Abstract
SerpinB2, a member of the serine protease inhibitor family, is expressed by macrophages and significantly upregulated by inflammation. Recent studies implicated a role for SerpinB2 in the control of Th1 and Th2 immune responses, but the mechanisms of these effects are unknown. In the current study, we used mice deficient in SerpinB2 (SerpinB2−/−) to investigate its role in the host response to the enteric nematode, Heligmosomoides bakeri (H. bakeri). Nematode infection induced a STAT6-dependent increase in intestinal SerpinB2 expression. The H. bakeri-induced up-regulation of IL-4 and IL-13 expression was attenuated in SerpinB2−/− mice coincident with an impaired worm clearance. In addition, lack of SerpinB2 in mice resulted in a loss of the H. bakeri-induced smooth muscle hyper-contractility and a significant delay in infection-induced increase in mucosal permeability. Th2 immunity is generally linked to a CCL2-mediated increase in the infiltration of macrophages that develop into the alternatively activated phenotype (M2). In H. bakeri-infected SerpinB2−/− mice, there was an impaired infiltration and alternative activation of macrophages accompanied by a decrease in the intestinal CCL2 expression. Studies in macrophages isolated from SerpinB2−/− mice showed a reduced CCL2 expression, but normal M2 development, in response to stimulation of Th2 cytokines. These data demonstrate that the immune regulation of SerpinB2 expression plays a critical role in the development of Th2-mediated protective immunity against nematode infection by a mechanism involving CCL2 production and macrophage infiltration.
Introduction
It is well established that enteric nematode infection induces a polarized Th2 cytokine response involving IL-4, IL-5, IL-9, IL-13, and IL-25 (1). In the early stages of infection, various innate and adaptive immune cells, including macrophages, basophils, eosinophils, and CD4+ T cells, are recruited to the site and play an essential role in host protective immunity (2). Macrophages undergo distinct pathways of activation depending on the cytokine microenvironment. In general, the bacterial product LPS and Th1 cytokine, IFNγ, induce classical activation (M1), while the Th2 cytokines, IL-4 and IL-13, induce alternative activation of macrophages (M2). Nematode infection also induces coordinated IL-4/−13 and STAT6-dependent changes in gut function that facilitate worm expulsion (3–5), which are also dependent in part on M2 (5). Although the sequelae of the Th2-mediated immune response following enteric nematode infection are well characterized, there is less information on the mechanisms involved in the initiation and maintenance of the response.
The urokinase plasiminogen activation system is composed of urokinase-type plasminogen activator (uPA), its receptor (uPAR), and plasminogen activator inhibitors, PAI-1 and SerpinB2 (also called PAI-2). SerpinB2 is a member of the clade B subgroup of the serine protease inhibitor superfamily, which are characterized by the lack of a classical secretory signal sequence and localization to cytoplasmic and/or nuclear compartments (reviewed in 6). Inhibition of uPA is important in the regulation of extracellular plasminogen-dependent proteolysis, but much of the SerpinB2 synthesized accumulates intracellularly, and this pool is considered to have a distinct role (6). SerpinB2 is one of the most abundant proteins of activated monocytes and macrophages and its expression is substantially up-regulated during most inflammatory processes by cytokines such as TNF-α (7,8). Recent studies have implicated SerpinB2 in the maintenance of TLR4-activated macrophages by preventing rapid macrophage death and premature cessation of the innate immune response (9). It is also linked to monocyte/macrophage proliferation and differentiation, as well as Th1 and Th2 immunity (9–11).
In the present study we infected mice with Heligmosomoides bakeri (H. bakeri; formerly polygyrus) because it is a natural murine pathogen with a strictly enteral life cycle. A primary infection is chronic lasting several weeks to months, however, a secondary infection induces a strong memory response and worms are cleared within 14 days (12). In consideration of the potential role of SerpinB2 in immune response, the present study was designed to investigate: (i) the immune-mediated regulation of SerpinB2 expression during nematode infection; (ii) the contribution of SerpinB2 in host Th2 protective immunity against H. bakeri; (iii) the role of SerpinB2 in the H. bakeri infection-induced alterations in intestinal smooth muscle and epithelial cell function; and (iv) the mechanisms by which SerpinB2 regulates host protective immunity against a gastrointestinal nematode infection. This study showed that SerpinB2 plays a critical role in the host protective immunity against nematode infection via a control of monocyte recruitment that impacts Th2 cytokine responses.
Materials and Methods
Mice
C57BL/6 wild-type (WT) mice were purchased from the Small Animal Division of the National Cancer Institute or Jackson laboratory (Bar Harbor, ME 04609). Mice deficient in SerpinB2 (SerpinB2−/−) backcrossed six times onto a C57BL/6 background were obtained from Dr. David Ginsburg (University of Michigan Medical School, Ann Arbor, MI) and were then further backcrossed for these studies a total of 12 times onto the C57BL/6 background. Mice deficient in STAT6 (STAT6−/−) were obtained from The Jackson Laboratory. These studies were conducted in accordance with principles set forth in the Guide for Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, Health and Human Services Publication (National Institutes of Health 85–23, revised 1996), Beltsville Area Animal Care and Use Committee (#07–003), and University of Maryland School of Medicine IACUC.
Enteric nematode infection and worm expulsion
Heligmosomoides bakeri (H. bakeri) infection with L3 (specimens on file at the U.S. National Parasite Collection, U.S. National Helminthological Collection, Collection 81930, Beltsville, MD) was described previously (4,13). Infective, third stage larvae (L3) of Nippostrongylus brasiliensis (N. brasiliensis) were propagated and stored at room temperature in fecal/charcoal/peat moss culture plates until use (4). Groups of mice were inoculated subcutaneously with 500 L3 and studied 10 days later. The timing of the studies following infection with N. brasiliensis correlated with the time of the maximal effects on gut function and coincided with worm expulsion (4). Appropriate age-matched controls were performed for each infection. Adult worms were detected quantitatively by scanning the intestinal surface with a dissecting microscope.
In vitro smooth muscle and epithelial cell function
In vitro smooth muscle contractility was measured as described previously (4). Smooth muscle responses to electric field stimulation (EFS, 20Hz, 100V) or acetylcholine (10nM-0.1mM), and the amplitude of spontaneous contractions were determined. Tension was expressed as force per cross sectional area (14). For in vitro epithelial cell ion transport in Ussing chambers, muscle-free segments of small intestine were mounted in Ussing chambers as described (15). After a 15-minute period, concentration-dependent changes in short-circuit current (Isc) were determined for the cumulative addition of acetylcholine to the serosal side or glucose to the mucosal side. Responses from all tissue segments exposed to acetylcholine from an individual animal were averaged to yield a mean response per animal.
Micro-snapwell assay for mucosal transepithelial electrical resistance (TEER)
The modified micro-snap well system is a miniaturized version of the standard Ussing chamber that has been engineered to measure mucosal TEER (16) where a decrease in TEER reflects increased tissue permeability. Briefly, segments of mouse intestine taken from control or H. bakeri-infected WT and SerpinB2−/− mice were stripped of both muscle and serosal layers and placed in the micro-snap well system. Two hundred fifty µl of DMEM containing 4.5g/L glucose, 4mM L-glutamine, and MEM with 1mM non-essential amino acids were added to the mucosal side. Three milliliter of the same medium was added to the serosal side. The system was incubated at 37°C with 5%CO2 for 30 minutes to stabilize the pH and the TEER was measured every 30 minutes for 90 minutes.
