Abstract
In humans, Th1/17 cells, identified by co-expression of the chemokine receptors CCR6 and CXCR3, have been proposed to be highly pathogenic in several autoimmune disorders due in part to their expression of the pro-inflammatory cytokines IL-17, IFN-γ and GM-CSF. However, their developmental requirements, relationship with “classic” Th17 and Th1 cells and physiological role in normal immune responses are not well understood. Here, we examined CCR6+CXCR3+ Th1/17 cells from healthy individuals, and found that ex vivo those cells produced the effector cytokines IL-17, IL-22 and IFN-γ in all possible combinations, and were highly responsive to both IL-12 and IL-23. Moreover, although the antigen specificity of CCR6+CXCR3+ Th1/17 cells showed substantial overlap with that of Th1 and Th17 cells, this population was enriched in cells recognizing certain extracellular bacteria and expressing the intestinal homing receptor integrin β7. Finally, we identified IL-1β as a key cytokine that renders Th17 cells sensitive to IL-12, and both cytokines together potently induced the differentiation of cells that produce IL-17, IFN-γ and GM-CSF. Therefore, interfering with IL-1β and IL-12 signaling in Th17 cells during inflammation may be a promising therapeutic approach to reduce their differentiation into “pathogenic” CCR6+CXCR3+ Th1/17 cells in patients with autoimmune diseases.
Introduction
Upon activation, naïve CD4 T cells differentiate into different T helper (Th) cell subsets depending on the nature of the antigen, the type of antigen-presenting cell (APC), the cytokines present in the microenvironment and the location where the APC/T cell encounter takes place (1). During this differentiation, T cells acquire specific functional characteristics such as the production of effector cytokines and the up-regulation of adhesion molecules and chemokine receptors whose expression are governed by so-called ‘master’ transcription factors. As a result, specialized Th cell subsets migrate to distinct anatomical locations, and this ensures that Th cells with the appropriate effector functions are mobilized during infection with different types of pathogens.
The association of specific chemokine receptors with distinct Th cell subsets has been used to identify Th17, Th1, Th2 and Th22 cells directly ex vivo in human peripheral blood (2–5). In addition to these Th subsets, Th1/17 cells are characterized by their ability to co-produce IL-17 and IFN-γ, together with co-expression of the Th17 and Th1 lineage-specifying transcription factors RORγt and T-bet (6). Accordingly, in humans, Th1/17 cells have been identified by the co-expression of T-bet and RORγt target genes CXCR3 and CCR6 (2,7), which allow them to migrate to sites of both Th1- and Th17-mediated inflammation. Although Th1/17 cells are found in healthy donors, interest in these cells has peaked due to their presence in cellular infiltrates observed in inflammatory bowel disease (IBD), multiple sclerosis, and juvenile idiopathic arthritis, where they are thought to contribute to disease pathogenesis (8–10). Recently, their pathogenic property was associated with the production of GM-CSF in addition to IL-17 and IFN-γ. Moreover, GM-CSF production by T cells has been linked to several autoimmune diseases, including multiple sclerosis, myocarditis and rheumatoid arthritis (11–14).
The mixed character of Th1/17 cells raises important questions regarding their differentiation, specificity and functional stability. Recent studies have shown that Th1/17 cells can differentiate from Th17 cells when stimulated via their TCR in the presence of IL-12, leading to cells producing only IFN-γ, the so-called ex-Th17 cells (8,15,16). However, in contrast to in vitro differentiated Th17 cells, in vivo generated mouse and human Th17 cells are largely unresponsive to IL-12 due to their lack of expression of the IL-12 receptor component IL-12Rβ2 (17). A more recent study reported that IL-23, signaling via the IL-23R and phosphorylation of STAT3 and STAT4, was required for the differentiation of Th17 cells into IL-17+IFN-γ+ Th cells in EAE, a mouse model for multiple sclerosis (18), but the mechanisms of Th1/17 cell development in other settings are still poorly understood. In addition, although Th17 cells and Th1 cells show differential specificity for commonly encountered infectious agents such as C. albicans, S. aureus and influenza virus (2,19), little is known about how the antigen specificity of Th1/17 cells relates to that of Th1 and Th17 cells in healthy donors.
In this study, we examined the functional characteristics, specificity and development of ex vivo purified CCR6+CXCR3+ “Th1/17” cells in healthy donors. We show that while sharing many features with Th1 and Th17 cells, this population has unique phenotypic and functional properties, and are broadly reactive with a variety of commonly encountered microorganisms. Additionally, we show that IL-1β, together with TCR stimulation renders Th17 cells responsive to IL-12 and thereby helps promote their differentiation into Il-17+IFN-γ+ Th cells. These data provide new insights into the development and function of this important T cell population, and will help in determining how Th1/17 responses are dysregulated during development of autoimmune and inflammatory diseases.
Materials and Methods
Cell purification and sorting
Samples were obtained from healthy donors participating in the Benaroya Research Institute Immune Mediated Disease Registry. Informed consent was obtained from all subjects according to IRB approved protocols at Benaroya Research Institute. CD4+CD25− cells were enriched from PBMCs by positive selection with CD4-specific microbeads (Miltenyi Biotec). Memory cell subsets were sorted to over 97% purity as CD4+CD45RA-CD45RO+CD127+CD25− using anti-CD45RA (eBioscience), anti-CD45RO (Biolegend), anti-CD127 (BD Horizon), anti-CD25 (Biolegend) and anti-CD4 (Invitrogen). Antibodies used for sorting of memory cell subsets were: anti-CCR6 (eBioscience); anti-CCR10 (R&D Systems), anti-CCR4 (Biolegend), anti-CXCR3 (BD Pharmingen), anti-IL1R1 (R&D Systems). CD14+ monocytes were isolated from PBMCs by positive selection with CD14-specific microbeads (Miltenyi Biotec). Cells were cultured in RPMI 1640 medium supplemented with 2 mM glutamine, 1% (vol/vol) nonessential amino acids, 1% (vol/vol) sodium pyruvate, penicillin (50 U/ml), streptomycin (50 µg/ml) (all from Invitrogen) and 5% heat-inactivated human serum. In some experiments, recombinant human IL-1β (10 ng/ml; R&D Systems), IL-12 (10 ng/ml; R&D Systems) or IL-23 (25 ng/ml; eBioscience) were added to the culture.
Intracellular staining
Intracellular staining for IL-22, IL-17, IFN-γ, GM-CSF and IL-10 was performed on cells stimulated for 5 h with PMA and ionomycin in the presence of brefeldin A (all from Sigma-Aldrich) for the final 2.5 h of culture. Cells were fixed and permeabilized with Fix and Perm buffer (BD Biosciences) according to manufacturer’s instructions. Cells were stained with anti-IL-17 (eBioscience), anti-IL-22 (eBioscience), anti-IL-10 (Biolegend), anti-IFN-γ (Biolegend and BD Pharmingen), anti-GM-CSF (eBioscience). FACS data were analyzed with FlowJo (Tree Star). Intracellular staining for RORγt and T-bet were performed directly ex vivo or after in vitro cell expansion using anti RORγt (eBioscience) and anti-T-bet (Biolegend and eBioscience).
