Summary
In layer 6 (L6), a principal output layer of the mammalian cerebral cortex, a population of excitatory neurons defined by the NTSR1-Cre mouse line inhibit cortical responses to visual stimuli. Here we show that of the two major types of excitatory neurons existing in L6, the NTSR1-Cre line selectively targets those whose axon innervate both cortex and thalamus and not those whose axons remain within the cortex. These cortico-thalamic neurons mediate widespread inhibition across all cortical layers by recruiting fast-spiking inhibitory neurons whose cell-body resides in deep cortical layers yet whose axons arborize throughout all layers. This study reveals a circuit by which L6 modulates cortical activity and identifies an inhibitory neuron able to regulate the strength of cortical responses throughout cortical depth.
Introduction
Layers are major subdivisions of cortical architecture whose identity is defined in terms of cell density, cellular specificity, afferent and efferent selectivity, molecular characteristics and differences in their responses to sensory stimulation. Cortical layers are strongly interconnected through excitatory axonal projections (Binzegger et al., 2004; Callaway, 1998; Dantzker and Callaway, 2000; Douglas and Martin, 2004; Gilbert and Wiesel, 1979; Lefort et al., 2009; Lorente de No, 1922; Lund et al., 1979; Thomson and Bannister, 2003). Through these projections it is believed that layers influence each other's activity (Gilbert and Wiesel, 1979; Hubel and Wiesel, 1962). Indeed, past and current work, using electrophysiological and pharmacological approaches, cooling methods and more recently the combination of cell-specific Cre mouse lines with optogenetic tools is beginning to unravel the functional impact that distinct layers have on one another (Adesnik and Scanziani, 2010; Beltramo et al., 2013; Constantinople and Bruno, 2013; Ferster and Lindstrom, 1985; Grieve and Sillito, 1991; Malpeli, 1983; Olsen et al., 2012; Schwark et al., 1986). This impact can be facilitatory (Adesnik and Scanziani, 2010; Beltramo et al., 2013; Schwark et al., 1986), suppressive (Olsen et al., 2012) mixed or neutral (Constantinople and Bruno, 2013; Ferster and Lindstrom, 1985), yet still very little is known about the cellular mechanisms that mediate these interactions. Revealing the neuronal circuits orchestrating the interactions between layers is fundamental for our understanding of how these major subdivisions of cortical architecture contribute to information processing.
Layer 6 (L6) of the primary visual cortex (V1) has attracted the attention of many investigators because a large fraction of its pyramidal cells (PCs) project back to the thalamic nucleus from which V1 receives visual information, the dorsolateral geniculate nucleus (dLGN) (Bourassa and Deschenes, 1995; Jones, 2007; Thomson). Indeed, several studies have demonstrated that through this feedback projection, neurons in L6 can modulate the response of dLGN to visual stimuli (for reviews see (Briggs and Usrey, 2008; Guillery and Sherman, 2002; Sillito and Jones, 2002)). L6 neurons however, not only modulate dLGN activity, but have also been shown to affect the response of the cortex to visual stimuli in both cats and rodents (Bolz and Gilbert, 1986; Grieve and Sillito, 1991; Olsen et al., 2012): Pharamacological silencing of L6 facilitates visually evoked responses in more superficial layers (Bolz and Gilbert, 1986) (but see Grieve and Sillito, 1991). Furthermore recent work taking advantage of the NTSR1-Cre mouse line, a line that targets a subpopulation of L6 PCs (L6PCs), demonstrated that optogenetic activation or silencing of this subpopulation, leads to a suppression or facilitation, respectively, of visual evoked activity in more superficial layers (Olsen et al., 2012). Through its suppressive effect on visually evoked activity, L6 has been implicated in controlling gain and modulating size tuning during visual processing (Bolz and Gilbert, 1986; Olsen et al., 2012). Although it was long believed that L6's impact on cortical responses to sensory stimuli was mediated indirectly, via its action on the dLGN, electrophysiological evidence suggests that at least part of the suppressive effect of L6 on visually evoked cortical activity may actually be mediated directly, via intracortical projections (Bolz and Gilbert, 1986; Ferster and Lindstrom, 1985; Olsen et al., 2012). Furthermore, connectivity studies and anatomical data indicate the presence circuit elements that could account for the intracortical suppression mediated by L6: L6PCs strongly innervate cortical inhibitory neurons (West et al., 2006) and inhibitory neurons in L6 have axons that can span several cortical layer (Kisvarday et al., 1987; Kumar and Ohana, 2008; Lund et al., 1988). If L6 indeed directly modulates cortical activity independently of its impact on dLGN, what is the precise nature of the neural circuits through which it exerts its action? Answering this question is a crucial step in understanding the functional impact of a cortical layer based on the underlying cellular-architecture. There are two large categories of L6PCs subdivided based on their axonal projections: intracortical L6PCs (L6ICs) whose axonal projections are restricted to the visual cortex and cortico-thalamic L6PCs (L6CTs) who, in addition to cortical projections, also send an axonal collateral to the thalamus (Zhang and Deschenes, 1997). Which one of these two major classes of L6PCs is targeted by the NTSR1-Cre mouse line and thus contributes to the L6 mediated suppression of cortical activity: L6ICs or L6CTs? Furthermore, because L6PCs are excitatory they cannot exert their cortical suppressive action without recruiting cortical GABAergic inhibitory neurons. What is the nature of the inhibitory neurons recruited by NTSR1-Cre neurons? Where are they located, and what are their morphological and physiological properties?
Here we show that in V1 the subpopulation of neurons targeted by the NTSR1-Cre line are all L6CTs and that the majority of L6CTs are targeted in the NTSR1-Cre line. Thus, L6CTs generate the suppression of cortical activity observed upon activation of neurons targeted by the NTSR1-Cre (Olsen et al., 2012). Although this suppression affects all cortical layers, it is mediated by the recruitment of inhibitory neurons whose cell bodies are located predominantly in L6. Widespread inhibition is achieved through a massive translaminar axonal arborization originating from these L6 inhibitory neurons and spanning throughout even the most superficial layers of the cortex. The identification of a circuit involving a large translaminar inhibitory neuron driven by L6CTs reveals a key mechanism by which L6 contributes to cortical sensory processing.