Preparation of bone marrow derived macrophages (BMDM)
Macrophages were prepared from bone marrow mononuclear cells as described (Zhonghan Yang et al, eCAM, in press). Briefly, mice mononuclear cells were obtained by flushing the marrow from femurs, tibias, and humeri with HyClone alpha MEM medium (Thermo) pre-equilibrated at 37°C. Cells were cultured overnight in alpha MEM medium containing 10% Fetal Bovine Serum (FBS) and 1% penicillin/streptomycin in humidified incubator at 37°C with 5% CO2. The non-adherent cells were collected by centrifugation after lysis of red blood cells using lysis buffer (Sigma) and, mononuclear cells were counted. Mature macrophages were generated by differentiating the isolated mononuclear cells with 20ng/ml recombinant M-CSF (R & D Systems, Minneapolis, MN) for 7 days. Cells were then treated with IL-4, IL-13, or LPS for 24 hrs to determine the ability of macrophages to differentiate into classically (M1) or alternatively activated (M2) phenotype.
CCL2 elicited peritoneal monocyte recruitment and preparation of peritoneal exduate cells
Mice were injected intraperitoneally with 10µg of CCL2 in 200µl of NaCl (0.9%, LPS-free) and euthanized at 18 hours post-injection as described previously 17. The peritoneal cavity was washed twice with 8 ml of PBS and the number of peritoneal exduate cells (PECs) was counted from the total lavage pooled from individual mice. The percentage of macrophages in PECs was determined by counting the macrophages on PEC smear prepared by cytospin and stained with Giemsa.
RNA extraction, cDNA synthesis and real-time quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from whole intestinal tissue or BMDM as described previously (18). RNA samples (2 µg) were reverse-transcribed to cDNA using random hexamer primers and RevertAid™ reverse transcriptase. Real-time quantitative PCR (qPCR) was performed using an iCycler detection system (Bio-Rad, Hercules, CA) as described19. Primers for qPCR were designed using Beacon Designer 7.0 software (Premier Biosoft International, Palo Alto, CA) and synthesized by Sigma. The primer sequences are: SerpinB2, ACTTAATGGGCTTTATCCTTTCC (sense), TGCGTCCTCAATCTCATCG (anti-sense); CCL2, TTTGAATGTGAAGTTGACCCGTAAATC (sense), GAAGTGCTTGAGGTGGTTGTGG (anti-sense). Primer sequences for other genes have been described previously (5).
Immunofluorescence staining
Frozen blocks of mid-jejunum were prepared using the Swiss-roll technique and stored at −80°C. Tissue sections (4 µm) were cut from frozen blocks using an HM505E cryostat (Richard-Allan Scientific, Kalamazoo, MI). For immunofluorescence staining, tissue slides were fixed in cold acetone for 30 min and blocked with 5% normal donkey serum in PBS for 1 hr at room temperature. The slides were then incubated with rat anti-F4/80 antibody (1:50, Biolegend, San Diego, CA) and goat anti-mouse CCL2 (1:40, Biolegend, San Diego, CA) overnight at 4°C. After being washed, the slides were stained with Dylight 488-Donkey anti-rat IgG and Dylight 649-Donkey anti-goat-IgG (1:400, Jackson ImmunoResearch, West Grove, PA) for 2 hr and then digitally photographed with a Nikon TE 2000-E microscope (Melville, NY) using MetaVue version 6.1 software (Universal Imaging Corporation, Downington, PA). The images were taken by establishing settings for the samples from individual vehicle groups and using the same conditions to evaluate the samples from infected groups. Comparisons were made only among slides prepared on the same day. Smooth muscle thickness was determined using Giemsa-stained sections for each treatment group.
Western blot and ELISA
Tissue lysates were prepared by homogenizing the intestine strips in T-PER (Thermo Scientific Pierce, Rockford, IL) with protease inhibitor cocktail. Proteins were separated on 4–12% Bis-Tris NuPage gels (Invitrogen), and transferred to PVDF membranes. The membranes were blocked with 5% milk in PBST and then incubated with primary antibodies overnight. Affinity purified rabbit-anti-mouse SerpinB2 antibody was prepared after immunization with a purified recombinant GST-murine SerpinB2 fusion protein produced in E.coli as described 20. The Western blot was reprobed with anti-GAPDH (Cell Signaling Technology) as a loading control. Immunoreactive bands were detected by HRP-conjugated secondary antibodies using standard techniques. In situ intestinal production of IL-13 was analyzed by ELISA in tissue lysates per manufacturer’s instruction (eBioscience, San Diego, CA).
Data analysis
Agonist responses were fitted to sigmoid curves (Graphpad, San Diego, CA). Statistical analysis was performed using one-way ANOVA followed by Bonferroni tests to compare the responses and gene expression among the different treatment groups.
Results
Enteric nematode infection up-regulates SerpinB2 expression in the intestine
SerpinB2 expression is up-regulated in response to microbe infection or inflammation, and is implicated in regulation of the Th1 cytokine response (9–11). To determine whether SerpinB2 also plays a role in Th2 immunity, we used enteric nematode infection in mice, a well-characterized model featuring a highly polarized Th2 cytokine response that is essential for host defense. We first infected groups of mice with H. bakeri to analyze the kinetics of SerpinB2 gene expression in the small intestine. Heligmosomoides bakeri is a strictly enteral infection with larvae developing first in the submucosa of the duodenum followed by release of adult worms into the lumen around day 8 after inoculation. This infection significantly up-regulated SerpinB2 mRNA expression post infection (PI), with the highest levels observed at day 4 (Figure 1A); a time when the L3 encyst in the intestinal mucosa, and persistent high levels of SerpinB2 are present during the entire course of infection. The infection-induced increase in SerpinB2 expression was further confirmed by Western-blot, as the specific 46-kDa SerpinB2 protein band was significantly stronger in samples from infected versus uninfected WT mice and was not seen in samples from SerpinB2−/− mice (Figure 1B). Notably, H. bakeri infection did not affect SerpinB2 expression in mice with STAT6 deficiency (Figure 1A), demonstrating that the increased expression of SerpinB2 in WT mice is dependent on IL-4/IL-13. Moreover, this infection-induced up-regulation of SerpinB2 was not specific to H. bakeri since N. brasiliensis, a nematode that migrates parenterally before adults develop in the intestinal lumen, also increased the expression of SerpinB2 in the small intestine of WT, but not STAT6−/−, mice (Figure 1C). The infection-induced elevation of SerpinB2 expression was independent of the genetic background of mouse because similar effects were detected in both BALB/c and C57BL/6 mice (data not shown).
Figure 1.
