 21.3 (3.8) | 14.9 (4.8) | 14.6 (4.2) | 48.0 (10.8) |
| Other | 125.9 (16.8) | 18.0 (6.1) | 20.6 (3.6) | 17.3 (5.1) | 13.6 (4.5) | 54.7 (5.8) |
| Health insurance | ||||||
| Private | 120.5 (18.0) | 18.4 (5.9) | 21.8 (3.3) | 15.6 (5.5) | 13.6 (4.3) | 51.1 (10.8) |
| Other | 125.6 (12.7) | 19.9 (5.9) | 19.2 (4.0) | 16.9 (3.7) | 15.1 (4.3) | 51.6 (5.1) |
| Income, annual, h | ||||||
| <$100,000 | 123.8 (13.3) | 18.8 (6.2) | 22.5 (3.5) | 17.5 (3.3) | 15.5 (3.9) | 49.3 (9.1) |
| ≥$100,000 | 123.5 (18.1) | 19.7 (4.7) | 19.9 (3.8) | 16.0 (5.5) | 13.4 (4.8) | 54.6 (8.6) |
| Type of surgical resection | ||||||
| Gross/total | 123.2 (20.7) | 18.3 (6.7) | 21.2 (3.0) | 15.6 (5.6) | 14.7 (4.5) | 53.4 (10.4) |
| Subtotal/biopsy | 120.8 (11.9) | 19.4 (3.3) | 20.7 (4.3) | 16.5 (4.5) | 13.7 (4.3) | 49.2 (7.8) |
| Laterality | ||||||
| Left | 122.1 (17.4) | 18.6 (5.6) | 20.7 (4.1) | 15.5 (5.5) | 14.6 (3.5) | 51.5 (10.4) |
| Other | 121.9 (16.4) | 19.3 (4.7) | 21.3 (3.3) | 16.7 (4.4) | 13.6 (5.2) | 50.9 (7.9) |
| Primary site | ||||||
| Frontal/temporal | 124.6 (17.2) | 19.9 (4.0)c | 20.6 (3.5) | 15.9 (5.4) | 14.9 (4.2)c | 52.3 (9.8) |
| Other | 112.2 (9.6) | 14.8 (7.3) | 22.6 (4.3) | 16.6 (2.6) | 11.0 (3.5) | 47.2 (5.2) |
| Histology | ||||||
| Glioblastoma | 121.4 (16.9) | 18.6 (5.2) | 21.4 (4.0) | 16.3 (5.3) | 14.4 (4.0) | 50.6 (10.0) |
| Other | 124.6 (17.0) | 20.0 (5.0) | 19.3 (1.6) | 15.0 (3.4) | 12.8 (5.8) | 53.7 (4.9) |
| Tumor size (mm) | ||||||
| ≤36 | 122.0 (17.1) | 18.3 (5.6) | 21.2 (3.7) | 16.8 (4.7) | 13.8 (4.8) | 50.9 (9.2) |
| >37 | 122.0 (16.9) | 20.3 (3.8) | 20.5 (3.9) | 14.5 (5.4) | 14.8 (3.0) | 52.1 (9.8) |
| Length of hospital stay | ||||||
| 0–3 days | 125.6 (15.9) | 19.4 (3.3) | 21.6 (3.4) | 15.4 (3.9) | 13.0 (4.1) | 53.6 (9.2) |
| ≥4 days | 123.6 (18.6) | 18.5 (4.6) | 21.7 (3.4) | 16.4 (6.8) | 15.8 (4.9) | 51.3 (11.2) |
| Disposition discharge | ||||||
| Home, indep. | 128.1 (17.1) | 19.5 (4.4) | 21.2 (3.7) | 14.7 (4.9) | 15.1 (5.2) | 55.7 (7.7) |
| Other | 123.0 (19.4) | 19.3 (4.5) | 22.1 (1.6) | 18.8 (6.1) | 13.5 (4.5) | 49.3 (12.8) |
| Postneurodiagnostic results | ||||||
| Evidence of tumor | 123.7 (18.3) | 18.5 (6.1) | 21.2 (3.3) | 17.3 (5.8) | 14.6 (4.4) | 52.0 (9.3) |
| No evidence of tumor | 119.1 (17.2) | 18.7 (4.7) | 20.9 (3.2) | 14.9 (4.2) | 14.4 (4.5) | 50.1 (10.7) |
| Comorbid condition | ||||||
| None | 114.5 (16.7) | 15.3 (6.3) | 20.9 (3.0) | 14.1 (6.8) | 12.9 (3.8) | 51.4 (8.8) |
| ≥1 | 125.7 (15.7) | 20.6 (3.4) | 21.0 (4.0) | 16.9 (3.7) | 14.7 (4.5) | 51.2 (9.7) |
| Chemotherapy ended by survey completion | ||||||
| Yes | 119.8 (21.4) | 17.5 (9.7) | 23.5 (7.0) | 16.7 (1.9) | 12.8 (6.8) | 49.3 (8.5) |
| No | 122.4 (16.1) | 19.2 (4.1) | 20.5 (2.7) | 15.9 (5.4) | 14.4 (3.9) | 51.6 (9.5) |
| Physical therapist | ||||||
