Abstract
Although Alzheimer's disease (AD) is usually sporadic, in a small proportion of cases it is familial and can be linked to mutations in β-amyloid precursor protein (APP). Unlike the other genetic defects, the mutation [alanine-673→valine-673] (A673V) causes the disease only in the homozygous condition with enhanced amyloid β (Aβ) production and aggregation; heterozygous carriers remain unaffected. It is not clear how misfolding and aggregation of Aβ is affected in vivo by this mutation and whether this correlates with its toxic effects. No animal models over-expressing the A673V–APP gene or alanine-2-valine (A2V) mutated human Aβ protein are currently available. Using the invertebrate Caenorhabditis elegans, we generated the first transgenic animal model to express the human Aβ1–40 wild-type (WT) in neurons or possess the A2V mutation (Aβ1–40A2V). Insertion of an Aβ-mutated gene into this nematode reproduced the homozygous state of the human pathology. Functional and biochemical characteristics found in the A2V strain were compared to those of transgenic C. elegans expressing Aβ1–40WT. The expression of both WT and A2V Aβ1–40 specifically reduced the nematode's lifespan, causing behavioral defects and neurotransmission impairment which were worse in A2V worms. Mutant animals were more resistant than WT to paralysis induced by the cholinergic agonist levamisole, indicating that the locomotor defect was specifically linked to postsynaptic dysfunctions. The toxicity caused by the mutated protein was associated with a high propensity to form oligomeric assemblies which accumulate in the neurons, suggesting this to be the central event involved in the postsynaptic damage and early onset of the disease in homozygous human A673V carriers.
Keywords: Alzheimer's disease, Amyloid β peptides, Toxic oligomers, Caenorhabditis elegans, A673V mutation
Highlights
-
•
We generated the first transgenic animal model expressing in neurons the human Aβ1–40 wild-type or has the A2V mutation.
-
•
Aβ1–40 expression reduced the worm's lifespan, caused behavioral and neuronal defects which were worse in the A2V strain.
-
•
The behavioral defects of mutant worms were specifically linked to postsynaptic dysfunctions.
-
•
The toxicity of Aβ1–40A2V was associated with its high propensity to form oligomers which accumulate in the neurons.
-
•
These transgenic strains represent an attractive tools for an in vivo screening of compounds interfering with oligomers.
Introduction
Alzheimer's disease (AD) is a chronic neurodegenerative disorder and one of the leading causes of death among the elderly in western countries (Hardy and Selkoe, 2002, Holtzman et al., 2011).
AD is usually sporadic, but a small proportion of cases are familial. Neuropathological hallmarks of familial AD are the same as in sporadic forms (Bertram and Tanzi, 2005). However, familial cases usually have an earlier age of onset and show an autosomal-dominant pattern of inheritance with virtually complete penetrance linked to mutations in the β-amyloid precursor protein (APP) or presenilins genes. Genetic defects may result in overproduction of amyloid β (Aβ) or in the generation of mutated Aβ forms with a greater propensity for aggregation (Rocchi et al., 2003).
Recently we identified a novel human APP mutation at codon 673 (A673V) in the N-terminal part of the Aβ sequence at position 2. Unlike the other genetic defects inducing AD, this mutation only has a pathogenic role in the homozygous condition (Di Fede et al., 2009). The neuropathological picture of the recently deceased A673V homozygous proband of this family revealed hallmarks consistent with degenerative dementia of AD type but also showed several distinct characteristics compared with sporadic or familial AD inherited as a dominant trait (Giaccone et al., 2010). The major specific features are the morphology, structural properties, topographic distribution and composition of Aβ deposits (Giaccone et al., 2010).
Studies conducted on the patients and control fibroblasts, as well as in cells transiently transfected with mutant or wild-type (WT) APP cDNA, indicated that the insertion of the A673V mutation promoted a shift in APP processing towards the amyloidogenic pathway, resulting in enhanced Aβ production (Di Fede et al., 2009). In vitro investigations indicated that the alanine-2-valine (A2V) substitution in Aβ synthetic peptides increased their aggregation propensity and cellular neurotoxicity compared with WT (Di Fede et al., 2012). The insertion of the A2V mutation into the Aβ1–40 peptide increased its in vitro propensity to form soluble oligomeric species which act as “stressors” in Caenorhabditis elegans, significantly inhibiting pharyngeal pumping (Beeg et al., 2013, Stravalaci et al., 2012). In addition, interaction between the A2V mutant and WT peptides hindered in vitro amyloidogenesis and Aβ-mediated neurotoxicity, accounting for the absence of disease in the A673V heterozygous carriers (Di Fede et al., 2009, Di Fede et al., 2012).
It is important to clarify how misfolding and aggregation patterns of Aβ are affected by mutation and whether this correlates with disease onset and progression. An animal model over-expressing the A673V-APP gene or A2V mutated human Aβ protein could help unveil the mechanisms underlying the marked pathogenic effects of this mutation.
Using the invertebrate C. elegans, we generated the first transgenic animal to express human Aβ1–40 carrying the A2V mutation (Aβ1–40A2V) in neurons. This peptide was selected on the basis of neuropathological observations of the homozygous proband where the deposition of Aβ40 species was over-represented and the effects of the A673V genetic mutation were more pronounced on Aβ40 than on Aβ42 (Giaccone et al., 2010). Since this nematode naturally lacks endogenous Aβ proteins (Ewald and Li, 2010, Link, 1995), insertion of the Aβ1–40A2V gene reproduced the homozygous state of the human pathology.
Although Link and colleagues generated various transgenic C. elegans strains expressing Aβ1–42 in muscle or at the pan-neuronal level (Dosanjh et al., 2010, Ewald and Li, 2010), no models constitutively expressing Aβ1–40 in neurons are currently available. Thus, to understand whether the insertion of the mutant gene causes gene-specific disease hallmarks, a transgenic C. elegans expressing the Aβ1–40 WT (Aβ1–40WT) peptide was also generated.
We report here that the pathological behavior and neurotransmission dysfunction caused by the pan-neuronal expression of the Aβ1–40WT protein are worsened by the insertion of an A2V mutation. In addition, Aβ1–40A2V has a greater propensity than the WT to form in vivo soluble oligomeric species which accumulate within neuronal cells, suggesting this was a central event in the postsynaptic damage and early disease progression in A673V homozygous carriers (Di Fede et al., 2009).
Methods
Construction and characterization of C. elegans transgenic strains
Pan-neuronal Aβ1–40 expression was achieved by cloning a construct for the expression of human Aβ1–40WT, Aβ1–42WT and A2V mutated Aβ1–40 (Aβ1–40A2V) under the control of the aex-3 neuronal promoter. aex-3 was subcloned into a pPD49.26 plasmid, resulting in the pPD49.26 + aex-3 plasmid. A minigene consisting of a synthetic modified signal peptide (MHKYLLALFFIFLAPAGT) and Aβ peptide was subcloned into the pPD49.26 + aex-3 plasmid (kindly provided by Prof. T. Tsubata). A minigene with the synthetic modified signal peptide, but without the Aβ peptide, was also subcloned into the pPD49.26 + aex-3 plasmid and used to generate control worms (Vector). The sequence of the constructs was confirmed by DNA sequencing.
Transgenes were introduced into the MT309 multivulva C. elegans strain (Caenorhabditis Genetics Center, CGC, University of Minnesota, USA) by gonad microinjection of a DNA solution containing 10 ng/μl of the Aβ construct together with 2.5 ng/μl of ttx-3::rfp and 2.5 ng/μl of plin-15(+) as marker plasmids. Multiple extrachromosomal lines were established based on the fluorescent marker and the disappearance of the multivulva phenotype. The transgenic animals maintained the injected DNA as an extrachromosomal element. The transmitting lines established for this study have 60–70% meiotic stability. Thus transgenic animals produced both transgenic and non-transgenic progeny. At least three independent transmitting lines were produced for each variant. All nematode strains were grown at 20 °C on Escherichia coli OP50.
To check the Aβ genotype of transgenic strains, reverse transcription was performed on RNA extracted from each transgenic line. The cDNA fragment codifying human Aβ was amplified by PCR and sequenced using the following primers: forward 5′-GAACATTTTCAGGAGGACC-3′ and reverse 5′-TTAGAAGTCAGAGGCACGGG-3′. Quantitative real-time PCR was also performed. Briefly, RNA was extracted using the RNeasy Plus Universal Mini Kit (Qiagen) and its yield was measured using a NanoDrop 2000 Spectrophotometer (Thermo Scientific). Retro-transcription was carried out using SuperScript III First-Strand (Invitrogen), according to manufacturer's instructions. Quantitative real-time PCR was performed on 10 μl reactions containing 300 nM of primer (CeAβF 5′-GAATTCCGACATGACTCAGG-3′ and CeAβR 5′-GCTCACGCTATGACAACACC-3′ for the amplification of Aβ), 5 μl of 2 × Sso Fast Eva Green Supermix (Bio-Rad) and 7.5 ng of each cDNA. PCR included amplification of peripheral myelin gene pmp-22/GAS-3 (CePmp3F: 5′-GTTCCCGTGTTCATCACTCAT-3′ and CePmp3R: 5′-ACACCGTCGAGAAGCTGTAGA-3′) as normalizing control. The initial denaturation step was performed at 98 °C for 30 s and was followed by 40 cycles at 98 °C for 8 s and 60 °C for 25 s. Each reaction was carried out in triplicate using the Viia7 System (Applied Biosystems) and the data were analyzed using the ΔΔCT method (Livak and Schmittgen, 2001).