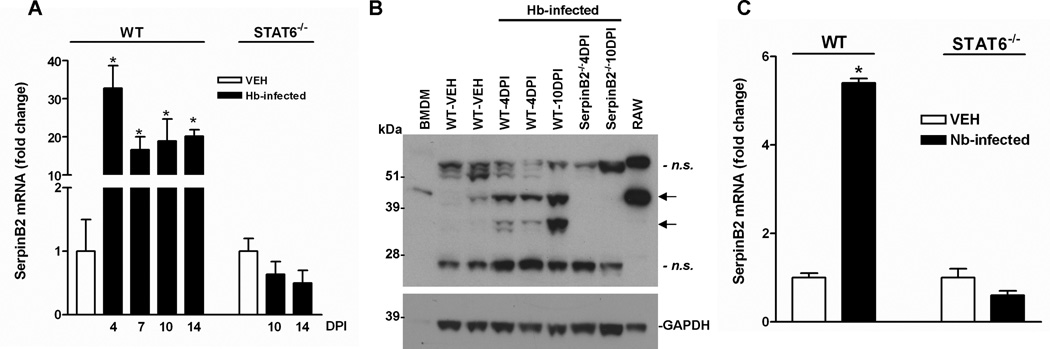
Nematode infection induced up-regulation of SerpinB2 expression in the small intestine dependent on STAT6. C57BL/6 mice (WT) mice were infected with H. bakeri (Hb) or N. brasiliensis (Nb) third stage larvae. (A) The kinetics of SerpinB2 mRNA expression in the small intestine of mice at days 4, 7, 10, and 14 post Hb infection (DPI) was analyzed by qPCR. The fold changes were relative to the vehicle (VEH) group after normalization to 18S rRNA. (B) The intestinal lysates (60µg) were analyzed on 4–12% Bis-Tris NuPage gels and probed with anti-mouse SerpinB2. The 46-kDa SerpinB2 protein and a cleavage product of SerpinB2 with lower molecular weight (~37 kDa) were detected (arrows). Negative controls: identically prepared intestinal lysates from SerpinB2−/− mice. Positive controls: LPS-treated RAW 264.7 murine macrophages (12µg) and bone marrow-derived macrophages (BMDM, 40 µg). Immunoblot was reprobed with anti-GAPDH as a loading control. n.s. indicates nonspecific immunoreactive bands as evidenced by their presence in intestinal lysates from SerpinB2−/− mice. (C) qPCR analysis of the SerpinB2 expression in intestines from WT and STAT6−/− mice at day 10 post Nb infection. *p<0.05 versus VEH (n≥5 for each group).
Worm expulsion was impaired in mice with SerpinB2 deficiency
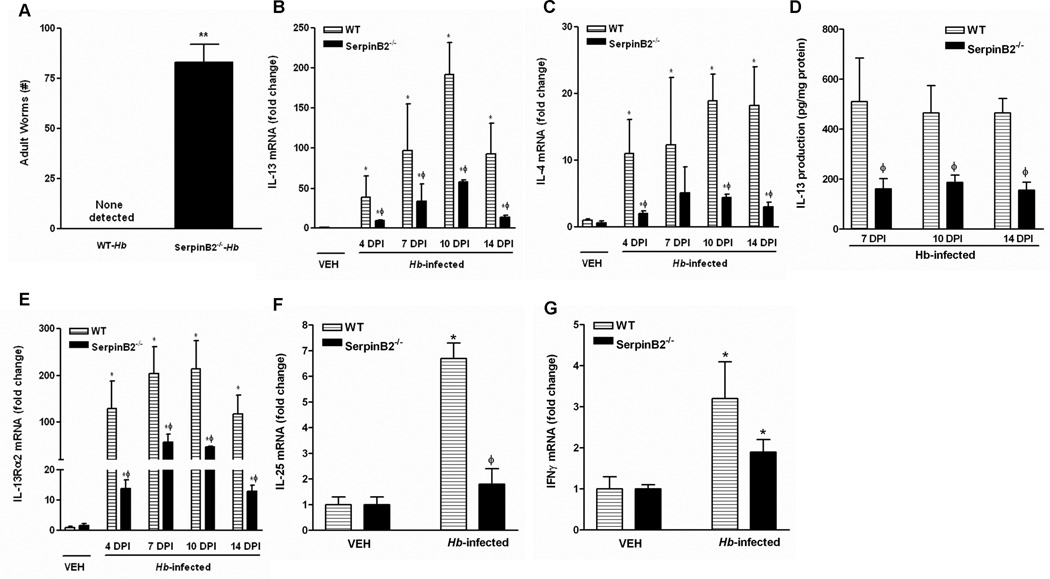
As described above, H. bakeri infection induced up-regulation of SerpinB2 expression early in the post infection period and preceded the peak of Th2 cytokine response implicating a role of SerpinB2 in host protective immunity against the infection. To explore this further, we monitored worm expulsion during the memory response to a secondary infection with H. bakeri. In general, C56BL/6 WT mice cleared adult worms by day 14 after a secondary inoculation of H. bakeri. At day 10 PI, WT and SerpinB2−/− mice harbored a significant, yet comparable number of adult worms in the intestinal lumen (data not shown), indicating that SerpinB2 deficiency did not alter the development of larvae into adult worms. There were no adult worms present in WT mice at day 14 PI; however there were significant numbers of worms remaining in the small intestine of SerpinB2−/− mice consistent with impaired worm expulsion (Figure 2A).
Figure 2.
Impaired Th2 protective immunity against H. bakeri infection in mice deficient in SerpinB2. WT or SerpinB2−/− mice were infected with H. bakeri (Hb). (A) Numbers of adult worms in the lumen of small intestine of C57BL/6 mice (WT) mice at day 14 post infection (DPI). qPCR was performed to measure the mRNA expression of (B) IL-13, (C) IL-4, (E) IL-13Rα2, (F) IL-25, and (G) IFNγ in the small intestine of mice at day 4, 7, 10, and 14 DPI. The fold changes were relative to the WT-vehicle (WT-VEH) groups after normalization to 18S rRNA. (D) ELISA analysis of the in situ IL-13 production of the intestines. **p<0.01 versus WT-Hb; *p<0.05 versus the respective VEH; ϕp<0.05 versus the respective WT (n≥5 for each group).
Defective Th2 cytokine response in mice deficient in SerpinB2 during nematode infection
Th2 cytokines, especially IL-4, and IL-13, are the major effector molecules in host protective immunity against nematode infection. To dissect the underlying mechanisms by which SerpinB2 deficiency impairs worm expulsion, we analyzed the kinetics of Th2 cytokine expression in the intestine following H. bakeri infection. The mRNA expression of IL-13 or IL-4 in WT mice was elevated significantly at day 4 PI, peaked at day 10 PI, and remained elevated at day 14 PI even after worm expulsion (Figure 2B & C). The level of IL-13 expression in infected SerpinB2−/− mice was significantly lower than that in infected WT mice at all time points examined, while the expression of IL-4 was increased transiently on day 7 and was significantly lower than WT at all other time points. Correspondingly, in situ intestinal IL-13 production in infected SerpinB2−/− mice was considerably lower than that of WT mice at day 7, 10, 14 PI (Figure 2D). Increased levels of IL-13 up-regulate expression of IL-13Rα2, the decoy receptor for IL-13, through its binding to IL-13Rα1 that, with the IL-4Rα, is part of the type II IL-4 receptor that activates STAT6 (18). Expression of IL-13Rα2 was increased significantly by infection in both WT and SerpinB2−/− mice, but levels were reduced significantly in SerpinB2−/− mice at all times points post infection corresponding to the decreased IL-13 expression (Figure 2E). IL-25 is an epithelial-derived cytokine that plays a key role in Th2 immunity. We showed previously that up-regulation of IL-25 in nematode infection is dependent on IL-13 and STAT6 (19). Of interest is that H. bakeri infection up-regulated IL-25 mRNA expression in WT, but not in SerpinB2−/− mice indicating a role for SerpinB2 in the IL-13-driven epithelial cell response to nematode infection (Figure 2F). Taken together, these data support a role for SerpinB2 in the full development of a Th2 immune response.
A defect in Th2 cytokine response may lead to an exaggerated Th1 cytokine response as described previously in STAT6−/− mice infected with N. brasiliensis (5). To determine if this occurred during H. bakeri infection in SerpinB2−/− mice, we further analyzed the expression of Th1 and Th17 cytokines in the intestine. IFNγ was slightly, but significantly, up-regulated at day 14 post H. bakeri infection in both WT and SerpinB2−/− mice (Figure 2G), but other major Th1/Th17 cytokines including IL-17A, IL-1β, IL-12a, IL-12b, IL-23a, and TNFα were not significantly altered by infection in either strain (data not shown). These data indicated that the impaired Th2 immune response due to SerpinB2 deficiency did not result in an elevated pro-inflammatory Th1 or Th17 response.