| Yes | 122.6 (20.5) | 17.9 (7.0) | 23.5 (3.7)c | 15.8 (4.1) | 13.4 (5.6) | 49.7 (10.8) |
| No | 121.7 (15.4) | 19.4 (4.1) | 19.8 (3.1) | 16.1 (5.4) | 14.5 (3.6) | 52.0 (8.6) |
| Occupational therapist | ||||||
| Yes | 123.5 (17.5) | 17.1 (7.6) | 22.2 (4.8) | 17.1 (4.8) | 14.7 (5.4) | 49.1 (8.4) |
| No | 121.6 (16.8) | 19.5 (4.2) | 20.6 (3.3) | 15.7 (5.1) | 13.9 (4.0) | 51.9 (9.6) |
| Speech pathologist | ||||||
| Yes | 128.5 (15.2) | 20.3 (3.4) | 21.5 (3.8) | 18.3 (4.8) | 17.3 (2.9) | 51.3 (14.4) |
| No | 120.7 (16.9) | 18.6 (5.4) | 20.9 (3.8) | 15.6 (5.0) | 13.6 (4.3) | 51.2 (8.4) |
| Time from diagnosis to survey | ||||||
| 3–7 months | 127.3 (15.6)c | 21.0 (3.5)c | 21.0 (4.1) | 18.0 (3.8)c | 14.7 (4.5) | 52.6 (9.3) |
| >8 months | 113.0 (14.8) | 15.8 (5.7) | 20.9 (3.1) | 13.1 (5.2) | 13.4 (4.0) | 49.1 (9.1) |
| Time from surgery and radiation therapy to survey | ||||||
| 1–4 months | 125.7 (14.8) | 20.9 (3.6) | 20.9 (4.3) | 17.6 (3.5)c | 14.6 (4.7) | 51.7 (8.9) |
| >5 months | 116.7 (18.2) | 16.3 (5.7) | 21.0 (2.9) | 14.0 (5.9) | 13.6 (3.9) | 50.7 (10.0) |
| Ease of travel to/from doctor visits/treatment | ||||||
| Very easy/easy | 131.0 (15.4)c | 20.6 (4.7) | 21.0 (3.4) | 16.7 (4.5) | 16.1 (4.8)c | 56.6 (6.2)c |
| Other | 114.3 (13.8) | 17.5 (5.2) | 20.9 (4.0) | 15.5 (5.4) | 12.7 (3.3) | 47.0 (9.2) |
| Perception of income | ||||||
| “I can save/I get enough for my needs” | 126.7 (17.2)c | 19.9 (4.1) | 21.6 (3.5) | 16.0 (5.2) | 15.4 (4.6)c | 53.7 (8.7) |
| Other | 115.3 (13.9) | 17.6 (6.1) | 20.2 (3.9) | 16.0 (4.9) | 12.6 (3.5) | 48.0 (9.3) |
| Out-of-pocket expenses | ||||||
| Very manageable | 133.2 (16.1)c | 21.7 (2.7)c | 22.4 (3.8) | 17.2 (3.8) | 17.3 (4.9)c | 54.6 (10.0) |
| Other | 117.3 (14.8) | 17.8 (5.4) | 20.4 (3.6) | 15.6 (5.4) | 13.0 (3.5) | 49.9 (8.8) |
| Treatment expectation | ||||||
| Be cured of my cancer | 121.9 (20.2) | 19.1 (6.5) | 21.0 (4.5) | 16.2 (4.7) | 14.5 (3.8) | 50.8 (10.8) |
| Other | 122.0 (14.2) | 18.8 (4.2) | 20.9 (3.2) | 15.9 (5.3) | 13.9 (4.7) | 51.5 (8.4) |
| Prognosis explained | ||||||
| Very well | 117.2 (11.4) | 18.3 (5.5) | 21.3 (3.2) | 16.3 (5.6) | 14.2 (2.5) | 47.0 (7.9)c |
| Other | 127.6 (20.3) | 19.4 (4.8) | 20.6 (4.2) | 15.8 (4.4) | 14.1 (5.9) | 55.9 (8.5) |
| Current health | ||||||
| Same/better | 115.3 (14.4) | 18.4 (3.9) | 19.9 (4.1) | 14.9 (4.3) | 12.9 (3.9) | 49.2 (9.2) |
| Worse | 125.3 (17.0) | 19.1 (5.7) | 21.5 (3.5) | 16.6 (5.3) | 14.8 (4.6) | 52.2 (9.3) |
FACT-Br Functional Assessment of Cancer Therapy—Brain, based on 24 questionnaires
The total FACT-Br score is the summation of the four general subscales and the brain subscale scores
Score range for FACT-Br: 0–28 (Physical, Social/Family, Functional well-being); 0–24 (Emotional well-being); 0–76 (Brain cancer subscale); 0–184 (TOTAL)
p <0.10
Table 4.