Results
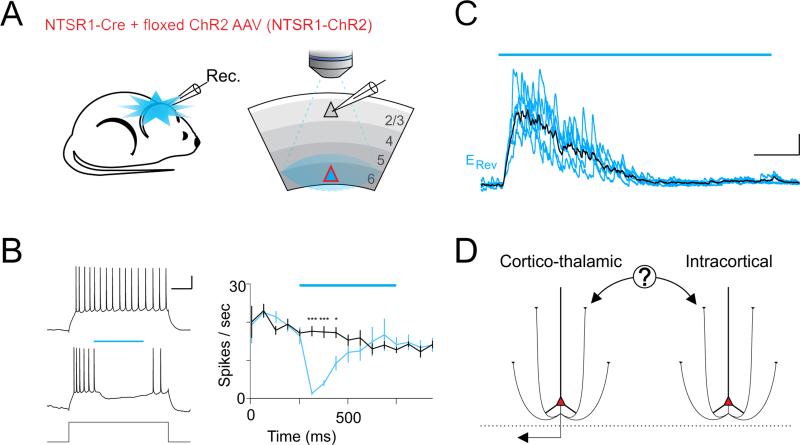
Optogenetic activation of neurons targeted by the NTSR1-Cre mouse line (from here on referred to as NTSR1 neurons) leads to a strong suppression of visually evoked activity in both dLGN and V1 (Olsen et al., 2012). Simultaneous extracellular recordings from these two structures suggest that at least part of the suppression of visually evoked activity in V1 is not indirectly due to the suppression of the dLGN (Olsen et al., 2012). If so, activation of NTSR1 neurons should also be able to suppress V1 activity that, unlike visually evoked activity, does not depend on dLGN input. We directly verified this possibility by performing in vivo whole-cell current clamp recordings from V1 neurons in L2/3 (209±13μm deep, n=10 cells, 6 mice) of anesthetized NTSR1-Cre mice (Gong et al., 2007; Olsen et al., 2012) that conditionally expressed Channelrhodopsin 2 (ChR2) (Boyden et al., 2005; Nagel et al., 2003) (Figure 1A). L2/3 neurons were depolarized with direct current injection above firing threshold (150-500pA for 1s), to trigger an average firing rate of 16.0±1.1Hz. Photostimulation of NTSR1 neurons (500ms) strongly decreased this dLGN independent firing of L2/3 neurons (8 of 10 cells were completely suppressed after 125ms photostimulation; Figure 1B), consistent with the notion that NTSR1 neurons exert a powerful and direct suppression of cortical activity, independently of their impact on dLGN. Furthermore, consistent with a direct intracortical suppression, photostimulation revealed a large inhibitory postsynaptic current (IPSC) in layer L2/3 neurons recorded in vivo and voltage clamped at the reversal potential for synaptic excitation (~ +9mV; IPSC peak amplitude: 251.7±68.2pA; peak conductance: 3.1±.3nS; n=9 cells, 5 mice; recording depth 235.0±16.1μm; Figure 1C). In vitro pharmacology confirmed this inhibition was disynaptic in all layers and therefore not the result of direct photostimulation of inhibitory neurons (Figure S1). These results thus definitively validate the notion that NTSR1 neurons in L6 suppress cortical activity in vivo independently of their impact on the dLGN. These results however also open a fundamental question: What is the nature of the intracortical circuit through which NTSR1 neurons exerts its suppressive action? This question is addressed below, first by establishing the type of L6PC whose activity leads to the observed cortical suppression and subsequently by revealing the cellular source of the observed cortical inhibition.
Figure 1. Photostimulation of NTSR1-Cre neurons suppresses thalamus independent cortical activity in vivo.
(A) Illustration of in vivo recording configuration from V1 in an adult NTSR1-Cre mouse conditionally expressing ChR2. The ChR2-expressing layer 6 pyramidal cell (L6PC; red triangle) was photoactivated while recording from a L2/3 neuron (grey triangle).
(B) Left traces: Response of a L2/3 neuron recorded in vivo in the whole-cell current clamp configuration (scale bar 200pA 20mV/250ms) to current injection (150pA; top) and to current injection with photoactivation of L6PCs (blue bar 0.5s). Right: The average firing rate is plotted against time (black: control; blue: with photostimulation; asterisks indicate significant difference; p=0.0002, 0.0002, 0.0074; n=10 cells, 6 mice; blue bar: duration of photostimulation).
(C) IPSC recorded in vivo in a L2/3 neuron voltage clamped at +7mV (scale bar 200pA/250ms) in response to photoactivation of L6PCs (blue bar 1.5s). 5 superimposed sweeps (blue). Average trace in black. See also Figure S1.
(D) Inhibition could be mediated by the activation of either cortico-thalamic or intracortical L6PCs.
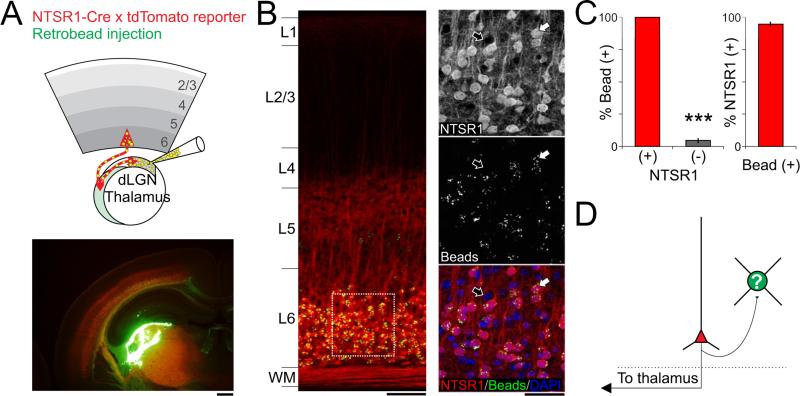
Which category of L6PC is responsible for the L6 mediated suppression of cortical activity: L6ICs or L6CTs (Zhang and Deschenes, 1997)? To answer this question we determined which of these two L6PC categories are labeled by the NTSR1-Cre line used to drive the intracortical suppression. The strong axonal labeling in thalamic nuclei (including the dLGN, the nucleus reticularis thalami (nRT), and the and the mediorostral part of the lateral posterior thalamic nuclei (LPMR)) observed in sections from NTSR1-Cre brains conditionally expressing the tdTomato reporter (Olsen et al., 2012) suggests that at least some L6CTs are labeled by this line. To directly verify this possibility and, more importantly, to determine whether L6ICs are also labeled in the NTSR1-Cre line we stereotactically injected fluorescent microspheres (RetroBeads, Lumafluor) in the dLGN and analyzed the distribution of RetroBeads in coronal sections of primary visual cortex 7-9 days following the injection (Figure 2). RetroBeads are taken up by axon terminals and retrogradely transported to the cell-body. Thus, the presence of RetroBeads in the cell-body of a L6PC identifies this cell as L6CT. We expected an underestimate of the actual percentage of cortico-thalamic neurons labeled by the line due to the unlikelihood that all axons that project to the dLGN pick up the beads. As such, we were surprised to find that all tdTomato expressing L6PCs contained beads (100±0%, n=154 cells, 4 mice, Figure 2B,C). Furthermore, very few non-tdTomato expressing L6PCs contained beads (4.7±1.8%, n=197 cells, 4 mice, Figure 2B,C). This finding not only validates the specificity of our bead labeling method, but also indicates the NTSR1-Cre line is highly specific for driving expression in the L6CTs. Because Cre-expressing cells in the NTSR1-Cre line represent about 65% of the entire excitatory cell population in L6 (Olsen et al., 2012), these results also indicate that approximately 65% of L6 excitatory neurons in mouse V1 are L6CTs. Thus, these data demonstrate that of the two major categories of L6PCs in visual cortex, L6CTs are responsible for the intracortical suppression of cortical activity observed with the NTSR1-Cre line.
