Abstract
Class 3 semaphorins are axonal guidance mediators and regulators of angiogenesis and tumor progression Semaphorin 3A and 3F (SEMA3A&F) act by depolymerizing F-actin, resulting in cytoskeleton collapse A key signaling step is that SEMA3A&F activates ABL2 tyrosine kinase, which activates p190RhoGAP, which in turn inactivates RhoA, thereby diminishing stress fiber formation and ensuing cell migration We now demonstrate that Gleevec (imatinib, STI571), an ABL2 tyrosine kinase inhibitor, abrogates SEMA3A&F-induced stress fiber loss in glioblastoma cells and endothelial cells and diminishes their ability to inhibit migration. On the other hand, Sutent (sunitinib), a receptor tyrosine kinase inhibitor, did not rescue SEMA3A&F-induced collapsing activity. These results describe a novel property of Gleevec, its ability to antagonize semaphorins.
Keywords: semaphorin, Gleevec, ABL2, stress fibers, cytoskeletal collapse, cell migration
1. Introduction
Class 3 semaphorins and their receptors, neuropilins and plexins, are important regulators of axon guidance during development of the central nervous system [1,2]. There are seven semaphorins (SEMA3A-G). Semaphorins were originally described to regulate axon guidance as a repulsive cue [3,4]. More recently, semaphorins also have been shown to be involved in vascular and tumor biology, mostly as inhibitors. For example, chromosome 3p21.3, which encodes SEMA3F, is deleted in small cell lung cancer, consistent with loss of a tumor suppressor [5,6]. Highly metastatic tumor cells down-regulate SEMA3F expression, again consistent with loss of a tumor suppressor [7,8]. Further evidence for vascular and tumor involvement is that SEMA3F inhibits migration of endothelial cells (EC) and tumor cells in vitro and tumor angiogenesis, progression and metastasis in vivo [7,9].
Some steps in the signaling pathway of SEMA3F have been identified [9]. First, SEMA3F makes a complex with neuropilin 2 (NRP2) and plexin A1. This complex attracts ABL2 tyrosine kinase, which activates p190RhoGAP and inactivates a small GTPase RhoA. Active RhoA phosphorylates cofilin, an actin depolymerizing factor. In response to SEMA3F, cofilin is dephosphorylated rapidly, leading to depolymerization of F-actin, loss of stress fibers and diminished EC and tumor cell migration [9]. Collectively, ABL2 is a key mediator of SEMA3F-induced RhoA inactivation and collapsing activity.
Gleevec (imatinib, STI571) is an ABL2 kinase inhibitor that has been used very successfully to treat chronic myeloid leukemia and gastrointestinal stromal tumors [10,11]. Recently, it has been demonstrated that ABL2/ARG controls dendritic spine and dendrite arbor stability via cytoskeletal control pathways [12,13]. Since Gleevec inactivates ABL2 kinase, we predicted that Gleevec would antagonize the SEMA3A&F signaling pathway. In this report, we show that Gleevec prevents SEMA3A&F-induced loss of stress fibers, as well as the inhibition of cell migration in U87MG glioblastoma cells and human umbilical vein endothelial cells (HUVEC). Rescue of semaphorin-induced activities would be a novel application of Gleevec with potential to abrogate adverse semaphorin effects [14,15,16].1
2. Materials and Methods
2.1. Antibodies and reagents
Mouse monoclonal anti-p190RhoGAP antibody and mouse monoclonal anti-phosphotyrosine antibody (clone 4G10) were purchased from BD Transduction Laboratories (cat. no. 610149) and EMD Millipore Chemicals, respectively. Gleevec (Imatinib methanesulfonate salt, cat. no. I-5508) was from LC Laboratories and the Dana-Farber Cancer Institute. Sutent (Sunitinib malate, cat. no. 3768) was purchased from TOCRIS Bioscience.
