Abstract
Background
Framingham risk score (FRS) underestimates risk in young adults. LV mass (LVM) relates to cardiovascular disease (CVD), with unclear value in youth. In a young biracial cohort, we investigate how FRS predicts CVD over 20 years and the incremental value of LVM. We also explore the predictive ability of different cut-points for hypertrophy.
Methods
We assessed FRS and echocardiography-derived LVM (indexed by BSA or height2.7) from 3980 African-American and white CARDIA participants (1990-1991); and followed over 20 years for a combined endpoint: cardiovascular death; nonfatal myocardial infarction, heart failure, cerebrovascular disease, and peripheral artery disease. We assessed the predictive ability of FRS for CVD and also calibration, discrimination, and net reclassification improvement for adding LVM to FRS.
Results
Mean age was 30±4 years, 46% males, and 52% white. Event incidence (n = 118) across FRS groups was, respectively, 1.3%, 5.4%, and 23.1% (p<0.001); and was 1.4%, 1.3%, 3.7%, and 5.4% (p<0.001) across quartiles of LVM (cut-points 117g, 144g, and 176g). LVM predicted CVD independently of FRS, with the best performance in normal weight participants. Adding LVM to FRS modestly increased discrimination and had a statistically significant reclassification. The 85th percentile (≥116 g/m2 for men; ≥96 g/m2 for women) showed event prediction more robust than currently recommended cut-points for hypertrophy.
Conclusion
In a biracial cohort of young adults, FRS and LVM are helpful independent predictors of CVD. LVM can modestly improve discrimination and reclassify participants beyond FRS. Currently recommended cut-points for hypertrophy may be too high for young adults.
Keywords: young adults, cardiovascular risk, left ventricular hypertrophy, echocardiography
Introduction
Global cardiovascular (CV) risk tools, such as the Framingham Risk Score (FRS)1, are recommended to assess risk in asymptomatic adults as young as age 20 years.2 However, the FRS alone tends to underestimate event prediction in youth, even when multiple risk factors are present.3 In addition, it is still unclear whether adding a risk marker to FRS may aid in young adults CV risk stratification.
Left ventricular mass (LVM) and hypertrophy (LVH) are markers of LV remodeling, recognized as important measures to assess clinical prognosis in hypertensive children, adolescents, and adults.4-6 Both measurements have shown predictive power for CV events in diverse clinical settings.7,8 Obesity is an important determinant of LVM and may interact with indexing methods, affecting the definition of LVH.7,9 The best way to integrate LVM measures and LVH into clinical algorithms, however, is not established; particularly in youth.2,10
In a biracial cohort of young adults followed over 20 years, we hypothesized that FRS would be a valuable tool to stratify CV risk and that adding information on LVM could aid in this risk stratification. Thus, in this study we aim: (1) to assess the occurrence of CV events as predicted by the FRS in youth alone; (1) to assess the ability of LVM to predict CV events independent of the FRS, exploring the interactions of the various indexing methods with obesity; and (3) to investigate if LVM improves discrimination and effectively reclassifies young adults by adding prediction power to the FRS. Additionally, we explore the performance of currently recommended LVH cut-points for long-term event prediction in this biracial young cohort.
Methods
Study design and sample
The Coronary Artery Risk Development in Young Adults (CARDIA) study was previously described.11 Briefly, 5,115 African-American and white adults, aged between 18 and 30 years, were enrolled in 4 field centers (Birmingham, AL; Oakland, CA; Chicago, IL; and Minneapolis, MN) in 1985-1986 and followed prospectively. The CARDIA exam year-5 (1990 – 1991) was defined as baseline for the present study, when the entire cohort underwent echocardiography assessment. All subjects with interpretable echocardiography exam and complete data on covariates at CARDIA exam year-5 were included in this study. From the 4352 participants who attended the year-5 exam, 109 did not have echocardiography data and one withdrew consent from the study, 132 were missing data on the Framingham risk covariates, 126 were missing information on LVM, and 4 were missing BSA, leaving 3980 in the analytic cohort. CARDIA exam Year-0 clinical characteristics for the analytic cohort and excluded participants are shown in Supplement Table S1. Informed consent was obtained from each participant and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the all centers' human research committee.
Echocardiography
All echocardiograms were performed on an Acuson cardiac ultrasound machine (Siemens)12 by trained professionals, using a standardized method previously designed and available at the CARDIA website (http://www.cardia.dopm.uab.edu/exam-materials2). All studies were interpreted at a single reading center (University of California, Irvine) at the time of year-5 examination. LVM was measured from short-axis views, using 2D-guided M-mode echocardiography, leading-edge-to-leading-edge technique, as recommended by the ASE.13,14 Reproducibility profile has been published for the original measurements and a recent reassessment.12,15 LVM was indexed (LVMi) by BSA or height2.7. BSA was computing using standardized weight / height measurements by the modified DuBois method.16,17 Weight was measured with balance beam scales (the same type of scale in all centers) and height with a wall mounted stadiometer or verticle ruler. Additionally, unindexed LVM and LVM/height1.7 were computed and reported in the supplemental material.