Study-based statistics and mean scores for FPQLI-C and patient factors
| Mean (SD) | |||||
|---|---|---|---|---|---|
| Characteristics | Total | Health/functioning | Socio-economic | Phychological/spiritual | Family |
| FPQLI-C | |||||
| Score rangea | 8–25 | 9–25 | 5–25 | 4–29 | 11–30 |
| Overall mean | 18.8 (4.3) | 16.6 (4.6) | 19.7 (4.3) | 18.0 (6.7) | 24.2 (4.9) |
| Cronbach's alpha | 0.84 | 0.88 | 0.92 | 0.88 | 0.90 |
| Patient | |||||
| Age at survey | |||||
| 33–49 years | 16.5 (3.9)b | 13.7 (5.2)b | 18.7 (2.7) | 13.5 (6.4)b | 24.3 (5.1) |
| >50 years | 19.6 (4.2) | 17.6 (4.1) | 20.1 (4.7) | 19.6 (6.2) | 24.2 (5.0) |
| Gender | |||||
| Male | 19.4 (3.2) | 17.0 (3.9) | 20.3 (2.8) | 18.5 (5.7) | 25.3 (3.8) |
| Female | 18.2 (5.2) | 16.1 (5.4) | 19.1 (5.4) | 17.5 (7.8) | 23.1 (5.7) |
| Race/ethnicity | |||||
| White | 18.4 (4.1) | 16.1 (4.2) | 19.6 (4.5) | 17.5 (6.5) | 23.5 (4.8)b |
| Other | 22.0 (4.7) | 20.3 (7.1) | 20.4 (0.8) | 21.5 (8.7) | 29.5 (0.9) |
| Education | |||||
| Completed college | 17.9 (4.5) | 15.5 (4.5) | 20.0 (5.1) | 15.9 (6.6)b | 23.8 (5.4) |
| Not college grad | 19.8 (4.0) | 17.8 (4.7) | 19.4 (3.3) | 20.4 (6.2) | 24.6 (4.5) |
| Handedness | |||||
| Right | 18.7 (4.3) | 16.4 (4.8) | 19.5 (4.4) | 18.0 (6.6) | 24.3 (4.7) |
| Other | 19.8 (5.7) | 18.1 (4.1) | 22.3 (2.0) | 17.4 (10.6) | 23.7 (9.0) |
| Living arrangement | |||||
| With others | 19.7 (3.6)b | 17.2 (4.7) | 20.8 (2.8)b | 19.1 (6.2)b | 25.5 (3.7)b |
| Alone | 13.6 (4.2) | 13.1 (2.7) | 13.9 (6.7) | 11.9 (6.7) | 16.9 (4.3) |
| Marital status | |||||
| Married/with a partner | 19.9 (3.7)b | 17.3 (4.8)b | 20.9 (2.7)b | 19.3 (6.3)b | 25.6 (3.8)b |
| Single/widowed | 14.2 (3.9) | 13.4 (2.4) | 14.6 (5.9) | 12.6 (6.0) | 18.3 (4.9) |
| Children (minors) | |||||
| Yes | 18.5 (3.7) | 16.2 (5.4) | 20.7 (2.5) | 15.6 (6.2) | 25.1 (4.4) |
| No | 18.9 (4.6) | 16.7 (4.5) | 19.2 (4.9) | 19.0 (6.8) | 23.8 (5.2) |
| Children (adults) | |||||
| Yes | 19.4 (4.4) | 17.5 (4.6) | 19.4 (4.6) | 19.4 (6.0)b | 24.2 (5.0) |
| No | 17.4 (4.2) | 14.1 (4.6) | 21.1 (3.0) | 14.1 (11.4) | 24.6 (5.2) |
| Lifetime occupation | |||||
| White collar | 18.8 (3.3) | 16.1 (4.1) | 20.7 (2.9)b | 17.6 (5.6) | 24.7 (4.1) |
| Blue collar | 19.1 (7.9) | 18.7 (7.1) | 17.2 (7.1) | 19.1 (5.6) | 23.0 (7.8) |
| Employment status | |||||
| Employed | 18.4 (2.7) | 15.9 (3.5) | 20.3 (2.7) | 17.0 (4.4) | 24.1 (3.6) |
| Other | 19.1 (5.5) | 17.3 (5.6) | 19.1 (5.4) | 18.9 (8.5) | 24.3 (6.1) |
| Health insurance | |||||
| Private | 19.4 (3.5) | 16.7 (4.7) | 20.9 (2.8)b | 18.4 (6.0) | 25.9 (3.4)b |
| Other | 17.5 (5.4) | 16.4 (4.9) | 17.5 (5.8) | 17.3 (8.2) | 21.0 (5.9) |
| Income, annual (h) | |||||
| <$100,000 | 18.7 (5.3) | 17.0 (5.4) | 18.5 (5.0)b | 18.1 (7.7) | 24.1 (6.0) |
| ≥100,000 | 19.0 (3.3) | 16.4 (4.0) | 21.5 (2.4) | 17.6 (6.3) | 24.1 (4.1) |
| Type of surgical resection | |||||
| Gross/total | 19.4 (4.2) | 17.3 (5.3) | 21.2 (2.6)b | 17.6 (7.1) | 24.6 (5.0) |
| Subtotal/biopsy | 18.3 (4.4) | 16.0 (4.2) | 18.4 (5.0) | 18.3 (6.6) | 23.8 (4.9) |
| Laterality | |||||
| Left | 19.3 (3.7) | 17.2 (4.6) | 20.0 (3.2) | 18.7 (6.2) | 24.7 (4.3) |
| Other | 18.2 (5.0) | 15.9 (4.8) | 19.4 (5.4) | 17.2 (7.4) | 23.7 (5.7) |
| Primary site | |||||
| Frontal/temporal | 18.7 (4.6) | 16.9 (4.9) | 19.4 (4.7) | 17.7 (7.0) | 23.5 (5.0) |
| Other | 19.3 (2.7) | 15.1 (3.4) | 20.8 (1.9) | 19.4 (5.8) | 27.4 (3.3) |
| Histology | |||||
| Glioblastoma | 19.0 (4.5) | 16.6 (4.9) | 20.2 (4.3) | 18.1 (7.1) | 24.4 (5.2) |
| Other | 18.1 (3.1) | 16.5 (3.9) | 17.7 (3.8) | 17.4 (5.2) | 23.4 (3.8) |
| Tumor size (mm) | |||||
| ≤36 | 19.0 (4.9) | 17.1 (5.1) | 19.9 (4.7) | 17.9 (7.7) | 23.9 (5.7) |
| ≥37 | 18.3 (2.4) | 15.3 (3.3) | 19.3 (3.5) | 18.1 (4.1) | 24.9 (2.1) |
| Length of hospital stay | |||||