SELDI-TOF MS analysis
After collection, washing and centrifugation, transgenic adult worms were resuspended in a lysis buffer (0.5 M urea, 0.5% (v/v) Triton X-100 and protease inhibitor mixture in 5 mM phosphate buffered saline (PBS), pH 7.4) and sonicated. Adult transgenic CL2006 worms, constitutively expressing Aβ3–42 in their body wall muscle cells, were also used (McColl et al., 2009). Samples were clarified by centrifugation at 13,500 ×g for 10 min, after which the supernatant was collected, put immediately in liquid nitrogen and stored at − 80 °C. Worm lysates were analyzed by an immunoproteomic assay for Aβ peptide detection, using SELDI-TOF MS analysis (Albertini et al., 2010). Specifically, 3 μl of a 0.125 mg/ml monoclonal antibody solution (6E10 and 4G8, Signet) was incubated for 2 h at room temperature in a humidity chamber to allow covalent binding to the PS20 ProteinChip Array (Bio-Rad Laboratories Inc.). Unreacted sites were blocked for 30 min at room temperature with 0.5 M Tris–HCl, pH 8.0, in a humidity chamber. Each spot was washed three times with PBS containing 0.5% (v/v) Triton X-100 then twice with PBS alone. Spots were coated with 5 μl of sample and incubated overnight in a humidity chamber before being washed three times with PBS containing 0.1% (v/v) Triton X-100, twice with PBS alone and finally with deionized water. One microliter of α-cyano-4-hydroxy cinnamic acid (Bio-Rad Laboratories, Inc.) was added to each spot and mass identification was performed using the ProteinChip SELDI System, Enterprise Edition (Bio-Rad Laboratories Inc.). Synthetic peptides homologous to residues 1–40 and 1–42 of Aβ, with or without the A2V substitution (Aβ1–40WT, Aβ1–40A2V, Aβ1–42WT, Aβ1–42A2V), and to residues 3–42 of Aβ (Aβ3–42WT) were prepared by solid-phase synthesis and purified as described (Di Fede et al., 2009). The purity and identity of peptides was determined by reverse-phase HPLC, amino acid sequencing, MALDI-TOF and SELDI-TOF MS; the purity was above 95%.
Immunoprecipitation
For immunoprecipitation experiments, 2 μg of the mouse monoclonal antibody to human Aβ17–24 (4G8, Covance) and 2 μg of the mouse monoclonal antibody to human Aβ1–16 (6E10, Covance) were immunocaptured by 250 ng of Dynabeads M-280 sheep anti-mouse IgG, according to the manufacturer's protocol (Invitrogen). Adult transgenic Vector, WT and CL2006 worms (~ 5000) were collected, washed, centrifuged and frozen in liquid-N2. Worms were homogenized using the TeSeE homogenizer (Bio-Rad) and acid-washed glass beads (Sigma Aldrich) in 2:1 (v:w) lysis buffer (90% acetonitrile, 1% formic acid, 9% H2O). The lysates were centrifuged twice at 15,000 ×g for 10 min and the supernatants were lyophilized, resuspended in 5 mM PBS, pH 7.4, and then neutralized with 10 M NaOH, 1:400 (v/v). Immunoprecipitation of Aβ was performed by adding the lysate to the Dynabeads and washing with 200 μl of lysis buffer. The eluates obtained were frozen, lyophilized and resuspended in 25 μl of 50% acetonitrile in water, and used for mass spectrometry analysis.
Mass spectrometry (MS)
MS analysis was performed on immunoprecipitated lysates by using an HPLC–MS/MS system consisting of a 1200 series pumps and autosampler (Agilent Technologies, CA), interfaced to an API 5500 triple quadrupole mass spectrometer, equipped with a turbo ion spray source (AB Sciex, Canada). Synthetic peptides, homologous to residues 1–40, 1–42 and 3–42 of Aβ (20 ng/μl in 50% acetonitrile in water), were used as standards. The HPLC separation was obtained with a Jupiter C4 300A column, 150 × 2 mm, 5 μm particle size (Phenomenex, CA), using an elution mixture composed of 0.1% formic acid in water (solvent A) and acetonitrile (solvent B). The injection volume was 5 μl and the flow rate was 200 μl/min. The elution gradient ran from 20% up to 60% of solvent B in 10 min, then to 98% of solvent B for 5 min and re-equilibration for 8 min at 20% of solvent B. The mobile phase was directly introduced into the ion source, which operated with a turbo ion gas at 600 °C. MS analysis was performed using positive ionization and selected reaction monitoring (SRM) mode, measuring the fragmentation products of the multiple-charged protonated pseudo-molecular ions, as shown in Supplementary Table 1. Peptide molecular weight and precursor ions were calculated or measured as the maximum height of the mass spectra peaks, at unit resolution. Precursor ions were + 6 and + 5 multiple charged ions, as originated in the electrospray ionization source. The choice of fragmentation products for the Aβ peptides and the optimization of source and collision energies were performed in continuous-flow mode, using synthetic Aβ1–40, Aβ1–42 and Aβ3–42 peptides at 1 ng/μl of 50% acetonitrile in water (Suppl. Fig. 1).
Aβ expression in transgenic strains
Transgenic worms were collected by washing plates with M9 buffer, transferred to tubes, centrifuged and washed twice to eliminate bacteria. The worm pellet was resuspended in a lysis buffer (5 mM NaCl, 5 mM EDTA, 1 mM dithiothreitol (DTT) and protease inhibitor mixture in 25 mM Tris, pH 7.5) and homogenized using the TeSeE homogenizer (Bio-Rad) and acid-washed glass beads (Sigma Aldrich) (Diomede et al., 2010). For dot-blot analysis, equal amounts of proteins from homogenized samples (3–5 μg) were spotted in triplicate onto the same nitrocellulose membrane (Millipore), blocked with PBS, pH 7.4 containing 0.1% (v/v) Tween 20 (PBS-T), and incubated overnight with a mouse monoclonal antibody to Aβ1–40 (11A50-B10,1:500 dilution, Signet, Emeryville, CA), a mouse monoclonal antibody to human Aβ17–24 (4G8, 1:500 dilution, Sigma), a mouse monoclonal antibody to Aβ (Clone WO2, 1:1000 dilution, Millipore) or a rabbit polyclonal antibody recognizing high-molecular-weight oligomers (A11, 1:1000 dilution, Biosource, USA). To minimize background staining due to non-specific membrane-binding of the antibodies, the membranes were saturated for 1 h at room temperature by incubation with PBS-T containing 5% (w/v) low-fat dry milk powder and 2% (w/v) bovine serum albumin (BSA). The Aβ species in transgenic populations were identified by immunoblotting using Tris-Tricine gel and western blotting (Diomede et al., 2010). Equal amounts of protein lysate (100 μg) were fractionated by SDS-PAGE and, after blotting, were probed with WO2 (1:1000 dilution). Anti-mouse IgG peroxidase conjugate and anti-rabbit IgG peroxidase conjugate (1:5000 dilution, Sigma) were used as secondary antibodies. A 0.1% (w/v) Red Ponceau solution (Sigma Aldrich) was used to stain blotted membranes for visualization of total proteins. The mean volume of the Aβ-reactive spots and Red Ponceau spots were analyzed using Progenesis SameSpots software (Nonlinear Dynamics, UK). The data were expressed as the mean of the volume of the immunoreactive band/volume of total Ponceau proteins ± SD.
Immunofluorescence
Fluorescence microscopy on whole Vector, WT and A2V worms was performed according to Ruvkun et al. (Finney and Ruvkun, 1990, Ruvkun and Giusto, 1989). Briefly, worms were collected, rinsed and fixed in 2% paraformaldehyde solution containing 80 mM KCl, 20 mM NaCl, 10 mM EGTA, 5 mM spermidine-HCl (Sigma Aldrich), 15 mM sodium-piperazine-N,N′-bis[2-ethanesulfonic acid] (Sigma Aldrich), pH 7.4, and 25% (v/v) methanol. Worms were then rinsed in 100 mM Tris–HCl solution, pH 7.4, containing 1% (v/v) Triton X-100 and 1 mM EDTA, and incubated for 1 h at room temperature in the same buffer containing 1% β-mercaptoethanol. After washing, worms were incubated for 15 min at room temperature with 25 mM H3BO3 solution, pH 9.2, containing 12.5 mM NaOH and 10 mM DTT, then incubated for 15 min at room temperature with 1% H2O2. After extensive washing with borate buffer, worms were incubated overnight at 4 °C with 4G8 or A11 antibodies (1:100 dilution). Samples were washed for 1 h at room temperature with 5 mM PBS, pH 7.4, containing 1% BSA, 0.5% Triton X-100 and 1 mM EDTA, and incubated overnight at 4 °C with an anti-mouse IgG 488 goat or an anti-rabbit IgG 546 goat antibody (1:100 dilution, Sigma). The worms were then incubated with 4′,6-diamidino-2-phenylindole, mounted on slides for microscopy and observed with an inverted fluorescent microscope (IX-71 Olympus) equipped with a CDD camera (F-VIEWII) for acquiring images.