2.2. Cell culture
U87MG human glioblastoma cells were cultured in minimum essential medium (MEM, Invitrogen) containing 10% fetal bovine serum (FBS, Denville Scientific, Inc.) and 1% L-glutamine/penicillin G/streptomycin sulfate (Invitrogen) in a 10% CO2 incubator at 37°C. HUVEC were purchased from Lonza and cultured in EGM2 media. Cells were maintained in 5% CO2 incubator at 37°C.
2.3. Human recombinant SEMA3s
Recombinant SEMA3s were purified as in our previous reports [9,17]. The full-length, His-Myc-tagged, chicken SEMA3A or human SEMA3F construct was transfected into 293T cells using FuGENE HD Transfection Reagent (Roche). Semaphorins secreted into culture medium were purified on HiTrap HP Chelating columns (GE Healthcare Bio-Sciences Corp.).
2.4. Immunoprecipitation
Cell lysates were immunoprecipitated with anti-p190RhoGAP antibody at 4°C overnight. Protein G-Sepharose 4 Fast Flow beads (GE Healthcare) were added to each sample, followed by mixing for 1 hour at 4°C. The samples were dissolved in sodium dodecyl sulfate sample buffer and boiled for 5 minutes.
2.5. Western blotting
Western blotting was performed as previously described by us [9]. Each sample was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and the gels were transferred to nitrocellulose membranes. The membranes were blocked with 4% skim milk in TBS-T (0.1% Tween 20 in tris-buffered saline [TBS]) for 30 minutes, followed by incubation with primary antibodies. After washing with TBS-T, the membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies. Immunoreactivity was detected by using ECL detection reagents.
2.6. F-actin staining/Confocal microscopy
U87MG cells and HUVEC were fixed with 4% paraformaldehyde. Cells were permeabilized with 0.2% Triton X-100 in phosphate buffered saline (PBS). F-actin and nuclei were stained with Alexa Fluor 488 phalloidin (Invitrogen) and 4’, 6-diamidino-2-phenyl-indole, dihydrochloride (DAPI, Invitrogen), respectively. The mounted samples on slide glasses were imaged on a Leica TCS SP2 confocal laser-scanning microscope equipped with a 63X objective (NA 1.4), 488 nm argon ion laser (F-actin), and 405 nm diode (nuclei).
2.7. Migration assay
Migration assays were performed in Transwells® (Corning Glass) with an 8.0-μm pore size. For HUVEC, the polycarbonate membrane of the upper Transwell insert was coated with 0.5% gelatin solution. U87MG cells (2.5 × 104) and HUVEC (7.5 × 104) were added into the upper wells with serum-free MEM or in DMEM containing 0.1% FBS, respectively. MEM or DMEM containing 1% FBS, SEMA3A and SEMA3F were added to the lower wells. Gleevec was added to upper and lower wells 1 hour prior to SEMA3A&F treatment. Cells that had migrated through the filter after 20 hours at 37°C were stained with Diff-Quick cell staining kit (Dade Behring, Inc.) and 4 fields were counted by phase microscopy.
3. Results and Discussion
Semaphorins, SEMA3A&F in particular, are potent inhibitors of tumor cell and EC functions such as cell adhesion, cell migration, angiogenesis, tumor progression and metastasis [7,9,18]. There are exceptions, such as SEMA3E [19,20]. NRPs are receptors of SEMA3A&F. NRP1 binds SEMA3A but not SEMA3F, whereas NRP2 binds SEMA3F with higher affinity than SEMA3A [21]. The signaling pathways of SEMA3A&F have been intensively studied, mostly in the neuronal system [1,2]. It was found that semaphorin collapsed axonal growth cones; thus, semaphorin was originally named collapsin-1 [3,4]. To be active, SEMA3A&F need to form complexes with NRPs and plexins. An important feature of this pathway is that the signals mediated by these interactions are transduced by small GTPases of the Rho family. Cytoplasmic domains of plexins have GTPase-activating protein activity and bind other small GTPase regulators [22,23].