Phospho-STAT staining and Ikbα degradation by flow cytometry
Cells were stimulated for 25 min in the presence or absence of 25 ng/ml recombinant human IL-12 (R&D Systems), 50 ng/ml IL-23 (eBioscience) or 25 ng/ml IL-1β (R&D Systems). Cells were then washed two times in FACS buffer and fixed for 20 min in BD Fix and Perm buffer at room temperature (BD Biosciences), washed in FACS buffer, and fixed in 90% ice-cold methanol for 30 min on ice. Cells were washed two times with BD Perm/Wash and stained with antibodies against intracellular markers, including pSTAT4 (Y693) and pSTAT3 (pY705) (both from BD Biosciences) or Ikbα (Cell Signaling), in BD Perm/Wash for 40 min at room temperature. Cells were then washed two times, resuspended in BD Perm/Wash and analyzed by flow cytometry.
In vitro cell expansion
Sorted cells were activated with anti-CD3/anti-CD28-coated microbeads (Invitrogen) at a 5:1 ratio (5 T cells per bead) and cultured for 13 days. IL-2 (20 U/ml) was added at day 4 and then every 2 to 3 days. After 13 days of culture, cells were analyzed for cytokine production by intracellular cytokine staining and for transcription factor expression by real-time quantitative RT-PCR and flow cytometry.
Real-time quantitative RT-PCR
Total RNA from sorted or expanded T cell subsets was extracted using the RNeasy kit (Qiagen) and treated with DNaseI (Qiagen) to avoid genomic DNA contamination. cDNA were synthesized with M-MuLV reverse-transcriptase and oligo(dT) primers (Fermentas) and gene expression was examined with ABI 7500 Fast Real-Time PCR system (Applied Biosystem) using a SYBR green real-time PCR kit (Fermentas). The data were normalized to β-Actin (ACTB) gene expression. The primers used were (5’-3’): RORC-F: GCATGTCCCGAGATGCTGTC and -R: CTGGGAGCCCCAAGGTGTAG; TBX21-F: CCGTGACTGCCTACCAGAAT and -R: ATCTCCCCCAAGGAATTGAC; IL12RB2-F: ACATTCTTGGACATAGTGAGGCC and -R: GTACATCTGCTCACAGAAGCC; IL23R-F: AACAACAGCTCGGCTTTGGT and -R: GGAATATCTGGCGGA TATCC; IL12RB1-F: CATTCCTGCCGACACCCACACA and -R: CTGTTTGCTGTCTTCATCTCGG; IL1R1-F: GTGATTGTGAGCCCAGCTA and -R: TGTTTGCAGGATTTTCCACA; ACTB-F: GGACTTCGAGCAAGAGATGG and -R: AGCACTGTGTTGGCGTACAG.
Antigen-specific proliferation assays
Sorted cells (5 × 104) we co-cultured in flat-bottomed 96-well plates with irradiated autologous monocytes (1.5 × 105) pulsed for 3h with C. albicans (Greer laboratories), S. aureus, S. pneumoniae, E. coli, L. rhamnosus (all from Invivogen) or Influenza virus (H3N2 Brisbane 10/07, Prospecbio) antigens. Proliferation was measured on day 4 after 16 h incubation with 5µCi/ml [3H]thymidine. In some experiments, sorted cells were labeled with CFSE (Sigma-Aldrich), stimulated with autologous monocytes pulsed with antigens for 6 days, followed by intracellular staining after restimulation with PMA and ionomycin for 4 h.. The analysis of cytokine-producing T cells was performed by flow cytometry on CFSElo cells.
Statistics
Statistical tests were performed using Prism software (GraphPad, San Diego, CA). Significance was determined by paired two-tailed Student’s t-test or one-way ANOVA analysis with Tukey correction, as noted in figure legends.
Results
CCR6+CXCR3+ Th1/17 cells share functional characteristics with Th17 and Th1 cells
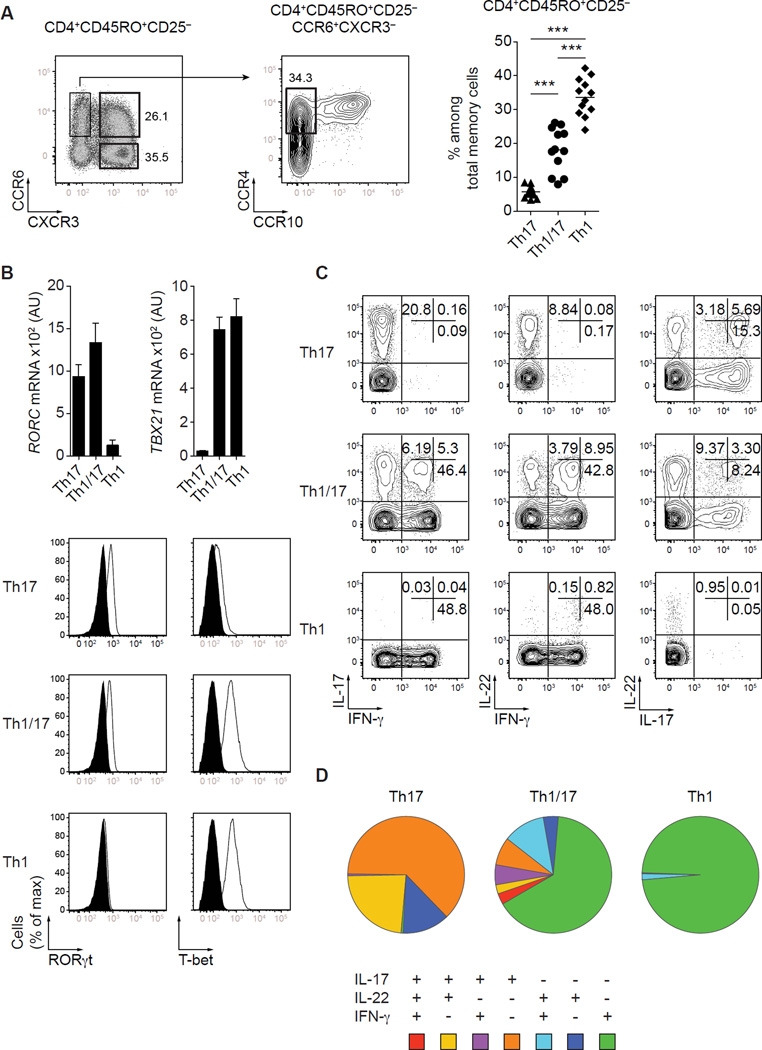
As we and others have previously reported (2,7,20), expression of the chemokine receptors CCR6 and CXCR3 defines 4 subsets of CD4+CD45RO+CD25-CD127+ memory Th cells in the peripheral blood of healthy donors: CCR6+CXCR3− IL-17-producing Th17 cells, CCR6+CXCR3+ cells containing Th1/17 cells, CCR6-CXCR3+ Th1 cells and CCR6-CXCR3− cells that express CCR4 and are enriched in IL-4-producing Th2 cells (Fig. 1A and data not shown). In most of our experiments, we further enriched in IL-17-producing T cells by selecting CCR6+CXCR3− T cells that were also CCR4+CCR10-, which distinguishes them from CCR6+CXCR3-CCR4+CCR10+ Th22 cells (3). For clarity, we will refer to CCR6+CXCR3+ T cells as “Th1/17 cells” throughout the paper. Among total memory CD4+ T cells in peripheral blood, Th17 cells were present at the lowest frequency, Th1 cells were the most abundant, and the frequency of Th1/17 cells was intermediate between the two other subsets. Th1/17 cells did not merely represent an activated subset of Th1 or Th17 cells, as expression of markers associated with T cell activation/proliferation such as ICOS and Ki-67 was low and did not substantially differ between the Th17, Th1 and Th1/17 cell populations (data not shown).