Follow-up and Endpoint
Details of outcomes ascertainment processes have been described.18 For this analysis, a combined endpoint of CV events, including cardiovascular death and nonfatal heart failure (HF), myocardial infarction (MI), stroke, transient ischemic attack (TIA), and peripheral artery disease (PAD) was the dependent variable.
The total follow-up period was 20 years, with median follow-up among those without CV events of 19.9 years. Participants were interviewed during their scheduled study examinations and by telephone yearly; vital status was checked by participant or proxy interview or by database searches at 6 month contacts between annual interviews. Participants were asked about overnight hospitalizations and outpatient procedures for treatment of cardiovascular conditions.
Medical records were requested for all suspected cardiovascular events. Death certificates were requested for all deaths; the protocol required requests for emergency services and emergency department records, next-of-kin and physician interviews for outpatient suspected cardiovascular deaths. Two members of the end-points committee reviewed each record, applying standard outcomes definitions contained in a detailed adjudication manual, to classify events; disagreements were resolved by committee consensus.
MI was classified based on an algorithm using symptoms, cardiac biomarkers, and ECG findings.19 HF required admission for new or decompensated heart failure and classification was based on symptoms, signs, and imaging according to criteria developed by the Atherosclerosis Risk in Communities Study. 20 Stroke was adjudicated based on symptoms, physical findings, and imaging results, and published guidelines were used for subclassification.21-23 TIA required one or more episodes of focal neurologic deficit, and imaging must have been negative for stroke regardless of symptom duration.23 PAD was adjudicated based on symptomatic disease, ischemic ulcers, gangrene, and/or requiring intervention. Cardiovascular death included mortality with an underlying cause of atherosclerotic coronary heart disease, stroke, atherosclerotic disease other than coronary or stroke (eg, abdominal aortic aneurysm), and non-atherosclerotic cardiac disease (eg, non-ischemic cardiomyopathy and including hypertensive heart disease). Fatal atherosclerotic coronary heart disease included fatal MI and coronary heart disease using published recommendations.19
Statistical Analysis
Cox regression analysis assessed the performance of LVMi as an independent predictor of CV events, computing hazard ratios (HR) for the overall cohort and according to BMI groups (normal weight, overweight, and obese). For the analysis, we computed the first event in each participant. Statistical significance of the HRs was assessed with the Wald chi-square test. Areas under the receiver-operating characteristic curves (AUC) were also computed.24 A nonparametric statistical test developed by DeLong et al24 was used to determine whether the AUCs for different models were significantly different . For the “FRS covariates” models, all covariates present in the calculation of the Framingham 10-year global cardiovascular risk score (FRS)1 were individually included in multivariable models, adjusting also for race and gender. For the “calculated FRS” models, we modified the score as first described by D'Agostino et al.1 to include age as a continuous variable and race. Net reclassification improvement was calculated to evaluate the added predictive ability for LVMi to the FRS.25 Statistical significance of the net reclassification improvement was tested with equation 9 in Pencina et al.25 Calibration was assessed by the Hosmer-Lemeshow test and indicated good calibration for all models (data not shown). In an exploratory additional analysis, we calculated HR and AUC for diverse LVH cut-points predicting events, using models adjusted for age, sex, and race. LVH cut-points included the currently ASE-recommended cut-points14, gender-specific percentiles in our entire population, 95th race-specific percentiles of a healthy reference subgroup, and additional cut-points previously shown in the literature.26 Additional information on statistical analysis is reported in the Supplements.
Results
Participant age ranged from 22 to 36 years at the CARDIA examination year 5. According to BMI classification, 49.9% of the participants were normal weight; 29.0% were overweight; 18.7% were obese; and 2.5% were underweight. Patient characteristics are shown in Table 1, according to the BMI group.
Table 1. Participant characteristics at CARDIA exam year-5 (1990-91), overall and according to the BMI group.