Attenuated intestinal smooth muscle and epithelial function in response to H. bakeri infection in mice deficient in SerpinB2
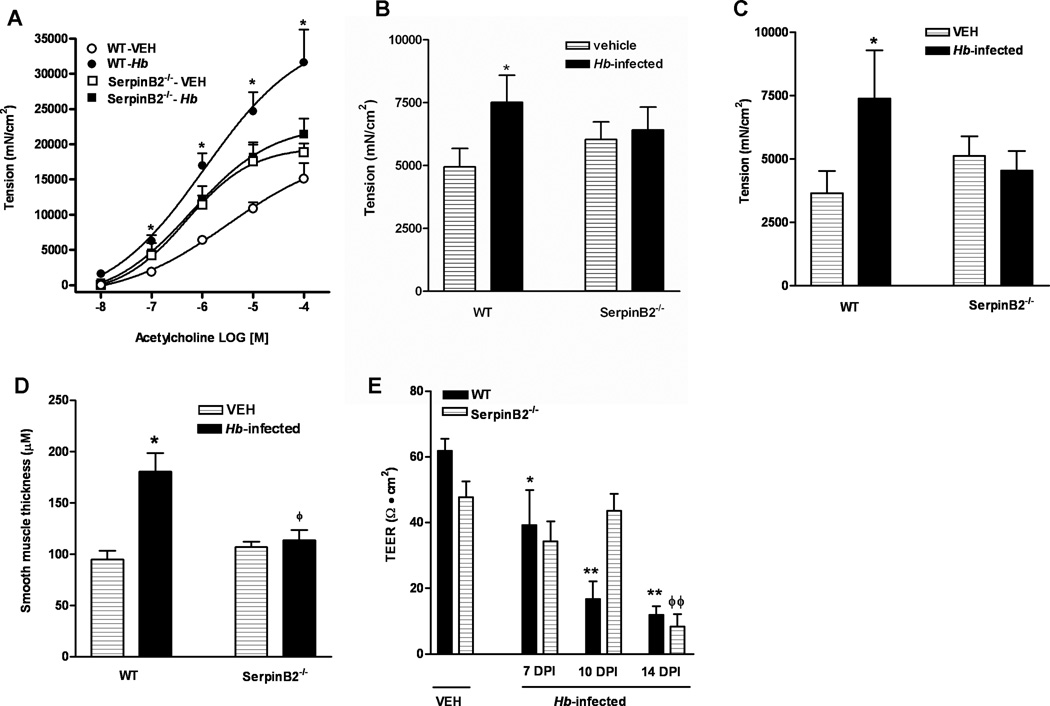
Changes in intestinal function are a hallmark feature of enteric nematode infection and facilitate worm clearance; therefore, we compared smooth muscle and epithelial cell function in WT and SerpinB2−/− mice. Based on the results from time-course experiments, we selected day 14 PI as the time point for comparison when the infection-induced smooth muscle hyper-contractility is maximal in WT mice. As expected, H bakeri infection in WT mice induced a characteristic smooth muscle hyper-contractility of the intestine including elevated responses to acetylcholine (Figure 3A) and electric field stimulation (Figure 3B), as well as an increase in the amplitude of spontaneous contractions (Figure 3C). Notably, this smooth muscle hyper-contractile response was absent in the infected SerpinB2−/− mice (Figure 3A–C). Intestinal smooth muscle hypertrophy/hyperplasia is often associated with enteric nematode infection and is attributed to the increased production of macrophage-derived growth factors. There was a significant increase in smooth muscle thickness in H baker-infected WT, but not in infected SerpinB2−/− mice (Figure 3D).
Figure 3.
Abolished intestinal smooth muscle hyper-contractility/hypertrophy and delayed increase in mucosal permeability in mice deficient in SerpinB2 in response to H. bakeri infection. C57BL/6 mice (WT) or mice with SerpinB2 deficiency (SerpinB2−/−) were infected with H. bakeri (Hb) and studied at day 14 post infection. Intestinal strips were suspended longitudinally in organ baths for in vitro contractility studies in response to (A) acetylcholine (ACH, 10nM-0.1mM), (B) EFS (20Hz, 100V), or (C) the amplitude of spontaneous contraction. (D) Thickness of the smooth muscle layer was measured by microscopic examination of tissue sections of intestine cut from frozen blocks and Giemsa stained. *p<0.05 versus the respective vehicle (VEH). (E) Muscle-free mucosa from mice at days 7, 10, 14 post infection (DPI) was mounted in micro snapwell for the measurement of trans-epithelial electrical resistance (TEER). *p<0.05, **p<0.01 versus WT-VEH; ϕϕp<0.01 versus SerpinB2−/−-VEH (n≥5 for each group).
Other characteristics of the intestinal functional response to nematode infection are decreased glucose absorption, hyposecretion to acetylcholine, decreased net ion flux and increased mucosal permeability (3,15,21). There were no differences in the basal Isc across the tissue or in the intestinal mucosal responses of WT or SerpinB2−/− mice to glucose or acetylcholine (data not shown). In contrast, during the course of H. bakeri infection there was a gradual decrease in TEER at both day 7 and 10 PI in WT mice but not in SerpinB2−/− mice (Figure 3E). By day 14 PI, however, TEER was similarly low in both strains of mice. These data indicate a specific role for SerpinB2 in the initiation of infection-induced changes in mucosal permeability.
Nematode infection failed to recruit monocytes to the intestine of mice with SerpinB2 deficiency
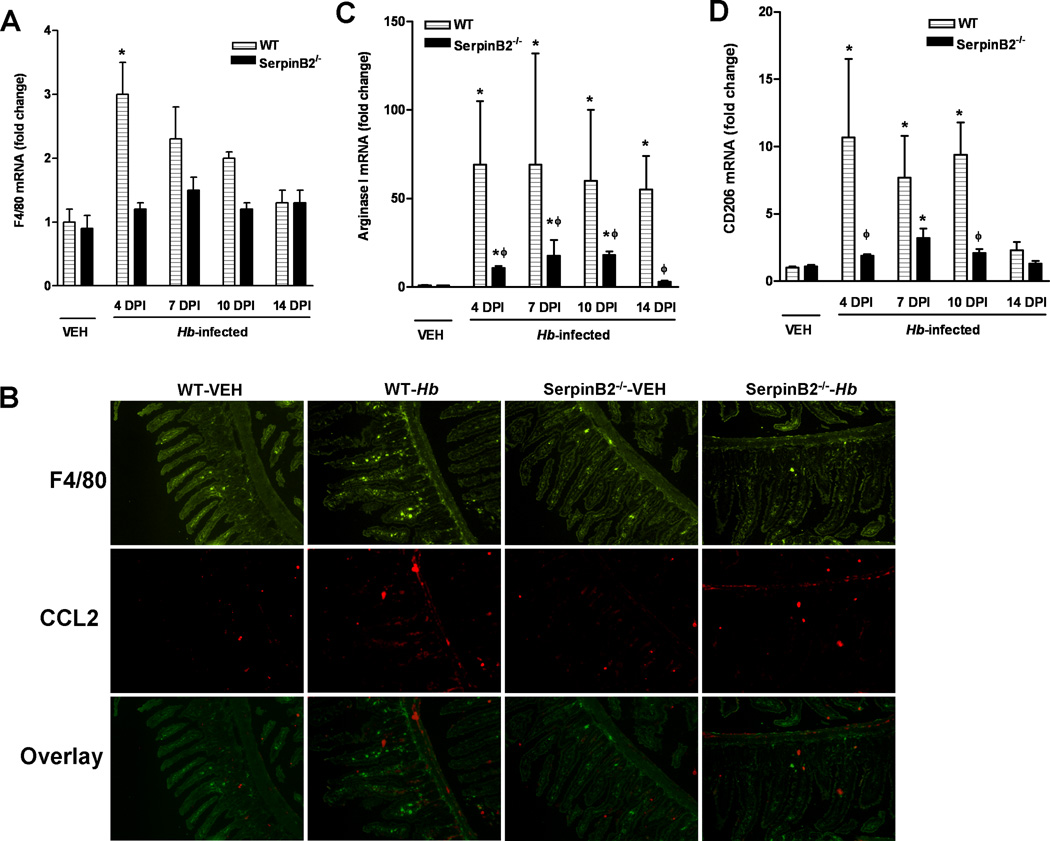
During enteric nematode infection, monocytes are recruited to the intestine and differentiated into macrophages that play a crucial role in host protective immunity (2, 9). To determine if the impaired host immunity to nematode infection in SerpinB2−/− mice is associated with changes in the number/function of macrophages, we monitored various macrophage markers in whole intestinal tissue. The constitutive expression level of F4/80, a general marker for macrophages, was not different in WT and SerpinB2−/− intestine, suggesting that the number of resident macrophages were similar in the two strains of mice. The mRNA expression of F4/80 in WT intestine was up-regulated transiently at day 4 PI, but declined thereafter (Figure 4A). Heligmosomoides bakeri infection, however, did not induce significant changes in F4/80 expression in SerpinB2−/− mice, suggesting a failure to recruit monocytes to the site of infection. This defect in monocyte recruitment was further confirmed by immunofluorescence staining of intestinal sections (Figure 4B).