Figure 1. Phenotype, gene expression and cytokine production by human CCR6+CXCR3+ T cells.
(A) Expression of CCR6, CXCR3, CCR4 and CCR10 by gated CD4+CD45RO+CD25-CD127+ Th cells from peripheral blood (left panels). Summary of several different donors with 6+3− Th17 cells, 6+3+ Th1/17 cells and 6−3+ Th1 cells (right panel). ***P<0.001 (ANOVA). Each symbol represents one donor; horizontal bars indicate mean. Data are from 12 donors. (B) Quantitative RT-PCR analysis of RORC and TBX21 gene expression by the indicated Th cell subsets (top panel). AU, arbitrary units. Data are mean ± SEM of 7 donors. Expression of RORγt and T-bet by sorted 6+3− Th17 cells, 6+3+ T cells and 6−3+ Th1 cells directly ex vivo (lower panel). Data are representative of 4 independent experiments. (C) Production of IL-17, IL-22 and IFN-γ by sorted Th cell subsets stimulated for 5h with PMA/ionomycin. (D) Boolean gating analysis showing each possible combination of IL-17, IL-22 and IFN-γ production representative of 6 different donors.
CXCR3 and CCR6 expression by Th cells is controlled by the transcription factors T-bet and RORγt, respectively (21,22). Consistent with this, the Th1/17 cell population homogenously co-expressed these transcription factors in amounts similar to that observed in conventional Th1 and Th17 cells (Fig. 1B). However, despite their uniform expression of T-bet and RORγt, Th1/17 cells were highly polyfunctional, and produced the cytokines associated with Th1 or Th17 cells, IFN-γ, IL-17 and IL-22, in all possible combinations (Fig. 1C, 1D). Notably, like ‘conventional’ Th1 cells, the majority of Th1/17 cells produced IFN-γ in the absence of either IL-17 or IL-22. Consistent with IFN-γ being the dominant cytokine produced by the Th1/17 cell population, most of the IL-22-producing 6+3+ T cells co-produced IFN-γ, but not IL-17. Th1/17 cells also contained the highest frequency of GM-CSF-producing cells as compared to Th17 and Th1 cells, and within the Th1/17 subset, production of GM-CSF was produced in association with IFN-γ, IL-17 and IL-22 (Supplemental Fig. 1).
CCR6+CXCR3+ Th1/17 cells are highly responsive to IL-12 and IL-23
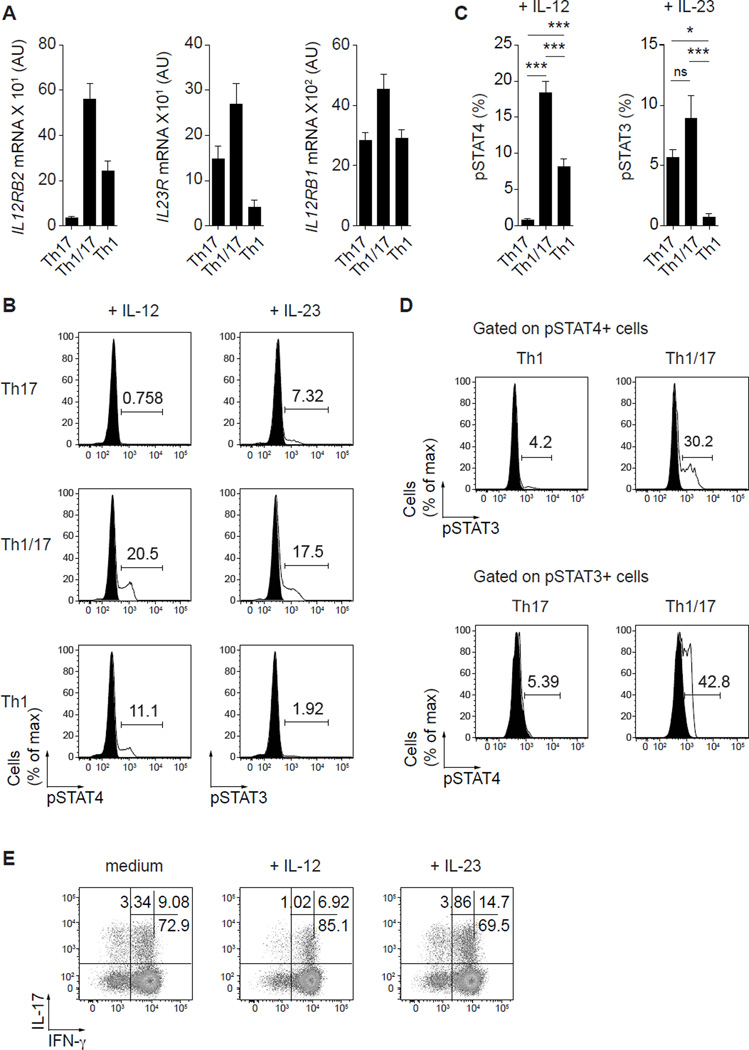
Given their expression of both T-bet and RORγt, we examined the ability of Th1/17 cells to respond to IL-12 and IL-23, which are associated with Th1 and Th17 cell differentiation, respectively. Surprisingly, Th1/17 cells expressed both IL12RB2 (IL-12Rβ2) and IL23R (IL-23R) cytokine receptors at levels significantly higher than either Th1 or Th17 cells and there was also a trend toward higher expression of IL12RB1 (IL12Rβ1), which is a shared component of the IL-12 and IL-23 receptors (Fig. 2A). Consistent with the expression results, Th1/17 cells displayed robust functional responses to both IL-12 and IL-23 as measured by phosphorylation of STAT4 and STAT3, respectively (Fig. 2B, 2C). Interestingly, Th1/17 cells that respond to IL-12 or IL-23 expressed the highest levels of T-bet or RORγt, respectively (data not shown). In addition, nearly 30% of the cells that phosphorylated STAT4 in response to IL-12 also responded to IL-23 by phosphorylating STAT3 in Th1/17 cells, whereas less than 5% of Th1 cells could respond to both cytokines. Similarly, around 40% of the cells that phosphorylated STAT3 in response to IL-23 also responded to IL-12 by phosphorylating STAT4 in Th1/17 cells, whereas only 5% or less of Th17 cells phosphorylating both STATs (Fig. 2D). Thus, a significant fraction of Th1/17 cells can respond to IL-12 and IL-23, indicating that these cytokines may help modify the survival, proliferation and/or functional characteristics of these cells depending on the inflammatory environment they encounter. To address this, we expanded Th1/17 cells with CD3/CD28 beads in the presence of IL-12 or IL-23 and analyzed their cytokine profile after 13 days (Fig. 2E). Whereas IL-12 increased the proportion of IFN-γ-only-producing cells while slightly reducing the number of cells producing IL-17, addition of IL-23 substantially increased the frequency of IL-17/IFN-γ-producing T cells without affecting the number of cells producing IL-17 alone. Additionally, IL-23, but not IL-12, slightly augmented the proliferation of anti-CD3/CD28-activated Th1/17 cells (data not shown). Thus, by their co-expression of IL-12Rβ2 and IL-23R, Th1/17 cells can adjust their functional characteristics in different inflammatory environments.