Immuno-electron microscopy
Individual nematodes were cut in a transverse plane with a razor blade just below the pharyngeal terminal bulb and fixed overnight in 2% paraformaldehyde and 1% glutaraldehyde in 120 mM PIPES, 50 mM HEPES, 8 mM MgCl2 and 20 mM EGTA buffer. They were then quickly washed in a drop of 7.5% gelatin (Sigma Aldrich), placed on a pre-warmed slide and embedded in 15% gelatin in 0.5 ml tubes at 37 °C. The tubes were transferred to an ice-bucket and when the gelatin had solidified, the embedded samples were dissected into 0.5–1 mm3 blocks and processed overnight at 4 °C with 2.3 M sucrose on a rotating wheel. After removing the excess sucrose, the blocks were placed on holders and immediately frozen in liquid nitrogen. Each holder with frozen tissue was then mounted on the arm of a Leica EM UC6 ultramicrotome equipped with a cryochamber (Leica EM UC6) and an antistatic device (Diatome, Switzerland). The samples were trimmed, sectioned to 50 nm thickness, collected on formvar/carbon-coated slot copper grids and incubated overnight with A11 antibody(1:100) at 4 °C, followed by 10-nm colloidal gold-conjugated protein A incubation (Cell Microscopy Center, Utrecht, The Netherlands) for 30 min (Diomede et al., 2010). After labeling, sections were treated with 1% glutaraldehyde, counterstained with uranyl acetate and examined with an Energy Filtered Transmission Electron Microscope (EFTEM, ZEISS LIBRA® 120) equipped with a YAG scintillator slow-scan CCD camera. The density of gold particles labeling A11 was measured by iTem software (Olympus Soft Imaging Solutions, Germany). Immunoreactive oligomeric inclusions in the neuronal cytoplasm area of WT (N = 10) and A2V (N = 25) nerve ring neurons were identified in a number of sections, ranging from 3 up to 8 in 3 nematodes for each experimental group.
Surface plasmon resonance (SPR)
SPR studies were carried out with the ProteOn XPR36 Protein Interaction Array System (Bio-Rad Laboratories) (Bravman et al., 2006) based on SPR technology. The anti-Aβ antibody 4G8 was immobilized on three parallel chambers of the same GLC sensor chip (Bio-Rad Laboratories) by standard amine coupling chemistry, as previously described (Stravalaci et al., 2012). An irrelevant IgG was immobilized in parallel and used as reference. After rotation of the microfluidic system, lysates from transgenic WT and A2V C. elegans strains (prepared as previously described, without protease inhibitors) were diluted to a final protein concentration of 0.05 μg/μl and flowed over immobilized 4G8 for 3–5 min at 30 μl/ml; dissociation was measured in the following 10–15 min. The sensorgrams (time course of the SPR signal in RU) were normalized to a baseline value of 0. The signal in the surfaces immobilizing 4G8 was corrected by subtracting the nonspecific response in the reference surface.
Behavioral studies
To perform lifespan experiments, gravid worms were allowed to lay eggs for 6–8 h to produce an age-synchronized population which was cultured at 20 °C. Once the worms reached adulthood (at reproductive maturity), they were transferred daily to fresh NMG plates seeded with E. coli OP50, in absence of fluorodeoxyuridine, until they stopped laying eggs. To avoid overlapping generations, worms were then transferred daily until all nematodes were dead. Worms were scored as dead when there was no touch-provoked movement. The first day of adulthood is day 3 in survival curves.
Body bends were recorded at room temperature using a stereomicroscope. L3–L4 worms were picked and transferred into a well of a 96-well ELISA plate containing 100 μl of ddH2O. The number of left–right movements in a minute was recorded. To determine the effect of epigallocatechin gallate (EGCG) in preventing the locomotor defect caused by Aβ expression, egg-synchronized transgenic worms were placed at 20 °C on fresh NMG plates seeded with E. coli OP50 (100 worms/plate). After 36 h, when worms were at L3/L4 larval stage, they were fed 50–100 μM EGCG (100 μl/plate). Body bends in liquid were scored 24 h later. EGCG was a kind gift from INDENA (Milan, Italy) and was freshly dissolved in water before use.
To evaluate pharyngeal pumping, individual L3–L4 worms were placed on NMG plates seeded with OP50 E. coli, and pumping was scored by counting the number of times the terminal bulb of the pharynx contracted in 1 min. For lifespan, body bends and pumping rate experiments, N2 ancestral worms (Caenorhabditis Genetics Center, CGC, University of Minnesota, USA) were used as a reference strain.
Aldicarb and levamisole sensitivity was evaluated on synchronized L4-young adult worms. Nematodes (30–50/plate) were transferred to freshly made NGM agar plates with a small spot of OP50 E. coli solution and 1 mM aldicarb or 1 mM levamisole was added. The worms were prodded on the nose after 30 min and for the next 2 h to determine when they had reached complete paralysis (Mahoney et al., 2006, Nguyen et al., 1995).
Fluorescent staining of β-amyloid
Two day old transgenic worms were fixed in 4% paraformaldehyde in PBS, pH 7.4, for 24 h at 4 °C. Nematodes were stained with 1 mM 1,4-bis(3-carboxy-hydroxy-phenylethenyl)-benzene (X-34) in 10 mM Tris, pH 8.0, for 4 h at room temperature (Diomede et al., 2010), destained, then mounted on slides for microscopy and observed with an inverted fluorescent microscope (IX-71 Olympus); images were acquired using a CDD camera.
Statistical analysis
The data were analyzed using GraphPad Prism 4.0 software (CA, USA) by an independent Student's t-test and one-way ANOVA and Bonferroni's post-test analysis. A p-value < 0.05 was considered significant.
Results
Pan-neuronal expression of A2V-mutated Aβ1–40 induced behavioral defects and neurotransmission impairment
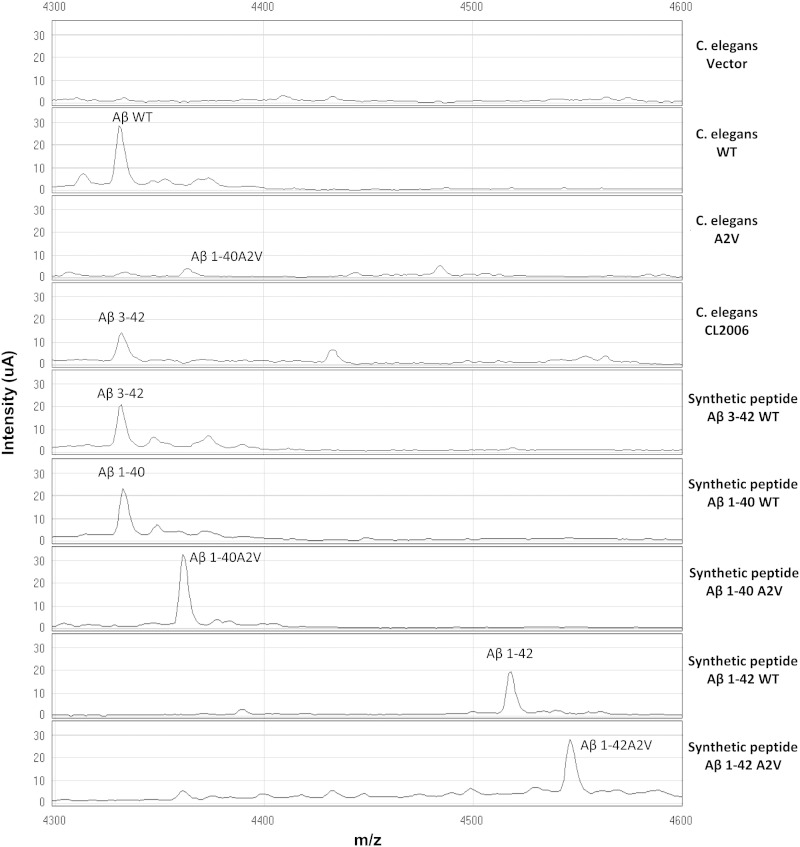
We investigated whether the A2V substitution in Aβ resulted in protein aggregation and toxicity in vivo in a new transgenic animal model generated using the nematode C. elegans. The human Aβ1–40 peptide containing the recessive A2V mutation (Aβ1–40A2V) was constitutively expressed in the nematodes' neurons, mimicking human homozygous carriers. Amyloid formation and proteotoxicity caused by the mutated protein was compared to that induced by the expression of Aβ1–40WT. The molecular weight of the Aβ protein expressed in transgenic worms was determined using SELDI-TOF MS analysis, in which proteins are selectively adsorbed to a chemically modified array surface prior to the addition of an energy-absorbing matrix solution (Albertini et al., 2010). As shown in Fig. 1, worms transfected with the empty vector (Vector) did not express Aβ peptide, as expected. C. elegans injected with the mutated Aβ1–40A2V construct did express the predicted peptide, as indicated by the SELDI-TOF MS spectra, which showed a peak with the m/z of 4360 Da (Fig. 1), consistent with the synthetic Aβ1–40A2V peptide (Fig. 1). The injection of Aβ1–40WT construct gave no stable lines, but the injection of the Aβ1–42 transgene resulted in the generation of a WT stable line. Although the generated transgenic WT strain has an Aβ1–42 genotype, as confirmed by reverse transcription of RNA and by PCR amplification and sequencing of the cDNA fragment coding for human Aβ, it did not express the predicted Aβ1–42 (m/z = 4515 Da) (Fig. 1). However, a major species with an m/z of 4332 Da, compatible with either Aβ1–40 or Aβ3–42 expression, was observed (Fig. 1). SELDI-TOF MS analysis was then performed on lysates from WT transgenic worms, analyzing in parallel, as controls, the Aβ3–42 and Aβ1–40 synthetic peptides, as well as lysates from CL2006 transgenic worms expressing Aβ3–42 (McColl et al., 2009) (Fig. 1). Due to the difference of less than 2 Da between Aβ1–40 and Aβ3–42, the ultimate identity of the peptide expressed by the transgenic WT worms could not be resolved by using SELDI-TOF MS. In addition, due to the low levels of Aβ related to neuronal expression, western blotting was not able to discriminate between the presence of Aβ1–40 from Aβ3–42 in the lysates from WT worms, neither after Aβ immunoprecipitation nor precipitation of proteins with methanol (data not shown).