| 0–3 days | 18.0 (4.9) | 15.9 (4.4) | 18.2 (5.9) | 17.3 (6.7) | 24.2 (6.2) |
| ≥4 days | 20.1 (4.7) | 17.9 (5.7) | 22.0 (2.5) | 19.4 (8.3) | 24.0 (4.8) |
| Disposition discharge | |||||
| Home, indep. | 20.0 (4.1) | 17.8 (5.3) | 21.2 (3.5) | 19.2 (6.6) | 25.1 (4.3) |
| Other | 17.7 (6.5) | 16.2 (5.2) | 18.1 (7.3) | 17.3 (9.7) | 21.4 (7.5) |
| Postneurodiagnostic results | |||||
| Evidence of tumor | 19.0 (4.9) | 16.8 (5.2) | 19.5 (5.2) | 18.4 (7.4) | 25.0 (5.1) |
| No evidence of tumor | 18.1 (3.9) | 16.1 (4.2) | 21.3 (2.4) | 15.4 (6.6) | 22.3 (5.3) |
| Comorbid condition | |||||
| None | 18.7 (4.3) | 15.2 (5.5) | 21.1 (3.3) | 17.1 (7.3) | 26.1 (4.7) |
| ≥1 | 18.8 (4.4) | 17.1 (4.3) | 19.1 (4.6) | 18.4 (6.6) | 23.4 (4.9) |
| Chemotherapy ended by survey completion | |||||
| Yes | 19.2 (4.2) | 16.1 (5.4) | 20.7 (3.3) | 18.3 (6.5) | 26.1 (3.6) |
| No | 18.7 (4.4) | 16.6 (4.7) | 19.5 (4.5) | 17.9 (6.9) | 23.9 (5.1) |
| Physical therapist | |||||
| Yes | 18.2 (5.0) | 16.1 (4.5) | 18.9 (6.1) | 17.6 (6.7) | 23.4 (6.3) |
| No | 19.1 (4.0) | 16.8 (4.9) | 20.1 (3.1) | 18.2 (7.0) | 24.6 (4.1) |
| Occupational therapist | |||||
| Yes | 18.5 (6.2) | 17.3 (5.9) | 18.0 (6.7) | 18.2 (6.6) | 22.8 (7.3) |
| No | 18.9 (3.5) | 16.3 (4.3) | 20.3 (3.0) | 17.9 (6.1) | 24.7 (3.8) |
| Speech pathologist | |||||
| Yes | 21.4 (3.7) | 19.7 (4.1) | 21.7 (1.2) | 21.8 (5.8) | 24.5 (5.6) |
| No | 18.3 (4.3) | 16.0 (4.6) | 19.3 (4.5) | 17.3 (6.7) | 24.2 (4.9) |
| Time from diagnosis to survey | |||||
| 3–7 months | 20.2 (3.4)b | 18.1 (4.0)b | 20.8 (2.7) | 19.7 (5.8) | 25.2 (3.6) |
| >8 months | 16.9 (4.7) | 14.4 (4.8) | 18.3 (5.6) | 15.6 (7.3) | 22.8 (6.2) |
| Time from surgery & radiation therapy to survey | |||||
| 1–4 months | 19.9 (3.3) | 17.8 (4.0) | 20.6 (2.7) | 19.1 (5.5) | 25.2 (3.7) |
| >5 months | 17.5 (5.0) | 15.1 (5.1) | 18.7 (5.5) | 16.7 (8.0) | 23.0 (5.9) |
| Ease of travel to/from doctor visits/treatment | |||||
| Very easy/easy | 20.8 (3.7)b | 18.7 (5.1)b | 21.2 (2.6) | 20.7 (6.0)b | 25.6 (3.8) |
| Other | 17.3 (4.2) | 15.0 (3.8) | 18.6 (5.0) | 16.0 (8.0) | 23.2 (5.4) |
| Perception of incomea | |||||
| “I can save/I get enough for my needs” | 19.9 (3.6) | 17.3 (4.4) | 22.1 (2.0) | 19.0 (6.4) | 24.3 (4.5) |
| Other | 17.5 (4.8) | 15.7 (5.0) | 16.9 (4.5) | 16.8 (7.1) | 24.1 (5.5) |
| Out-of-pocket expenses | |||||
| Very manageable | 20.6 (2.9) | 18.1 (4.2) | 22.3 (2.1)b | 20.8 (3.9) | 24.5 (4.2) |
| Other | 18.1 (4.5) | 16.0 (4.8) | 18.8 (4.5) | 17.0 (7.3) | 24.1 (5.2) |
| Treatment expectation | |||||
| Be cured of my cancer | 17.6 (5.3) | 16.2 (5.5) | 18.0 (5.1)b | 15.9 (7.8) | 23.2 (5.6) |
| Some cancer left/symptoms left/no expectations | 19.6 (3.2) | 16.9 (4.1) | 21.0 (3.1) | 19.5 (5.5) | 25.0 (4.4) |
| Prognosis explained | |||||
| Very well | 17.0 (3.6)b | 14.6 (3.1)b | 18.5 (4.8) | 15.4 (5.8)b | 23.3 (5.4) |
| Other | 20.8 (4.2) | 18.9 (5.2) | 21.1 (3.3) | 20.9 (6.6) | 25.3 (4.2) |
| Current health | |||||
| Same/better | 19.3 (4.6) | 16.8 (5.7) | 19.3 (3.0) | 18.9 (6.8) | 26.2 (3.9) |
| Worse | 19.2 (3.3) | 16.9 (4.1) | 20.7 (3.2) | 18.4 (6.1) | 24.1 (4.2) |
FPQLI-C Ferrans and Powers Quality of Life Index–cancer; scores based on all 26 questionnaires
Score range for FP-QLIC: 0–30 for overall and all domains
p < 0.10
For all patients, the overall mean for the FACT-Br scale was 122.0 out of a possible maximum score of 184. Patients scored lowest on the functioning well-being scale and scores ranged from 50.7 to 52.1 % of the total subscale score. The overall alpha coefficients ranged from 0.70 to 0.82 which were higher or comparable to results based on the validation sample of newly diagnosed and recurrent brain cancer patients for the instrument [physical: 0.76; social/family: 0.69; emotional: 0.75; functional: 0.84; brain cancer concerns: 0.84] [11].
