Abstract
There is paucity of data on whether or not women can be re-infected with human papillomavirus (HPV) types to which they were exposed earlier in life and on the role of natural immunity. The observation of HPV infection at older ages may be explained by reactivation of a latent infection or new exposure from sexual activity. Our objective was to analyze the association between re-infection and sexual activity. We analyzed data from 2462 women enrolled in the Ludwig-McGill cohort and followed every 4-6 months for up to 10 years. We performed HPV typing and viral load measurements via polymerase chain reaction and determined HPV-16 seroreactivity at enrolment. Incidence of infection and re-infection were estimated for individual types. Adjusted relative risks (RR) for the association between infection/re-infection and new sexual partners were calculated using Cox regression. Rates of initial infection and re-infection post-clearance were statistically comparable. RRs of initial infection or re-infection were consistently associated with new sexual partners (2.4 [95%CI: 2.0-3.1] for first infection, 3.7 [1.1-13.8] for re-infection with the same type, and 2.3 [1.5-3.7] for re-infection with a different type). Re-infection in older women was also associated with new sexual partners (RR=2.8, 95%CI: 1.4-5.3) as were new infections with HPV-16 among women with serological evidence of prior HPV-16 exposure (RR=3.0, 95%CI: 1.6-5.3). Viral loads at initial infection and at re-infection were comparable. HPV infection and re-infection were strongly associated with sexual activity. This study suggests that natural immunity does not play a role in controlling the extent of re-infections.
Keywords: Human papillomavirus, re-infection, adult women, sexual behavior, natural immunity, epidemiology
INTRODUCTION
Human papillomavirus (HPV) infections are among the most common sexually-transmitted infections(1-2). Over 40 genotypes (types for short) infect the epithelial lining of the anogenital tract and other mucosal areas of the body(3). Although high incidence and prevalence are found in both females and males soon after the onset of sexual activity the vast majority of genital HPV infections are asymptomatic and clear within 1-2 years(4-5).
Although much is known about the molecular epidemiology of genital HPV infection in women, very little is known about the probability of re-infection with HPV, especially with the same type. There is also a paucity of data concerning the source of HPV infection in older women, e.g., those older than 40 years. There are two competing hypotheses to explain the occurrence of presumably incident HPV infection in such women(6-8). The first is based on the assumption that infections acquired at a young age never completely clear but become latent; infections appearing later in life would mostly represent reactivation of such latent infections acquired many years earlier. Such reactivations could result from one or more non-mutually exclusive reasons, such as hormonal changes during the peri-menopause or waning of cellular and humoral immunity against the HPV types that caused the original infections. The second hypothesis hinges on the notion that infections do clear following an initial immune response which does not completely protect against future infections by the same HPV type, following new exposure via sexual activity later in life. One of the justifications for vaccinating adult women, and especially older women, against HPV depends on understanding whether an infection represents a true new episode, or the re-activation of an earlier infection that has remained latent and undetected. Since vaccination is known to be mainly prophylactic (not therapeutic) it would have a benefit against new infections but not against latent infections.
In an effort to inform the debate about the cause of re-infection and to stimulate further research, we analyzed the association between infection/re-infection (with the same or different types that women had been exposed to in the past) and new sexual partners at the time the infection episodes were detected. We hypothesized that a latent infection that became detectable later in life is unlikely to be associated with new sexual partners, whereas new infections (with new types as well as for the same types a woman had before) would represent exposure to new sexual partners. We also analyzed HPV viral burden (viral load) at first infection and re-infection and the impact of the presence of HPV-16 antibodies at enrolment to better understand the role of natural immunity in preventing re-infection.
METHOD
Subject recruitment
The women included in this study were enrolled into the Ludwig-McGill cohort, a longitudinal investigation of the natural history of HPV infection and precursor lesions of cervical cancer. A detailed description of the design and methods of the study has been published previously(9). Briefly, women attending a maternal and child health program catering to a low-income neighborhood in São Paulo (Brazil) were recruited between 1993 and 1997 and followed for up to 10 years. Women were eligible to participate if they: 1) were between 18 and 60 years of age, 2) were permanent residents of São Paulo, 3) were not currently pregnant and had no intention of becoming pregnant during the first year of follow-up, 4) had an intact uterus and no current referral for hysterectomy, 5) reported no use of vaginal medication in the previous 2 days and 6) had no reported treatment for cervical disease in the previous 6 months. Subjects gave a signed informed consent. The study protocol was approved by institutional ethical and research review boards of the participating institutions in Canada and in Brazil.
The study enrolled 2528 women, corresponding to a 70% response rate and subsequently, 66 ineligible women were excluded. Follow-up for the remaining 2462 women consisted of 1 visit every 4 months for the first year and 2 visits per year thereafter. Cervical specimens were taken for HPV testing at every visit with a fixed-sampling area Accelon device. Blood samples taken at enrolment were tested for HPV-16 serology. Also, for the first 4 visits and for each annual visit thereafter, subjects answered a nurse-administered questionnaire designed to collect information on socio-demographic, lifestyle, sexual, reproductive and contraceptive characteristics.