Fig. 1.
Aβ1–40A2V expression in transgenic C. elegans. An immunoproteomic assay using SELDI-TOF MS was performed on lysates from the transgenic C. elegans strains expressing: empty vector (C. elegans Vector), AβWT (C. elegans WT) or Aβ1–40A2V (C. elegans A2V). Aβ1–40WT, Aβ1–40A2V, Aβ1–42WT, Aβ1–42A2V and Aβ3–42WT synthetic peptides were loaded and analyzed as reference standards. Lysates from transgenic CL2006 worms were also analyzed (CL2006).
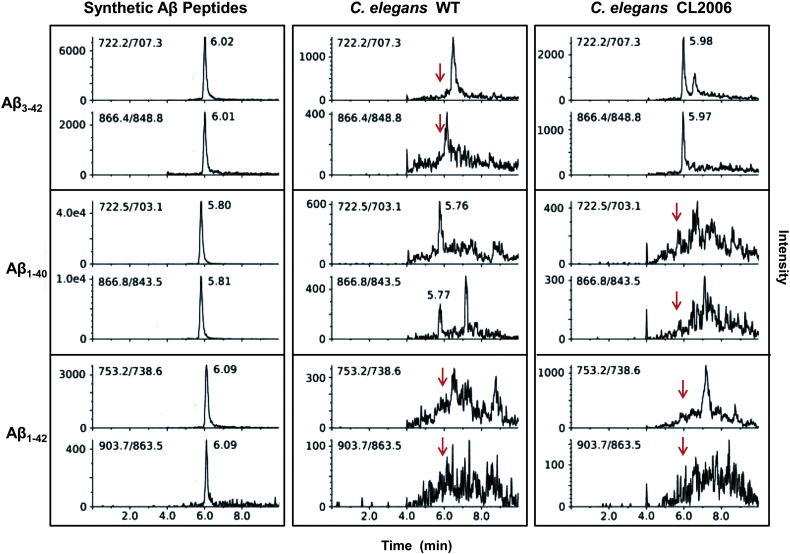
An original method was then developed involving the analysis of immunoprecipitated samples by using a triple quadrupole HPLC–MS/MS system with SRM acquisition. To this end, synthetic Aβ1–40, Aβ1–42 and Aβ3–42 peptides were characterized by positive electrospray ionization and the optimal SRM transitions and collision energies were determined (Table S1). The most abundant precursor ions of each peptide were found to be multiply charged ions with 6 and 5 charges. For Aβ1–40 and Aβ3–42 peptides, the m/z values of the precursor ions were not distinguishable at the unit resolution of the triple quadrupole instrument, however the two peptides had different and unique fragmentation products, which made their complete discrimination possible (Suppl. Fig. 1). An HPLC method was also set up, which allowed the separation and identification of the three peptides. As shown in Supplementary Fig. 2, no specific peaks corresponding to the Aβ peptides were observed in worms transfected with the empty vector (Vector). Lysates from WT and CL2006 transgenic worms were then analyzed; the recorded chromatograms are shown in Fig. 2. Only peaks corresponding to the retention time of Aβ3–42 were detected in lysate from CL2006 nematodes, as expected. In lysate from WT worms, no specific peaks were revealed at the retention time of Aβ3–42 and Aβ1–42, according to the SELDI-TOF spectra whereas peaks corresponding to the retention time of Aβ1–40 were observed (Fig. 2). These data clearly demonstrate that transgenic C. elegans WT strain only express the Aβ1–40 peptide.
Fig. 2.
HPLC-selected reaction monitoring (SRM) chromatograms relative to the analysis of Aβ in transgenic C. elegans strains. Synthetic Aβ3–42, Aβ1–40 and Aβ1–42 peptides were dissolved at 20 ng/μl in 50% acetonitrile in water and analyzed. The retention time of the peaks for synthetic Aβ3–42 (6.01 min), Aβ1–40 (5.80 min) and Aβ1–42 (6.09 min) were identified and labeled. In lysate from transgenic C. elegans WT, only peaks corresponding to the retention time of Aβ1–40 (5.77 min) were detected, whereas no specific peaks corresponding to the retention time of Aβ3–42 and Aβ1–42 (red arrows) were observed. In lysate from CL2006 worms, only peaks corresponding to the retention time of Aβ3–42 were observed (5.98 min). Red arrows indicate the retention times at which Aβ1–40 and Aβ1–42 peaks were expected. All chromatograms were obtained from the injection volume of 5 μl; instrumental parameters are reported in the Methods section.
The expression level of Aβ1–40 peptide in WT- and A2V-expressing transgenic worms was measured by quantifying mRNA. The two strains had similar relative quantities of Aβ mRNA, normalized to worm pmp-22/GAS-3 content (10.3 ± 1 vs. 15.0 ± 3.8 for WT and A2V worms, respectively).
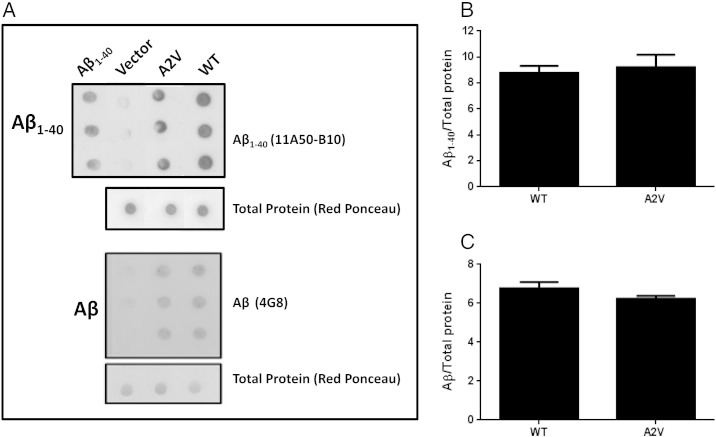
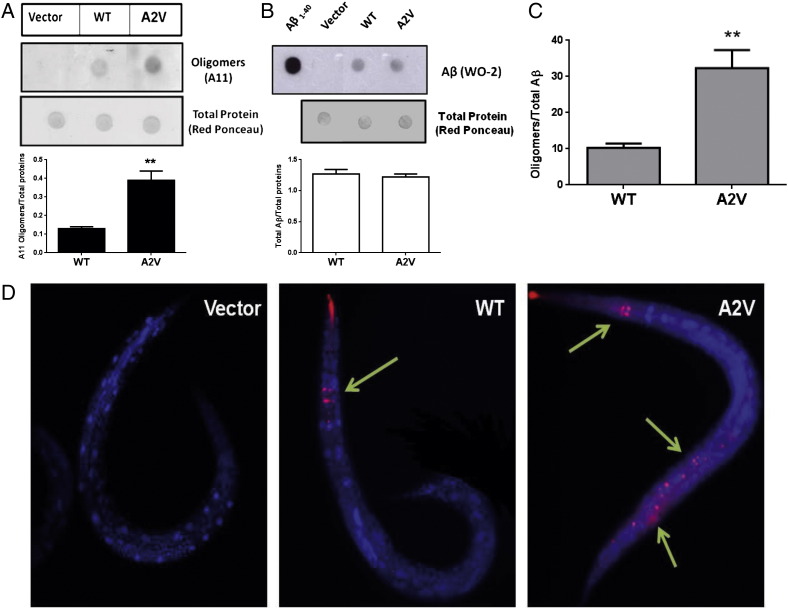
The Aβ species expressed in transgenic strains were also characterized by dot blotting using antibodies specific to Aβ1–40 (11A50-B10) or the anti-Aβ monoclonal antibody 4G8, and using as control the Aβ1–40 synthetic peptide as control. Lysates from WT and A2V strains were immunoreactive for both antibodies (Fig. 3A). The immunoreactivity of 11A50-B10 was quantified and the data obtained indicated that A2V and WT strains expressed comparable Aβ1–40 protein levels (p = 0.159, WT vs. A2V, Student's t-test) (Fig. 3B). Similar data were obtained using the anti-Aβ monoclonal antibody 4G8 (p = 0.537, WT vs. A2V, Student's t-test) (Fig. 3C).