Importantly, we found that the SEMA3 signaling pathways in axons were also relevant to other cell types, in particular, tumor cells and EC [9]. In these cells, SEMA3F induces a complex of NRP2 and plexin A1, activating p190RhoGAP, which inactivates RhoA, converting GTP to GDP, resulting in loss of stress fibers and collapse of the F-actin cytoskeleton. In addition, inactivation of RhoA activates cofilin, an actin depolymerization factor, further collapsing the cytoskeleton and inhibiting cell migration in glioblastoma cells and EC [9]. SEMA3A has similar interactions, but they are mediated by NRP1 rather than NRP2.
A key step in the SEMA3A&F signaling pathway is the involvement of ABL2, a tyrosine kinase that activates p190RhoGAP [9]. We predicted that an ABL2 kinase inhibitor would block the signaling pathways by inhibiting p190RhoGAP activity and preventing loss of stress fibers. To show that SEMA3A&F activated p190RhoGAP, cell lysates were immunoprecipitated with anti-p190RhoGAP antibody and immunoblotted with anti-phosphotyrosine antibody (Fig. 1). Western blot analysis showed that phosphorylated p190RhoGAP protein was enhanced by 124% and 146% for SEMA3A and SEMA3F, respectively; however, Gleevec reduced SEMA3A&F-induced p190RhoGAP phosphorylation by 65% (lane 2 vs. 5 and lane 3 vs. 6). Thus, it was concluded that Gleevec is a SEMA3A&F antagonist.
Figure 1.
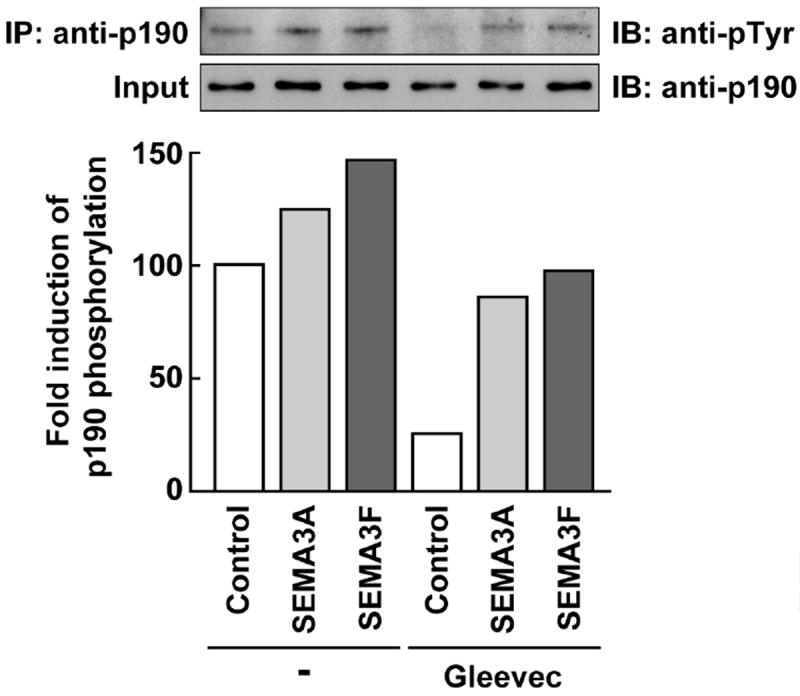
Gleevec blocks the phosphorylation of p190RhoGAP by SEMA3A and SEMA3F. U87MG cells were pre-treated with Gleevec (100 μM) 1 hour prior to SEMA3A and SEMA3F (320 ng/ml) treatment. After 10 minutes, cells were lysed and immunoprecipitated with anti-p190RhoGAP antibody. Western blotting analysis was carried out with anti-phosphotyrosine antibody or anti-p190RhoGAP antibody. The band intensity of phosphorylated p190RhoGAP bands was normalized to their respective total p190RhoGAP levels. The bar graph represents the fold-change in band intensity relative to control.