Figure 4.
Impaired monocyte recruitment, CCL2 production, and alternative activation of macrophages in the intestines of mice deficient in SerpinB2. C57BL/6 mice (WT) or mice with SerpinB2 deficiency (SerpinB2−/−) were infected with H. bakeri (Hb). Segments of small intestine were collected at days 4, 7, 10, 14 post infection (DPI) and analyzed for mRNA expression of (A) F4/80, (C) arginase I, or (D) CD206 by qPCR. The fold changes are relative to the WT-vehicle (WT-VEH) groups after normalization to 18s rRNA. (B) Tissue sections of intestine were cut from frozen blocks collected at 4 DPI, and stained with anti-F4/80 (blue) and anti-CCL2 (red) for visualizing macrophages and CCL2 production, respectively. The images are representative from 5 mice per group. *p<0.05 versus the respective vehicle (VEH); ϕp<0.05 versus the respective WT (n≥5 for each group).
Consistent with our previous results (5), H. bakeri infection increased the number of M2 macrophages in WT mice, as evidenced by the significant up-regulation of arginase I and CD206 (Figure 4C and D). SerpinB2 deficiency resulted in a severely attenuated expression of arginase I and CD206 (Figure 4C, D) consistent with impaired infiltration. The reduced M2 development in the intestine did not result in a more pronounced M1 response because the expression of NOS-2, a marker for M1, did not differ between the infected SerpinB2−/− and WT mice (data not shown).
CCL2 is the major chemoattractant protein responsible for monocyte recruitment during nematode infections (22). Heligmosomoides bakeri significantly up-regulated CCL2 expression in WT intestine at day 4 PI, and this increase was maintained through day 14 PI (Figure 5A). In contrast, CCL2 expression was increased significantly in SerpinB2−/− mice only at day 7 PI and levels were significantly lower than WT at all other time points (Figure 5A). Reduced CCL2 production in SerpinB2−/− mice was further confirmed by immunofluorescence staining of intestinal sections taken from mice at day 4 post infection (Figure 4B). These data suggested that an insufficient production of CCL2 might be one of the factors responsible for the impaired monocyte recruitment to the intestine of SerpinB2−/− mice during H. bakeri infection. Since CCL2 signals the recruitment of monocytes to the site of infection by binding the receptor, CCR2, we compared CCR2 expression levels in BMDM and macrophage-enriched PECs derived from WT and SerpinB2−/− mice. qPCR showed no significant differences in the CCR2 expression in either BMDM or PECs taken from WT and SerpinB2−/− mice (Figure 5B). In response to intraperitoneal injection of CCL2, however, the number of monocytes recruited to the peritoneal cavity and differentiated into macrophages in SerpinB2−/− mice was significantly less than that in WT mice (Figure 5C), indicating an intrinsic defect in the macrophage response to CCL2 stimulation due to SerpinB2 deficiency.
Figure 5.
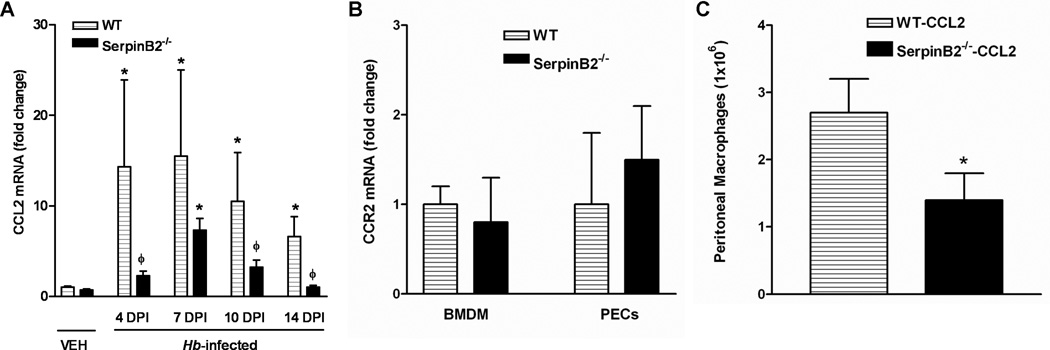
Attenuated up-regulation of CCL2 expression in the intestines in response to H. bakeri infection and a defect of monocyte recruitment in response to CCL2 stimulation in mice with SerpinB2 deficiency. (A) Segments of small intestine were collected at days 4, 7, 10, 14 post H. bakeri infection (DPI) for analyzing mRNA expression of CCL2 by qPCR. The fold changes were relative to the WT-vehicle (WT-VEH) groups after normalization to 18s rRNA. *p<0.05 versus the respective VEH; ϕp<0.05 versus the respective WT (n≥5 for each group). (B) Bone marrow-derived macrophages (BMDM) or enriched peritoneal exudate cells (PECs) were prepared from WT or SerpinB2−/− mice, and analyzed for CCR2 expression by qPCR. (C) WT or SerpinB2−/− mice were injected with CCL2 and peritoneal lavages were collected at 18 hours post injection for macrophage counting. (n=4 per group; *p<0.05)
SerpinB2 deficiency did not alter the abilities of macrophages to become M1 or M2 in response to the respective stimuli in vitro
SerpinB2 has been linked to monocyte proliferation and differentiation, and prevention of macrophage apoptosis induced by inflammatory stimuli such as LPS and TNF-α (9,11). It is possible, therefore, that the attenuated monocyte recruitment and development of the intestinal M2 macrophages in SerpinB2−/− mice resulted from a defect in macrophage survival or the ability of macrophages from SerpinB2−/− mice to differentiate into M1 or M2 phenotype. However, stimulation of BMDM from WT or SerpinB2−/− mice with IL-4 or IL-13 resulted in a similar up-regulation of arginase I (Figure 6A) and CD206 (data not shown), the major markers for M2. In addition, LPS stimulation of BMDM from WT or SerpinB2−/− mice also up-regulated the expression of NOS-2 to similar levels (Figure 6B). These data indicated that SerpinB2-deficiency did not restrict the ability of macrophage differentiation into M1 or M2 cells.