Figure 2. CCR6+CXCR3+ T cell respond to both IL-12 and IL-23.
(A) Quantitative RT-PCR analysis of IL12RB2, IL23R and IL12RB1 gene expression by the indicated Th cell subsets. AU, arbitrary units. Data are mean ± SEM of 6 donors. (B) Phosphorylation of STAT4 and STAT3 by the indicated Th cell subsets in response to IL-12 and IL-23, respectively; untreated cells (filled histograms) and cytokine-treated cells (solid line). (C) Proportion of cells that phosphorylate STAT4 or STAT3 in response to IL-12 and IL-23. Data are mean ±SEM of 7 donors. ns, P>0.05; *P<0.05; ***P<0.001 (ANOVA). (D) Phosphorylation of STAT4 and STAT3 by the indicated Th cell subsets in response to IL-12 + IL-23; cells treated with IL-12 (upper panels) or IL-23 (lower panels) (filled histograms) and cells treated with both cytokines together (solid line). Plots are gated on pSTAT4+ (top) and pSTAT3+ (bottom) cells. Data are representative of 4 independent experiments. (E) Production of IL-17 and IFN-γ by 6+3+ T cells activated with CD3/CD28 beads and cultivated for 13 days with either medium, IL-12 or IL-23. Data are representative of 6 donors analyzed.
CCR6+CXCR3+ Th1/17 cells are broadly reactive
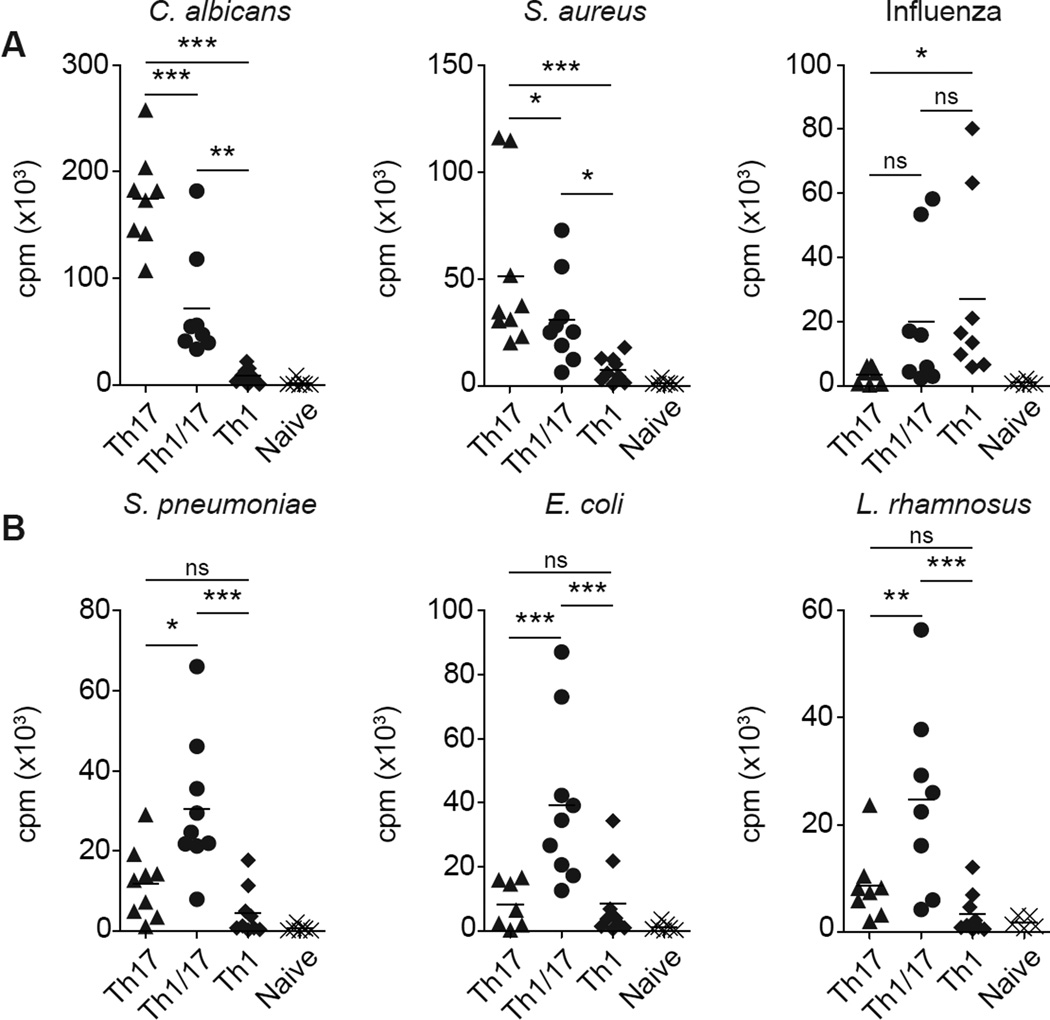
To further examine the functional relationship of Th1/17 cells with Th17 and Th1 cells, we compared their antigen specificities directly ex vivo. For this, we measured T cell proliferation in response to antigens from commonly encountered microorganisms. We focused first on two Th17-associated antigens (C. albicans and S. aureus) and one Th1-associated antigen (Influenza) (Fig. 3A). Consistent with their mixed phenotype, Th1/17 cells proliferated in response to all three antigens, but at a lower magnitude than Th17 or Th1 cells. However, T cells responding to extracellular bacteria associated with either the upper respiratory tract (S. pneumonia) or the gut (E. coli, L. rhamnosus) were consistently enriched in the Th1/17 cell subset (Figure 3B), with little or no response observed in the Th17 or Th1 cells. Given their polyfunctionality, we next assessed the cytokine production potential of Th1/17 cells specific for these different antigens (Supplemental Fig. 2). In contrast to Th17 and Th1 cells, antigen-specific Th1/17 cells displayed a highly polyfunctional cytokine response dominated by production of IFN-γ in conjunction with IL-17 and/or IL-22 as we observed in total Th1/17 cells. Therefore, our results show that even though Th1/17 cells shared antigen specificities with Th17 and Th1 cells, these T cells were enriched in cells recognizing certain extracellular bacteria, and present a very broad cytokine response capable of mobilizing multiple effector pathways that may contribute to pathogen clearance and control.