Confocal microscopy in conjunction with phalloidin staining was used to show the effects of Gleevec on SEMA3A&F-treated U87MG glioblastoma cells and HUVEC morphology and stress fiber formation (Fig. 2 and Fig. 3), respectively. U87MG cells express abundant stress fibers, of which about 18% remain after SEMA3A treatment (Fig. 2A, a vs. b). However, Gleevec rescues this inhibition so that 75% of the stress fibers are present upon SEMA3A administration (Fig. 2A, b vs. c). Similarly, SEMA3F inhibited stress fibers by 98% (Fig. 2A, a vs. d), whereas Gleevec restored stress fiber formation to about 64% (Fig. 2A, d vs. e).
Figure 2.
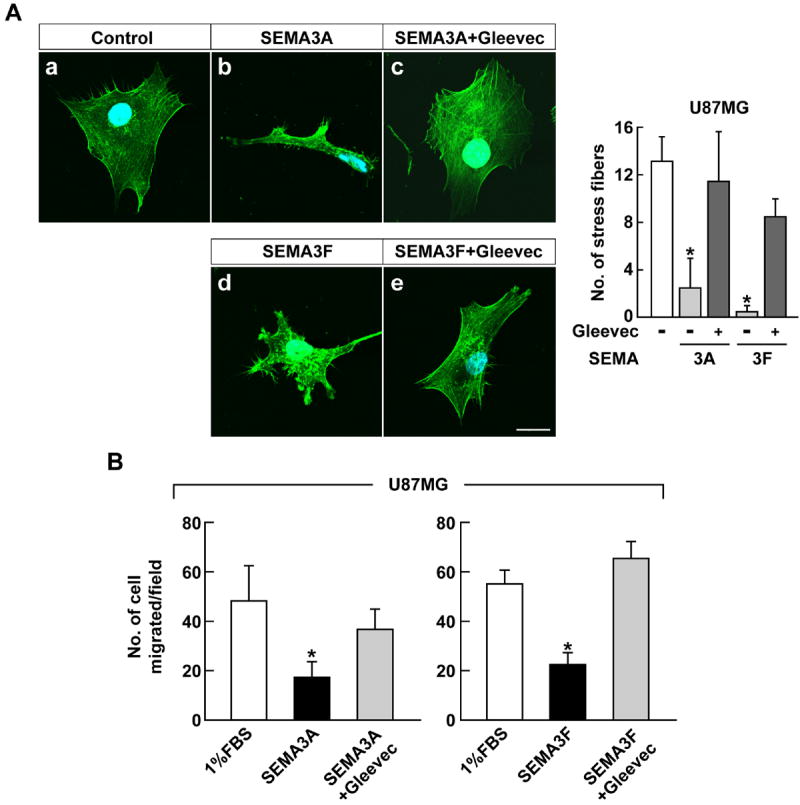
Gleevec abrogates the effect of SEMA3A and SEMA3F in U87MG cells. A, U87MG cells were pre-treated with 100 μM Gleevec for 1 hour before SEMA3A and SEMA3F (320 ng/ml) treatment. After 45 minutes, cells were fixed and stained with Alexa Fluor 488 phalloidin and DAPI and observed by confocal microscopy. The right panel shows the quantification of stress fibers. Data represent the mean ± SD (n = 3), * p < 0.05. The scale bar indicates 20 μm. B, U87MG cells were treated with Gleevec (50 μM) prior to 1 hour of SEMA3A or SEMA3F (320 ng/ml) treatment and assessed for their ability to migrate through transwells. Data represent the mean ± SD, * p < 0.05 vs. 1% FBS-treated group.
Figure 3.