Figure 6.
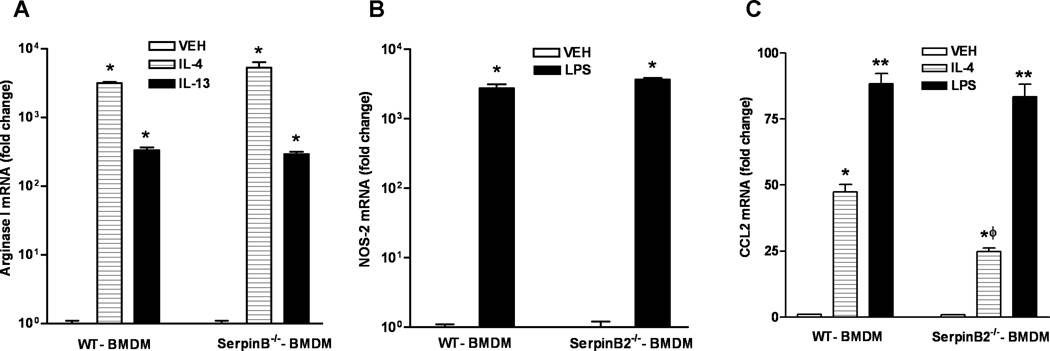
In vitro macrophage production of CCL2, but not the development of M1 or M2 phenotype, was impaired by SerpinB2 deficiency. Macrophages were generated from bone marrow mononuclear (BMDM) cells of WT or SerpinB2−/− mice. Cells were treated with IL-4, IL-13, or LPS for 24 hours, and analyzed for mRNA expression of (A) arginase I, (B) nitric oxide synthase 2, or (C) CCL2 by qPCR. The fold changes are calculated relative to the WT-BMDM-VEH after normalization to 18S rRNA. *p<0.05 versus the respective VEH; ϕp<0.05 versus the respective WT-BMDM. Data shown in bar graphs are the means ± SEM and are representative of two independent experiments performed in triplicate.
To evaluate whether SerpinB2 deficiency affects macrophage production of CCL2, we stimulated WT or SerpinB2−/− BMDM with either IL-4 or LPS. CCL2 expression was up-regulated significantly by both treatments; however, IL-4-induced CCL2 was significantly less in SerpinB2−/− macrophages than in WT macrophages (Figure 6C). These results indicated that SerpinB2 deficiency led to a defective CCL2 production specifically in a Th2 environment and this defect contributed to impaired monocyte recruitment during H. bakeri infection.
Discussion
In the present study, we demonstrated that SerpinB2 plays a critical role in host protective immunity against enteric nematode infection. The impaired worm expulsion after inoculation with H. bakeri in SerpinB2−/− mice was associated with a reduced Th2 cytokine response and a defect in monocyte recruitment and activation in the intestine. In addition, the infection-induced intestinal smooth muscle hyper-contractility and mucosal permeability, functions that contribute to worm expulsion (4,5), were absent or attenuated in SerpinB2−/− mice. Although SerpinB2 deficiency did not affect the ability of macrophages to differentiate into M1 or M2 phenotype in vitro, IL-4-stimulated CCL2 expression was significantly less in SerpinB2−/− macrophages, and may contribute to the failure of monocyte recruitment in SerpinB2−/− mice infected with H. bakeri. Moreover, SerpinB2 deficiency resulted in a defective monocyte recruitment elicited by CCL2. These results revealed a previously unrecognized role for SerpinB2 in the induction and maintenance of Th2 immunity against enteric nematode infection.
SerpinB2 is a single-chain protein with 415 amino acids and exists predominantly as a 47 kDa non-glycosylated intracellular form. There is also a small portion of SerpinB2 that can enter the secretory pathway and be released as a 60 Kda glycosylated protein (reviewed in 23). While up-regulation of the uPA system is associated generally with the transition from mucosa to adenoma in colorectal cancers (24), increased levels of SerpinB2 are associated also with prolonged survival and decreased tumor growth and metastasis (24,25). The biological activities of the extracellular form of SerpinB2 are linked to inhibition of the plasminogen activators uPA and tPA, however, the functional role of intracellular SerpinB2 remains unclear and is thought not to be related to these protease inhibitory activities. Expression of SerpinB2 in the healthy gastrointestinal tract is considered to be limited to macrophages, although there is evidence for induction of SerpinB2 in response to H. pylori infection in chief and mucous cells, as well as in metastatic tissue and transformed intestinal cancer cell lines (26).
As a highly regulated gene, SerpinB2 expression is modulated at multiple levels by different factors ranging from cytokines, hormones, growth factors, toxins, and bacterial products. In the current study, enteric nematode infection induced a significant STAT6-dependent up-regulation of SerpinB2 expression in the small intestine of WT mice. Thus, SerpinB2 can be added to the list of IL-4/IL-13 dependent genes that play a key role in the Th2 immune response. Abnormal expression of SerpinB2 is associated with a number of Th1 dominant inflammatory pathologies including diabetes mellitus (27) and colorectal cancer (25) as well as in Th2-dominant diseases such as asthma (28). Its role in inflammation in the gastrointestinal tract, however, is relatively unexplored. SerpinB2−/− mice showed an exaggerated Th1 cytokine response after immunization with OVA in complete Freund’s adjuvant suggesting that a general function of SerpinB2 is the control of pro-inflammatory Th1-mediated immune responses (11). In addition, infection of SerpinB2−/− mice with the Th2-inducing trematode, Schistosoma japonicum, resulted in diminished hepatic Th2 cytokine responses associated with low levels of IL-13 and arginase I expression, but increased levels of the Th1 cytokines, IL-6, TNFα, and NOS-2 (10). Our studies in H. bakeri-infected mice indicated that SerpinB2 is important for host protective immunity evidenced by delayed worm expulsion and a significantly attenuated Th2 cytokine response. This occurred in the absence of compensatory changes in the expression of pro-inflammatory Th1 or Th17 cytokines observed in other systems (10) and may be due to differences either in the pathogen (S. japonica vs H. bakeri) or in the affected region (liver versus small intestine).
Nematode infection induces stereotypic STAT6-dependent changes in epithelial function leading to increased intraluminal fluid as well as smooth muscle hyper-contractility, all of which promote worm expulsion (3,4). The infection-induced increase in permeability observed in WT mice was delayed significantly in SerpinB2−/− mice, yet TEER was similar in both strains at day 14 PI, the time of worm expulsion. Changes in permeability are linked to both worm-generated products as well as STAT6-dependent gene transcription (12). The deferred increase in barrier function in the absence of SerpinB2, therefore, was likely attributed to decreased Th2 cytokines and STAT6 gene transcription. Surprisingly, there were no differences in the infection-induced inhibition of epithelial secretion or glucose absorption between WT and SerpinB2−/− mice suggesting that SerpinB2 lacks a direct effect on epithelial cell function. In contrast, the infection-induced increase in smooth muscle hyper-contractility was absent in SerpinB2−/− mice, indicating a major role for SerpinB2. We showed previously that post infection hyper-contractility was due to a direct effect of IL-13/STAT6-dependent transcription of genes involved in smooth muscle function as well as an indirect effect mediated by macrophages (4,5,18,29,30). The absence of the smooth muscle responses to adult H. bakeri in SerpinB2−/− mice may be due to impaired monocyte recruitment and/or function.