Fig. 3.
Aβ species in transgenic C. elegans. (A) Representative dot blot of Aβ1–40(11A50-B10) and total Aβ (4G8) in WT and A2V transgenic worms. Equal amounts of proteins from worm lysates (5 μg) were spotted in triplicate. Nematodes injected with the empty vector (Vector) were used as control strain. Aβ1–40 synthetic peptide (2 μg) was spotted as an internal control. Total proteins on the blotted membranes were stained using a 0.1% Red Ponceau solution and were used as housekeeping control. The mean volume of the Aβ-reactive and Red Ponceau spots was analyzed using Progenesis SameSpots software (Nonlinear Dynamics, UK). Immunoreactivity of (B) 11A50-B10 and (C) 4G8 from three independent experiments (N = 9) were expressed as the mean of the volume of the immunoreactive band / volume of total Ponceau proteins ± SD.
The lysates of the different transgenic C. elegans strains were then analyzed via western blot using the anti-Aβ antibody WO2. The total immunoreactivity of WO2, indicative of the total Aβ, and the immunoreactivity specifically related to the 56 kDa and 20 kDa bands were quantified and normalized to the protein content. As shown in Supplementary Fig. 3, a similar total Aβ level was produced by WT and A2V worms, whereas a significant increase in the 20 kDa Aβ oligomeric species and a decrease in the band at 56 kDa were observed in lysates from A2V worms. The absence of monomeric Aβ in the western blot of WT and A2V nematodes can be attributed to the low Aβ levels following the neuronal expression.
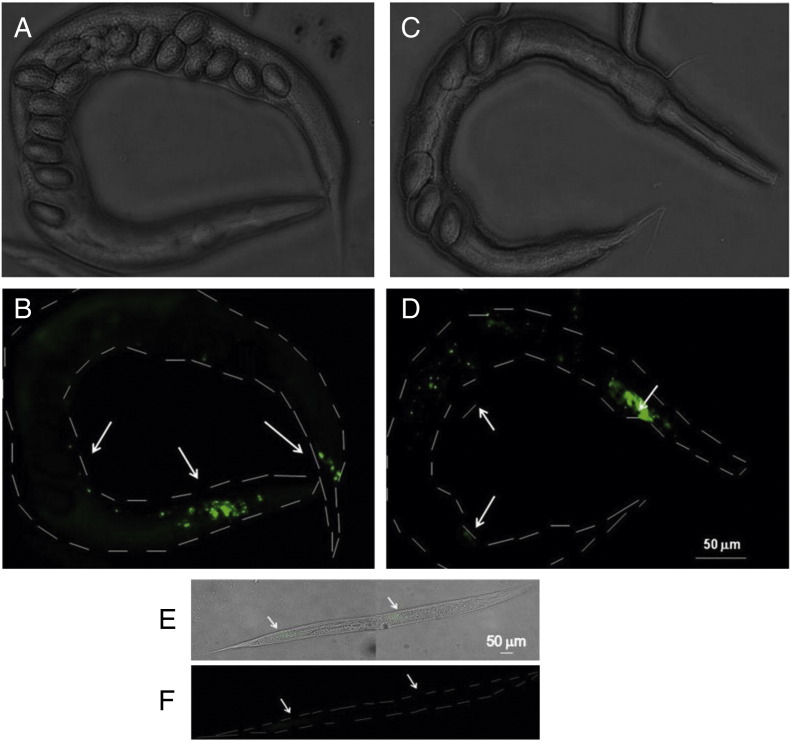
Immunofluorescence studies showed a 4G8-positive signal in animals expressing the WT or mutated Aβ1–40 starting from the larval stage L2 (Suppl. Fig. 4). At this stage and in 2-day-old worms fluorescence was localized in the nerve ring of the head region, in the ventral cord and lateral body wall neurons, and in the tail ganglia, in both WT and mutated worms (Fig. 4). There was no 4G8-specific signal in worms transfected with the empty vector (Figs. 4E–F).
Fig. 4.
Location of Aβ1–40 in transgenic C. elegans strains. Representative images obtained in bright field (A, C, E) and by immunofluorescence analyses (B, D, F) of Aβ1–40WT (A, B) and Aβ1–40A2V-expressing worms (C, D). The 4G8 antibody immunostaining of worms expressing empty vector is reported in panel F. All animals are 2-day-old adults. A 4G8 positive signal (green) was observed in the head region (in the nerve ring area), ventral cord and lateral body wall neurons and in the tail ganglia (white arrows). Scale bar, 50 μm.
We then evaluated whether the constitutive expression of WT or A2V led to the neuronal accumulation of fibrillar Aβ deposits. To this end, 2-day-old adult transgenic worms were stained with X-34 dye, which specifically recognizes Aβ fibrillar aggregates but not oligomers (Diomede et al., 2010, Link et al., 2001). The co-localization of the TTX-3::RFP and X-34 fluorescence in the heads of the A2V nematodes indicated that the dye can enter the neurons (Suppl. Fig. 6). However, no X-34 positive spots were observed in neurons of either WT or A2V nematodes (Suppl. Fig. 5), indicating that the expression of Aβ1–40 in these transgenic worms did not result in amyloid deposition. This might be due to the low neuronal peptide concentration.
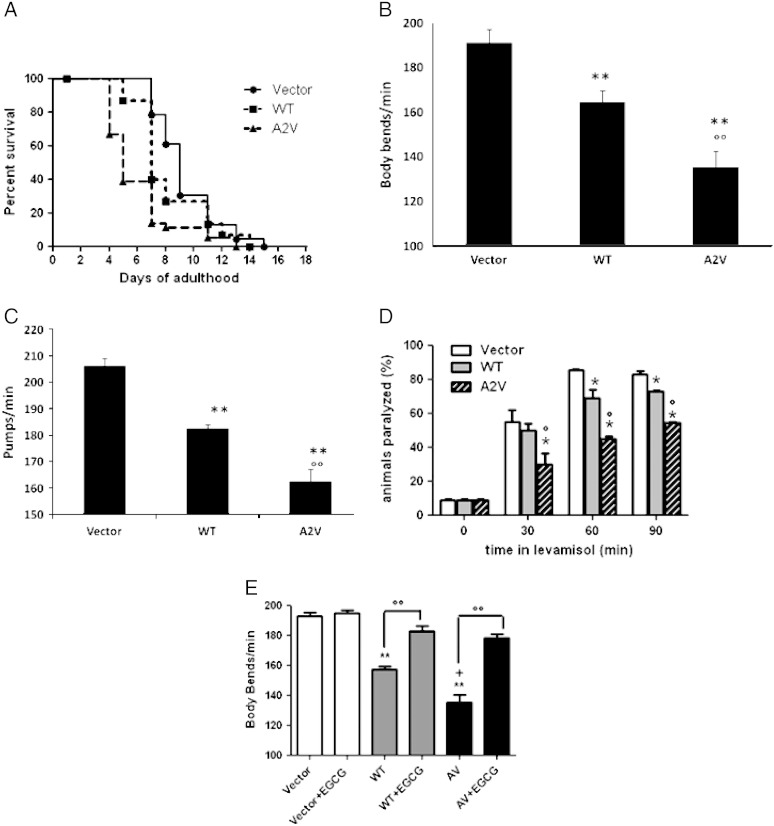
The effect of WT and mutated protein expression on overall nematode survival was also investigated. The insertion of the transgene alone, without the Aβ construct, did not affect the nematodes' survival (median survival was 10 and 9 days for N2 and Vector worms, respectively). Aβ1–40WT expression significantly reduced the lifespan of transgenic worms by 22%, compared to nematodes injected with the empty vector (Fig. 5A, median survival respectively: 9 and 7 days for Vector and WT, p = 0.009, log-rank analysis). Insertion of the A2V mutated gene shortened survival by 44% (median survival 5 days for A2V, p = 0.0004 vs. Vector, log-rank analysis) (Fig. 5A). The median lifespan of worms expressing the A2V mutated protein was 29% shorter than WT (p = 0.0005, log-rank analysis).
Fig. 5.