Figure 2. The NTSR1-Cre line selectively targets layer 6 cortico-thalamic pyramidal cells.
(A) Top left: Schematic of thalamic injection of fluorescent microspheres into adult NTSR1-Cre X tdTomato reporter mouse in vivo. Green fluorescent microspheres are retrogradely transported to the soma of cortico-thalamic neurons. Bottom left: Confocal image illustrating the thalamic injection site on a coronal section of the brain (Red: tdTomato; Green: fluorescent microspheres; Yellow: superimposition of Red and Green; scale bar 500μm). (B) Left: confocal image illustrating a coronal section through V1. Note the accumulation of microspheres in L6 (Yellow fluorescence; scale bar 100μm). Right: Magnification of area delineated by white square to left. Top right: Red channel: confocal image of L6PCs expressing tdTomato (NTSR1+; scale bar 50μm; the white and black arrows indicate cell bodies that express or do not express tdTomato, respectively). Right middle: microspheres. Right bottom: overlay of red tdTomato expression, green microspheres and blue nuclear counter stain (DAPI). Note that while all tdTomato expressing cell bodies contained microspheres, most cell bodies lacking tdTomato expression do not contain beads.
(C) Summary histogram: Left: 154 of 154 tdTomato expressing cells (red column; 4 mice) contained microspheres while only 9 of 197 non-expressing cells contained microspheres (4 mice; p<0.0001). Right: 154 of 163 cells that contained microspheres expressed tdTomato (4 mice).
(D) What inhibitory interneurons are being recruited by L6CTs to suppress the visual cortex?
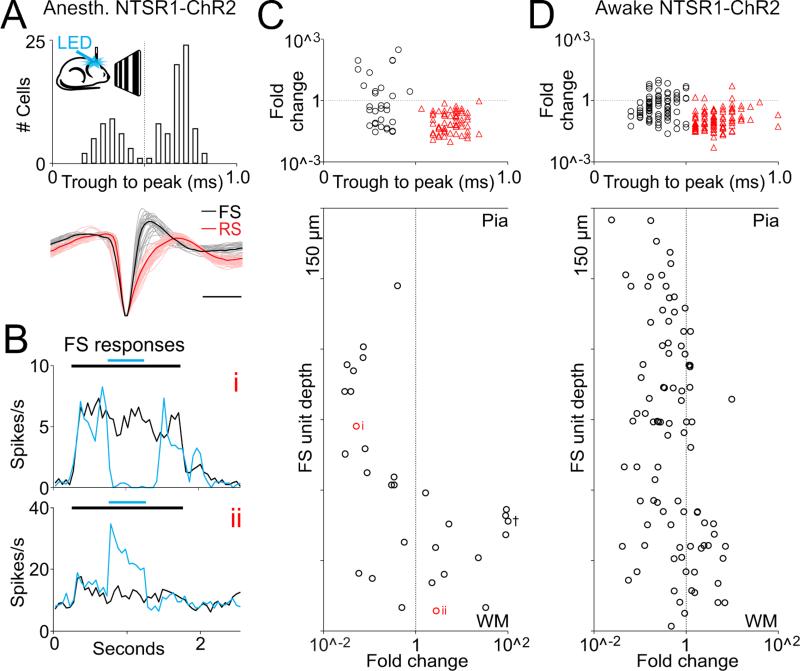
Through what inhibitory circuits do L6CTs operate to suppress cortical activity (Figure 2D)? We used linear probes to perform in vivo extracellular recordings throughout the depth of V1 in anesthetized NTSR1-Cre mice conditionally expressing ChR2 and photo-stimulated L6CTs. While the photostimulation suppressed the activity of most cortical neurons throughout layers, it also increased the firing of a small fraction of neurons (11.7%, n=12/102 cells, 9 mice Figure 3A-C; part of this dataset (n= 90) was collected during a previous study (Olsen et al., 2012)). Interestingly, these neurons invariably showed fast-spiking (FS) properties, that is, their extracellularly recorded action potential had a fast time-course with a trough to peak time less than 0.5 ms (0.31±0.03ms, n=12, as compared with regular spiking neurons 0.69 ±0.01ms, n=68). Such fast spikes represent the electrophysiological signature of a large category of GABAergic cortical inhibitory neurons that includes basket and chandelier cells. Importantly, not all recorded FS cells showed an increase in firing rate upon L6CT photostimulation (37.5%, n=32, Figure 3B,C). Where are the FS cells that increase their firing rate in response to L6CT photostimulation located? We determined the distribution of all extracellularly recorded FS cells across cortical depths. FS cells were distributed throughout the cortical radial axis yet, strikingly, those FS cells whose firing was increased upon photostimulation of L6CTs were selectively located in the deeper layers of the cortex. Specifically, from the pial surface to 600μm deep 0% (0 out of 14) of FS cells were recruited by L6CT activation, while below 600μm 66.7% of FS units were recruited (12 of 18). Similar results were obtained when photostimulating L6CT in non-anesthetized animals (Figure 3D). A large fraction of FS cells below 600μm was facilitated (45.9%; 17 of 37 cells, 4 mice) while only 16.4% of all FS cells above 600μm (9 of 55 cells) increased their firing rate upon photostimulation of L6CT in non-anesthetized animals. Furthermore, the small fraction of FS cells above 600μm whose firing rate was facilitated increased their firing rate significantly less than deeper FS cells (2.1±0.9 and 3.4±0.5 fold change, respectively; p=0.0031). Consistent with these results, suppressing L6CTs activity using the conditional expression of Arch/Halo in the NTSR1-Cre line significantly reduced the firing rate of most FS cells located below 600μm (88.9%, p=0.0392, n=8 cells, 5 mice; average decrease 34.0±11.7%). Together these data indicate a preferential recruitment of FS cells in the deeper layers upon activation of L6CTs.
Figure 3. Selective recruitment of deep layer fast spiking cells by layer 6 cortico-thalamic pyramidal cells in vivo.
(A) Schematic illustrates in vivo extracellular recording from V1 in NTSR1-ChR2 anesthetized mouse during visual stimulation and photo-activation of L6 cortico-thalamic pyramidal cells (L6CTs). Histogram shows separation of fast spiking (FS) from regular spiking (RS) units based on trough to peak latency (102 units, 9 mice). Dotted line indicates the chosen divider for defining a unit as FS or RS. Bottom: 30 FS units (grey) and 30 RS units (pink) shown on bottom with representative example shown in bold (scale bar 0.5ms).