We found that H. bakeri infection resulted in an early increase in CCL2 expression (day 4 PI) that is important for recruitment of monocytes to the site of infection and also coincides with the early elevation in tissue macrophage expression of F4/80. These recruited monocytes develop into the M2 macrophages that are essential to Th2 host protective immunity. Since macrophages are the primary cells that express SerpinB2 in the intestine, it is likely that the infection-induced up-regulation of SerpinB2 is attributed to newly recruited and differentiated macrophages. M2 macrophages are characterized by a STAT6-dependent increase in the expression of arginase-1 and CD206 (5,31) and are emerging as an important source of IL-13 during infection (32). The M2 phenotype is also critical for the smooth muscle hyper-contractility and hypertrophy mediated by arginase-1 activity as well as growth factors such as IGF-1 (5). In SerpinB2−/− mice, H. bakeri infection failed to increase the number of macrophages and therefore, resulted in fewer M2 macrophages in the SerpinB2−/− intestine. Consequently, these mice were unable to develop the changes in smooth muscle morphology and function required for efficient worm expulsion.
The lowered infiltration of macrophages in the small intestine in SerpinB2−/− mice could be due to impaired generation of chemokines or to a defect in the macrophage itself. Resident intestinal macrophages have a specific non-reactive tolerogenic phenotype. In response to nematode infection, recruitment of monocytes relies on the chemoattractant proteins produced by intestinal mucosal epithelial cells or lamina propria immune cells, mostly resident macrophages. CCL2 is the major chemokine for increased monocyte infiltration in mice during infection and elevated expression inhibits macrophage differentiation into non-reactive tissue macrophages. These data suggest that during infection, SerpinB2 functions to maintain CCL2 expression thereby preventing the development of the newly recruited monocytes into the tolerogenic macrophage phenotype (33) and allowing them to become M2. Indeed, mice with CCL2 deficiency had fewer macrophages in the intestine and mesenteric lymph nodes in response to infection with Trichuris muris, a cecal and proximal colon dwelling nematode, and failed to expel these worms (22). In the current study, H. bakeri-induced up-regulation of CCL2 was reduced markedly in SerpinB2−/− mice implicating insufficient production of CCL2 as one of the mechanisms for the impaired protective immune response in these mice. Interestingly, majority of the CCL2 staining was not co-localized with F4/80 staining cells. As a secreted protein, CCL2 is released rapidly from the producing cells soon after they sense the danger signal (Hb infection in this case), which may explain why much of the CCL2 staining was located in the intercellular space. The proximity of CCL2 staining to F4/80 positive cells, however, suggests that it is released from macrophages. It is also true that cells other than macrophages produce CCL2 as indicated by the staining in submucosal area and myenteric plexus. The ability of SerpinB2 deficiency to impact CCL2 production in cells other than macrophages or during other types of infection remains to be elucidated. Additional experiments demonstrated that significantly less number of monocytes were recruited to peritoneal cavity of SerpinB2−/− relative to WT mice after administration of CCL2. Notably, loss of SerpinB2 function did not alter the CCR2 expression in macrophages. It remains to be determined whether SerpinB2 is involved in regulating the intracellular signaling of CCL2 that might contribute to the intrinsic defect of macrophage response to CCL2.
An alternate explanation for the decreased number of macrophages in SerpinB2−/− mice may be the loss of the protective effects of SerpinB2 on macrophage survival (9). SerpinB2 deficiency renders macrophages more susceptible to apoptosis induced by LPS and TNFα (9,11). In addition, the attenuated Th2 cytokine production in response to H. bakeri in SerpinB2−/− mice similarly impacts M2 development. In vitro experiments, however, revealed that SerpinB2−/− macrophages have normal development into M1 in response to LPS or into M2 in response to IL-4 or IL-13. Of interest was that IL-4-stimulated CCL2 expression was significantly less in SerpinB2−/− macrophages than in WT macrophages. These data indicate that SerpinB2 is important for macrophage production of CCL2 and this likely contributes to the decreased recruitment of monocytes to the small intestine in response to H. bakeri infection in vivo.
In conclusion, mice deficient in SerpinB2 exhibit an attenuated up-regulation of Th2 cytokines in response to H. bakeri infection. This effect was associated with an absence of macrophage infiltration that can be attributed to a decreased expression of CCL2 demonstrated both in vivo and in vitro, as well as a defect in CCL2-elicited monocyte recruitment. There is an emerging role for macrophages as a source of IL-4/IL-13 cytokines (32). SerpinB2−/− mice exhibited a reduced expression of intestinal M2 markers in vivo, and this is a likely mechanism for the lack of smooth muscle responses to infection. The lack of M2 markers in SerpinB2−/− mice in vivo was not an inherent defect in the macrophage since isolated BMDM from WT and SerpinB2−/− mice had a similar up-regulation of arginase-1 expression in response to IL-4 or IL-13 and NOS-2 expression in response to LPS. These studies show that the immune regulation of SerpinB2 expression plays an important role in the development of the Th2 protective host immunity against gastrointestinal parasitic nematodes.
Acknowledgments
This work was supported by NIH grants R01-DK083418 (AZ), R01-AI/DK49316 (T.S-D), R01-DK081376 and R56-CA098369 (TMA), USDA CRIS project #1235–51000–055 (JFU), and Maryland Stem Cell Research Fund grant 0145–00 (S.N-A.) The opinions and assertions in this article are those of the authors and do not necessarily represent those of the U. S. Department of Agriculture or the Department of Defense. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The USDA is an equal opportunity provider and employer.
Abbreviations
- N. brasiliensis
Nippostrongylus brasiliensis
- H. bakeri
Heligmosomoides bakeri
- STAT6−/−
STAT6-deficient
- WT
wild type
- TEER
transepithelial electrical resistance
- PI
post infection
Footnotes
The authors have no conflicts of interest to disclose.