Survival and behavioral phenotype of transgenic C. elegans. (A) Kaplan–Meier survival curves of hermaphrodite adult worms expressing Aβ1–40WT (WT, squares) and Aβ1–40A2V (A2V, triangles). Vector (circles) and transgenic animals were placed on plates seeded with OP50 starting from L4, cultured at 20 °C and transferred to fresh plates on consecutive days. Survival was scored every day and is expressed as a percentage. Plots are representative of three independent experiments (N = 100). WT expression significantly reduced the lifespan of transgenic worms by 22% compared to nematodes injected with the empty vector (median survival: 9 and 7 days for empty vector and WT respectively, p = 0.009, log-rank analysis). Insertion of the A2V mutated gene further shortened survival by 44% (median survival is 5 days for A2V, p = 0.0004 vs. empty vector, log-rank analysis). The median lifespan of worms expressing the A2V mutated protein was 29% shorter than WT (p = 0.0005, log-rank). (B) Locomotor defects were scored in L3–L4 transgenic worms by measuring the number of body bends in liquid. At least three independent assays were performed (N = 50 animals in each group). Data are expressed as the mean number of body bends/min ± SD. (C) Pharyngeal pumping rate was scored in L3–L4 transgenic worms by determining the number of pumps/min. At least three independent assays were performed (N = 30 animals per group) and the data are expressed as the mean pumps/min ± SD. **p < 0.01 vs. Vector, °°p < 0.01 vs. WT, by one-way ANOVA and Bonferroni's post-test analysis. (D) Levamisole sensitivity of transgenic strains. WT and A2V synchronized L4-young adult transgenic worms were exposed for different times (0–90 min) to 1 mM levamisole and the animals not moving were scored as paralyzed. At each time point, the percentage of paralyzed worms was calculated. At least three independent assays were performed. Data are the mean ± SD (N = 30). *p < 0.01 vs. Vector and °p < 0.01 vs. WT at each time point, by one-way ANOVA and Bonferroni's post-test analysis. (E) Effect of epigallocatechin gallate (EGCG) on Aβ-induced locomotory defect in transgenic C. elegans strains. Egg-synchronized control worms (Vector), WT and A2V nematodes were placed at 20 °C on fresh NMG plates seeded with OP50. When worms were at the L3–L4 larval stage, animals were fed 50 μM EGCG (100 μl/plate). Body bends in liquid were scored 24 h after. At least three independent assays were performed. Data are the mean of the number of body bends/min ± SD. **p < 0.01 vs. Vector, p < 0.01 vs. WT, °°p < 0.01 vs. the respective untreated group, according to one-way ANOVA (N = 40 animals for each group).
The coordination and motility of C. elegans is controlled by numerous neurons innervating body wall muscle cells; misfolded proteins can cause their dysfunction (Dosanjh et al., 2010, Sengupta and Samuel, 2009, Wang et al., 2009). The motility of Aβ-expressing worms was evaluated by counting body bends to quantify their locomotion in liquid. Worms transfected with the empty vector had a motility similar to ancestral N2 animals (Vector, 191.2 ± 6 body bends/min; N2, 186.8 ± 10 body bends/min, N = 50 worms/group) indicating that insertion of only the transgene, without the Aβ construct, did not affect neuronal function. Body bends were significantly decreased by expression of the Aβ peptide (135.5 ± 7 and 164.6 ± 5 bends/min for A2V and WT, respectively, p < 0.01 one-way ANOVA vs. Vector, N = 50 worms/group) (Fig. 5B). The loss of motility was greater in A2V mutants, which showed significantly fewer body bends than WT worms (Fig. 5B).
We then investigated whether the expression of Aβ1–40 affected pharyngeal contraction rates, which are controlled by neurons in the pharyngeal nervous system. The pharyngeal pumping rate in worms transfected with the empty vector was similar to N2 worms (205.9 ± 3.1 and 215.4 ± 5.1 pumps/min, respectively, N = 30 worms/group) whereas nematodes expressing Aβ1–40WT and Aβ1–40A2V showed significantly lower rates (− 11% and − 21% respectively vs. Vector) (Fig. 5C). The expression of the mutated protein caused more defective pumping than the expression of Aβ1–40WT (182.5 ± 2 pumps/min of WT vs. 162.4 ± 5 pumps/min of A2V, p < 0.01 one-way ANOVA, N = 30 worms/group) (Fig. 5C).
Locomotion in nematodes, like pharyngeal pumping, is controlled by acetylcholine receptors which mediate synaptic functions (Mahoney et al., 2006). We explored whether the neuronal expression of Aβ1–40, with or without the A2V mutation, affected cholinergic transmission by recording the paralyzing effect of a cholinergic agonist (levamisole) and an acetylcholinesterase inhibitor (aldicarb). Levamisole induces muscle hypercontraction and paralysis, by activating postsynaptic, levamisole-sensitive acetylcholine receptors (Mahoney et al., 2006). Aβ-expressing worms were significantly more resistant to levamisole than the corresponding animals transfected with the empty vector (Fig. 5D). Those expressing Aβ1–40A2V were more resistant than WT, indicating that the mutated protein caused a worse impairment in postsynaptic function. The expression of WT or mutated Aβ1–40 also resulted in resistance to aldicarb-induced paralysis, an indication of a synaptic transmission defect. However, no significant differences were observed between transgenic WT and A2V animals (Suppl. Fig. 6).
The consistency of these new transgenic C. elegans strains in recapitulating important clues to the amyloidosis was also investigated by studying the response to EGCG, a polyphenolic green tea constituent. This is a prototypical inhibitor of oligomerization (Stravalaci et al., 2012), emerging as an anti-amyloidogenic compound, which counteracts aggregation and fibrillogenesis of various amyloidogenic proteins, including Aβ (Bieschke et al., 2010, Ehrnhoefer et al., 2008). It is currently under investigation in a Phase II–III Clinical Trials (ClinicalTrials.gov Identifier: NCT00951834).
Worms were fed for 24 h with either vehicle or 50–100 μM EGCG, and the body bends were scored. EGCG did not affect the motility of Vector worms at any of the doses tested (192.0 ± 3 and 193.2 ± 3 body bends/min for Vector fed vehicle and 100 μM EGCG, respectively, N = 50 worms/group). As shown in Fig. 5E, 50 μM EGCG completely reversed the reduction in body bends caused by Aβ expression in both WT and A2V worms. Similar data were obtained by feeding worms with 100 μM EGCG (data not shown). This protective effect was paralleled by the ability of EGCG to reduce the oligomer levels in WT and A2V worms (Suppl. Fig. 7).
Aβ1–40A2V expression resulted in higher production of oligomeric species
Introduction of the A2V substitution in the synthetic Aβ1–40 peptide enhances its in vitro aggregation and fibrillogenic properties (Di Fede et al., 2009), as well as its propensity to form oligomers (Stravalaci et al., 2012). To investigate whether the A2V mutation modifies the Aβ assembly in vivo, lysates from adult transgenic C. elegans were analyzed using different experimental approaches. First, lysates were analyzed by dot blotting, using the anti-oligomer antibody A11. As shown in Fig. 6A, no immunoreactivity was observed in worms injected with the vector; however the expression of Aβ1–40WT was accompanied by the appearance of A11-positive signal which was stronger in A2V mutants. In agreement with the data reported in Fig. 3 and in Supplementary Fig. 3, the total amount of Aβ, quantified from the dot blot with the WO2 antibody (Fig. 6B), was similar in the two transgenic strains. The A11-immunoreactivity was then normalized against WO2-immunoreactivity and the results obtained indicated that the presence of the A2V mutation caused a three-fold increase in the proportion of A11-stained oligomers (p < 0.01, Student's t-test, N = 9) (Fig. 6C). Such a feature is consistent with the increase in the 20 kDa band reported in Supplementary Fig. 3 and with the well-established in vitro propensity of mutated Aβ1–40A2V to misfold and self-aggregate (Di Fede et al., 2009, Stravalaci et al., 2012).
Fig. 6.
Aβ oligomers in transgenic C. elegans strains. (A) Representative dot blots of Aβ oligomers (A11) in transgenic worms. (B) Representative dot blots of total Aβ (WO2) in transgenic worms and (C) the A11-immunoreactivity as a function of the total Aβ quantified from the dot blot obtained using the WO2 antibody. Data are the mean of the volume of A11-immunoreactive bands/volume of WO2-immunoreactive bands ± SD from three independent experiments (N = 9). **p < 0.01 vs. WT (Student's t-test). (D) Representative images of Vector, WT and A2V transgenic worms after immunostaining with A11 antibody. All animals are 2-day-old adults. Arrows indicate the A11-positive signal (red). DNA was probed using 4′,6-diamino-2-phenylidole (blue). In A2V worms, oligomers (green arrows) were associated with the nerve ring in the head region, in the ventral and lateral body regions, and in the tail, whereas in WT animals the A11-positive signal was only in the head. Scale bar, 50 μm.
Immunofluorescence studies with the A11 antibody were then carried out to look for differences in oligomer deposition between transgenic WT and A2V worms (Fig. 6D). WT nematodes showed a specific fluorescence signal only in neurons in the head, lower than that found in animals expressing the mutated peptide. The latter also showed in the ventral cord and lateral body wall (Fig. 6D). No A11-positive staining was observed in Vector nematodes (Fig. 6D).