For FPQLI-C, the mean scores for all patients ranged from 16.6 to 24.2 on a 30-point scoring scale. Patients scored the lowest on the health/functioning subscale and the highest on the family subscale. The validation study for the FPQLI-C, based on a sample of breast cancer patients, reported alpha coefficients for the total scale and the four subscales that ranged from 0.66 to 0.95; the family subscale had the lowest internal consistency [12]. Based on all patients in this study, the alpha coefficient for the total scale was 0.84 with coefficients for the subscales ranging from 0.88 to 0.92.
Association between FACT-Br and patient characteristics
Significant associations (p <0.10) between FACT-Br scores and various patient factors are shown in Table 3. Having adult children was associated with higher physical well-being (p =0.0084) and functional well-being scores (p =0.0348). Conversely, having minors was associated with worse emotional well-being [yes=13.2 (SD 4.2) vs. no=17.4 (SD 4.8), p =0.0479]. Longer duration from time of diagnosis to survey completion was associated with lower QoL scores in three of the six outcome measures (overall [127.3 (SD 15.6) vs. 113.0 (SD 14.8), p =0.0372], physical well-being [21.0 (SD 3.5) vs. 15.8 (SD 5.7), p =0.0085], emotional well-being [18.0 (SD 3.8) vs. 13.1 (SD 5.2), p = 0.0108]). There was a negative association (lower QoL) between how well chances for survival was explained and the brain subscale [very well=47.0 (SD 7.9) vs. somewhat well, not very well=55.9 (SD 8.5), p =0.0123]. Patients that perceived they “could save” or “had enough for their needs” had higher overall QoL scores [126.7 (SD 17.2) vs. 115.3 (SD 13.9), p =0.0978] and functional well-being scores [15.4 (SD 4.6) vs. 12.6 (SD 3.5), p =0.0978] than patients that were “just able to make ends meet”, “didn't get enough for their needs”, or were “in desperate need”.
Association between FPQLI-C and patient characteristics
The FPQLI-C was significantly associated (p <0.10) with many nonclinical patient characteristics and two clinical characteristic, type of surgical resection and time from diagnosis to survey (Table 4). There was a positive association between being married and higher QoL scores (indicating better QoL), overall [19.9 (SD 3.7) vs. 14.2 (SD 3.9), p =0.0054] and for every domain. Similar to the FACT-Br, patients with longer duration from time of diagnosis (≥8 months) to survey completion had lower QoL than patients who were more recently diagnosed when surveyed [health/functioning 18.1 (SD 4.0) vs. 14.4 (4.8), p =0.0446]. Having adult children was associated with higher QoL scores for the psychological/spiritual domain (p =0.0912). Gross resection rather than subtotal or biopsy only was associated with higher socioeconomic scores [gross resection=21.2 (SD 2.6) vs. 18.4 (SD 5.0), p =0.0891].
Content analysis
Twenty-four of the 26 patients (92.3 %) responded to the question, “What does quality of life mean to you?” and selected responses are displayed in Table 5. Patients’ responses encompassed five major themes, or aspects of life: functional/functioning, emotional/psychological, physical/health, economic, and social/family, with no one identifying QoL with spiritual well-being. The two most common QoL themes were functional/functioning (75 %) and emotional/psychological (58.3 %), of which more than half of respondents were females [functional/functioning (57.1 %); and emotional/psychological (61.1 %)]. Responses related to emotional/psychological QoL aspects had three subthemes relating to fear/worry, coping, and happiness. Other subthemes included active/energy, independence, pain, and good health. Seventy-five percent of respondents who identified QoL with economic aspects were men. As expressed by one male patient, QoL meant the: “Ability to continue providing for my family and participating in family activities. I am the family sole provider”. For one patient quality of life meant: “(having) good compassionate doctors”. While many patients’ responses covered multiple themes, some responses were focused on a single theme: “Live happy” and “Being able to take care of myself—i.e.: preparing food, shopping, dressing myself, basic needs”. When asked, “What type of brain tumor do you have?”, only 69.2 % correctly identified their tumor type (data not shown).
Table 5.
Themes, patterns, and selected responses based on response to “What does quality of life mean to you?”
| Theme | Selected response |
|---|---|
| Functional/functioning | |
| n = 18 (75 %) | Active/energy |
| “Being active” | |
| “Freedom in motility” | |
| “To be active and helpful to others” | |
| Independence | |
| “To be able to do things by yourself” | |
| “That I have the ability to function as a husband” | |
| “Being independent” | |
| “To be able to function as normal as I can...” | |
| Emotional/psychological | |
| n = 14 (58.3 %) | Fear/worry |
| “not being afraid” | |
| “...living without the fear of dying soon” | |
| “...no trouble about the future” | |
| Coping | |
| “...how you feel and try you get through it” | |
| “how well I can live emotionally” | |
| Happiness | |
| “Being happy most of the time” | |
| “to have a positive outlook on life” | |
| “I would like to be ...emotionally content” | |
| Physical/health | |
| n = 12 (50 %) | Pain |
| “living without pain” | |
| “being pain free” | |
| Good health | |
| “to feel good and healthy” | |
| “I would like to be physically well” | |
| Economic | |
| n = 8 (30.8 %) | “...have no economic concerns of any type” |
| “Financial stability” | |
| “...able to afford what I want to do and have” | |
| Social/family | “how you affect the rest of your family” |
| n = 8 (30.8 %) | “I also feel sorry for my husband who has to carry the load while I recuperate” |
| “...ability to do as many things as possible with my wife and to help her a small percentage of how she helps me” | |
| “Being able to be with my wife, family and friends (and dog)” | |
Discussion
Overview of results
Quality of life, a patient-reported outcome, provides a subjective assessment of different aspects of an individual's life and is becoming an increasingly common tool in cancer management. In this study, several significant associations with non-clinical patient characteristics (age, living arrangement, educational status, children, health insurance, explanation of prognosis) were observed for the overall scale or at least one of the domains for both FACT-Br and FPQLI-C. Of note, living alone and having underage children were associated with poorer QoL while having adult children was associated with better QoL. QoL scores as measured by FPQLI-C were most often affected by living arrangement while those for FACT-Br were most often affected by time from diagnosis to survey. Of the 19 individual variables identified in this study as being significantly associated with the QoL measures, several variables (age, race/ethnicity, educational status, adult children, lifetime occupation, time from diagnosis to survey, ease of travel to/from medical visits, manageability of out-of-pocket expense, prognosis explained) were identified with both measures. Patients’ meaning of QoL differed by gender, however most patients viewed it as affecting multiple aspects of their lives.