Figure 3. CCR6+CXCR3+ T cells recognize certain extracellular bacteria in addition to Th1- and Th17-associated antigens.
(A and B) Antigen-specific proliferation of the indicated Th cell subsets stimulated with autologous monocytes pulsed with different antigens. Proliferation was measured after 16h pulse with [3H]thymidine on day 4 of the culture. Data are from 8 or 9 healthy donors.
Integrin α4β7 expression in Th17 cells and CCR6+CXCR3+ Th1/17 cells discriminates between C. albicans and E. coli responses
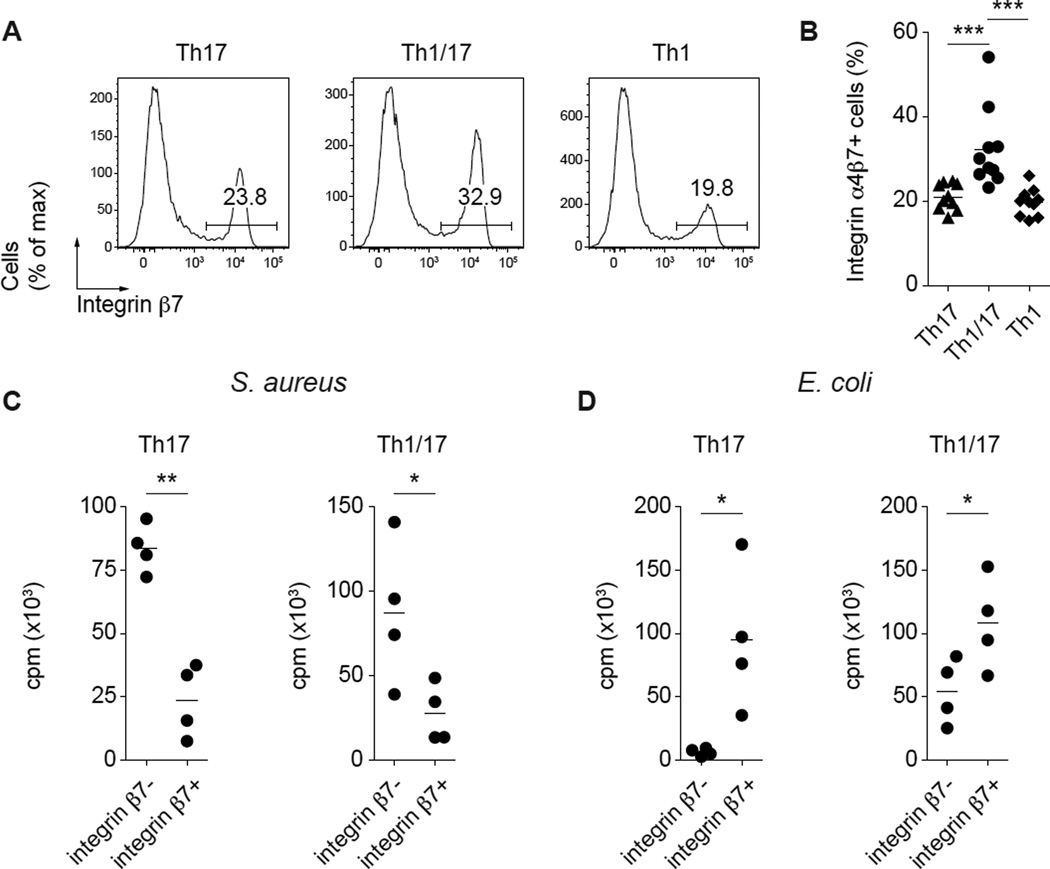
Because Th1/17 cells were enriched in cells directed towards bacteria associated with mucosal surfaces such as E. coli and L. rhamnosus, we asked if this was related to the presence of a higher frequency of cells expressing integrin β7, which in conjunction with the α4 and αe integrin subunits helps mediate T cell migration to the intestinal lamina propria and epithelium. Indeed, compared with Th1 and Th17 cells, Th1/17 cells contained a higher proportion of integrin β7-expressing cells (Fig. 4A, 4B). Moreover, integrin β7 expression segregated antigen-specific responses to S. aureus, a bacterium associated with the human respiratory tract and the skin, and E. coli, which generally colonizes the intestines, in both Th17 cells and Th1/17 cells (Fig. 4C, 4D). Thus, Th1/17 cells are enriched in cells capable of migrating to the intestines and reactive for gut-tropic microorganisms, suggesting an association between antigen-recognition in the intestinal tissues and acquisition of the unique Th1/17 cell phenotype.
Figure 4. Integrin α4β7 expression in Th17 cells and 6+3+ T cells discriminates between C. albicans and E. coli responses.
(A) Expression of integrin β7 by the indicated Th cell subsets. (B) Proportion of cells that express integrin β7 among the different Th cell subsets. Data are from 10 donors. ***P<0.001 (ANOVA). Antigen-specific proliferation of 6+3− Th17 and 6+3+ Th1/17 cell subsets sorted based on integrin β7 expression and stimulated with autologous monocytes pulsed with S. aureus (C) or E. coli (D). Proliferation was measured after 16h pulse with [3H]thymidine on day 4 of the culture. Data are from 4 donors. *P<0.05; **P<0.01 (two-tailed paired t test).