(B) Peristimulus time histogram of the response of two example FS units to visual stimulation (black bar, 1.5s) with (blue) and without (black) photo-activation of L6CTs (blue bar, 0.5s). Note that while the top FS unit (i) is suppressed the lower one (ii) is facilitated by photo-activation of L6CTs.
(C) Fold change in firing rate (LED on/LED off, log scale; black circles denotes FS units, red triangles RS units) in response to photoactivation of L6CTs during visual stimulation shown for all units in A. Note that the only units whose firing rate increases during photo-activation of L6CTs are FS units. Recording depth of individual FS units are shown in bottom panel against the log scale of their fold change. Example units from B are labeled next to their corresponding depths (red circles). Cross indicates outlier moved from 296 to 100 fold change.
(D) In vivo unit recordings from awake mouse (n=146 RS units 92 FS units; 4 mice) presented as in C.
Is the recruitment of these deep FS cells responsible for the suppression of cortical activity throughout all cortical layers or is L6 photostimulation also recruiting additional cortical inhibitory neurons that are not detected by our recording electrodes? If our extracellular recordings do not provide an unbiased sample of the different types of inhibitory neurons present throughout cortical depth, recruited inhibitory neurons located in more superficial layers could have been missed.
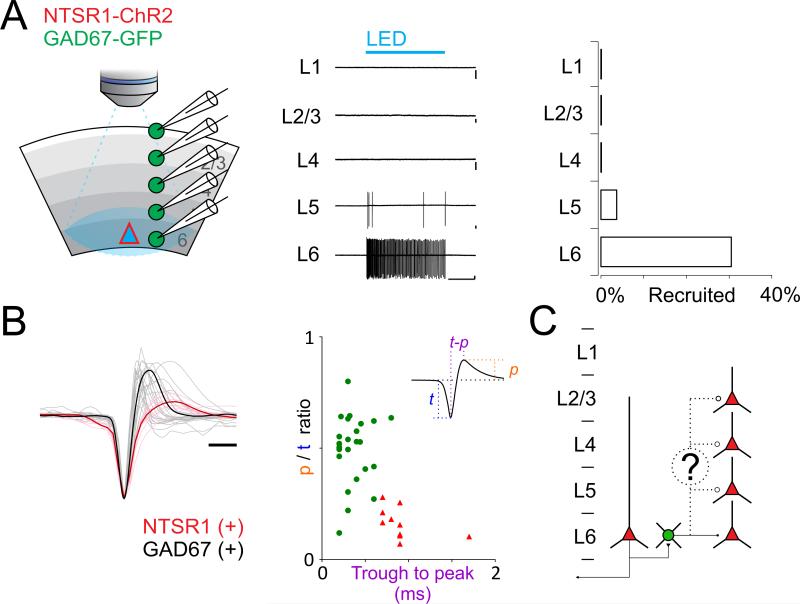
To directly assess the distribution and type of cortical GABAergic neurons recruited by L6CTs, we performed recordings from visual cortex in vitro. Consistent with the in vivo data reported above full field photostimulation (1.5s) of acute visual cortical slices from the NTSR1-Cre line conditionally expressing ChR2 generated large IPSCs in PCs cells throughout all layers (Figure S1). Therefore, as in vivo, in vitro photostimulation of L6CTs also generates widespread cortical inhibition. To identify the GABAergic neurons recruited by the activation of L6CTs we crossed the NTSR1-Cre line with the GAD67-GFP line, a mouse line that expresses GFP in all GABAergic neurons (Tamamaki et al., 2003), and performed targeted loose patch recordings from GFP expressing cells (Figure 4A). Loose patch recordings allow one to record the spiking activity of a neuron without perturbing its physiological cytosolic composition. Strikingly, while no GFP expressing neuron in layers 1-4 (0 out of n=128 cells, 7 mice) and only 3.7% (2 out of 54) in L5 fired an action potential in response to photoactivation of L6CTs almost a third (30.6% 22 out of 72, Figure 4A) of L6 GFP expressing neurons responded to the stimulus. Furthermore, the GFP-expressing neurons recruited by L6CTs had action potentials with FS waveforms (Figure 4B). Thus, the specific firing of FS cells in deeper layers in response to the activation of L6CTs is not due to a unit isolation bias of our in vivo recording configuration, but represents a genuine selectivity in the recruitment of cortical inhibitory neurons by L6CTs. The preferential recruitment of inhibitory neuron in deep cortical layers was not due to the specific photostimulation protocol used here (a ramp of LED intensity see methods). Photo-stimulating layer 6 with brief pulses of light (2 ms duration; see methods), also preferentially recruited inhibitory neurons in deeper layers (L1: 0%, n=42; L2/3: 0%, n=45; L4: 2%, n=41; L5: 30%, n=54; L6: 42%, n=72; 7 mice; Figure S2). These data demonstrate that despite the widespread inhibition generated across cortical layers by activation of L6, the source of this inhibition appears to be mediated by GABAergic neurons whose somatic location is restricted to the deep cortical layers (Figure 4C).
Figure 4. Selective recruitment of layer 6 fast spiking cells by layer 6 cortico thalamic pyramidal cells in vitro.
(A) Left: Schematic illustration of loose patch recordings from GFP expressing neurons in V1 slice from a NTSR1-ChR2 x GAD67-GFP mouse. A single loose-patch recording was made on a GFP-positive inhibitory neuron in either L2/3, L4, L5 or L6 while photoactivating L6CTs. Center: Example recordings from GFP expressing neurons in each layer of one example slice during photo-activation of L6CTs (blue bar, 1.5s). Note that only neurons in deeper layers fire in response to photo-activation (Scale bars 50pA/500ms). Right: Summary histogram showing percentage of GFP expressing neurons recruited by photo-activation of L6CTs (L1 n=42, L2/3 n=45, L4 n=41, L5 n=54, L6 n=72; 7 mice). See also Figures S2 and S3.
(B) Left: Waveforms of action potentials (average of first 5 spikes; recorded in loose patch) of all responding GFP expressing neurons (GAD67(+); gray) and of directly photo-activated L6CTs (NTSR1(+); red, for comparison; scale bar 0.5ms). Bold lines are averages. Middle: Peak-to-trough height ratio is plotted against trough-to-peak latency for GFP+ cells (green circles) and NTSR1+ cells (red triangles).
(C) Do L6 FS cells extend their axons throughout layers to inhibit also superficial neurons?