HPV DNA testing
An Accelon biosampler (Medscand) was used to collect an ecto- and endocervical cell sample which was then placed in a tube containing Tris-EDTA buffer (pH 7.4). DNA was extracted, purified by spin column chromatography, and amplified by polymerase chain reaction (PCR), using the MY09/11 and PGMY protocols(10-11) for detection of HPV DNA. Typing of amplified products was performed by hybridization with individual oligonucleotide probes and by restriction fragment length polymorphism (RFLP) analysis. This method identified more than 40 HPV genital types. Amplified products that hybridized only with a generic probe and were unidentifiable in RFLP analysis were classified as positive for unknown types. The genotypes tested included high oncogenic risk (HR-) HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73 and 82, and low oncogenic risk (LR-) HPV types 6, 11, 26, 32, 34, 40, 42, 44, 53, 54, 57, 61, 62, 67, 69, 70, 71, 72, 81, 83, 84 and 89 (unknown types considered LR-HPVs) (12-13). We included more than 30 type-specific positive controls in hybridization membranes. DNA specimen quality was checked by amplification of a 268-bp human β–globin gene region(10). Specimens were tested blindly and precautions were taken to prevent contamination. Samples that were negative for both HPV and β–globin were considered inadequate for analysis.
HPV viral load
All cervical specimens found to be positive were retested by a quantitative, lowstringency PCR to measure viral burden in cervical cells(14-15). General primers (GP5/6) from a well-known PCR protocol that detects a broad spectrum of HPVs are used with this method (16). Low-stringency conditions are used by the quantitative PCR protocol to co-amplify the specific HPV DNA fragment along with DNA sequences from the human genome present in the starting PCR mixture. Standards consisting of mixtures containing varying amounts of reference HPV-16 plasmid were included in duplicate in every assay added to a constant background of normal human DNA (corresponding to 4, 20, 100, 500, and 2500 viral copies/cell). Control samples consisting of DNA from 2 cervical carcinoma cell lines with known quantities of HPV 16 or HPV 18 copies (Caski and HeLa, respectively) were also included in duplicate in every assay. Quantification by densitometry was done for the silver-stained gel bands corresponding to the HPV and to the constant human genome fragments(14). The logarithm of the ratio between these 2 bands is directly proportional to the logarithm of the amount of HPV DNA in the individual samples and linear interpolation in a standard curve constructed with the results from the control mixtures allow proper quantification of viral load.
HPV-16 Serology
Serum samples were separated from the clotted blood specimens and stored at −20°C until testing. An ELISA technique was used for semi-quantitative measurement of IgG antibodies to HPV 16 VLPs constructed with both L1 and L2 capsid proteins (17). Recombinant HPV-16 VLPs were prepared in the baculovirus system (18). An initial batch was kindly donated by Dr. John Schiller, Laboratory of Cellular Oncology, US National Institutes of Health and subsequent batches were prepared at the Ludwig Institute for Cancer Research under strict adherence to Dr. Schiller's protocols. Polystyrene ELISA microtiter plates were coated with 50 μl aliquots of a preparation containing 2 mg of VLP particles per 100 ml of phosphate-buffered saline (PBS) and then incubated for 1.5 hours at 37°C. Plates were washed three times by flooding with calcium-and magnesium-free PBS. Plates were washed again as above after non-specific reactive sites in the wells were blocked for 2 hours at room temperature with PBS containing 0.5% skim milk and 0.1% newborn calf serum (PBS-MNCS). Plates were incubated with serum samples diluted 1:10 and 1:50 in PBS-MNCS for 2.5 hours at 37°C. Following repeated washings, 50 μl aliquots of a conveniently diluted (by prior block titration) peroxidase-labelled anti-IgG conjugate were added to the wells. Plates were incubated for 1 hour at room temperature. Following an additional washing cycle, a chromogen-substrate mixture (0.1 mg/ml O-phenylenediamine and 0.003% hydrogen peroxide in 0.15 M PBS, pH 6.0) was added to the wells to reveal the bound enzyme. Absorbances were read at 490 nm in a colorimetric plate reader after 45 minutes. Replicate blank wells that substituted PBS-MNCS for the diluted serum samples were included in all plates and processed as above in all assay steps. A control human serum pool in triplicate diluted in the same way as the specimens was included in every ELISA plate to control for the inter- and intra-assay variation in reactivity that is inherent to immunoenzymatic techniques. A single batch of this serum pool (aliquoted and kept frozen at −20°C) was prepared beforehand from dozens of blood bank and normal clinical laboratory specimens from female adult donors at the AC Camargo Hospital in Sao Paulo. An aliquot from this pool was thawed and processed in the same manner as all study serum samples included in each ELISA run and the same serum pool was used throughout the study. Absorbances were corrected for the fluctuation in seroreactivity of this serum pool as previously described (19).