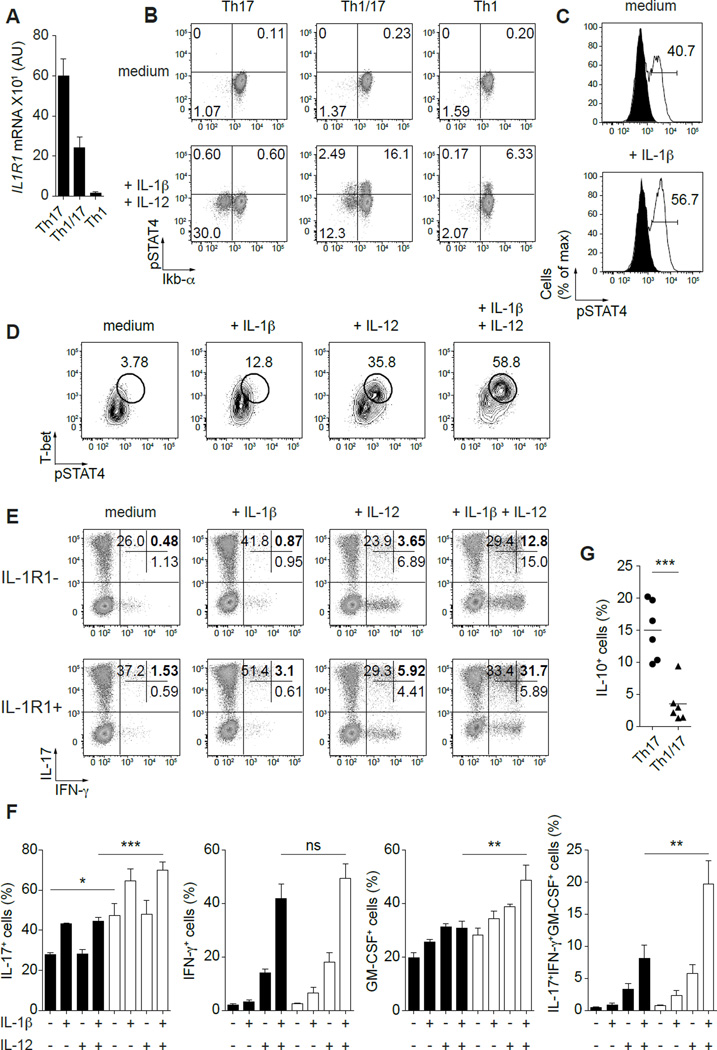
TCR stimulation and IL-1β render Th17 cells responsive to IL-12 and favor differentiation into Th1/17 cells
Recent fate-mapping studies in murine systems have demonstrated that Th1/17 cells can be derived from Th17 cells (16). However, the cytokines that promote their differentiation in different circumstances remain unclear. Although IL-12 can potently induce expression of genes associated with Th1/17 cells such as T-bet, IFN-γ and CXCR3, we and others have shown that Th17 cells are not responsive to IL-12 directly ex vivo (17). Notably, a subset of Th17 cells expressed the IL-1 receptor (IL-1R1) (Fig. 5A and Supplemental Fig. 3A), and responsiveness to IL-1β can be measured by assessing IL-1β-dependent IKbα degradation (Fig. 5B and Supplemental Fig. 3A). Moreover, IL-12 and IL-1β are both produced upon pathogen sensing via toll-like receptors and intracellular inflammasomes, respectively, raising the possibility that these cytokines may be co-produced following infection or tissue injury (23,24). Therefore, we evaluated the effect of IL-1β treatment on the capacity of Th17 cells to respond to IL-12, and subsequently to differentiate into IL-17+IFN-γ+ Th cells.
Figure 5. TCR stimulation and IL-1β render Th17 cells responsive to IL-12 and favor the differentiation into IFN-γ+IL-17+ Th cells.
(A) Quantitative RT-PCR analysis of IL1R1 gene expression by the indicated Th cell subsets. AU, arbitrary units. Data are mean ± SEM of 9 donors. (B) STAT4 phosphorylation and Ikbα degradation in response to IL-12 and IL-1β by the indicated Th cell subsets directly ex vivo. (C) STAT4 phosphorylation in response to IL-12 by 6+3− Th17 cells activated for 24h with CD3/CD28 beads in the presence or absence of IL-1β; untreated cells (filled histograms) and cytokine-treated cells (solid line). (D) STAT4 phosphorylation and T-bet expression by 6+3− Th17 cells activated for 24h with CD3/CD28 beads in the presence of absence of IL-1β and/or IL-12. (E) Production of IL-17 and IFN-γ by IL-1R1 – and + 6+3− Th17 cells expanded for 13 days with the indicated cytokines and stimulated for 5h with PMA/ionomycin. (F) Proportion of IL-1R1 – and + 6+3− Th17 cells producing IL-17, IFN-γ, GM-CSF or all three cytokines together after expansion as indicated above. Data are mean ±SEM of 4 donors. *P<0.05; **P<0.01; ***P<0.001 (ANOVA). (G) Production of IL-10 by expanded 6+3− Th17 and 6+3+ T cells. Data are mean ±SEM of 6 donors. ***P<0.001 (two-tailed paired t test).
Interestingly, overnight TCR stimulation itself induced Th17 cell responses to IL-12 as illustrated by STAT4 phosphorylation. However, the addition of IL-1β to the culture dramatically increased this response (Fig. 5C). Furthermore, Th17 cells activated in the presence of IL-1β + IL-12 strongly upregulated T-bet expression and displayed an enhanced capacity to respond to IL-12 (Fig. 5D). These data correlated with the up-regulation of TBX21 (T-bet) and IL12RB2 (IL-12Rβ2) at the gene expression level, while slightly increasing RORC (RORγt) (Supplemental Fig. 3B and C). To determine if this led to induction of IFN-γ expression, we expanded Th17 cells with IL-1β, IL-12 or both and analyzed cytokine production after 13 days. Because IL1-R1 is not homogeneously expressed on Th17 cells (Supplemental Fig. 3A), we analyzed IL-1R1-negative and positive Th17 cells separately. As previously published, IL-1R1-positive Th17 cells produced the highest levels of IL-17 (25). Addition of IL-1β alone to the culture increased the percentage of IL-17-producing cells without inducing IFN-γ, whereas IL-12 had only a small impact on the proportion of IFN-γ-producing cells. However, IL-1β and IL-12 together displayed a strong synergistic effect, inducing a large proportion of Th17 cells to express IFN-γ in conjunction with IL-17, and this synergistic effect was observed with amounts of IL-1β that were ~10-fold lower than that conventionally used in T cell differentiation cultures (Fig. 5E, 5F and Supplemental Fig. 4). Moreover, the proportion of cells with a proinflammatory and potentially pathogenic cytokine profile (coproduction of IL-17, IFN-γ and GM-CSF), was strongly increased by IL-1β + IL-12 treatment in IL-1R1-positive Th17 cells. Interestingly, more T cells lost IL-17 expression to become IFN-γ-only producing cells in the IL-1R1-negative Th17 cell population compared to the IL-1R1-positive cells.
IL-1β exposure was recently shown to potently inhibit IL-10 production by Th17 cells (19), and consistent with a potential role for IL-1β in the development of Th1/17 cells in vivo, the fraction of IL-10-producing Th1/17 cells was much smaller than that observed in conventional Th17 cells (Fig. 5G). Collectively, these data indicate that IL-1β is a key cytokine that, together with TCR stimulation, renders in vivo generated Th17 cells sensitive to IL-12, and both cytokines potently induce the differentiation of IFN-γ+IL-17+ Th1/17 and IFN-γ+IL-17− “ex-Th17 cells” that also produce GM-CSF.
Discussion
To adequately respond to biologically diverse pathogens, CD4 T cells undergo functional specialization into distinct Th cell subsets. However, recent data indicate that there is substantial plasticity among these populations, and that cells with ‘blended’ phenotypes can be identified (26). In this study, we performed an in depth characterization of the function, antigen specificity and developmental requirements of ex vivo purified CCR6+CXCR3+ Th1/17 cells that have been associated with multiple inflammatory and autoimmune diseases, and which display unique functional and gene-expression profiles (27). By focusing on ex vivo sorted T cells, our study brings critical insights into the phenotypic and functional characteristics of Th1/17 cells, and avoids bias that can be introduced by working on in vitro expanded T cells. This is illustrated by the lack of detectable levels of IL12RB2 on freshly isolated peripheral blood Th17 cells as compared to precedent work where human Th17 clones express T-bet and IL-12RB2 at levels similar to Th1 and Th1/17 clones (8). In addition, the notion that Th1/17 cells express higher levels of IL-12RB2 than Th1 cells was not previously appreciated. Moreover, by comparing the antigen specificities of Th17, Th1 and Th1/17 cells directly ex vivo, we could show that Th1/17 cells, in addition to sharing antigen specificities with Th17 and Th1 cells, have increased frequencies of cells recognizing certain types of bacteria and this was linked to their higher integrin β7 expression. Finally, we report for the first time that IL-1β can potently synergize with IL-12 to induce human IL-1R1+ Th17 cells to upregulate IFN-γ and GM-CSF expression, therefore generating cells with a pro-inflammatory and potentially pathogenic cytokine profile.