Sociodemographic factors
Sociodemographics as possible QoL determinants are understudied in this population. Some brain cancer studies failed to find an association with age and overall QoL [10, 17] but we found a trend towards poorer QoL in the younger cohort. Our findings of no association between QoL and gender agree with Brown et al. [18] but contradict another study that reported a negative association for females [10]. However, we did find a trend towards poorer QoL for females for most domains. This phenomenon may be explained by the tendency for females to continue their daily duties (or feel obligated to) despite illness [19]. Two major findings from Glantz et al. [20] concluded that females were more likely to be abandoned by their partner after a serious medical illness than males and that regardless of gender, separation or divorce adversely affected quality of life and quality of care. None of our study participants were separated or divorced at the time of survey (range 3-19 months) which may explain why study participants scored within the mid to high range of the scales. We found that patients with adult children scored significantly higher on physical and functional well-being and psychological/ spiritual subscales while those with underage children scored significantly lower on emotional well-being scales. These findings may be explained by adult children's capacity to provide support to their parent during this difficult time and the patients’ inability to cope with the prospect of leaving their underage children without a parent. Although these findings have not been replicated in other studies, two breast cancer studies found that having underage children was a significant predictor of negative changes in QoL after surgery [21] and those with unmarried children had significantly lower QoL scores [22], which lends credence to our hypothesis.
Clinical factors
There is reasonable consensus that clinical factors are associated with survival but less evidence that these factors are associated with QoL. Unlike Giovagnoli et al. [23] who found no association between primary site and QoL, our results showed that primary site was significantly associated with functional well-being (FACT-Br). Shorter length of time from diagnosis to survey (<8 months) had a significant positive effect on several QoL domains. Similarly, shorter length of time from completion of radiation to survey was associated with better physical well-being (FACT-Br). Progressive decline in neurological and cognitive function followed by death is the disease course for malignant gliomas [24]. Perhaps patients that were more recently diagnosed or completed radiation therapy earlier reported better QoL because their disease course had not yet reached a progressive and debilitating state unlike longer-term survivors or they had yet to experience the late effects of radiation. In agreement with some brain tumor studies [25, 26], but contradictory to another study [27], was our finding of no association between QoL and lateralization of the tumor (left, right, or midline symmetry). Gross surgical resection was associated with better QoL as measured by the socioeconomic subscale (FPQLI-C). No other clinical characteristics were associated with QoL (i.e., histology, tumor size). Determining QoL probably has more to do with clinical status (stable vs. unstable) than tumor malignancy or location [23].
Perception/belief factors
To what extent personal beliefs and perceptions effect quality of life is unclear. Studies have demonstrated a direct relationship between annual income and QoL [8, 28, 29]. While this was not supported in the current study, results did show a significant positive association between QoL and higher satisfaction with manageability of out-of-pocket expenses on physical and functional well-being (FACT-Br) subscales and with income perception on functional well-being (FACT-Br) and socioeconomic (FPQLI-C) subscales, respectively. Believing that prognosis was well explained had a significant negative effect (worse QoL) on health/functioning and psychological/spiritual scores (FPQLI-C). High-grade gliomas are associated with markedly short length of survival. Therefore, faced with the realization of a poor prognosis, it is understandable that patients who perceived their prognosis was well explained would have lower QoL scores.
Limitations
The major constraint to this study is the small sample size, which may have limited our ability to detect weaker associations. Because significant tests are sensitive to sample size, a larger sample size would have been needed to detect a weak association. Selection bias is inherent in this study due to nonrandomization. Lack of heterogeneity in treatments completed at time of survey precluded an assessment of the association between time from different therapies and QoL. Our use of a convenience patient sample is surely not representative of patients with high-grade gliomas. While participants and nonparticipants did not differ in terms of age, gender, race/ethnicity, or histology, participants may have been healthier than those that refused. Participants were clinically stable at time of consent and may have still been clinically stable at time of survey. If one were to assume that extremely ill patients would report lower QoL scores then our reported scores may be overestimated. By definition, this cross-sectional study provided information at one point in time. Repeated evaluations at regular intervals, such as in a longitudinal study would be more informative and allow us to assess QoL throughout the disease course.
Conclusion
This study confirms some earlier findings but also identifies new factors that have not been previously explored in high-grade glioma patients. While the FACT-Br is one of the most widely used instruments for reporting QoL in brain tumor patients [30], the FPQLI-C has rarely been used in a brain tumor-specific study. However, we identified QoL determinants using both assessment tools, thus adding validity to our findings. Because the FPQLI-C includes additional domains which proved important in this population, using FPQLI-C may prove valuable in future brain tumor studies. This study also sheds light on the emotional supportive needs of patients with underage children (FACT-BR) and the multiple needs of patients who live alone (FPQLI-C), which is important knowledge for clinicians to have while managing this disease. Finally, inclusion of the qualitative variable, “What does quality of life mean to you?” adds depth to our findings and provides insight into what high-grade glioma patients deem important.