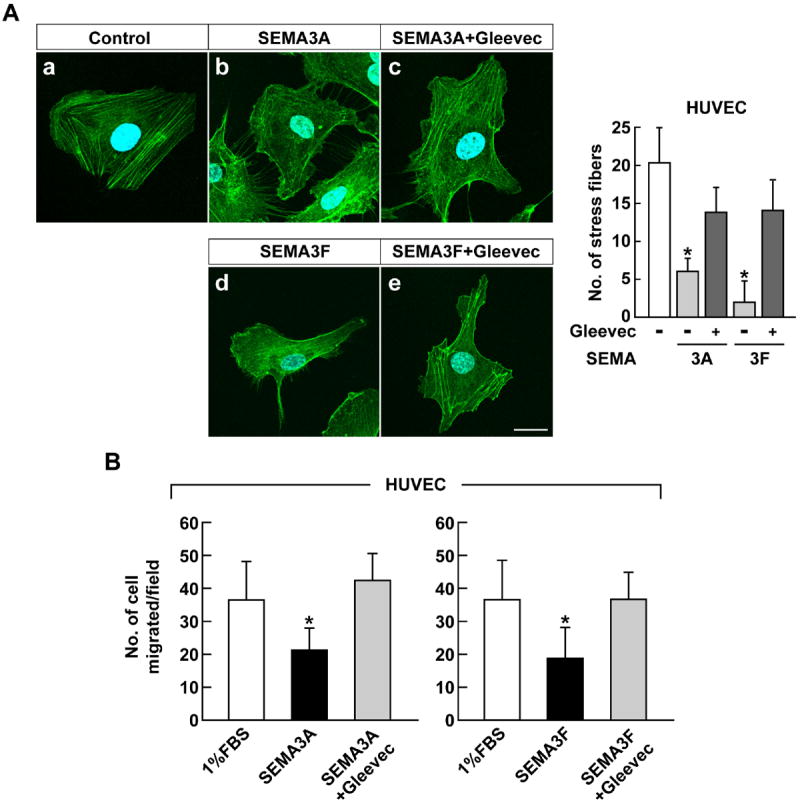
Gleevec abrogates the effect of SEMA3A and SEMA3F in HUVEC. A, HUVEC were pre-treated with 50 μM Gleevec for 1 hour before SEMA3 (320 ng/ml) treatment. After 45 minutes, cells were fixed and stained with Alexa Fluor 488 phalloidin and DAPI and observed by confocal microscopy. The right panel shows the quantification of stress fibers. Data represent the mean ± SD (n = 3), * p < 0.05. The scale bar indicates 20 μm. B, HUVEC were treated with Gleevec (10 μM) prior to 1 hour of SEMA3A or SEMA3F (160 ng/ml) treatment and assessed for their ability to migrate through transwells. Data represent the mean ± SD, * p < 0.05 vs. 1% FBS-treated group.
SEMA3A (Fig. 2B, left) and SEMA3F (Fig. 2B, right) inhibited U87MG cell migration in transwells by 35% and 40%, respectively. The loss of migration by SEMA3A&F was rescued by Gleevec treatment by about 75% and 120%, respectively, compared to control (Fig. 2B). We tested migration and collapse assays with Gleevec obtained from LC Laboratories and Dana-Farber Cancer Institute, and they showed the same anti-SEMA3 effect on tumor and EC (data not shown).
Similar results were obtained with HUVEC, which has abundant stress fibers (Fig. 3Aa). SEMA3A (Fig. 3Ab) and SEMA3F (Fig. 3Ad) treatment reduced stress fiber formation by 80% and 90%, respectively. However, unlike U87MG cells, HUVEC morphology was not as greatly affected. Gleevec restored HUVEC stress fiber formation by 70% (Fig. 3Ac,e). Gleevec also rescued the anti-migration activity of SEMA3A&F by 115% and 100%, respectively (Fig. 3B). Together, these results indicate that Gleevec is a novel semaphorin inhibitor.