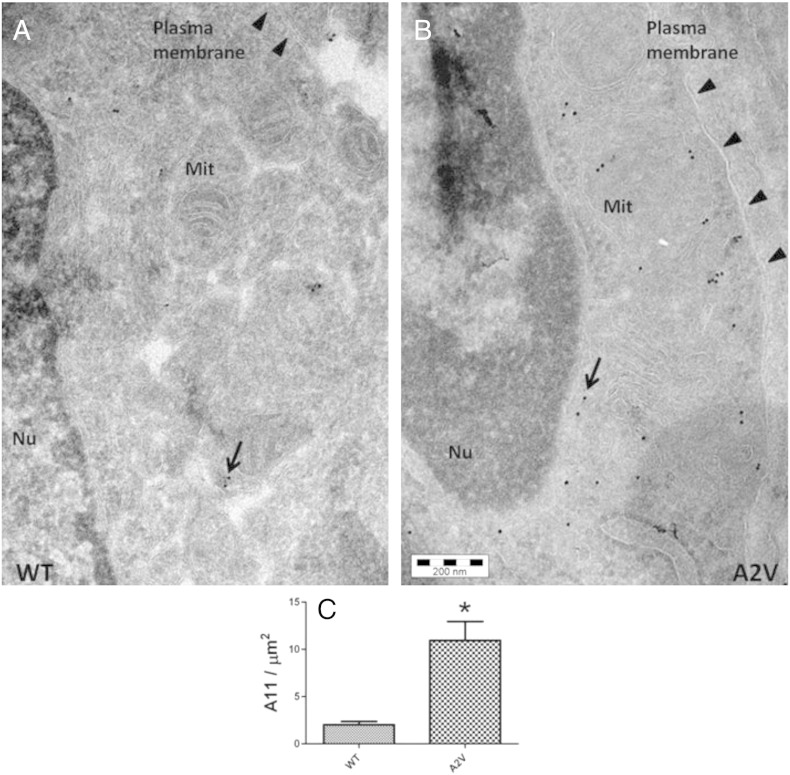
We also performed immuno-electron microscopy studies with the A11 antibody to see whether oligomers localized in neurons (Figs. 7A–B). Quantification of A11-binding with a gold-labeled secondary antibody indicated a 5.4 fold significant increase (p < 0.001, Student's t-test) in immunoreactive oligomeric inclusions in the cytoplasm of A2V nerve ring neurons (10.94 ± 2.00 gold particles/μm2) compared to WT worms (2.02 ± 0.35 gold particles/μm2) (Fig. 7C). In addition, there were some morphological abnormalities in neurons of Aβ1–40A2V expressing worms, including swollen and enlarged mitochondria (Fig. 7B). No immunoreactive signal was observed in worms transfected with the empty vector (data not shown).
Fig. 7.
Immuno-electron microscopy of transgenic C. elegans strains. Transmission electron microscopy representative images of the nerve ring neurons in the head region of WT (A) and A2V (B) 4-day-old adult transgenic C. elegans. A11-positive oligomers (arrows), indicated by the interaction with the 10 nm gold-conjugated protein A, accumulated in the cytosol of neurons. Nu: nucleus, Mit: mitochondria, Scale bar 200 nm. (C) The quantification of A11 immunolabeling in WT (N = 10) and A2V (N = 25) nerve ring neurons was expressed as the number of neuronal gold particles divided by cytoplasmic area (*p < 0.001 vs. WT, Student's t-test).
Finally, we used a novel immunoassay based on surface plasmon resonance (SPR), which specifically recognizes biologically active Aβ oligomers (Stravalaci et al., 2012), The method exploits the fact that soluble oligomers bind to chip-immobilized 4G8 in a pseudo-irreversible manner (very slow dissociation rates, likely due to multivalent binding), whereas monomers bind with significantly faster dissociation rates, and higher-order aggregates are not detectable. We note that the possibility to use 4G8 to discriminate between different Aβ species, and to selectively detect soluble oligomers, is due to the specific features of SPR, e.g. the fact that it measures the binding events in real time on a time scale of seconds. This assay has been already applied to detect the A11-positive Aβ1–42 oligomers present in lysates from transgenic worms expressing Aβ1–42 (Stravalaci et al., 2012). As shown in Fig. 8, the analysis of lysates from A2V nematodes resulted in a much higher SPR signal than that observed in WT, indicating a greater amount of 4G8-binding species. As expected, no signal was observed with lysates from worms transfected with the Vector (Fig. 8). Importantly, most of the SPR signal observed with WT lysates was due to quickly-dissociating species (i.e. monomers), whereas most of the binding observed with mutant lysates was due to slowly-dissociating species (i.e. oligomers). These SPR data indicate that the lysates from Aβ1–40A2V-expressing worms contained more oligomeric species than lysates from WT worms, in agreement with the data obtained via dot-blotting, western blotting, immunofluorescence and immune-electron microscopy analyses.
Fig. 8.
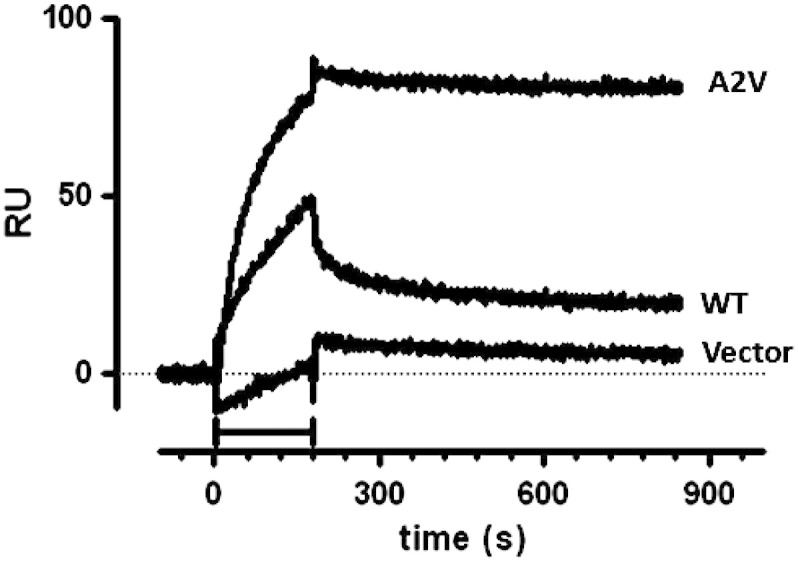
Aβ1–40 oligomers in transgenic C. elegans strains recognized by SPR-based immunoassay. Lysates from transgenic worms expressing Aβ1–40WT (WT) or Aβ1–40A2V (A2V) were flowed for 3 min (bar) onto a sensor chip on which we previously immobilized 4G8. Lysates from worms transfected with the empty vector were used as control (Vector). The three-min association phase was followed by 11 min of dissociation. The figure shown the obtained sensorgrams, i.e. the time course of the SPR signal expressed in resonance units (RU), obtained in a single experimental session. Very similar results were found in four independent sessions, with new sensor-chips and ex novo preparations of worm lysate.
Discussion
It has been previously shown that the occurrence of A673V mutation in the APP gene only results in an early-onset dementia in humans in the homozygous state (Di Fede et al., 2012). In vitro studies also showed that the corresponding A2V mutation in the Aβ peptide enhances its aggregation and fibrillogenic properties (Di Fede et al., 2009, Di Fede et al., 2012). To address specific questions on the proteotoxicity of the A2V protein variant, specifically the correlation of its aggregation state with the dysfunctional phenotypes underlying the neurodegenerative pathology, we have generated a new transgenic C. elegans strain expressing human Aβ1–40 which carries the recessive A2V mutation in its neurons. Since this nematode naturally lacks endogenous Aβ proteins, introduction of the mutated gene recapitulates the human homozygous state of the disease. A transgenic C. elegans strain producing Aβ1–40WT in neurons was also created. To definitively demonstrate that WT strain worms produce Aβ1–40, but not Aβ1–42 and Aβ3–42, we developed an innovative and highly sensitive method involving the use of HPLC–MS/MS which, based on the separation of different and unique fragmentation products, completely discriminates among the three Aβ peptides. An N-terminal truncation of Aβ was already reported in the transgenic CL2006 C. elegans strain created with an Aβ1–42 construct but actually expressing Aβ3–42 peptide (McColl et al., 2009). This represents the first description of a C-terminus trimming of Aβ in C. elegans and we hypothesized that it was due to a C-terminal truncation during protein processing, e.g. by neutral dipeptidases or carboxypeptidases. The possibility of Aβ truncation or modifications must therefore be taken into account when new transgenic strains are generated, and an accurate protein analysis must be conducted.
Nematodes expressing the mutant gene had a shorter lifespan than WT, indicating that Aβ1–40A2V has a greater in vivo toxicity than Aβ1–40WT. Accordingly, the behaviors of transgenic mutated worms were specifically altered by the A2V substitution in the Aβ1–40 peptide. In particular, we demonstrated a worse impairment of locomotor activity and pharyngeal pumping rates in nematodes expressing Aβ1–40A2V rather than Aβ1–40WT.