Our findings demonstrate that nonclinical characteristics are associated with QoL in patients with high-grade gliomas. Sociodemographic factors as determinants of QoL have not been examined extensively in this population and individual beliefs have not been examined at all. Given the exploratory and limited nature of this study, it is imperative that further studies on a larger scale be conducted to confirm results identified in this study. A better understanding of the relationship between QoL and sociodemographic and belief factors may have aid in directing prevention and management strategies. Improving quantity of life in patients with high-grade gliomas may currently pose a challenge but efforts should be continually made to improve their QoL.
Acknowledgments
The authors sincerely thank the patients and their families for their participation. We thank the medical and research staff for their assistance, particularly Nina Paleologos, MD; Ayman Omar, MD; Patricia Lada, RN; Mara Motley; Christina Papirnik; and Candice Zahora, RHIA. The authors would also like to thank Bridget J. McCarthy, PhD for her contributions to the development of this study.
Usha Menon is currently at the College of Nursing, Ohio State University, Columbus, OH 43210. John L. Villano is currently at the Division of Medical Oncology, University of Kentucky, Lexington, KY 40506. Faith G. Davis is currently at the Department of Public Health Sciences, School of Public Health, University of Alberta, Edmonton AB T6G 1C9.
Funding Kimberly R. Porter was supported by a National Cancer Institute pre-doctoral fellowship through the cancer prevention and control training grant at the University of Illinois at Chicago (2 R25 CA057699, co-Is Davis, FG and Fitzgibbon, ML).
Abbreviations
- QoL
Health-related quality of life
- FACT-Br
Functional Assessment of Cancer Therapy for brain tumor patients
- FPQLI-C
Ferrans and Powers Quality of Life Index Cancer
Footnotes
Present Address: K. R. Porter Department of Research and Evaluation, Kaiser Permanente Southern California, 100 South Los Robles, 2nd Fl, Pasadena, CA 91101, USA
Conflict of Interest The authors declare that they have no competing interests.
Disclosures None.
Contributor Information
Kimberly R. Porter, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois at Chicago, Chicago, IL 60612, USA
Usha Menon, College of Nursing and Health Innovation, Arizona State University, Phoenix, AZ 85004, USA.
Nicholas A. Vick, Neurology, NorthShore University HealthSystem, Evanston, IL 60201, USA
John L. Villano, Department of Medicine, University of Illinois at Chicago, Chicago, IL 60612, USA
Michael L. Berbaum, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois at Chicago, Chicago, IL 60612, USA
Faith G. Davis, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois at Chicago, Chicago, IL 60612, USA
References
- 1.Koshy M, Villano JL, Dolecek TA, Howard A, Mahmood U, Chmura SJ, Weichselbaum RR, McCarthy BJ. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neuro-Oncol. 2012;107(1):207–212. doi: 10.1007/s11060-011-0738-7. doi:10.1007/s11060-011-0738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. doi:10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Fox SW, Lyon D, Farace E. Symptom clusters in patients with high-grade glioma. J Nurs Scholarsh Off Publ Sigma Theta Tau Int Honor Soc Nurs Sigma Theta Tau. 2007;39(1):61–67. doi: 10.1111/j.1547-5069.2007.00144.x. [DOI] [PubMed] [Google Scholar]
- 5.Taphoorn MJ, Stupp R, Coens C, Osoba D, Kortmann R, van den Bent MJ, Mason W, Mirimanoff RO, Baumert BG, Eisenhauer E, Forsyth P, Bottomley A. Health-related quality of life in patients with glioblastoma: a randomised controlled trial. Lancet Oncol. 2005;6(12):937–944. doi: 10.1016/S1470-2045(05)70432-0. doi:10.1016/S1470-2045(05)70432-0. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda T, Aptel I, Exbrayat C, Grosclaude P. Determinants of quality of life of bladder cancer survivors five years after treatment in France. Int J Urol Off J Jpn Urol Assoc. 2003;10(8):423–429. doi: 10.1046/j.1442-2042.2003.00657.x. [DOI] [PubMed] [Google Scholar]
- 7.Sehlen S, Hollenhorst H, Lenk M, Schymura B, Herschbach P, Aydemir U, Duhmke E. Only sociodemographic variables predict quality of life after radiography in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;52(3):779–783. doi: 10.1016/s0360-3016(01)02600-1. [DOI] [PubMed] [Google Scholar]
- 8.Dapueto JJ, Servente L, Francolino C, Hahn EA. Determinants of quality of life in patients with cancer. Cancer. 2005;103(5):1072–1081. doi: 10.1002/cncr.20870. doi:10.1002/cncr.20870. [DOI] [PubMed] [Google Scholar]
- 9.Giovagnoli AR, Tamburini M, Boiardi A. Quality of life in brain tumor patients. J Neuro-Oncol. 1996;30(1):71–80. doi: 10.1007/BF00177445. [DOI] [PubMed] [Google Scholar]
- 10.Weitzner MA, Meyers CA, Byrne K. Psychosocial functioning and quality of life in patients with primary brain tumors. J Neurosurg. 1996;84(1):29–34. doi: 10.3171/jns.1996.84.1.0029. doi:10.3171/jns.1996.84.1.0029. [DOI] [PubMed] [Google Scholar]