Our Gleevec studies are based on rationale design by targeting ABL2 [24], a key step in SEMA3F signaling [9]. Several small SEMA3 inhibitors that block interaction of SEMA3A and NRP1 have been isolated. SM-216289 isolated from a fungal strain inhibited growth cone collapse by SEMA3A, but not by SEMA3F [16,25]. This specificity might be due to SEMA3F binding to NRP2 rather than to NRP1 [21]. SM-216289 promoted axonal regeneration and enhanced functional recovery in rats with spinal cord injury [16]. Screening a peptoid library for small molecules identified SICHI (semaphorin induced chemorepulsion inhibitor), which inhibited the chemorepulsive effect of SEMA3A [26]. SICHI blocked growth cone collapse by SEMA3A but not by SEMA3F or netrin-1. It also promoted the regeneration of damaged axons in mice. In contrast, we show that Gleevec, widely used as a tyrosine kinase inhibitor, blocks both SEMA3A and SEMA3F. These results are consistent with ABL2 inhibiting signaling through both NRP1 and NRP2.
The specificity of Gleevec was tested by comparison to Sutent, a tyrosine kinase that inhibits c-Kit, platelet-derived growth factor receptor (PDGFR) and vascular endothelial growth factor receptor (VEGFR). Unlike Gleevec, Sutent did not rescue the collapsing activity induced by SEMA3A&F in glioblastoma cells (Fig. 4, b vs. c, d vs. e) and HUVEC (Fig. 4, g vs. h, i vs. j).
Figure 4.
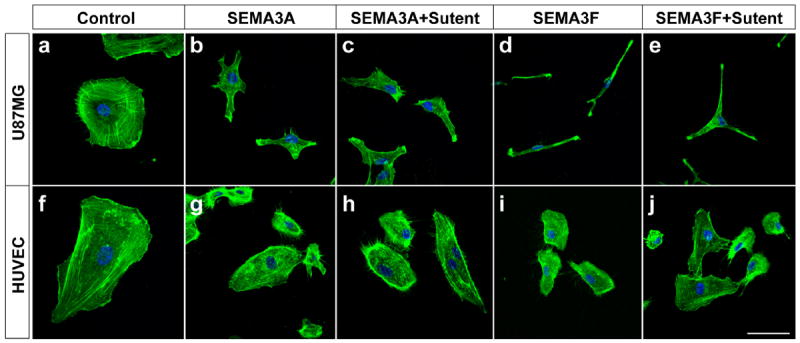
Unlike Gleevec, Sutent does not rescue SEMA3A&F effects on stress fibers in U87MG cells and HUVEC. U87MG cells and HUVEC were pre-treated with Sutent (0.5 μM) 1 hour prior to SEMA3A and SEMA3F (320 ng/ml) treatment. After 45 minutes, cells were fixed and stained with Alexa Fluor 488 phalloidin and DAPI and observed by confocal microscopy. The scale bar indicates 20 μm.
Antagonizing semaphorins might be beneficial; for example, SEMA3A&F are involved in inhibiting axonal regeneration in the injured adult central nervous system [27,28,29,30]. In addition, SEMA3A&F that are secreted from neurons in the avascular hypoxic retina enhance aberrant neovascularization and impair neuroretinal function [14,15]. These results indicate that a semaphorin inhibitor could be therapeutic for vascular regenerative failure.
In summary, Gleevec is an ABL2 tyrosine kinase inhibitor, first shown to be therapeutic for human leukemia. We have found now that Gleevec has a novel function as an antagonist of SEMA3A&F in tumor and endothelial cells, with possible ramifications for neuronal and vascular impairment. Future studies will examine the role of Gleevec in vascular and tumor biology.
Highlights.
SEMA3A&F inactivates RhoA with subsequent loss of stress fibers and cell motility.
Gleevec is an ABL2 kinase inhibitor that antagonizes SEMA3A&F-dependent activities.
Overcoming SEMA3A&F inhibitory activity is a novel Gleevec function.
Gleevec may be a potent inhibitor of SEMA3s’ adverse activity in vivo.