By what mechanism do L6 cortico-thalamic neurons selectively recruit deep FS cells? We can hypothesize two extreme scenarios: in the first, L6CTs exclusively form synaptic contacts with FS cells located in deep layers; in the second scenario, L6CTs indiscriminately contact GABAegic cells throughout cortical layers but only deep FS cells receive sufficiently strong synaptic input to be depolarized above action potential threshold. We tested these two scenarios by systematically performing whole-cell voltage clamp recordings from GAD67-GFP expressing neurons throughout cortical layers while photostimulating L6CTs in visual cortical slices. Consistent with the second scenario, all recorded GAD67-GFP expressing neurons received direct excitation from L6CTs (n=19 cells, 10 mice) indicating that they do not only contact deep layer FS cells (Figure S3A). We thus tested whether recruited FS cells receive larger synaptic excitation from L6CTs as compared to the other contacted inhibitory neurons. We first identified GAD-EGFP expressing neurons that fired in response to photostimulation of L6CTs. Consistent with above results, these cells were invariably FS cells located in L6. We then compared the excitatory postsynaptic currents (EPSCs) evoked in these neurons with the EPSCs recorded in non spiking GAD-EGFP expressing neurons located either in L6 or in L2/3 of the same slice. Almost all GAD-EGFP expressing neurons that fired in response to photostimulation of L6CTs received significantly larger EPSCs as compared to neighboring L6 or more distal L2/3 GABAergic neurons that did not fire (248.6±50.8pC L6 spiking vs. 19.2±5.7pC non-spiking L2/3, n=7 cells 5 mice, p = 0.0029, Figure S3B left graph; and 317.1±54.6pC L6 spiking vs. 84.8±16.9pC non-spiking L6, n=6 cells 3 mice, p = 0.0155, Figure S3C left graph). Furthermore, among recruited L6 FS cells, there was a significant correlation between the amount of excitatory charge received and the firing rate (p=0.0008, n=19 cells, 8 mice, Figure S3D right). As a consequence of the larger excitation received by recruited L6 FS cells also the ratio between excitation and inhibition was larger in these neurons (35.0±6.0% L6 spiking vs. 15.1±3.1% non-spiking L2/3, n=7, p=0.0333, Figure S3B right; and 42.1±8.1% L6 spiking vs. 21.7±3.2% non-spiking L6, n=6, p=0.0396, Figure S3C right). Thus, despite the fact that L6CTs contact inhibitory neurons throughout cortical layers, deep FS cells receive stronger excitation, a likely mechanism for their selective recruitment.
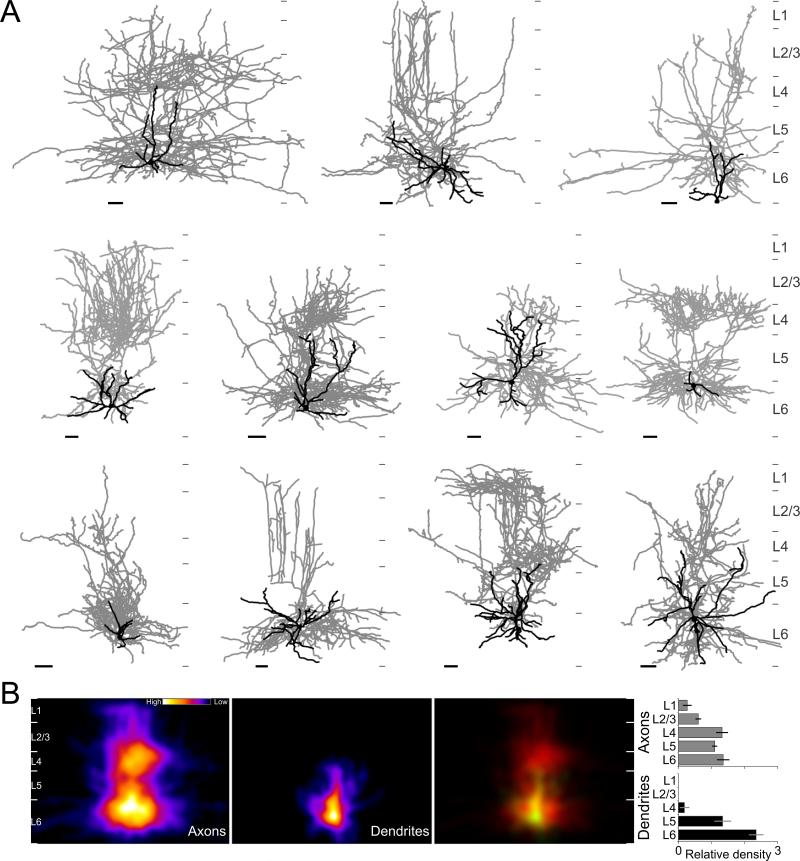
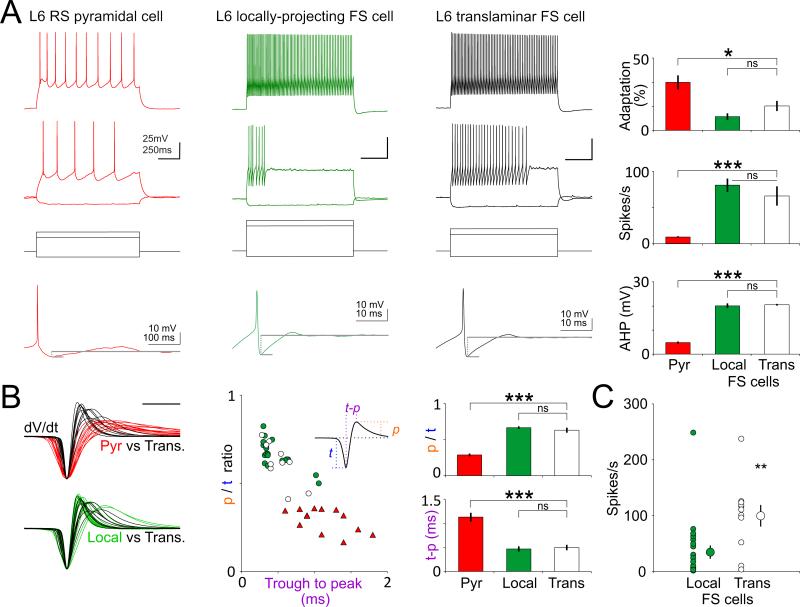
By what mechanism could deep FS cells generate inhibition throughout cortical layers? One possibility is that at least some of the cells recruited by cortico-thalamic L6PCs send an axonal projection that spans the entire cortical depth (Figure 4C). To test this hypothesis, we first identified GABAergic neurons that fired action potentials in response to L6CTs photostimulation, using loose patch recordings of GFP expressing inhibitory neurons (see above and methods). We then obtained whole-cell current clamp recordings from these neurons to determine their intrinsic membrane properties and to dialyze the neurons with an intracellular solution containing biocytin for subsequent morphological reconstruction. Initial anatomical reconstructions indicated the presence of at least two types of inhibitory neurons that were recruited by the activation of L6CTs: those whose axon arborized locally and remained confined within deep layers (Figure S4) and those that, consistent with our hypothesis, had axons that arborized throughout the entire cortical depth (Figure 5). We reconstructed the axonal arborization of eleven large translaminar neurons (9 mice), whose axonal arborization reached across all cortical layers containing excitatory neurons, from L6 to L2/3 (9 of 11 spanned from L6 to L1; Figure 5A). These 11 translaminar neurons were the result of filling 58 GFP-expressing interneurons or 19.0%. The average density of the axonal arborization peaked in L6 and L4 yet, the exact distribution varied between neurons (Figure 5B). In contrast to the axonal arborization the dendrite of these neurons were largely restricted to the deep cortical layers. Importantly, these dendrites lacked spines, consistent with the aspiny nature of cortical interneurons (Figure S5). Furthermore, consistent with in vivo and in vitro extracellular recordings, these large translaminar neurons showed properties typical of FS cells (Figure 6): high firing rates (66.0±13.0Hz; n=11), little adaptation in response to current injections (16.9±3.5%), pronounced after-hyperpolarization after every action potential (20.5±0.7mV, n=10) and narrow action potentials (0.49±0.06ms, n=11) with high peak to trough ratios (0.63±0.04). While these characteristic FS properties were not significantly different between the two FS cell type recruited by L6CTs, i.e between the translaminar and the local FS cells (Figure 6A,B), translaminar neurons reached significantly higher firing rates in response to L6CT photo-stimulation (99.7±19.4Hz translaminar interneurons vs 37.2±11.7Hz locally projecting, p=0.0037).