- 11.Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75(5):1151–1161. doi: 10.1002/1097-0142(19950301)75:5<1151::aid-cncr2820750515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Ferrans CE. Development of a quality of life index for patients with cancer. Oncol Nurs Forum. 1990;17(3 Suppl):15–19. discussion 20-11. [PubMed] [Google Scholar]
- 13.Cella D, Hernandez L, Bonomi AE, Corona M, Vaquero M, Shiomoto G, Baez L. Spanish language translation and initial validation of the functional assessment of cancer therapy quality-of-life instrument. Med Care. 1998;36(9):1407–1418. doi: 10.1097/00005650-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Mezzich JE, Ruiperez MA, Perez C, Yoon G, Liu J, Mahmud S. The Spanish version of the quality of life index: presentation and validation. J Nerv Mental Dis. 2000;188(5):301–305. doi: 10.1097/00005053-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Ferrans CE. Cancer Nursing: Principles and Practice. 6th edn Jones and Bartlett; Sudbury: 2005. Quality of life as an outcome of cancer care. [Google Scholar]
- 16.Nunnally J. Psychometric theory. 2nd edn. McGraw-Hill; New York: 1978. [Google Scholar]
- 17.Bhat SR, Goodwin TL, Burwinkle TM, Lansdale MF, Dahl GV, Huhn SL, Gibbs IC, Donaldson SS, Rosenblum RK, Varni JW, Fisher PG. Profile of daily life in children with brain tumors: an assessment of health-related quality of life. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(24):5493–5500. doi: 10.1200/JCO.2005.10.190. doi:10.1200/JCO.2005.10.190. [DOI] [PubMed] [Google Scholar]
- 18.Brown PD, Ballman KV, Rummans TA, Maurer MJ, Sloan JA, Boeve BF, Gupta L, Tang-Wai DF, Arusell RM, Clark MM, Buckner JC. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neuro-Oncol. 2006;76(3):283–291. doi: 10.1007/s11060-005-7020-9. doi:10.1007/s11060-005-7020-9. [DOI] [PubMed] [Google Scholar]
- 19.Guner P, Isikhan V, Komurcu S, Il S, Ozturk B, Arpaci F, Ozet A. Quality of life and sociodemographic characteristics of patients with cancer in Turkey. Oncol Nurs Forum. 2006;33(6):1171–1176. doi: 10.1188/06.ONF.1171-1176. doi:10.1188/06.ONF.1171-1176. [DOI] [PubMed] [Google Scholar]
- 20.Glantz MJ, Chamberlain MC, Liu Q, Hsieh CC, Edwards KR, Van Horn A, Recht L. Gender disparity in the rate of partner abandonment in patients with serious medical illness. Cancer. 2009;115(22):5237–5242. doi: 10.1002/cncr.24577. doi:10.1002/cncr.24577. [DOI] [PubMed] [Google Scholar]
- 21.Salonen P, Kellokumpu-Lehtinen PL, Tarkka MT, Koivisto AM, Kaunonen M. Changes in quality of life in patients with breast cancer. J Clin Nurs. 2011;20(1–2):255–266. doi: 10.1111/j.1365-2702.2010.03422.x. doi:10.1111/j.1365-2702.2010.03422.x. [DOI] [PubMed] [Google Scholar]
- 22.Pandey M, Thomas BC, SreeRekha P, Ramdas K, Ratheesan K, Parameswaran S, Mathew BS, Rajan B. Quality of life determinants in women with breast cancer undergoing treatment with curative intent. World J Surg Oncol. 2005;3:63. doi: 10.1186/1477-7819-3-63. doi:10.1186/1477-7819-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giovagnoli AR. Quality of life in patients with stable disease after surgery, radiotherapy, and chemotherapy for malignant brain tumour. J Neurol Neurosurg Psychiatry. 1999;67(3):358–363. doi: 10.1136/jnnp.67.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remer S, Murphy ME. The challenges of long-term treatment outcomes in adults with malignant gliomas. Clin J Oncol Nurs. 2004;8(4):368–376. doi: 10.1188/04.CJON.368-376. doi:10.1188/04.CJON.368-376. [DOI] [PubMed] [Google Scholar]
- 25.Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, Halperin EC. Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. Int J Radiat Oncol Biol Phys. 2003;55(4):992–999. doi: 10.1016/s0360-3016(02)04205-0. [DOI] [PubMed] [Google Scholar]
- 26.Klein M, Taphoorn MJ, Heimans JJ, van der Ploeg HM, Vandertop WP, Smit EF, Leenstra S, Tulleken CA, Boogerd W, Belderbos JS, Cleijne W, Aaronson NK. Neurobehavioral status and health-related quality of life in newly diagnosed high-grade glioma patients. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(20):4037–4047. doi: 10.1200/JCO.2001.19.20.4037. [DOI] [PubMed] [Google Scholar]
- 27.Salo J, Niemela A, Joukamaa M, Koivukangas J. Effect of brain tumour laterality on patients’ perceived quality of life. J Neurol Neurosurg Psychiatry. 2002;72(3):373–377. doi: 10.1136/jnnp.72.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang FM, Tsai WL, Lee TF, Liao KC, Chen HC, Hsu HC. Multivariate analysis of quality of life outcome for nasopharyngeal carcinoma patients after treatment. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2010;97(2):263–269. doi: 10.1016/j.radonc.2010.05.022. doi:10.1016/j.radonc.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Penson DF, Stoddard ML, Pasta DJ, Lubeck DP, Flanders SC, Litwin MS. The association between socioeconomic status, health insurance coverage, and quality of life in men with prostate cancer. J Clin Epidemiol. 2001;54(4):350–358. doi: 10.1016/s0895-4356(00)00312-7. [DOI] [PubMed] [Google Scholar]
- 30.Cheng JX, Zhang X, Liu BL. Health-related quality of life in patients with high-grade glioma. Neuro-Oncol. 2009;11(1):41–50. doi: 10.1215/15228517-2008-050. doi:10.1215/15228517-2008-050. [DOI] [PMC free article] [PubMed] [Google Scholar]