Statistical analysis
For every individual HPV type, we estimated the incidence rate of first infection as well as of re-infection. We hypothesized that if natural immunity played a role in preventing second infections with the same type the type-specific incidence rates of second infections would be lower than those for the first, presumably incident infections. We used Cox regression modeling with time-dependent covariates to estimate rate ratios (RR) and respective 95% confidence intervals (CI) for the association between new sexual partners and presumably incident infections and re-infections for all women as well as for older women (40+) exclusively. To further study the role of natural immunity against HPV, we compared viral loads at the time of first infection and of re-infection (with the same type or with a different type). We hypothesized that in the presence of a functional natural immune response viral loads for re-infections would be lower than those for incident infections with the same type. We also analyzed incident HPV-16 infections in the course of follow-up according to HPV-16 serological status at baseline. We hypothesized that if natural HPV-16 specific immunity provides protection against subsequent HPV-16 infections, women with a high titer of HPV-16 antibodies at enrolment should have a lower incidence rate of HPV-16 infections in the course of follow-up.
We analyzed infection and re-infection outcomes for every type individually and also grouped HPV types per their phylogenetic relationship within the genus Alpha-papillomavirus (species 3, 5, 6, 7, 9, 10) (3) and grouped HPV per their general tropism for the cervix or the vagina (20) such as HPV group 1 (benign; cervical and vaginal species 1, 8, 10, which include HPV types 6, 11, 32, 40, 42, 44), HPV group 2 (cervical species 5, 6, 7, 9, 11, which include HPV types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70 , 82) and HPV group 3 (vaginal species 3, 4, 15, which includes HPV types 57, 61, 71, 72, 62, 81, 83, 84, 89).
To study re-infections with a given HPV type we had to define what constituted clearance of the initial infection. We tested several different definitions of clearance (the results were similar) but presented only the results obtained with the most conservative definition. Clearance after first infection with a given HPV type was defined as 3 consecutively negative visits for that type following the last positive visit (a minimum of 1.5 years without evidence of cervical DNA positivity for the type in question). Only then could a woman be considered at risk of re-infection with a given type that she had before and thus contribute person-time for the analysis of re-infections. For grouped HPV data (species or grouped species defined above) clearance was defined in the same way, i.e., a woman had to be negative for at least 3 consecutive visits for any type in that group.
Covariate-adjustment for statistical models included all empirical confounders for each association between new sexual partners and the specific virological outcome. We used a conservative 5% change-in-estimate rule to identify empirical confounders suitable for inclusion in the models. In other words, if the incorporation of the potential confounder in the model changed the RR of the specific association by more than 5%, this variable was kept in the model and assumed to be a confounder; otherwise the variable was excluded because no adjustment was required. The following variables were considered to be potential empirical confounders: age group at infection (18-24, 25-34, 35-44, 45+), race (white, non-white), education (<elementary, elementary, high school, college/university), religion (catholic, others), income (low, medium, high), smoking (yes, no), alcohol drinking at enrolment (yes, no), age at menarche (≤12, 12+), hygienic tampon use (yes, no), sexually-transmitted diseases (ever, never), lifetime number of Pap smears (0, 1-3, 4-7, 8+) and time since last Pap at enrolment (within a year, more than 1 year), age at first sexual intercourse (≤15, 16-18, 19+), number of pregnancies (0, 1-2, 3-4, 5+), number of deliveries (0, 1-2, 3+), oral contraception use (yes, no), duration of oral contraception (never, <6 years, 6 years and more), condom use (yes, no), menopausal status (yes or no at the time of infection), and lifetime number of sexual partners at enrolment (0-1, 2-3, 4+). All analyses were done using Stata 9.2 (Stata Corp., College Station, TX).
RESULTS
A total of 2455 women provided at least one adequate sample for HPV testing. Altogether, these women contributed 23719 visits for which the cervical specimens were typed for HPV during the first 7 years of follow-up (first 16 visits). The mean follow-up time was 59.0 months (SD=36.3), the mean age at enrolment was 32.7 years (SD=8.8; median=32, range: 18-59) and most of the women were white (64%). Of the 2455 women included in the analysis, 472 (19.3%) reported having at least one new sexual partner at some point in the course of follow-up. Among the latter, 21.2% remained HPV negative, whereas 57.3% of those reporting no new sexual partners remained HPV negative. Among the 568 women who were 40 years and over at enrolment 63 (11%) reported having at least one new sexual partner during follow-up. Among those who had no new sexual partners 60.8% remained HPV negative compared with 28.6% of those who had at least one new sexual partner.