Acknowledgments
We thank Melissa Anderson for preparation of the manuscript, and Dr. Tomoshige Akino and Ryan P. Kelly for migration assays. This research was supported by the National Cancer Institute of the National Institutes of Health R56CA37392 and P01CA45548 (to M.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. H.N. is supported by the Strategic Young Researcher Overseas Visiting Program for Accelerating Brain Circulation (No. S2207 to Dr. Shigeki Higashiyama, Ehime University Proteo-Medicine Research Center) and Postdoctoral Fellowships for Research Abroad from Japan Society for the Promotion of Science, Japan.
Footnotes
Abbreviations: SEMA3A&F, semaphorin 3A and 3F; EC, endothelial cell; HUVEC, human umbilical vein endothelial cell; NRP, neuropilin; PDGFR, platelet-derived growth factor receptor; VEGFR, vascular endothelial growth factor receptor; SICHI, semaphorin induced chemorepulsion inhibitor
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klagsbrun M, Eichmann A. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor Rev. 2005;16:535–548. doi: 10.1016/j.cytogfr.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- 3.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 4.Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol. 2000;10:88–94. doi: 10.1016/s0959-4388(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 5.Roche J, Boldog F, Robinson M, Robinson L, Varella-Garcia M, Swanton M, Waggoner B, Fishel R, Franklin W, Gemmill R, Drabkin H. Distinct 3p21.3 deletions in lung cancer and identification of a new human semaphorin. Oncogene. 1996;12:1289–1297. [PubMed] [Google Scholar]
- 6.Xiang RH, Hensel CH, Garcia DK, Carlson HC, Kok K, Daly MC, Kerbacher K, van den Berg A, Veldhuis P, Buys CH, Naylor SL. Isolation of the human semaphorin III/F gene (SEMA3F) at chromosome 3p21, a region deleted in lung cancer. Genomics. 1996;32:39–48. doi: 10.1006/geno.1996.0074. [DOI] [PubMed] [Google Scholar]
- 7.Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, Klagsbrun M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest. 2004;114:1260–1271. doi: 10.1172/JCI21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coma S, Amin DN, Shimizu A, Lasorella A, Iavarone A, Klagsbrun M. Id2 promotes tumor cell migration and invasion through transcriptional repression of semaphorin 3F. Cancer Res. 2010;70:3823–3832. doi: 10.1158/0008-5472.CAN-09-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu A, Mammoto A, Italiano JE, Jr, Pravda E, Dudley AC, Ingber DE, Klagsbrun M. ABL2/ARG tyrosine kinase mediates SEMA3F-induced RhoA inactivation and cytoskeleton collapse in human glioma cells. J Biol Chem. 2008;283:27230–27238. doi: 10.1074/jbc.M804520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen JL, Rousselot P, Reiffers J, Saglio G, Shepherd J, Simonsson B, Gratwohl A, Goldman JM, Kantarjian H, Taylor K, Verhoef G, Bolton AE, Capdeville R, Druker BJ I. Investigators. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 11.Tuveson DA, Willis NA, Jacks T, Griffin JD, Singer S, Fletcher CD, Fletcher JA, Demetri GD. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20:5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 12.Kerrisk ME, Greer CA, Koleske AJ. Integrin alpha3 Is Required for Late Postnatal Stability of Dendrite Arbors, Dendritic Spines and Synapses, and Mouse Behavior. J Neurosci. 2013;33:6742–6752. doi: 10.1523/JNEUROSCI.0528-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin YC, Yeckel MF, Koleske AJ. Abl2/Arg controls dendritic spine and dendrite arbor stability via distinct cytoskeletal control pathways. J Neurosci. 2013;33:1846–1857. doi: 10.1523/JNEUROSCI.4284-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buehler A, Sitaras N, Favret S, Bucher F, Berger S, Pielen A, Joyal JS, Juan AM, Martin G, Schlunck G, Agostini HT, Klagsbrun M, Smith LE, Sapieha P, Stahl A. Semaphorin 3F forms an anti-angiogenic barrier in outer retina. FEBS Lett. 2013 doi: 10.1016/j.febslet.