| Variable | Mean(SD) | |||
|---|---|---|---|---|
|
| ||||
| Normal (n = 1,986) | Overweight (n = 1,153) | Obese (n = 743) | Overall (n = 3,980) | |
| Age | 29.8 (3.7) | 30.1 (3.6) | 30.2 (3.7) | 30.0 (3.6) |
| Height (m) | 1.71 (0.09) | 1.72 (0.10) | 1.69 (0.09) | 1.71 (0.09) |
| Weight (Kg) | 64.9 (9.1) | 80.3 (9.8) | 101.0 (17.4) | 75.7 (18.1) |
| BSA (m2) | 1.74 (0.18) | 1.90 (0.18) | 2.06 (0.22) | 1.84 (0.23) |
| Heart rate (beats/30 sec) | 33.8 (5.0) | 33.7 (4.8) | 35.0 (4.7) | 34.1 (5.0) |
| Total cholesterol (mg/dL) | 173.2 (33.0) | 182.2 (34.1) | 185.9 (35.3) | 177.9 (34.2) |
| HDL cholesterol (mg/dL) | 56.7 (14.0) | 50.9 (13.5) | 47.2 (12.5) | 53.4 (14.2) |
| LDL cholesterol (mg/dL) | 102.9 (30.9) | 114.5 (31.6) | 118.3 (32.3) | 108.8 (32.0) |
| SBP (mmHg) | 105.6 (11.0) | 109.1 (10.7) | 111.6 (12.3) | 107.7 (11.4) |
| DBP (mmHg) | 67.3 (9.5) | 69.9 (9.5) | 73.3 (10.4) | 69.1 (9.9) |
| Cigarette/day | 3.8 (7.6) | 3.8 (8.0) | 3.4 (7.0) | 3.7 (7.6) |
| BMI (kg/m2) | 22.3 (1.7) | 27.1 (1.4) | 35.4 (5.2) | 26.0 (5.7) |
| LVMi/height2.7 (g/m2.7) | 32.4 (8.0) | 36.5 (8.2) | 41.7 (10.0) | 35.2 (9.2) |
| LVMi/BSA (g/m2) | 78.5 (18.3) | 83.1 (18.8) | 83.6 (19.1) | 80.6 (18.8) |
| Variable | Number of participants (%) | |||
|---|---|---|---|---|
|
| ||||
| Normal (n = 1,986) | Overweight (n = 1,153) | Obese (n = 743) | Overall (n = 3,980) | |
| African-American Ethnicity | 782 (39.4) | 584 (50.7) | 512 (68.9) | 1919 (48.2) |
| Male Gender | 878 (44.2) | 640 (55.5) | 278 (37.4) | 1813 (45.6) |
| Diabetic participants | 12 (0.6) | 8 (0.7) | 10 (1.4) | 30 (0.8) |
| Use of anti-hypertensive medication | 11 (0.6) | 18 (1.6) | 31 (4.2) | 61 (1.5) |
Legend: BMI – body-mass index; SD – standard deviation; SBP – systolic blood pressure; DBP – diastolic blood pressure; LVM – left ventricular mass; LVMi – left ventricular mass index.
The combined endpoint of CV events was registered in 118 participants; 29 (24.6%) had cardiovascular death, 26 (22.0%) developed congestive heart failure, 29 (24.6%) myocardial infarction, 21 (17.8%) stroke, 9 (7.6%) TIA, and 4 (3.4%) participants developed PAD. Cardiovascular death was due to hypertension (8 participants); ischemic heart disease (7 participants); pulmonary heart disease (3 participants); cardiomyopathy (2 participants); cardiac dysrhythmias (3 participants); cerebrovascular disease (4 participants); and complication of heart diseases (2 participants). Information on participant characteristics according to the presence of events is shown in Supplement Table S2. Events were incident in 83 African-American participants (4.3% of the total) and in 35 white participants (1.7% of the total). Normal BMI participants had 26 CV events (1.3%), while the overweight had 38 (3.3%), and the obese 34 CV events (4.6%). Unadjusted cumulative event rates according to the FRS point score and to LVM indexed by BSA and height2.7 are shown in Figure 1, demonstrating increasing event rates across the variables, with a tendency for steeper slopes at the higher levels of both FRS and indexed LVM.
Figure 1. Cubic spline fitted to show event rates for computed Framingham risk score plus age and across left ventricular mass deciles, according to indexation method.
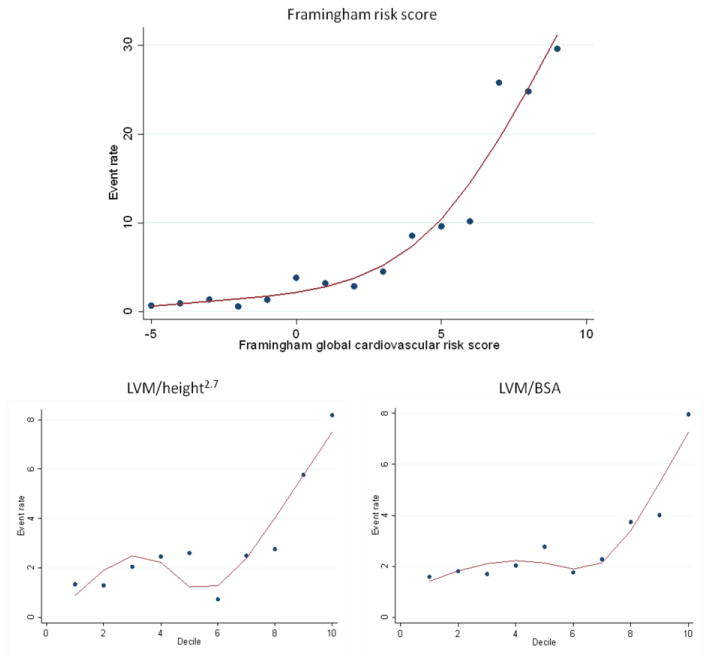
Legend: Framingham global cardiovascular risk score following D'Agostino et al (2008)1; scores of ≥9 are pooled. Sample sizes in the Framingham point score categories are (point score: sample size): (-5: 141), (-4: 543), (-3: 441), (-2: 533), (-1: 592), (0: 439), (1: 409), (2: 278), (3: 216), (4: 173), (5: 103), (6: 49), (7: 23), (8: 20), and (≥9: 20), with maximum point score 13. LVM category refers to deciles of the distribution in the cohort; LVM – left ventricular mass; BSA – body-surface area.