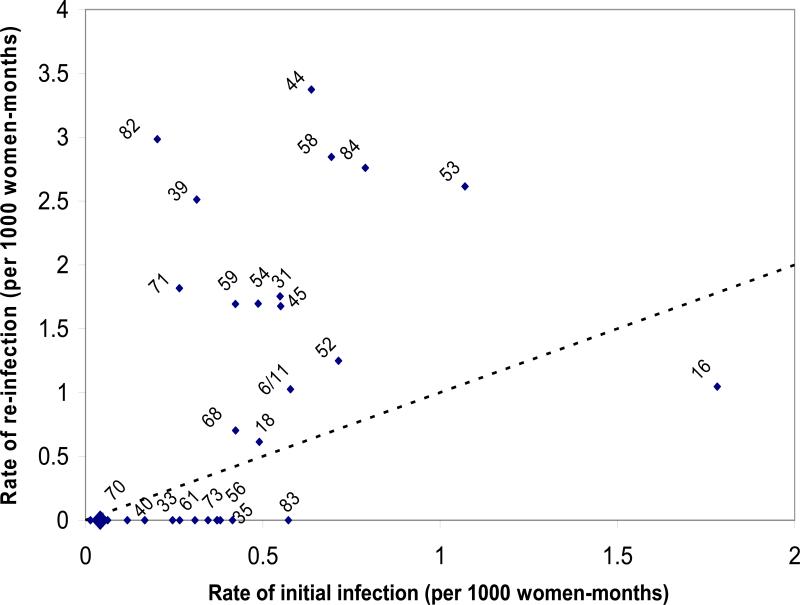
Figure 1 shows the correlation between infection rates and re-infection rates for all individual HPV types. For example, the rate of first infection with HPV 16 was 1.78 cases per 1000-women-months whereas the rate of re-infection for HPV-16 was 1.05 per 1000-women-months. The diagonal dotted line on the figure denotes the equivalency in rates of first infections and re-infections. Types were randomly distributed about the equivalency line, with no statistical evidence that the magnitude of the initial infection rate for a given type was higher than that for re-infection.
Fig 1.
Correlation between HPV type-specific rates of initial infection and re-infection. HPV types are indicated atop of the rate coordinates. Diagonal dotted line represents equivalency in rates of initial and re-infection. Large diamond near origin denotes clustered rates for HPV types 89/82/69/67/57/34/32/26. There was no significant difference between rates of initial and of re-infection (p=0.500 via non parametric sign test). Ludwig-McGill Cohort, Brazilian women, 1993-2004.
Table 1 shows the rates of initial infection and re-infection according to grouped HPV types. For most HPV groups rates of initial infection were comparable to or significantly lower than those of re-infection with a different type, suggesting that natural immunity is not sufficient to prevent a new infection with a different type. Expectedly, because of the need to restrict to same-type infections, the rate of re-infection with the same type tended to be lower than the initial infection rate with that type.
Table 1.
Incidence rates of initial infection and of re-infection*, overall and for grouped HPV types. Ludwig-McGill Cohort, Brazilian women, 1993-2004.
| HPV Group | Categories | Initial infection | Initial infection | Re-infection | |||||
|---|---|---|---|---|---|---|---|---|---|
| With the same type | With a different type | ||||||||
| N‡ | women-months | Rate (95CI%) | N§ | women-months | Rate (95CI%) | women-months | Rate (95CI%) | ||
| All types | 1814 | 83632 | 9.6 (9.0-10.3) | 566 | 13062 | 1.5 (0.9-2.3) | 16155 | 9.7 (8.3-11.3) | |
| Species | 3 | 2129 | 131304 | 2.1 (1.9-2.4) | 161 | 4179 | 1.9 (1.0-3.8) | 4472 | 5.1 (3.4-7.7) |
| 5 | 2146 | 135545 | 1.3 (1.1-1.5) | 119 | 3747 | 0.8 (0.3-2.5) | 3737 | 1.1 (0.4-2.9) | |
| 6 | 2123 | 132596 | 1.7 (1.5-1.9) | 146 | 4570 | 1.8 (0.9-3.5) | 4482 | 0.9 (0.3-2.4) | |
| 7 | 2102 | 129851 | 2.1 (1.8-2.3) | 182 | 5322 | 1.3 (0.6-2.8) | 5600 | 2.9 (1.8-4.7) | |
| 9 | 2047 | 117208 | 3.9 (3.5-4.3) | 298 | 8247 | 1.0 (0.5-1.9) | 9109 | 4.4 (3.2-6.0) | |
| 10 | 2146 | 136744 | 1.2 (1.0-1.4) | 100 | 3020 | 1.7 (0.7-4.0) | 2939 | 0.7 (0.2-2.7) | |
| Grouped species† | 1 | 2139 | 134590 | 1.6 (1.4-1.8) | 121 | 3595 | 1.4 (0.6-3.3) | 3535 | 1.1 (0.4-3.0) |
| 2 | 1911 | 96969 | 7.0 (6.5-7.5) | 484 | 12514 | 1.2 (0.7-2.0) | 14513 | 6.8 (5.5-8.2) | |
| 3 | 2123 | 130036 | 2.3 (2.1-2.6) | 173 | 4438 | 1.8 (0.9-3.6) | 4834 | 5.4 (3.7-7.9) | |
Per 1000 women-months
Species grouping based on phylogenetic and biological relatedness (Group 1: benign; cervical and vaginal; Group 2: cervical; and group 3: vaginal) according to Schiffman et al. 2005 (20). See text for details.
N=number of women. Because incidence rates are reported, women included in the analysis were originally free of infection with HPV that belongs to that group (N varies according to HPV group).
N=number of women. To be included in the analysis, women must have been infected with a type that belongs to that group and then clear the infection for at least 3 consecutive visits (see text for details).