Figure 5. Translaminar axonal projections from fast spiking cells recruited by layer 6 cortico-thalamic pyramidal cells.
(A) Morphological reconstructions of 11 FS cells with translaminar axonal arborization that were recruited above threshold for spike generation upon photostimulation of L6CTs in vitro. Nine of the eleven translaminar FS cells were recorded in the GAD67-GFP line and expressed GFP. The remaining two FS cells (top row, 1st cell; Bottom row, 2nd cell) were recorded in the G42 line and also expressed GFP. Dendrites and somas are shown in black with axons in grey (scale bars 50μm, medial is to the right). Thin grey tics to right of each cell indicate layer boundaries.
(B) Average heat map of axons (left) and dendrites (middle) of the eleven reconstructed translaminar FS cells after normalizing for differences in layer depths. Right panel shows overlay of axons (red) and dendrites (colored green; yellow where overlapping with axons). Left: shows the relative density of neurite length for each layer for dendrites (black) and axons (grey) of all 11 cells. The relative density is the fraction of total neurite length divided by the fractional layer thickness; the fractional layer thickness is computed as the thickness of a layer divided by the cortical thickness, measured along the radial axis from the pia to the layer 6 white matter border.
Figure 6. Electrophysiological properties of layer 6 translaminar FS cells.
(A) Responses to steps of current injection are shown for L6CTs, locally-projecting L6 FS cells and translaminar FS cells. Translaminar FS cells (n=11) did not significantly differ from locally-projecting FS cells (n=16) with respect to firing rate adaptation, firing rate or afterhyperpolarization following an action potential (bottom traces), although they did significantly differ from regular spiking pyramidal cells (n=10) in all these characteristics (p=0.0124, p<0.0001 and p=0.0002, respectively; see methods for analysis parameters).
(B) Left: dV/dt of action potentials recorded in current clamp in translaminar FS cells (black traces; n = 11), L6CTs (red races; n = 13) and locally projecting FS cells (green traces; n = 16). Traces from translaminar FS cells are superimposed with those of L6CTs (top) and from locally projecting FS cells (bottom) for comparison. Center: The peak to trough ratio (p/t ratio) is plotted against the trough to peak latency of the dV/dt waveform (inset illustrates parameters measured). Right: averages and statistical comparison to right. No statistically significant difference was noted between locally-projecting and translaminar FS cells. Translaminar FS cells did significantly differ from L6CTs in the peak-to-trough ratio (p<0.0001), and in the trough to peak latency (p=0.0002).
(C) Firing rate of FS cells in response to L6CT photo-activation: Translaminar FS cells fired at significantly higher rates than locally projecting FS cells (p=0.0037).
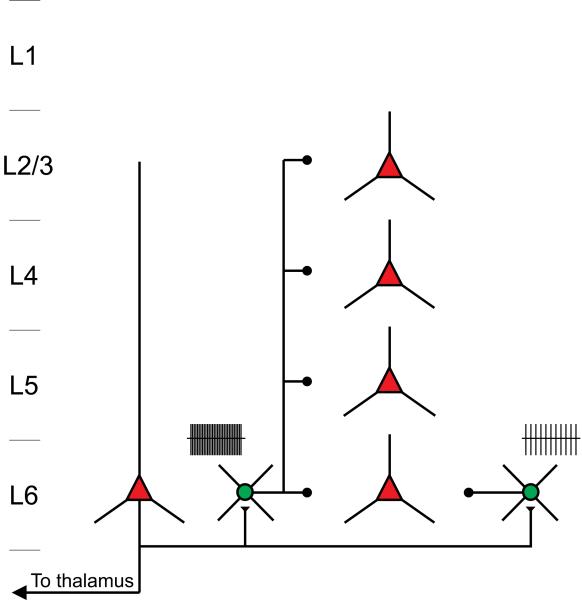
Taken together, these results indicate that the activity of L6CTs can generate cortex-wide inhibition by recruiting FS cells whose soma is located in L6, yet whose translaminar axon spans all cortical layers (Figure 7).
Figure 7. Model.
L6CTs suppress responses in the visual cortex by recruiting FS cells located in L6 some of which extend large translaminar axon throughout all cortical layers.
Discussion
Whether and how one of the major cortical outputs, the cortico-thalamic L6 pyramidal cells, directly impact cortical function has been a long-standing question. While previous work of this and other labs provided evidence that L6PCs may directly suppress cortical activity (Bolz and Gilbert, 1986; Olsen et al., 2012), it was not clear whether this effect was due to L6ICs or L6CTs and how these PCs may mediate the suppression. By discovering that the NTSR1-Cre line selectively targets L6CTs, we have established that this cell type directly affects cortical excitability, independent of its thalamic projection. Furthermore, by identifying a large translaminar L6 FS cell whose axons span the entire cortical depth we have revealed the mechanisms through which L6CTs exert their suppressive action on V1.
Through the present and previous work we can now begin to understand the impact that L6CTs exert on their two main targets, the cortex and the thalamus: Through their cortical projections, L6CTs provide excitation to most excitatory and inhibitory neurons across cortical layers, however, due to their particularly strong excitation of translaminar FS cells, the resulting disynaptic inhibition swamps the modest direct excitation mediated by L6CTs. These results are consistent with the report that L6CTs preferentially innervate cortical inhibitory neurons (West et al., 2006). Via their feedback projections to the thalamus, L6CTs target the nRT (the main inhibitory nucleus in the thalamus) the dLGN and the LPMR. Because nRT neurons strongly inhibit dLGN neurons and because L6CT axons also innervate local inhibitory neurons in the dLGN, the overall impact of L6CTs onto dLGN relay neurons is, similar to cortex, suppressive (Olsen et al., 2012). The overall suppressive action of L6CTs on V1 and dLGN, however, should not be understood as a homogeneous suppression of cortical and thalamic neurons. L6CTs provide direct excitation to PCs throughout all cortical layers and to dLGN relay neurons. If this excitation differs in its spatial distribution from that of the disynaptic inhibition generated by the recruitment of L6 FS cells, nRT cells and local inhibitory neurons in the dLGN, neurons receiving direct excitation could be suppressed less than those receiving only disynaptic inhibition. In other words, the amount of suppression exerted by L6CTs in V1 and dLGN, may have a spatial profile, reflecting the ratio of direct excitation and disynaptic inhibition received by each neuron (Murphy and Sillito, 1987). Furthermore, because of the disynaptic nature of inhibition, the onset of L6CT activity may transiently excite the target neurons before the onset of inhibition.