Considering the entire cohort and adjusting for FRS covariates, the hazard ratios for CV events were slightly higher for LVM/BSA compared to LVM/height2.7 (Table 2). Of note, African-American ethnicity was associated with higher hazard ratios for both LVMi: 2.28 (95% CI: 1.51, 3.45) for LVM/height2.7 and 2.33 (95% CI: 1.55, 3.52) for LVM/BSA. Similar results were found for unindexed LVM or LVM/height1.7 (Supplement Table S3). When the models were adjusted for the calculated FRS, race, and age, LVM and indices showed statistically significant independent event prediction ability. Both LVMi had modest increases in the AUC when added to the calculated FRS or the FRS covariates (Table 2). When the hazard ratios were computed according to the BMI group, the best performance was found for normal weight individuals, with similar performance for LVM/BSA or height-derived LVM indexing (Table 3; Supplement Table S3).
Table 2. Cox regression hazard ratios (HR) and areas under the receiver-operating characteristic curves (AUC) for LVM and the Framingham risk score (FRS).
| Predictor | FRS covariates | Calculated FRS | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) p-value |
AUC | HR (95% CI) p-value |
AUC | |
| LVM/height2.7 | 1.15 (0.99, 1.35) 0.07 |
0.80† | 1.18 (1.03; 1.35) 0.02 |
0.80† |
| LVM/BSA | 1.18 (1.01, 1.38) 0.04 |
0.80† | 1.21 (1.05; 1.39) 0.007 |
0.80¥ |
Legend: LVM – left ventricular mass; BSA – body surface area; CI – confidence interval. HR refers to 1 standard-deviation increase. The “FRS covariates” models included: race, gender, age, HDL-cholesterol, total cholesterol, systolic blood pressure, treatment for hypertension, smoking status, and presence of diabetes. In the calculated FRS, the score is calculated as initially described by D'Agostino et al modified to include age as a continuous variable and with further adjustment to race. 1 AUC for FRS covariates alone = 0.79 and for calculated FRS alone = 0.79.
p-value < 0.05 and
p-value = 0.07, in both cases when comparing AUC between FRS alone and adding LVM index.24
Table 3. Cox regression hazard ratios (HR) and areas under the receiver-operating characteristic curves (AUC) for cardiovascular events combined endpoint in normal, underweight, and obese participants.
| Predictor | FRS covariates | Calculated FRS | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) p-value |
AUC | HR (95% CI) p-value |
AUC | |
| LVM/height2.7 | ||||
| Normal | 1.55 (1.07; 2.22) 0.02 |
0.87 | 1.54 (1.13; 2.10) 0.006 |
0.85 |
| Overweight | 1.11 (0.79; 1.57) 0.56 |
0.80 | 1.10 (0.79; 1.53) 0.58 |
0.80 |
| Obese | 1.05 (0.82; 1.36) 0.70 |
0.72 | 1.15 (0.91; 1.45) 0.24 |
0.69 |
| LVM/BSA | ||||
| Normal | 1.43 (1.03; 1.98) 0.03 |
0.87 | 1.51 (1.12; 2.02) 0.006 |
0.85 |
| Overweight | 1.07 (0.77; 1.49) 0.67 |
0.80 | 1.07 (0.80; 1.45) 0.64 |
0.80 |
| Obese | 1.14 (0.88; 1.48) 0.33 |
0.73 | 1.24 (0.98; 1.55) 0.07 |
0.70¥ |
Legend: BMI – body-mass index; LVM – left ventricular mass; BSA – body surface area; CI – confidence interval. HR refers to 1 standard-deviation increase. The “FRS covariates” models included: race, gender, age, HDL-cholesterol, total cholesterol, systolic blood pressure, treatment for hypertension, smoking status, and presence of diabetes. In the calculated FRS, the score is calculated as initially described by D'Agostino et al modified to include age as a continuous variable and with further adjustment to race. 1 AUC or FRS covariates alone were 0.86, 0.80, and 0.72 for normal, overweight, and obese respectively. AUC for calculated FRS alone were 0.85, 0.80, and 0.68 for normal, overweight, and obese respectively.