Table 2 shows the RRs for the association between new sexual partners and infections. Not surprisingly, initial infections were invariably associated with new sexual partners (adjusted RR for any HPV types: 2.4; 95%CI: 2.0-3.1). It is noteworthy also that, apart from the loss in precision that comes from the restriction in the subcohort analyses, re-infections (whether with the same or different types) were also strongly associated with new sexual partners (adjusted RRs: 3.7 and 2.3, respectively; both significantly greater than unity). These findings were consistent across all HPV species or HPV species groups.
Table 2.
Rate ratios (RR)* and 95% confidence intervals (CI) for the association between infection with different grouped HPV types and new sexual partners. Ludwig-McGill Cohort, Brazilian women, 1993-2004.
| HPV group | Categories | Initial infection | Re-infection | ||||
|---|---|---|---|---|---|---|---|
| Crude RR (95%CI) | Adjusted RR (95%CI)† | With the same type | With a different type | ||||
| Crude RR (95%CI) | Adjusted RR (95%CI)† | Crude RR (95%CI) | Adjusted RR (95%CI)† | ||||
| All types | 2.6 (2.1-3.3) | 2.4 (2.0-3.1) | 6.0 (2.0-18.6) | 3.7 (1.1-13.8) | 2.7 (1.7-4.3) | 2.3 (1.5-3.7) | |
| Species | 3 | 2.8 (2.0-3.9) | 2.5 (1.8-3.5) | 3.6 (0.8-15.4) | 4.7 (0.5-46.3) | 1.1 (0.3-3.6) | 1.3 (0.4-5.0) |
| 5 | 3.2 (2.2-4.8) | 2.5 (1.6-3.8) | 3.2 (0.3-35.5) | 2.0 (0.1-31.8) | -- | -- | |
| 6 | 3.7 (2.7-5.2) | 3.4 (2.5-4.8) | 4.1 (0.8-22.4) | 3.6 (0.3-52.9) | -- | -- | |
| 7 | 3.1 (2.3-4.2) | 2.5 (1.8-3.4) | -- | -- | 3.6 (1.2-10.3) | 2.1 (0.7-6.4) | |
| 9 | 2.9 (2.2-3.7) | 2.4 (1.8-3.2) | 1.5 (0.2-12.6) | 1.5 (0.2-14.0) | 3.1 (1.4-6.7) | 2.7 (1.2-6.1) | |
| 10 | 3.3 (2.3-5.0) | 2.9 (1.9-4.3) | -- | -- | |||
| Group | 1 | 3.6 (2.6-5.1) | 3.2 (2.2-4.5) | -- | 2.2 (0.2-21.1) | 2.9 (0.1-63.5) | |
| 2 | 2.8 (2.3-3.6) | 2.6 (2.1-3.3) | 2.7 (0.6-12.1) | 2.5 (0.5-20.6) | 2.6 (1.5-4.5) | 2.0 (1.1-3.6) | |
| 3 | 2.5 (1.8-3.4) | 2.2 (1.6-3.1) | 4.0 (0.9-17.0) | 4.2 (0.7-25.3) | 0.9 (0.3-3.0) | 0.8 (0.2-2.8) | |
Rate ratio (RR) and respective 95% confidence intervals (CI) obtained via Cox regression modeling with time-dependent covariates. Referent group: women who reported not having a new sexual partner.
Adjusted for empirical confounders. See text for details.
Unlike true new infections, latent infections acquired earlier in life and no longer detectable (but later reactivated because of immunosenescence or hormonal changes) would not be expected to be associated with the acquisition of new partners. To test this hypothesis we analyzed this association among women with a high likelihood of HPV infection exposure earlier in life by including only those who were 40 years or older and reported two or more lifetime sexual partners at enrolment (N=251). As shown (table 3), HPV infections among older and presumably pre-exposed women were also mostly associated with new sexual partners, even after extensive covariate adjustment for potential and empirical confounders.
Table 3.
Incidence rate of new HPV infections (per 1000 women-months) and rate ratios (RR)* for the association between new sexual partners and new infections among women aged 40 years or older who reported 2 or more lifetime sexual partners at enrolment (N=251). Ludwig-McGill Cohort, Brazilian women, 1993-2004.
| HPV group | Categories | Incidence rate (95%CI) | Crude RR (95%CI) | Adjusted RR (95%CI)† |
|---|---|---|---|---|
| All types | 9.8 (8.1-11.7) | 2.5 (1.3-4.8) | 2.8 (1.4-5.3) | |
| Species | 3 | 1.9 (1.3-2.7) | 5.2 (2.2-12.0) | 6.1 (2.6-14.4) |
| 5 | 1.0 (0.7-1.6) | 2.1 (0.5-9.0) | 2.1 (0.5-9.6) | |
| 6 | 1.5 (1.1-2.2) | 3.9 (1.5-10.4) | 6.1 (2.2-16.7) | |
| 7 | 1.6 (1.1-2.3) | 3.4 (1.3-9.1) | 4.4 (1.2-15.5) | |
| 9 | 4.0 (3.1-5.1) | 2.6 (1.2-5.8) | 3.4 (1.5-7.7) | |
| 10 | 0.9 (0.6-1.5) | 4.5 (1.5-13.9) | 5.1 (1.6-16.1) | |
| Group | 1 | 1.4 (1.0-2.1) | 4.2 (1.6-11.1) | 3.5 (1.3-9.7) |
| 2 | 6.9 (5.6-8.4) | 1.7 (0.8-3.6) | 1.7 (0.8-3.6)‡ | |
| 3 | 2.4 (1.8-3.3) | 4.9 (2.3-10.7) | 6.2 (2.7-14.0) | |
Rate ratio (RR) and respective 95% confidence intervals (CI) obtain via Cox regression modeling with time-dependent covariates. Referent group: women who reported not having a new sexual partner.