Reference List
- 1.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 2.Anthony RM, Rutitzky LI, Urban JF, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madden KB, Yeung KA, Zhao A, Gause WC, Finkelman FD, Katona IM, Urban JF, Jr, Shea-Donohue T. Enteric nematodes induce stereotypic STAT6-dependent alterations in intestinal epithelial cell function. J. Immunol. 2004;172:5616–5621. doi: 10.4049/jimmunol.172.9.5616. [DOI] [PubMed] [Google Scholar]
- 4.Zhao A, McDermott J, Urban JF, Jr, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J. Immunol. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 5.Zhao A, Urban FJ, Anthony RM, Jr, Sun R, Stiltz J, van Rooijen N, Wynn TA, Gause WC, Shea-Donohue T. Th2 Cytokine-Induced Alterations in Intestinal Smooth Muscle Function Depend on Alternatively Activated Macrophages. Gastroenterology. 2008;135:217–225. doi: 10.1053/j.gastro.2008.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medcalf RL, Stasinopoulos SJ. The undecided serpin. The ins and outs of plasminogen activator inhibitor type 2. FEBS Journal. 2005;272:4858–4867. doi: 10.1111/j.1742-4658.2005.04879.x. [DOI] [PubMed] [Google Scholar]
- 7.Kruithof EK, Tran-Thang C, Gudinchet A, Hauert J, Nicoloso G, Genton C, Welti H, Bachmann F. Fibrinolysis in pregnancy: a study of plasminogen activator inhibitors. Blood. 1987;69:460–466. [PubMed] [Google Scholar]
- 8.Jang WG, Kim HS, Park KG, Park YB, Yoon KH, Han SW, Hur SH, Park KS, Lee IK. Analysis of proteome and transcriptome of tumor necrosis factor alpha stimulated vascular smooth muscle cells with or without alpha lipoic acid. Proteomics. 2004;4:3383–3393. doi: 10.1002/pmic.200400972. [DOI] [PubMed] [Google Scholar]
- 9.Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, Otsu K, Hoffmann A, Montminy M, Karin M. Signaling Pathways and Genes that Inhibit Pathogen-Induced Macrophage Apoptosis-- CREB and NF-[kappa]B as Key Regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 10.SCHRODER WA, GARDNER J, LE TT, DUKE M, BURKE ML, JONES MK, McMANUS DP, SUHRBIER A. SerpinB2 deficiency modulates Th1/Th2 responses after schistosome infection. Parasite Immunology. 2010;32:764–768. doi: 10.1111/j.1365-3024.2010.01241.x. [DOI] [PubMed] [Google Scholar]
- 11.Schroder WA, Le TTT, Major L, Street S, Gardner J, Lambley E, Markey K, MacDonald KP, Fish RJ, Thomas R, Suhrbier A. A Physiological Function of Inflammation-Associated SerpinB2 Is Regulation of Adaptive Immunity. J Immunol. 2010;184:2663–2670. doi: 10.4049/jimmunol.0902187. [DOI] [PubMed] [Google Scholar]
- 12.Shea-Donohue T, Notari L, Stiltz J, Sun R, Madden KB, Urban JF, Jr, Zhao A. Role of enteric nerves in immune-mediated changes in protease-activated receptor 2 effects on gut function. Neurogastroenterol. Motil. 2010;22:1138–e291. doi: 10.1111/j.1365-2982.2010.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urban J, Fang H, Liu Q, Ekkens MJ, Chen SJ, Nguyen D, Mitro V, Donaldson DD, Byrd C, Peach R, Morris SC, Finkelman FD, Schopf L, Gause WC. IL-13-mediated worm expulsion is B7 independent and IFN-gamma sensitive4. J. Immunol. 2000;164:4250–4256. doi: 10.4049/jimmunol.164.8.4250. [DOI] [PubMed] [Google Scholar]
- 14.Zhao A, Bossone C, Pineiro-Carrero V, Shea-Donohue T. Colitis-induced alterations in adrenergic control of circular smooth muscle in vitro in rats. J. Pharmacol. Exp. Ther. 2001;299:768–774. [PubMed] [Google Scholar]
- 15.Shea-Donohue T, Sullivan C, Finkelman FD, Madden KB, Morris SC, Goldhill J, Pineiro-Carrero V, Urban JF., Jr The role of IL-4 in Heligmosomoides polygyrus-induced alterations in murine intestinal epithelial cell function. J. Immunol. 2001;167:22342239. doi: 10.4049/jimmunol.167.4.2234. [DOI] [PubMed] [Google Scholar]
- 16.El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- 17.Handel TM, Johnson Z, Rodrigues DH, dos Santos AC, Cirillo R, Muzio V, Riva S, Mack M, D+¬ruaz M, Borlat Fdr, Vitte PA, Wells TNC, Teixeira MM, Proudfoot AEI. An engineered monomer of CCL2 has anti-inflammatory properties emphasizing the importance of oligomerization for chemokine activity in vivo. J Leukoc Biol. 2008;84:1101–1108. doi: 10.1189/jlb.0108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morimoto M, Zhao A, Sun R, Stiltz J, Madden KB, Mentink-Kane M, Ramalingam T, Wynn TA, Urban JF, Jr, Shea-Donohue T. IL-13 Receptor {alpha}2 Regulates the Immune and Functional Response to Nippostrongylus brasiliensis Infection. J Immunol. 2009;183:1934–1939. doi: 10.4049/jimmunol.0804299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao A, Urban JF, Sun R, Stiltz J, Morimoto M, Notari L, Madden KB, Yang Z, Grinchuk V, Ramalingam TR, Wynn TA, Shea-Donohue T. Critical Role of IL-25 in Nematode Infection-Induced Alterations in Intestinal Function. J Immunol. 2010;185:6921–6929. doi: 10.4049/jimmunol.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dougherty KM, Pearson JM, Yang AY, Westrick RJ, Baker MS, Ginsburg D. The plasminogen activator inhibitor-2 gene is not required for normal murine development or survival. PNAS. 1999;96:686–691. doi: 10.1073/pnas.96.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Jr, Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J. Immunol. 2002;169:4417–4422. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 22.deSchoolmeester ML, Little MC, Rollins BJ, Else KJ. Absence of CC chemokine ligand 2 results in an altered Th1/Th2 cytokine balance and failure to expel Trichuris muris infection. J. Immunol. 2003;170:4693. doi: 10.4049/jimmunol.170.9.4693. [DOI] [PubMed] [Google Scholar]
- 23.Medcalf RL. Plasminogen activator inhibitor type 2: still an enigmatic serpin but a model for gene regulation. Methods Enzymol. 2011;499:105–134. doi: 10.1016/B978-0-12-386471-0.00006-7. [DOI] [PubMed] [Google Scholar]
- 24.Saucy F, Bachmann D, Peterman O, Sordat B, Sordat I, Dorta G. The Plasminogen System in Microdissected Colonic Mucosa Distant from an Isolated Adenoma. Pathology & Oncology Research. 2011;17:25–31. doi: 10.1007/s12253-010-9268-4. [DOI] [PubMed] [Google Scholar]
- 25.Croucher DR, Saunders DN, Lobov S, Ranson M. Revisiting the biological roles of PAI2 (SERPINB2) in cancer. Nat Rev Cancer. 2008;8:535–545. doi: 10.1038/nrc2400. [DOI] [PubMed] [Google Scholar]
- 26.Varro A, Noble P-JM, Pritchard DM, Kennedy S, Hart CA, Dimaline R, Dockray GJ. Helicobacter pylori Induces Plasminogen Activator Inhibitor 2 in Gastric Epithelial Cells through Nuclear Factor-+¦B and RhoA. Cancer Research. 2004;64:1695–1702. doi: 10.1158/0008-5472.can-03-2399. [DOI] [PubMed] [Google Scholar]
- 27.Kardesler L, Buduneli N, Biykoglu B, Cetinkalp S, Kutukculer N. Gingival crevicular fluid PGE2, IL-1beta, t-PA, PAI-2 levels in type 2 diabetes and relationship with periodontal disease. Clinical Biochemistry. 2008;41:863–868. doi: 10.1016/j.clinbiochem.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, Erle DJ, Yamamoto KR, Fahy JV. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proceedings of the National Academy of Sciences. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morimoto M, Morimoto M, Zhao A, Madden KB, Dawson H, Finkelman FD, Mentink-Kane M, Urban JF, Jr, Wynn TA, Shea-Donohue T. Functional Importance of Regional Differences in Localized Gene Expression of Receptors for IL-13 in Murine Gut. J Immunol. 2006;176:491–495. doi: 10.4049/jimmunol.176.1.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao A, Urban JF, Jr, Morimoto M, Elfrey JE, Madden KB, Finkelman FD, Shea-Donohue T. Contribution of 5-HT2A receptor in nematode infection-induced murine intestinal smooth muscle hypercontractility. Gastroenterology. 2006;131:568–578. doi: 10.1053/j.gastro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van RN, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, Benoit LA, Byers DE, Alevy Y, Tucker J, Swanson S, Tidwell R, Tyner JW, Morton JD, Castro M, Polineni D, Patterson GA, Schwendener RA, Allard JD, Peltz G, Holtzman MJ. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spoettl T, Hausmann M, Herlyn M, Gunckel M, Dirmeier A, Falk W, Herfarth H, Schoelmerich J, Rogler G. Monocyte chemoattractant protein-1 (MCP-1) inhibits the intestinal-like differentiation of monocytes. Clinical & Experimental Immunology. 2006;145:190–199. doi: 10.1111/j.1365-2249.2006.03113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]