p-value = 0.07, when comparing AUC between FRS alone and adding LVM index.24
Both LVM indexing methods showed similar positive and statistically significant net reclassification improvement when added to FRS covariates (Table 4). Adding LVM/height2.7 correctly downgraded risk in 189 (5%) participants that did not have events, and correctly reclassified 7 (6%) of those that had events to a higher risk group. Adding LVM/BSA moved 188 (5%) of participants that did not have events to a lower risk group, and reclassified 8 (7%) participants that had events to a higher risk group. The net reclassification improvement for LVM/height2.7 was 0.13 (p < 0.01) and for LVM/BSA was 0.11 (p = 0.02).
Table 4. Reclassification table: absolute number of participants classified in each strata for Framingham risk score (FRS) components plus race vs. adding information on left ventricular mass (LVM) index.
| Risk Category | No event (n = 3862) | Events (n = 118) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| FRS | FRS | ||||||||
| <2.5% | 2.5 – 4.9% | 5.0 – 9.9% | ≥ 10% | <2.5% | 2.5 – 4.9% | 5.0 – 9.9% | ≥ 10% | ||
| FRS + LVM/height2.7 | <2.5% | 2517 | 92 | 2 | 0 | 24 | 8 | 0 | 0 |
| 2.5 - 5.0% | 112 | 583 | 64 | 1 | 1 | 18 | 7 | 0 | |
| 5.0 - 10% | 0 | 60 | 259 | 30 | 0 | 6 | 15 | 7 | |
| > 10% | 0 | 0 | 23 | 119 | 0 | 0 | 0 | 32 | |
| FRS + LVM/BSA | <2.5% | 2514 | 94 | 3 | 0 | 24 | 8 | 0 | 0 |
| 2.5 - 5.0% | 117 | 576 | 66 | 1 | 3 | 16 | 7 | 0 | |
| 5.0 - 10% | 0 | 72 | 253 | 24 | 0 | 5 | 18 | 5 | |
| > 10% | 0 | 0 | 21 | 121 | 0 | 0 | 0 | 32 | |
Legend: LVM – left ventricular mass; BSA – body surface area. Cut-points for risk groups were defined according to logistic regression models (see Methods for full description).
The prevalence of LVH varied with the indexing process (Table 5). The results of the exploratory analysis regarding the best cut-point value to define LVH in our population are shown in Table 5 Compared to the current ASE-recommended values for LVM/height2.7 and for LVM/BSA, overall, the 85th percentile achieved the highest AUC values (0.716 and 0.726, respectively) though they did not reach statistical significance (p=0.20 and p=0.08, respectively). The 85th percentile also had the highest HRs (2.89 and 3.00, respectively) overall.
Table 5. Age-, race, and sex-adjusted hazard ratios (HR) and areas under the receiver-operating characteristic curves (AUC) for current American Society of Echocardiography (ASE)-recommended cut-points for left ventricular hypertrophy (LVH) and for 85th, 90th, and 95th percentile cut-points of left ventricular mass (LVM) index.
| LVH parameter (unit) | LVH cut-point value | Prevalence of LVH (%) | HR (95% CI) | AUC (p-value) |
|---|---|---|---|---|
| LVM/ height2.7 (g/m2.7) | ||||
| ASE-recommended | ≥49 M, ≥45 W | 378 (9.5) | 2.35 (1.51, 3.67) | 0.705 (NA) |
| Liao,26 1997 (sex specific) | ≥ 50 M, 47 W | 299 (7.5) | 2.31 (1.44, 3.71) | 0.702 (0.55) |
| Liao,26 1997 | ≥ 51 M/W | 216 (5.4) | 2.24 (1.33, 3.78) | 0.700 (0.51) |
| 95% Reference group (race-specific) | ≥44.6 B, ≥44.5 C | 551 (13.8) | 2.70 (1.84, 3.97) | 0.716 (0.16) |
| 85th Percentile | ≥45.1 M, ≥42.9 W | 587 (15.0) | 2.89 (1.98, 4.22) | 0.716 (0.20) |
| 90th Percentile | ≥47.3 M, ≥45.9 W | 399 (10.0) | 2.90 (1.93, 4.37) | 0.715 (0.07) |
| 95th Percentile | ≥51.6 M, ≥51.2 W | 200 (5.0) | 2.26 (1.32, 3.87) | 0.698 (0.39) |
| LVM/BSA (g/m2) | ||||
| ASE-recommended | ≥116 M, ≥96 W | 318 (8.0) | 2.53 (1.60, 4.01) | 0.706 (NA) |
| Liao,26 1997 (sex specific) | ≥117 M, ≥104 W | 197 (5.0) | 2.26 (1.31, 3.90) | 0.699 (0.38) |
| Liao,26 1997 | ≥ 125 M/W | 75 (1.9) | 2.34 (1.08, 5.08) | 0.698 (0.35) |
| 95% Reference group (race-specific) | ≥103.6 B, ≥104.5C | 395 (9.9) | 2.70 (1.76, 4.14) | 0.709 (0.77) |
| 85th Percentile | ≥105.4 M, ≥89.5 W | 598 (15.0) | 3.00 (2.06, 4.37) | 0.726 (0.08) |
| 90th Percentile | ≥111.1 M, ≥94.8 W | 399 (10.0) | 2.06 (1.31, 3.24) | 0.702 (0.31) |
| 95th Percentile | ≥119.4 M, ≥101.8 W | 200 (5.0) | 2.12 (1.21, 3.73) | 0.697 (0.25) |
Legend: LVM – left ventricular mass; BSA – body surface area; HR – hazard ratio; CI – confidence interval; NA – not applicable; M – men; W – women; B – blacks; C - caucasians. AUC p values refer to the difference in AUC from ASE-recommended cut-points.24
Discussion