Adjusted for empirical confounders. See text for details.
Crude and adjusted estimates equivalent because no empirical confounders were identified.
Table 4 shows that viral loads for first infection, re-infections with the same type, and infections with different types than those initially found were comparable with largely overlapping 95%CIs. Finally, for the women who were HPV-16 DNA negative at baseline, we examined the strength of the association between new sexual partners and incidence of HPV-16 infection according to the level of serological response against HPV-16 at enrolment (table 5). The incidence rate was highest among women with the highest level of HPV-16 antibodies at baseline. Most importantly, however, having a new sexual partner was associated with newly detected HPV-16 infections even among women with clear evidence of serological exposure to HPV-16 at the onset of follow-up.
Table 4.
Viral load (number of HPV genome copies per cell) at initial infection and re-infection with a new or a different type. Ludwig-McGill Cohort, Brazilian women, 1993-2004.
| Viral load measure | Initial infection (N*=779) | Re-infection | |
|---|---|---|---|
| With the same type (N=19) | With a different type (N=153) | ||
| Geometric mean (95%CI) | 1.8 (1.5-2.1) | 0.9 (0.4-1.9) | 1.5 (1.0-2.1) |
| Median (inter-quartile range) | 0.5 (0.5-3.0) | 0.5 (0.5-0.5) | 0.5 (0.5-1.0) |
| Range | 0.5 - 29195 | 0.5 - 242 | 0.5 - 6267 |
Number of women
Table 5.
Incidence of new HPV-16 infection and its association with new sexual partners stratified by level of IgG serological response against HPV-16 at enrolment. Analysis restricted to women who were HPV-16 DNA negative at enrolment. Ludwig-McGill Cohort, Brazilian women, 1993-2004.
| Tertile of seroreactivity | N* | Women-months | Incidence rate per 1000 women-months (95%CI) | Crude RR† (95%CI) | Adjusted RR‡ (95%CI) |
|---|---|---|---|---|---|
| Lowest | 646 | 44772 | 1.6 (1.3-2.1) | 1.7 (0.8-3.6) | 1.2 (0.5-2.6) |
| Middle | 645 | 44004 | 1.7 (1.3-2.1) | 2.6 (1.4-4.9) | 2.1 (1.1-4.0) |
| Highest | 611 | 37996 | 2.1 (1.7-2.6) | 3.0 (1.6-5.3) | 3.0 (1.6-5.3) |
N=number of women. Serological data at enrolment were available for 2034 women, of whom 1974 were HPV-16 DNA negative at enrolment. The analysis was restricted to 1902 women who had multiple follow-up visits. Imbalance in frequencies by seroreactivity is because proportionally more women who were HPV-16 DNA positive were excluded from the highest tertile of seroreactivity.
Rate ratio (RR) and respective 95% confidence intervals (CI) obtain via Cox regression modeling with time-dependent covariates. Referent group: women who reported not having a new sexual partner.
Adjusted for empirical confounders. See text for details.
DISCUSSION
A long-standing, yet unanswered question in HPV epidemiology is whether or not infections clear completely or become latent to reappear later in life. Even with today's highly sensitive PCR protocols to detect HPV DNA the possibility remains that the apparent clearance of HPV infections may simply reflect that viral load dipped below the threshold of detectability. Moreover, HPV DNA testing in women enrolled in repeated-measurement cohort studies is done in exfoliated cell scrapes, which tend to oversample the upper cellular layers of the ectocervix and thus they may miss low viral load infections confined to the basal layer. Because of this dual methodological limitation (test sensitivity and sampling inadequacy) it is impossible to verify whether or not an infection has cleared and whether or not such putatively latent infections exist and may be the source of reactivation later in life. Epidemiological studies have found that prevalence of HPV infection is highest soon after the onset of sexual activity during late adolescence or early adulthood. It then falls rapidly with age, presumably due to the development of immune response, and in many populations a second peak in prevalence occurs after the age of 45 or 50 years, coinciding with the peri-menopausal years (21). Prospective studies that measured incidence of HPV infection have also shown a second infection peak in older women (22). Although the reason for this second, menopausal peak is not clear, it could be plausibly attributed to one or more non-mutually exclusive mechanisms, such as reactivation of the aforementioned latent infections due to a gradual loss of type-specific immunity, or to acquisition of new infections due to contacts with new sexual partners later in life. Also plausible is a cohort effect: age-related variations in prevalence may reflect the diverse HPV exposure of successive birth cohorts. Sexual mores have changed during the last few decades, which may have influenced the HPV exposure of different age cohorts (5).