The L6 translaminar FS cell described here has no dendritic spines and expresses GFP in the GAD67-GFP mouse-line, consistent with its hypothesized GABAergic nature. Furthermore, consistent with the strong correlation existing between FS cells and the expression of the protein parvalbumin (PV), we reconstructed 2 of the large translaminar FS cells from mice where the NTSR-1 Cre line was crossed with the G42 line, a mouse line that selectively labels PV-expressing, somatostatin-negative cells (Chattopadhyaya et al., 2004). The Martinotti cell is an anatomically, physiologically and molecularly well described cortical inhibitory neuron whose axonal projection crosses layers to reach the most superficial ones (Markram et al., 2004; Wang et al., 2004). However, several properties of the translaminar FS cell described here distinguish it from Martinotti cells: The fast and non-adapting spike pattern, the complete lack of dendritic spines and the GFP labeling in the G42 line, a line that excludes interneurons expressing somatostatin (Chattopadhyaya et al., 2004), a molecular marker of Martinotti cells (Kawaguchi and Kubota, 1996; Wang et al., 2004).
Approximately 20% of the FS cells recruited upon L6CT photostimulation and successfully reconstructed had a translaminar axonal arborization (Figure 5) while the rest had an axon that remained confined within the deep layers (Figure S4). While this percentage may reflect an actual prevalence of translaminar interneurons recruited by L6CTs, the proportion may be biased by our experimental protocols. A translaminar axon would be more likely to be cut than local axons. Additionally, the visually guided targeting of neurons may generate further biases possibly leading to an enrichment or a reduction in our sampling of this population, for example if the GFP expression is stronger or weaker, respectively, in translaminar as compared to other inhibitory neurons (Suzuki and Bekkers, 2010).
We attribute most of L6CT mediated intracortical suppression of visually evoked activity in the superficial layers of V1 (Bolz and Gilbert, 1986; Olsen et al., 2012) to the recruitment of FS cells with translaminar axons. Additional mechanisms, however, cannot be ruled out: For example if activity in L6IC facilitates the firing of PCs in superficial layers, the inhibition of L6ICs by FS cells whose axons are confined in the deep layers may contribute to the observed suppression.
The existence of non-Martinotti cortical inhibitory cells with translaminar axonal arborization is not novel in and of itself. In both supra and infragranular layers inhibitory neurons have been reported whose axonal arbors span several layers (Helmstaedter et al., 2009; Jiang et al., 2013; Kisvarday et al., 1987; Lorente de No, 1922; Lund, 1988; Somogyi and Cowey, 1981; Somogyi et al., 1981; Thomson and Bannister, 2003; Thomson et al., 2002). In particular, studies in rodents (Kumar and Ohana, 2008), carnivores (Kisvarday et al., 1987) and primates (Lund et al., 1988) have identified inhibitory neurons whose cell bodies are located in layer 6 and whose axons span several layers yet have morphological and physiological properties that differentiate them from Martinotti cells. Furthermore, a recent study (Buchanan et al., 2012) described an FS, PV expressing L5 inhibitory neuron type that receives excitation from L5 PCs and whose ascending translaminar axonal arborization reaches L2/3. While the location of this L5 FS cell and the lack of axonal arborization in L1 distinguishes it from the one we describe here, the translaminar L5 and L6 FS cells may represent a “family” of PV expressing neurons with the ability of suppressing cortical activity across layers. Whether, like a Martinotti cell, translaminar FS cells also inhibit the dendritic compartment of PCs or whether, more like a PV expressing FS basket cell, they inhibit the somatic and perisomatic compartments of PCs across cortical layers will be answered by additional anatomical work. The possibility of perisomatic inhibition mediated by translaminar axonal projections is well documented by work in cat visual cortex, where basket cells whose cell body is located in deep layers contact, through an ascending axonal arborization, the perisomatic compartment of PCs in more superficial layers (Kisvarday et al., 1987). Identifying molecular markers or combinations thereof that selectively label the L6 translaminar FS cells will be of great help for future anatomical and functional studies.
The results reported here also open new questions with regard to the function of L6: Do L6ICs differently impact cortical activity as compared to L6CTs? Do they also recruit the translaminar FS cells or do they exert their action through entirely distinct circuits? Furthermore, L6CTs labeled by the NTSR1-Cre line belong to two categories based on their dendritic arborization: Those that send their dendrites to the most superficial layers and those whose apical dendrites end in L4 (Olsen et al., 2012) consistent with previous subivisions of L6 pyramidal cells into tall and short (Briggs and Usrey, 2008). Do both of these L6CTs types recruit the translaminar FS cells? Either systematic electrophysiological paired recordings followed by morphological identification or the advent of new Cre-lines selectively labeling L6ICs, or one of the two sub-types of L6CTs will provide the answer.
We have described an intracortical circuit by which L6CTs suppress V1. It would be of great interest to know if other cortical layers, regions or even other brain areas that send their projections to L6 also utilize this circuit to regulate the activity of V1.
Experimental Procedures
All experiments were carried out in accordance with the animal care and handling guidelines set forth by the University of California.
Mouse lines
The following mouse lines were used: NTSR1-Cre (strain B6.FVB(Cg)-Tg(Ntsr1-cre)GN220Gsat/Mmcd, stock number 030648-UCD), which was generated by the GENSAT project (Gong et al., 2007) and acquired from the Mutant Mouse Regional Resource Centers; tdTomato reporter (Hongkui Zeng, Jax number 007908); GAD67-GFP (Δneo, Takeshi Kaneko); G42 GAD67-GFP (Z. Josh Huang, Jax number 007677).