Phenotypically, IL-17 and IFN-γ co-producing Th cells were previously shown to co-express CCR6 and CXCR3 (2,7). However, we found that despite homogenous expression of the transcription factors T-bet and RORγt, Th1/17 cells displayed a highly polyfunctional cytokine profile. This degree of functional heterogeneity was not observed in classical Th1 or Th17 cells, and cautions against using the Th1/17 phenotype to definitely identify IFN-γ/IL-17 co-producing cells. Although this may reflect an inherent functional instability or flexibility in the Th1/17 cells, their cytokine profiles were relatively stable during in vitro activation and expansion in the absence of added polarizing cytokines, suggesting that they are not innately less stable that those of Th1 or Th17 cells. Alternatively, recent epigenetic data has demonstrated that the ‘enhancer landscape’ available to T-bet and RORγt is dictated by TCR- and cytokine-dependent activation of AP-1 and STAT-transcription factors (28). Thus, heterogeneity in availability of specific promoter/enhancer elements to T-bet and RORγt caused by differences in TCR/cytokine signals the cells have been exposed to during differentiation may underlie the various patterns of cytokine secretion present in the Th1/17 cell population. The relatively high frequency of Th1/17 cells observed in peripheral blood of healthy donors, and the presence of Th1/17 cells specific for commonly encountered microorganisms such as C. albicans and S. aureus and influenza virus indicates that the differentiation of cells into this “hybrid” Th cell population is a fairly common mechanism happening most likely during an ongoing immune response. However, the imbalance between these different T cell populations might be perturbed during an autoimmune disease.
Interestingly, despite the strong association of IL-22 with Th17 responses, most IL-22-producing cells in the Th1/17 cell population co-produced IFN-γ and not Il-17. However, Th1 cells were the first cell type reported to express IL-22 (29), and IL-22 production in human CD4 T cells is more strongly associated with production of IFN-γ than IL-17 (3). Co-production of IL-17 and IL-22 is beneficial in coordinating innate epithelial cell responses against extracellular pathogens. Whereas IL-17 is largely pro-inflammatory and causes tissue destruction, IL-22 has a regenerative and protective effect on epithelial cells (30). Similarly, IL-22 may help balance the pro-inflammatory functions of IFN-γ with important wound healing and tissue repair functions. For instance, in the intestine IFN-γ and IL-22 can have opposing effects in maintaining epithelial barrier function during inflammation caused by food sensitivities, toxins or infection (31,32). Furthermore, the genomic proximity of the IFNG and IL22 gene loci suggest that shared regulatory regions may contribute to the co-expression of IFN-γ and IL-22 in Th1/17 cells (31). It will therefore be interesting to examine the epigenetic status of the IL-22 locus to define how expression of this important cytokine is controlled in different IL-22-producing Th cell populations.
Cells specific for both Th17-associated antigens C. albicans and S. aureus, and the Th1-associated antigen influenza were contained in the Th1/17 cell population, suggesting that some of these cells may originate from classical Th17 cells that upregulate T-bet and CXCR3, whereas others may be derived from Th1 cells that turn on expression of RORγt and CCR6. Whereas a firm developmental link between Th1 and Th1/17 cells has not been established, several studies have demonstrated that Th1/17 cells can differentiate from Th17 cells. For instance, TCR sequence analysis demonstrated that at least a fraction of the CD161+ Th1 cells (which are predominantly found in the Th1/17 cell population) were clonally related to Th17 cells (33). Additionally, several reports indicated that Th17 cells are plastic and can differentiate into cells producing both IL-17 and IFN-γ or IFN-γ only (8,15,17,18). Further analysis, including comprehensive TCR sequence analysis on antigen-specific T cells, is needed to help determine the developmental relationships between classical Th1 or Th17 cells, and different populations of Th1/17 cells.
Although IL-12 is capable of promoting Th1/17 cell development from in vitro generated Th17 cells, Th17 sorted directly from human blood do not efficiently respond to IL-12 due to low expression of IL-12Rβ2. We found that IL-1β, together with TCR stimulation renders Th17 cells responsive to IL-12, and that there is a strong synergy between IL-1β and IL-12 in promoting the differentiation of IL-1R1-positive Th17 cells into cells that coproduce IL-17 and IFN-γ without impacting RORγt expression. Moreover, most of the IL-17+IFN-γ+ cells also co-produced GM-CSF, and production of these three cytokines together has been associated with pathogenicity in autoimmune diseases. Thus, we hypothesize that IL-1β might help stabilize T-bet expression, and thereby potentiate IL-12R signaling on a subset of Th17 cells, rendering them more susceptible to functional reprogramming. As a result, co-production of IL-1β and IL-12 during bacterial or fungal infection (19,34,35), or during inflammation in patients with active autoimmune diseases (36,37), could participate in the differentiation of Th17 cells into highly pro-inflammatory Th1/17 cells in vivo. Furthermore, inhibition of Th1/17 cell differentiation may partially underlie the therapeutic efficacy of treatments such as Anakinra that target IL-1β activity. In addition to IL-1β + IL-12, other pathways have been identified to induce IFN-γ production by Th17 cells. Indeed, similar to IL-1β, IFN-γ itself can render Th17 cells sensitive to IL-12 (17). Moreover, although IL-23 failed to synergize with IL-1β to promote Th1/17 differentiation, repeated stimulation of Th17 cells in the presence of IL-23 can directly upregulate IFN-γ independently of T-bet (18). Therefore, Th17 cell plasticity may be governed in vivo by multiple pathways depending on the cytokine microenvironment and interfering with this mechanism might prove more challenging than previously anticipated.
In addition to their shared antigenic specificities with Th1 and Th17 cells, we found that Th1/17 cells present a unique reactivity profile. Indeed, this T cell population was enriched in cells responding to E. coli, L. rhamnosus, and S. pneumonia, and a previous study demonstrated that responses to Mycobacterium tuberculosis PPD are found almost exclusively in the Th1/17 cell population (2). The unique properties of Th1/17 cells were also illustrated by the high frequency of integrin α4β7+ cells in this population, and by the enrichment of T cell specific for E. coli in the integrin α4β7+ cell fraction. This suggests that the intestinal tissue may be a particularly potent site of Th1/17 cell differentiation. Consistent with this, compared to peripheral blood, the intestinal mucosa of IBD patients contains a high frequency of Th1/17 cells (8). Indeed, several studies have reported high levels of IL-1β secretion by colonic lamina propria monocytes from patients with active IBD (38,39), and although IL-12 production is tightly controlled in the intestines it can be induced during infection with certain enteric pathogens (40). Thus, Th17 cells present at sites of intestinal inflammation can be exposed to both IL-1β and IL-12, triggering their differentiation into Th1/17 cells. Whereas this may be beneficial in combating enteric pathogens and maintaining proper intestinal homeostasis, when dysregulated it may also contribute to development of inflammatory and autoimmune diseases. The unique reactivity profile of Th1/17 cells supports the idea that these cells are in some degree different from Th17 and Th1 cells. This was further enforced by a recent study showing that Th1/17 cells in human peripheral blood displayed a somewhat distinct gene signature. This is illustrated by the expression of the gene ABCB1 specifically in Th1/17 cells (data not shown and (27)).