Ongoing cohort studies of the natural history of HPV infection and cervical neoplasia have reached the stage where this question can be at least indirectly addressed by correlating information on HPV exposure at the outset with the history of re-infections in women with long-term follow-up. We found that the incidence rate of a first episode with a given HPV type was comparable to the equivalent rate of re-infections in our study. Re-infection with the same HPV type was a common finding. These results suggest that a woman is not fully protected by a first HPV infection and she continues to be at risk of being re-infected with different types, as well as with the same type she previously had and seemed to have cleared. Moreover, viral loads for infection or re-infection (same or different HPV types) were comparable indicating that natural immunity post-initial infection did not dampen the cervical viral burden in subsequent infections later in life. Specifically for HPV-16, seroreactivity at enrolment did not seem to protect against new cervical infections with this HPV type. These results suggest that in this population of adult women natural immunity does not seem to play a role in controlling the extent of re-infections, whether with the same type or with new types.
Of relevance to the latent infection hypothesis was our finding that risks of infection and re-infection (with the same or with a different type) were strongly (and comparably) associated with having a new sexual partner. If all instances in which women were found to have a new infection with a specific HPV type which they had been infected with previously and demonstrated to have cleared represented reactivated latent HPV infections (i.e., as defined above), sexual contact with new partners would be an unlikely risk factor for such reactivations. Biological changes stemming from endocrine or exogenous hormonal influences and waning of immunity would be more plausible triggers for the reactivation of such putatively latent infections. When we mimicked this situation in our cohort by restricting the analyses to women with a high probability of prior HPV exposure, i.e., those aged 40 years or older and with multiple sex partners at enrolment, we also observed strong associations with new sexual partners, even with extensive adjustment for lifestyle and reproductive history cofactors that would have changed around the peri-menopausal years. A related observation was the finding that the risk of HPV-16 infections (during follow-up) was most strongly associated with new partners among women with the highest levels of anti-HPV-16 antibodies at enrolment in the cohort.
In all, our findings indicate that natural immunity does not seem to mediate risk of HPV infections at any point during the span of female reproductive years that we observed in our cohort. More importantly, and germane to the issue of causality of repeated infection episodes with the same HPV type a woman harbored in her past, we observed that they are largely explained by the acquisition of new sexual partners. The magnitude of the association of re-infections with new sexual partners was comparable to that for initial infections, whether for types to which the woman was previously exposed or for new types. If a substantial proportion of the re-infections constituted reactivation of latent infections, we would have expected that the magnitude of the association with new partners would have been lower than that for types she did not harbor at the outset of the study because reactivations would likely have been triggered by biological events unrelated to sexual activity. Admittedly, some of these events could be correlated with the time-dependent changes in reproductive characteristics and hormonal intake history around menopause. Such information was collected during annual interviews and we used them as covariates to control for potential confounding of the association with new partners. However, these model-based adjustments did not change the interpretation that acquisition of new partners was the primary risk factor for infection episodes during follow-up.
Important limitations must be recognized. We called initial or first infections as any episode with a type the woman had not harbored before. We also used the term re-infections loosely, to denote infections that we qualified as “with the same type” or “with a different type” occurring in women with an initial infection at the early visits in the cohort. Proper characterization of a truly incident HPV infection event would have required that we observed the cohort from the onset of sexual exposure, an impractical proposition in most settings. Most importantly, however, is the definition of a re-infection with the same type because of the relevance it has for our objective of inferring the cause of reactivation of potentially latent infections. We used a conservative clearance definition that imposed a minimum of 3 consecutive visits and at least 1.5 years without detection of the original type with which a woman had been infected. In light of fixed endo-and ecto-cervical cervical area sampling that we systematically used in our study (via Accelon sampler) we believe that our inability to detect a given HPV type indicated credible clearance and was not due to the vagaries in sampling and cellularity of the consecutive specimens.
One could hypothesize that sexual practices with new partners might cause latent infections to become detectable via abrasion or other stimuli, which would thus explain our findings. However, these would be transient cellular exfoliation events that would have been unlikely to affect detectability of HPV DNA in the cervical specimens we collected every 6 months on average in our study. Furthermore, the magnitude of the association with new partners was comparable between putatively latent and new infections (with different types), which favors the notion of true transmission rather than reactivation. Unfortunately, we could not distinguish among women with new partners during follow-up the ones engaged in extra-marital relationships from those who had embarked in new, non-concurrent relationships, which would have helped us to address the issue of directionality in transmission. It is plausible to assume that the HPV infection risk for those in extramarital relationships was also affected by the extramarital activity of the husbands. Finally, we verified that infection risk was not associated with the reported frequency of sexual intercourse among all women in the cohort or restricted to those with a single partner, even after adjustment for number of previous partners (data not shown), which argues against the abrasion hypothesis.