Both FRS and LVM are widely used in decision-making on adult patients, although their value as a global cardiovascular risk marker when assessed in early adulthood is not established. In a population based study of biracial young healthy adults, we showed that FRS had good performance for risk stratification over a 20-year follow-up (as opposed to 10 years for the Framingham score in older individuals). LVM assessed by echocardiography showed a modest but consistent additional predictive power to FRS, particularly in normal weight participants. This suggests that LVMi may be adequate to complement the FRS information in young individuals with other risk factors, in which FRS alone typically underestimates the CV risk burden. Further, the current cut-points for LVH were explored in a long-term perspective for predicting CV events in young adults and showed that current ASE-recommended cut-points appear to be too high for young adults.
D'Agostino and colleagues followed 8,491 predominantly white subjects free of CV disease (mean age 49 years) over 12 years and described a more robust version of the FRS updated for global CV 10-year risk profile.1,3 However, age is the major determinant of risk in the FRS and many young individuals with hypertension, obesity, and other risk factors have therefore a low global FRS predicted risk.3 Since young individuals with chronic exposure to risk factors have a higher CV risk burden early in life, risk scores may underestimate risk in this age group.27
The rates of cardiovascular events in young adults are a major concern.28 Despite the low event rate (2.96% in 20 years) and the known racial- and age-related limitations, the calculated FRS performed well in CARDIA with relative risk of nearly 20 for the highest 1% of FRS values compared to those with risk below 2.5% (Figure 1). In this study, we computed the FRS in percentiles of risk as it is widely known and usually applied to patients in daily practice. To avoid statistical limitations, we also used the FRS covariates as independent variables in our models.
After adjustment for race, our findings support LVM as a risk marker that could add valuable information beyond the FRS in a young cohort of young adult Caucasian and African-American men and women. Prior studies investigating the predictive power of LVM including a biracial cohort were performed in older or sicker populations, have not used recently recommended risk reclassification methodology, and have a substantially shorter follow-up period when compared to the present report.7,29
Heart size scales with body size and definitions of normality range should take into account variation associated with anthropometrics. The ASE currently provides cut-points for the diagnosis of LVH when LVM is indexed to height2.7 or to BSA.14 Obesity relates to LV remodeling and may interact with the indexing method.30 Studies have reported that BSA indexing underestimates LVH prevalence among obese and overweight individuals.31,32 Height based indexing seems to predict CV events similarly to BSA indexing in studies with low prevalence of obesity, but becomes superior as the prevalence of obesity increases.33,34
Obesity plays a major role in cardiac geometry even in the absence of increased cardiometabolic risk and also influences LVM values early in life.35,36 However, it is not clear when an adaptive increase in LVM becomes pathologic. Indexing LVM for body size attempts to overcome this problem, however, the best LVM indexing method that could adjust for adaptive increases but not pathologic increases in LVM remains under debate.10 Indexing to height appears to show a better relation with lean body mass, but LVM/BSA is still used in the literature and is recommended by the ASE.7,14,37 It is possible that the relationship of indexed LVM to events might be different in obese and non-obese young adults. As previously reported,33 LVM indexing methods had similar success across BMI groups in our study. The most robust results for the LVMi predicting CV events were among participants in the normal BMI group (Tables 2 and S2). The adaptive increase in LVM mediated by obesity is not present in normal weight participants, thus increased LVM can be assumed to be pathologic rather than adaptive in these individuals.
Current cut-points for LVH are based on studies using middle-aged populations and do not use global CV event prediction as a parameter to define cut-points for LVH.14 Clear cut-points for LVH in young adults may aid the general clinician in daily decision-making and therapeutic approach.7 Our exploratory results suggest that the current ASE recommendation on LVH may not be the most appropriate for young adults. A more adequate cut-point could include lower values of LVM and be based on global events prediction ability.