Although extracellular aggregates are thought to play a primary role in the disruption of synaptic plasticity, numerous studies indicated that intraneuronal accumulation of oligomeric non-fibrillar Aβ may contribute to early postsynaptic dysfunction and neurodegeneration (Capetillo-Zarate et al., 2012, Gimenez-Llort et al., 2007, Wirths and Bayer, 2012). In both transgenic WT and A2V worms, Aβ1–40 was expressed intraneuronally, due to the presence of the signal peptide sequence in the construct. Thus the specific behavioral impairment was related to the intracellular toxicity of Aβ assemblies. Both WT and mutated strains showed resistance to aldicarb, indicating that the intracellular Aβ1–40 assembly affects cholinergic neurotransmission. The observation that the expression of Aβ1–40, in particular in the mutated form, makes the worms more resistant to the paralysis induced by levamisole, a potent cholinergic agonist, indicates postsynaptic defects involving alterations to acetylcholine receptors. Synaptic dysfunctions arising from the neuronal expression of Aβ1–42 in C. elegans had already been reported (Dosanjh et al., 2010).
Previous in vitro data using the synthetic peptides indicated that the A2V mutation boosts the formation of Aβ1–40 transient oligomeric species, as recognized by an SPR-based immunoassay using 4G8 antibody (Stravalaci et al., 2012). The data now obtained in transgenic C. elegans provide an in vivo confirmation that Aβ1–40A2V has a higher propensity to form oligomers than Aβ1–40WT. This was demonstrated by dot blot, western blot, immunohistochemistry and immune-electron microscopy studies with the anti-oligomer antibody A11, and by SPR immunoassay. We suggest that the worsened phenotype observed in the A2V worms, including neuronal dysfunction at postsynaptic level, is associated with the higher propensity of Aβ1–40A2V to form oligomeric assemblies.
The description of the A673V mutation in the APP gene led us to re-consider the role of the Aβ amino-terminal sequence in Aβ misfolding and toxic properties (Di Fede et al., 2012). The present data further confirm that this domain is involved in the assembly of Aβ1–40 into oligomeric conformations with proteotoxic and neurotoxic activity in vivo.
Despite the differences between nematodes and vertebrates, this new transgenic C. elegans model, in which the defect is gene-specific and disease-relevant, opens new avenues for in vivo study of the relation between the molecular assemblies generated by N-terminal mutated Aβ and synaptic dysfunctions. Moreover, these new transgenic C. elegans strains represent convenient and attractive tools for an in vivo screening of compounds interfering with oligomer formation and/or effects, as shown in the present study with EGCG.
Abbreviations
- AD
Alzheimer's disease
- Aβ
amyloid β
- APP
β-amyloid precursor protein
- Aβ1–40A2V
Aβ1–40 carrying the A2V mutation
- WT
wild-type
- Muv
multivulva
- NGM
nematode growth medium
- PBS
phosphate buffered saline
- DTT
dithiothreitol
- BSA
bovine serum albumin
- EGCG
epigallocatechin gallate
- MS
mass spectrometry
- SRM
selected reaction monitoring
- SPR
surface plasmon resonance
- X-34
1,4-bis(3-carboxy-hydroxy-phenylethenyl)-benzene
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LD, designed the research, analyzed data and wrote the paper; GDF designed the research; AP, MSt, AR performed research; MR, RG, FF, MS, MC, LB, MG, RB, performed research and analyzed data; and FT, MSalm, designed research and wrote the paper. All authors read and approved the final manuscript.
Acknowledgments
We thank Maria Grazia Malabarba for her help in microinjection of plasmid DNA into gonads of C. elegans, Gabriela Paroni for her assistance in molecular biology experiments, Ada De Luigi for her skill in immunofluorescence studies and X-34 synthesis and Laura Colombo and Alfredo Cagnotto for peptide synthesis and purification. Some C. elegans strains and E. coli OP50 were provided by CGC, which is funded by NIH Office of Research Infrastructure Program (P40 OD010440). This work was partly supported by: Cariplo Foundation Project no. 2009-2543, Telethon Foundation Project no. GGP10120, Progetto Strategico Alzheimer RF2007 of the Italian Ministry of Health, Alzheimer's Association Project NIRG-10-171655, Ricerca Corrente Italian Ministry of Health, Monzino Foundation and AFaR 2012 ID 13.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nbd.2013.10.024.
Appendix A. Supplementary data
Supplementary material.
References
- Albertini V. Optimization protocol for amyloid-beta peptides detection in human cerebrospinal fluid using SELDI TOF MS. Proteomics Clin. Appl. 2010;4:352–357. doi: 10.1002/prca.200900166. [DOI] [PubMed] [Google Scholar]
- Beeg M. Novel approaches for studying amyloidogenic peptides/proteins. Curr. Opin. Pharmacol. 2013;5:787–811. doi: 10.1016/j.coph.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Bertram L., Tanzi R.E. The genetic epidemiology of neurodegenerative disease. J. Clin. Invest. 2005;115:1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieschke J. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravman T. Exploring “one-shot” kinetics and small molecule analysis using the ProteOn XPR36 array biosensor. Anal. Biochem. 2006;358:281–288. doi: 10.1016/j.ab.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Capetillo-Zarate E. Intraneuronal Aβ accumulation, amyloid plaques, and synapse pathology in Alzheimer's disease. Neurodegener. Dis. 2012;10:56–59. doi: 10.1159/000334762. [DOI] [PubMed] [Google Scholar]
- Di Fede G. A recessive mutation in the APP gene with dominant-negative effect on amyloidogenesis. Science. 2009;323:1473–1477. doi: 10.1126/science.1168979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fede G. Good gene, bad gene: new APP variant may be both. Prog. Neurobiol. 2012;99:281–292. doi: 10.1016/j.pneurobio.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Diomede L. Tetracycline and its analogues protect Caenorhabditis elegans from beta amyloid-induced toxicity by targeting oligomers. Neurobiol. Dis. 2010;40:424–431. doi: 10.1016/j.nbd.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Dosanjh L.E. Behavioral phenotyping of a transgenic Caenorhabditis elegans expressing neuronal amyloid-beta. J. Alzheimers Dis. 2010;19:681–690. doi: 10.3233/JAD-2010-1267. [DOI] [PubMed] [Google Scholar]
- Ehrnhoefer D.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- Ewald C.Y., Li C. Understanding the molecular basis of Alzheimer's disease using a Caenorhabditis elegans model system. Brain Struct. Funct. 2010;214:263–283. doi: 10.1007/s00429-009-0235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney M., Ruvkun G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell. 1990;63:895–905. doi: 10.1016/0092-8674(90)90493-x. [DOI] [PubMed] [Google Scholar]
- Giaccone G. Neuropathology of the recessive A673V APP mutation: Alzheimer disease with distinctive features. Acta Neuropathol. 2010;120:803–812. doi: 10.1007/s00401-010-0747-1. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L. Modeling behavioral and neuronal symptoms of Alzheimer's disease in mice: a role for intraneuronal amyloid. Neurosci. Biobehav. Rev. 2007;31:125–147. doi: 10.1016/j.neubiorev.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Holtzman D.M. Alzheimer''s disease: the challenge of the second century. Sci. Transl. Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link C.D. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link C.D. Visualization of fibrillar amyloid deposits in living, transgenic Caenorhabditis elegans animals using the sensitive amyloid dye, X-34. Neurobiol. Aging. 2001;22:217–226. doi: 10.1016/s0197-4580(00)00237-2. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mahoney T.R. Analysis of synaptic transmission in Caenorhabditis elegans using an aldicarb-sensitivity assay. Nat. Protoc. 2006;1:1772–1777. doi: 10.1038/nprot.2006.281. [DOI] [PubMed] [Google Scholar]
- McColl G. The Caenorhabditis elegans A beta 1–42 model of Alzheimer disease predominantly expresses A beta 3–42. J. Biol. Chem. 2009;284:22697–22702. doi: 10.1074/jbc.C109.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M. Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics. 1995;140:527–535. doi: 10.1093/genetics/140.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi A. Causative and susceptibility genes for Alzheimer's disease: a review. Brain Res. Bull. 2003;61:1–24. doi: 10.1016/s0361-9230(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Ruvkun G., Giusto J. The Caenorhabditis elegans heterochronic gene lin-14 encodes a nuclear protein that forms a temporal developmental switch. Nature. 1989;338:313–319. doi: 10.1038/338313a0. [DOI] [PubMed] [Google Scholar]
- Sengupta P., Samuel A.D. Caenorhabditis elegans: a model system for systems neuroscience. Curr. Opin. Neurobiol. 2009;19:637–643. doi: 10.1016/j.conb.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stravalaci M. Specific recognition of biologically active amyloid-beta oligomers by a new surface plasmon resonance-based immunoassay and an in vivo assay in Caenorhabditis elegans. J. Biol. Chem. 2012;287:27796–27805. doi: 10.1074/jbc.M111.334979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. An ALS-linked mutant SOD1 produces a locomotor defect associated with aggregation and synaptic dysfunction when expressed in neurons of Caenorhabditis elegans. PLoS Genet. 2009;5:e1000350. doi: 10.1371/journal.pgen.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O., Bayer T.A. Intraneuronal Abeta accumulation and neurodegeneration: lessons from transgenic models. Life Sci. 2012;91:1148–1152. doi: 10.1016/j.lfs.2012.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.