The identification of new helper T cell subsets such as Th17 and Th22 cells has provided important insights into the high degree of T cell specialization required to efficiently respond to a microbial insult. However, recent evidence that Th subsets are more plastic that initially believed adds considerable complexity to this issue and highlights the need to better characterize “hybrid” Th cell populations such as Th1/17 cells that have been implicated in autoimmune disease pathogenesis. Our characterization of the functional properties, specificity and development of CCR6+CXCR3+ Th1/17 cells has revealed that these cells are highly polyfunctional, that they have a unique specificity profile and surface phenotype associated with activation in mucosal tissue sites, and that the cytokine IL-1β may play a key role together with IL-12 in their differentiation from IL-1R1-positive Th17 cell precursors in vivo. Given their polyfunctionality and broad antigen-reactivity, it will be interesting in future studies to examine the differentiation and functional behavior of different Th1/17 populations in settings of acute and chronic inflammation. In particular, analyses of inflamed tissue samples will help determine if certain functionalities and specificities are enriched during different types of inflammatory responses. This information will be useful in assessing the development and function of these cells in the contexts of normal and pathogenic immune responses, and for the identification of new therapeutic strategies to manipulate Th1/17 cells in immune-mediated diseases.
Supplementary Material
Acknowledgements
The authors thank K. Arumuganathan for his help with cell sorting and flow cytometry; the Benaroya Research Institute Translational Research Program Clinical Core for obtaining donor samples.
Footnotes
This work was in part supported by grants AR055695, DK072295, HL098067 and AI067750 to D.J.C. from the NIH. T.D. was the recipient of a postdoctoral fellowship from the Arthritis Foundation.
The authors have no conflicting financial interests.
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 3.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 4.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J. Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 5.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 6.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O'Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen CJ, Crome SQ, MacDonald KG, Dai EL, Mager DL, Levings MK. Human Th1 and Th17 cells exhibit epigenetic stability at signature cytokine and transcription factor loci. J. Immunol. 2011;187:5615–5626. doi: 10.4049/jimmunol.1101058. [DOI] [PubMed] [Google Scholar]
- 8.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosmi L, Cimaz R, Maggi L, Santarlasci V, Capone M, Borriello F, Frosali F, Querci V, Simonini G, Barra G, Piccinni MP, Liotta F, De PR, Maggi E, Romagnani S, Annunziato F. Evidence of the transient nature of the Th17 phenotype of CD4+CD161+ T cells in the synovial fluid of patients with juvenile idiopathic arthritis. Arthritis Rheum. 2011;63:2504–2515. doi: 10.1002/art.30332. [DOI] [PubMed] [Google Scholar]
- 10.Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, Duquette P, Prat A. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 2009;66:390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- 11.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 13.Sonderegger I, Iezzi G, Maier R, Schmitz N, Kurrer M, Kopf M. GM-CSF mediates autoimmunity by enhancing IL-6-dependent Th17 cell development and survival. J. Exp. Med. 2008;205:2281–2294. doi: 10.1084/jem.20071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell IK, Rich MJ, Bischof RJ, Dunn AR, Grail D, Hamilton JA. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J. Immunol. 1998;161:3639–3644. [PubMed] [Google Scholar]
- 15.Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, Crawford GE, Hatton RD, Weaver CT. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lexberg MH, Taubner A, Albrecht I, Lepenies I, Richter A, Kamradt T, Radbruch A, Chang HD. IFN-gamma and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur. J. Immunol. 2010;40:3017–3027. doi: 10.1002/eji.201040539. [DOI] [PubMed] [Google Scholar]
- 18.Duhen R, Glatigny S, Arbelaez CA, Blair TC, Oukka M, Bettelli E. Cutting Edge: the pathogenicity of IFN-gamma-producing Th17 cells is independent of T-bet. J. Immunol. 2013;190:4478–4482. doi: 10.4049/jimmunol.1203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 20.Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012;119:4430–4440. doi: 10.1182/blood-2011-11-392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lord GM, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–3439. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev. Immunol. 2011;31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- 25.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, Flavell RA, Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, McCauley JL, Abreu MT, Unutmaz D, Sundrud MS. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J. Exp. Med. 2014;211:89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vahedi G, Kanno Y, Sartorelli V, O'Shea JJ. Transcription factors and CD4 T cells seeking identity: masters, minions, setters and spikers. Immunology. 2013;139:294–298. doi: 10.1111/imm.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J. Immunol. 2002;168:5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 30.Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C. IL-17 and IL-22: siblings, not twins. Trends Immunol. 2010;31:354–361. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol. Rev. 2013;252:116–132. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- 32.Beaurepaire C, Smyth D, McKay DM. Interferon-gamma regulation of intestinal epithelial permeability. J. Interferon Cytokine Res. 2009;29:133–144. doi: 10.1089/jir.2008.0057. [DOI] [PubMed] [Google Scholar]
- 33.Nistala K, Adams S, Cambrook H, Ursu S, Olivito B, de JW, Evans JG, Cimaz R, Bajaj-Elliott M, Wedderburn LR. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14751–14756. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu L, Bray MD, Osorio M, Kopecko DJ. Campylobacter jejuni induces maturation and cytokine production in human dendritic cells. Infect. Immun. 2006;74:2697–2705. doi: 10.1128/IAI.74.5.2697-2705.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwak DJ, Augustine NH, Borges WG, Joyner JL, Green WF, Hill HR. Intracellular and extracellular cytokine production by human mixed mononuclear cells in response to group B streptococci. Infect. Immun. 2000;68:320–327. doi: 10.1128/iai.68.1.320-327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hessian PA, Highton J, Kean A, Sun CK, Chin M. Cytokine profile of the rheumatoid nodule suggests that it is a Th1 granuloma. Arthritis Rheum. 2003;48:334–338. doi: 10.1002/art.10776. [DOI] [PubMed] [Google Scholar]
- 37.Yilmaz M, Kendirli SG, Altintas D, Bingol G, Antmen B. Cytokine levels in serum of patients with juvenile rheumatoid arthritis. Clin. Rheumatol. 2001;20:30–35. doi: 10.1007/s100670170100. [DOI] [PubMed] [Google Scholar]
- 38.Mahida YR, Wu K, Jewell DP. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989;30:835–838. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinecker HC, Steffen M, Witthoeft T, Pflueger I, Schreiber S, MacDermott RP, Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin. Exp. Immunol. 1993;94:174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelsall BL, Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol. Rev. 2005;206:132–148. doi: 10.1111/j.0105-2896.2005.00292.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.