Study Limitations
We report a low event rate over the 20-year follow-up period, which may affect the statistical power of our survival assessment. However, the incidence rate seems adequate to the assessment of a healthy cohort of young individuals. LVM was calculated using an algorithm that computes M-mode echocardiography measurements, assuming that the heart is modeled as a prolate ellipsoid of revolution, limiting the use of this method in remodeled hearts. 7,14 However, remodeled hearts are rarely present in young healthy adults. Moreover, echocardiography is a validated and recommended method to assess LVM and LVH, with a reasonable profile for cost, versatility, acceptability, availability, and reproducibility. 4-6,14,38,39
Conclusion
In African-American and White adults at ages 22 to 36 years, the FRS showed good performance predicting global cardiovascular events over 20 years of follow-up. LVM can independently predict CV events, modestly improve discrimination, and also effectively reclassify participants beyond the FRS. Although modest, the additional value of LVM, particularly in those of normal weight may help to assess CV risk in young adults with multiple risk factors, typically underestimated by FRS alone. Different LVM indexing methods performed similarly for event prediction in our study. The results of our exploratory analysis for the 85th percentiles of LVM/height2.7 and for LVM/BSA suggest that the currently ASE-recommended cut-points for LVH might be lowered for CV event prediction in young generally healthy individuals.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH); CARDIA contract [grant numbers N01-HC-48047 – N01-HC-48050, N01-HC-95095] and a subcontract with the Echocardiography reading centers at year 5 [grant number N01-HC-45134] and at year 25 [grant number NIH NHLBI-HC-09-08]. Dr. Armstrong was supported by Universidade Federal do Vale do São Francisco (Petrolina, PE, Brazil) and by the Johns Hopkins University (Baltimore, MD, USA).
All CARDIA sites' ethics committees have approved the research protocol and informed consent has been obtained from all CARDIA participants. Dr. Lima and Dr. Armstrong had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
All authors have read and approved submission of the manuscript, which has not been published and is not being considered for publication elsewhere in whole or in part in any language. On behalf of all authors, there is no relevant disclosure related to this manuscript.
A preliminary version of these findings was presented on November 4th, during the 2012 American Heart Association scientific meetings (Los Angeles, CA), in the finals of the Elizabeth Barrett-Connor Investigator Award competition.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008 Feb 12;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010 Dec 21;122(25):e584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 3.Marma AK, Lloyd-Jones DM. Systematic examination of the updated Framingham heart study general cardiovascular risk profile. Circulation. 2009 Aug 4;120(5):384–390. doi: 10.1161/CIRCULATIONAHA.108.835470. [DOI] [PubMed] [Google Scholar]
- 4.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007 Jun;28(12):1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 5.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004 Aug;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003 Dec;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong AC, Gjesdal O, Wu C, Gidding S, Bluemke DA, Lima JA. Left ventricular mass assessed by Echocardiography and Cardiac Magnetic Resonance, cardiovascular outcomes, and medical practice. J Am Coll Cardiol Img. 2012 Aug 1;5(8):11. doi: 10.1016/j.jcmg.2012.06.003. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai CL, Chien KL, Hsu HC, Su TC, Chen MF, Lee YT. Left ventricular mass and risk of cardiovascular events and all-cause death among ethnic Chinese--the Chin-Shan Community Cardiovascular Cohort study. Int J Cardiol. 2011 Jun 16;149(3):347–352. doi: 10.1016/j.ijcard.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues SL, Angelo LC, Pereira AC, Krieger JE, Mill JG. Determinants of left ventricular mass and presence of metabolic risk factors in normotensive individuals. Int J Cardiol. 2009 Jul 10;135(3):323–330. doi: 10.1016/j.ijcard.2008.03.066. [DOI] [PubMed] [Google Scholar]
- 10.Gidding SS. Controversies in the assessment of left ventricular mass. Hypertension. 2010 Jul;56(1):26–28. doi: 10.1161/HYPERTENSIONAHA.110.153346. [DOI] [PubMed] [Google Scholar]
- 11.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 12.Gardin JM, Wagenknecht LE, Anton-Culver H, et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995 Aug 1;92(3):380–387. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- 13.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989 Sep-Oct;2(5):358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005 Dec;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Gidding SS, Liu K, Colangelo LA, et al. Longitudinal Determinants of Left Ventricular Mass and Geometry: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Circ Cardiovasc Imaging. 2013 Sep 1;6(5):769–775. doi: 10.1161/CIRCIMAGING.112.000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boothby WM, Standiford RB. Normographic charts for the calculation of the metabolic rate by the gasometer method. Boston Med Surg J. 1921 Sep 22;185:18. 1921. [Google Scholar]