In conclusion, the positive association between new sexual partners and re-infection thus indicates that true new infections with the same types harbored earlier in life are possible in older women. This finding has some support in the literature; others have also shown a strong relationship between new sexual partners and infection in older women (22). The implications of this finding with respect to a possible benefit of HPV vaccination in older women are obvious. The recent results from randomized controlled trials of HPV vaccination (23-24) have shown a benefit among older women and, in light of our results, it is possible that some of that protection may come from averting the re-acquisition of vaccine-targeted HPV types to which women may have been exposed before and cleared. However, we urge caution in concluding that a possible benefit against virological endpoints may ultimately translate into reduced cervical cancer risk. Very little is known about the risk of lesions following incident infections that occur at older ages. HPV infections in older women may be less likely to progress to precancerous lesions or to cancer. Clearly, these are key questions that should be prioritized for further research with cost effectiveness analyses, before public health implementation of HPV vaccination in older women.
ACKNOWLEDGMENTS
The authors are grateful to Maria L. Baggio and Lenice Galan for managing patients and specimens and to Romulo Myamura for DNA extraction work. Portions of the contents of this manuscript were presented in an oral presentation form at the International HPV Conference in Sweden, in May 2009 [Trottier H, et al, HPV in older women is associated with new sexual partners; 25th International Papillomavirus conference & Clinical Workshop. Malmo. Sweden. May 2009; abstract number: O-06.02].
FUNDING
This study was supported by an intramural grant from the Ludwig Institute for Cancer Research to LLV and ELF and by grants from the U.S. National Cancer Institute (CA70269) and the Canadian Institutes of Health Research (grant MOP-49396 and team grant 83320) to ELF. HT holds a salary award (chercheur-boursier) from the Fonds de la recherche en santé du Québec (FRSQ).
REFERENCES
- 1.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32:S16–24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Kjaer SK, Chackerian B, van den Brule AJ, et al. High-risk human papillomavirus is sexually transmitted: evidence from a followup study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev. 2001;10:101–6. [PubMed] [Google Scholar]
- 3.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman M, Castle PE. Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med. 2003;127:930–4. doi: 10.5858/2003-127-930-HPEAPH. [DOI] [PubMed] [Google Scholar]
- 5.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24:S1–15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 6.Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92:464–74. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- 7.Lazcano-Ponce E, Herrero R, Muñoz N, et al. Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer. 2001;91:412–20. doi: 10.1002/1097-0215(20010201)91:3<412::aid-ijc1071>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Castle PE, Schiffman M, Herrero R, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1808–16. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 9.Franco E, Villa L, Rohan T, Ferenczy A, Petzl-Erler M, Matlashewski G. Design and methods of the Ludwig-McGill longitudinal study of the natural history of human papillomavirus infection and cervical neoplasia in Brazil. Ludwig-McGill Study Group. Rev Panam Salud Publica. 1999;6:223–33. doi: 10.1590/s1020-49891999000900001. [DOI] [PubMed] [Google Scholar]
- 10.Bauer HM, Ting Y, Greer CE, et al. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991;265:472–7. [PubMed] [Google Scholar]
- 11.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Agency for Research on Cancer (IARC) Human Papillomaviruses. Vol. 90. Lyon; France: 2007. Monographs on the evaluation of carcinogenic risks to humans. [PMC free article] [PubMed] [Google Scholar]
- 13.Muñoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 14.Caballero OL, Villa LL, Simpson AJ. Low stringency-PCR (LS-PCR) allows entirely internally standardized DNA quantitation. Nucleic Acids Res. 1995;23:192–3. doi: 10.1093/nar/23.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlecht NF, Trevisan A, Duarte-Franco E, et al. Viral load as a predictor of the risk of cervical intraepithelial neoplasia. Int J Cancer. 2003;103:519–24. doi: 10.1002/ijc.10846. [DOI] [PubMed] [Google Scholar]
- 16.van den Brule AJ, Snijders PJ, Gordijn RL, Bleker OP, Meijer CJ, Walboomers JM. General primer-mediated polymerase chain reaction permits the detection of sequenced and still unsequenced human papillomavirus genotypes in cervical scrapes and carcinomas. Int J Cancer. 1990;45:644–9. doi: 10.1002/ijc.2910450412. [DOI] [PubMed] [Google Scholar]
- 17.Kirnbauer R, Hubbert NL, Wheeler CM, Becker TM, Lowy DR, Schiller JT. A virus like particle enzyme linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type. Journal of the National Cancer Institute. 1994;86:494–9. doi: 10.1093/jnci/86.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirnbauer R, Taub J, Greenstone H, et al. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol. 1993;67:6929–36. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramanakumar AV, Thomann P, Candeias JM, Ferreira S, Villa LL, Franco EL. Use of the normalized absorbance ratio as an internal standardization approach to minimize measurement error in enzyme-linked immunosorbent assays for diagnosis of human papillomavirus infection. J Clin Microbiol. 2010;48(3):791–6. doi: 10.1128/JCM.00844-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffman M, Herrero R, Desalle R, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 21.de Sanjosé S, Diaz M, Castellsagué X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 22.Muñoz N, Méndez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190:2077–87. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 23.Muñoz N, Manalastas R, Jr, Pitisuttithum P, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–57. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 24.Olsson SE, Kjaer SK, Sigurdsson K, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5:696–704. doi: 10.4161/hv.5.10.9515. [DOI] [PubMed] [Google Scholar]