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joyal JS, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, Shao Z, Polosa A, Zhu T, Hamel D, Djavari M, Kunik D, Honore JC, Picard E, Zabeida A, Varma DR, Hickson G, Mancini J, Klagsbrun M, Costantino S, Beausejour C, Lachapelle P, Smith LE, Chemtob S, Sapieha P. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood. 2011;117:6024–6035. doi: 10.1182/blood-2010-10-311589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, Okano HJ, Ikegami T, Moriya A, Konishi O, Nakayama C, Kumagai K, Kimura T, Sato Y, Goshima Y, Taniguchi M, Ito M, He Z, Toyama Y, Okano H. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- 17.Bielenberg DR, Shimizu A, Klagsbrun M. Semaphorin-induced cytoskeletal collapse and repulsion of endothelial cells. Methods Enzymol. 2008;443:299–314. doi: 10.1016/S0076-6879(08)02015-6. [DOI] [PubMed] [Google Scholar]
- 18.Maione F, Molla F, Meda C, Latini R, Zentilin L, Giacca M, Seano G, Serini G, Bussolino F, Giraudo E. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J Clin Invest. 2009;119:3356–3372. doi: 10.1172/JCI36308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, Risio M, Trusolino L, Weitz J, Schneider M, Mazzone M, Comoglio PM, Tamagnone L. Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Invest. 2010;120:2684–2698. doi: 10.1172/JCI42118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev. 2011;25:1399–1411. doi: 10.1101/gad.2042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, He Z, Bagri A, Tessier-Lavigne M. Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron. 1998;21:1283–1290. doi: 10.1016/s0896-6273(00)80648-0. [DOI] [PubMed] [Google Scholar]
- 22.Hota PK, Buck M. Plexin structures are coming: opportunities for multilevel investigations of semaphorin guidance receptors, their cell signaling mechanisms, and functions. Cell Mol Life Sci. 2012;69:3765–3805. doi: 10.1007/s00018-012-1019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negishi M, Oinuma I, Katoh H. Plexins: axon guidance and signal transduction. Cell Mol Life Sci. 2005;62:1363–1371. doi: 10.1007/s00018-005-5018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moresco EM, Koleske AJ. Regulation of neuronal morphogenesis and synaptic function by Abl family kinases. Curr Opin Neurobiol. 2003;13:535–544. doi: 10.1016/j.conb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi K, Kishino A, Konishi O, Kumagai K, Hosotani N, Saji I, Nakayama C, Kimura T. In vitro and in vivo characterization of a novel semaphorin 3A inhibitor, SM-216289 or xanthofulvin. J Biol Chem. 2003;278:42985–42991. doi: 10.1074/jbc.M302395200. [DOI] [PubMed] [Google Scholar]
- 26.Montolio M, Messeguer J, Masip I, Guijarro P, Gavin R, Antonio Del Rio J, Messeguer A, Soriano E. A semaphorin 3A inhibitor blocks axonal chemorepulsion and enhances axon regeneration. Chem Biol. 2009;16:691–701. doi: 10.1016/j.chembiol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 27.De Winter F, Oudega M, Lankhorst AJ, Hamers FP, Blits B, Ruitenberg MJ, Pasterkamp RJ, Gispen WH, Verhaagen J. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol. 2002;175:61–75. doi: 10.1006/exnr.2002.7884. [DOI] [PubMed] [Google Scholar]
- 28.McCormick AM, Leipzig ND. Neural regenerative strategies incorporating biomolecular axon guidance signals. Ann Biomed Eng. 2012;40:578–597. doi: 10.1007/s10439-011-0505-0. [DOI] [PubMed] [Google Scholar]
- 29.Pasterkamp RJ, Anderson PN, Verhaagen J. Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur J Neurosci. 2001;13:457–471. doi: 10.1046/j.0953-816x.2000.01398.x. [DOI] [PubMed] [Google Scholar]
- 30.Pasterkamp RJ, Giger RJ, Ruitenberg MJ, Holtmaat AJ, De Wit J, De Winter F, Verhaagen J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci. 1999;13:143–166. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]