- 17.Du Bois D, Du Bois EF. Clinical calorimetry. X. A formula to estimate the approximate surface area if height and weight are known. Arch Intern Med. 1916 Jun;17(6_2):9. 1916. [PubMed] [Google Scholar]
- 18.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009 Mar 19;360(12):1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003 Nov 18;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 20.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012 Mar 1;5(2):152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993 Jan;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Madden KP, Karanjia PN, Adams HP, Jr, Clarke WR. Accuracy of initial stroke subtype diagnosis in the TOAST study. Trial of ORG 10172 in Acute Stroke Treatment. Neurology. 1995 Nov;45(11):1975–1979. doi: 10.1212/wnl.45.11.1975. [DOI] [PubMed] [Google Scholar]
- 23.Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009 Jun;40(6):2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 24.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988 Sep;44(3):837–845. [PubMed] [Google Scholar]
- 25.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008 Jan 30;27(2):157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 26.Liao Y, Cooper RS, Durazo-Arvizu R, Mensah GA, Ghali JK. Prediction of mortality risk by different methods of indexation for left ventricular mass. J Am Coll Cardiol. 1997 Mar 1;29(3):641–647. doi: 10.1016/s0735-1097(96)00552-9. [DOI] [PubMed] [Google Scholar]
- 27.Oren A, Vos LE, Uiterwaal CS, Grobbee DE, Bots ML. Cardiovascular risk factors and increased carotid intima-media thickness in healthy young adults: the Atherosclerosis Risk in Young Adults (ARYA) Study. Arch Intern Med. 2003 Aug 11-25;163(15):1787–1792. doi: 10.1001/archinte.163.15.1787. [DOI] [PubMed] [Google Scholar]
- 28.O'Flaherty M, Allender S, Taylor R, Stevenson C, Peeters A, Capewell S. The decline in coronary heart disease mortality is slowing in young adults (Australia 1976-2006): a time trend analysis. Int J Cardiol. 2012 Jul 12;158(2):193–198. doi: 10.1016/j.ijcard.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009 May 5;119(17):2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rider OJ, Lewandowski A, Nethononda R, et al. Gender-specific differences in left ventricular remodelling in obesity: insights from cardiovascular magnetic resonance imaging. Eur Heart J. 2013 Jan;34(4):292–299. doi: 10.1093/eurheartj/ehs341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuspidi C, Meani S, Negri F, et al. Indexation of left ventricular mass to body surface area and height to allometric power of 2.7: is the difference limited to obese hypertensives? J Hum Hypertens. 2009 Nov;23(11):728–734. doi: 10.1038/jhh.2009.16. [DOI] [PubMed] [Google Scholar]
- 32.Chirinos JA, Segers P, De Buyzere ML, et al. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010 Jul;56(1):91–98. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Simone G, Devereux RB, Maggioni AP, Gorini M, de Divitiis O, Verdecchia P. Different normalizations for body size and population attributable risk of left ventricular hypertrophy: the MAVI study. Am J Hypertens. 2005 Oct;18(10):1288–1293. doi: 10.1016/j.amjhyper.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 34.de Simone G, Kizer JR, Chinali M, et al. Normalization for body size and population-attributable risk of left ventricular hypertrophy: the Strong Heart Study. Am J Hypertens. 2005 Feb;18(2 Pt 1):191–196. doi: 10.1016/j.amjhyper.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 35.Paneni F, Gregori M, Marra A, et al. Preclinical effects of healthy obesity on inappropriate left ventricular mass and systolic function. Int J Cardiol. 2012 Dec 8; doi: 10.1016/j.ijcard.2012.11.089. [DOI] [PubMed] [Google Scholar]
- 36.Chien KL, Tu YK, Hsu HC, Su TC, Lee YT, Chen MF. Partial least squares analysis of the association between metabolic factors and left ventricular mass among Taiwanese adolescents. Int J Cardiol. 2011 Mar 3;147(2):305–306. doi: 10.1016/j.ijcard.2010.12.060. [DOI] [PubMed] [Google Scholar]
- 37.Dewey FE, Rosenthal D, Murphy DJ, Jr, Froelicher VF, Ashley EA. Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation. 2008 Apr 29;117(17):2279–2287. doi: 10.1161/CIRCULATIONAHA.107.736785. [DOI] [PubMed] [Google Scholar]
- 38.Gardin JM, Brunner D, Schreiner PJ, et al. Demographics and correlates of five-year change in echocardiographic left ventricular mass in young black and white adult men and women: the Coronary Artery Risk Development in Young Adults (CARDIA) study. J Am Coll Cardiol. 2002 Aug 7;40(3):529–535. doi: 10.1016/s0735-1097(02)01973-3. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong AC, Gjesdal O, Almeida A, et al. Left ventricle mass by cardiac magnetic resonance and echocardiography: the multi-ethnic study of atherosclerosis. Echocardiography. 2013 doi: 10.1111/echo.12303. Ahead to print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