In vivo anesthetized extracellular recordings
In vivo multichannel silicon probe recordings were conducted as previously described (Olsen et al., 2012). Briefly, mice were anaesthetized with 5mg/kg chlorprothixene, and 1.2g/kg urethane. 0.5-1.0% isoflurane was used during surgery. A head-plate was mounted over V1 and the skull was thinned using a dental drill. PBS was then applied to the thinned skull and sharpened forceps were then used to make a hole large enough to allow the insertion of the NeuroNexus 16-channel linear probe (A1×16-3mm-50-177). The probe was inserted at a depth of 800-1000μm, which was estimated based on the depth and angle of the probe insertion. PBS was then applied to keep the craniotomy moist. After more than 20 minutes of probe insertion, visual stimuli were presented using a gamma corrected, Dell 52 ×32.5cm LCD monitor (60-Hz refresh rate, mean luminance 50cd/m2, 25cm from contralateral eye). Full field sinusoidal drifting gratings (2Hz, 0.04 cycles per degree, 100% contrast) were generated using Psychophysics ToolBox (Brainard, 1997). The visual stimulus was 1.5s with a 3-6s inter-trial interval during which a grey screen was presented. Visual stimulus trials were interleaved with trials presenting both visual stimulus and a 0.5s 20mW LED pulse (470nm, 1mm diameter, Doric Lenses). Black foil (Thor Labs) was used to prevent the LED from reaching the eyes. Recordings were amplified ×1000 and band-pass filtered between 0.3Hz and 5kHz using an AM systems 3500. Acquisition was done at 32kHz with a NIDAQ PCIe-6239 board using custom Matlab software (Mathworks).
In vivo awake extracellular recordings
Five to seven days before recording, head plates were implanted over V1. Isoflurane (2.5%) was used during the implantation procedure. The skin was removed and the head-plate was fixed in place with black dental cement. Kwik-Cast (WPI) was then used to cover the skull. Animals were injected with 0.1 mg/kg buprenorphine subcutaneously and monitored daily.
Prior to recording, mice were familiarized to head fixation for three 10-min daily sessions. During these training session the head-plate was clamped to a metal post allowing mice to run on a plastic circular treadmill (Fast-Trac from Bio-Serv).
For recordings, mice were anaesthetized using 1.5–2% isoflurane. A small craniotomy was then made over V1, a drop of PBS was placed in the well of a head-plate that was clamped to a metal post, and a NeuroNexus 32-channel linear probe (A1×32-Edge-5mm-20-177) was inserted into the craniotomy. A higher channel probe was used in the awake mouse because of the desire to use as few mice as possible for these experiments and technological improvements. Mice were given at least 30 min to recover from anesthesia before recordings began. Recording sessions were between 1 and 2 hours long. Acquisition was done at 20kHz to accommodate higher channel density.
Solutions
Sucrose solution (in mM: NaCl, 83; KCl, 2.5; MgSO4, 3.3; NaH2PO4, 1; NaHCO3, 26.2; d-glucose, 22; sucrose, 72; and CaCl2, 0.5, bubbled with 95% O2 and 5% CO2). Artificial cerebrospinal fluid (ACSF; in mM: NaCl, 119; KCl, 2.5; NaH2PO4, 1.3; NaHCO3, 26; d-glucose, 20; MgCl2, 1.3; CaCl2, 2.5; and mOsm, 305, bubbled with 95% O2 and 5% CO2). Cesium-based internal (in mM: CsMeSO4, 125; NaCl, 4; HEPES, 10; Na3GTP, 0.3; MgATP, 4; EGTA, 0.3; QX-314-Cl, 2.5; BAPTA(5Cs), 10; adjusted to pH 7.4 with CsOH (140mL; mOsm 295). Potassium-based internal solution (in mM: K-gluconate, 150; MgCl2, 1.5; HEPES, 5; EGTA, 1.1; phosphocreatine, 10; adjusted to pH 7.4 with KOH; mOsm 295). HEPES buffered ACSF (in mM: NaCl, 142; KCl, 5; HEPES Sodium-Salt, 10; d-Glucose, 10; MgCl2, 1.3; CaCl2, 3.1; carbogen free).
In vivo whole-cell recordings
Mice (5 to 10 weeks old) were anesthetized using chlorprothixene (5mg/kg mouse) and isoflurane (1-2.5%). Dexamethazone (0.5μL/g mouse) was given to reduce swelling. The head was fixed to a mounting plate using dental cement (Lang Dental Mfg, Inc.). A dremmel was used to thin the entire skull covering V1 and to make a small craniotomy (approximately 250μm diameter). The dura was removed and the craniotomy was covered with HEPES buffered ACSF. Recordings were obtained using the blind patch technique (Margrie et al., 2002).. Cortical depth was determined by using the angle and depth of insertion of the recording pipette. The depth boundaries of L2/3 were chosen by measuring the depth of the layer in slices of primary visual cortex (134±7.8μm to 333±15.2μm, n=6 mice). Conservative boundaries of 150-300μm were chosen based on these depth measurements. Pipettes (4-6MΩ tip resistance) filled with either cesium or potassium-based internals (see solutions) were quickly advanced to a cortical depth of 150μm with a positive pressure of 3psi. A 100Hz train of 5mV steps were applied to continuously measure the resistance of the pipette tip. Positive pressure was reduced to 0.5psi as the pipette was advanced in steps of 2μm through the depth of L2/3. Upon advancing, a sharp increase in pipette resistance accompanied with the appearance of an oscillation was taken as an indication of contact with the cell. Pressure was removed to obtain a GΩ seal. Neurons were voltage clamped at the reversal potential of inhibition (−73.5mV with cesium internal solution) to record excitatory postsynaptic currents (EPSCs). To record inhibitory postsynaptic currents neurons were voltage clamped at the reversal potential of excitation (approximately +7mV with cesium internal solution). Data was acquired as in in vitro slice preparation.
In vitro slice preparation and recordings
Mice (4 to 8 weeks old) were anesthetized using Ketamine (100mg/kg) and Xylazine (10mg/kg). The descending aorta was clamped and right atrium cut before perfusing 1 min with chilled sucrose solution. Coronal sections of V1 (300μm, Bregma −2.2 to −4) were made using a vibratome (DSK Microslicer DTK-1000) in a chilled sucrose solution. Slices were incubated in sucrose solution in a submerged chamber at 34°C for 45 min and then at room temperature (21°C) until used for recordings. Whole-cell recordings were done at 31.5°C in ACSF using pipettes with 3–5MΩ resistance. Excitatory and inhibitory synaptic currents were recorded using a cesium-based internal solution. Whole-cell current clamp recordings to monitor spiking activity were performed using a potassium-based internal solution. Loose patch recordings were performed using ACSF as an internal (>8MΩ seal). Biocytin filled cells included 0.2-0.5% biocytin in the internal solution. Filled cells were held for 10-20 min and immediately fixed in 4% PFA in PBS. A Vector ABC kit was used to process filled neurons, which were then traced using Neurolucida. Data were recorded with Multiclamp 700B amplifiers (Axon instruments). Current clamp recordings were filtered at 10kHz and digitized with a Digidata1440A (Axon instruments) at 50kHz. Voltage clamp recordings were filtered at 3kHz and digitized at 10kHz. Axon binary files were imported to Igor Pro (Wavemetrics) using DataAccess (Bruxton; Seattle, WA) and analyzed using custom-made routines. Charges represent the time integral of the synaptic current recorded during the first second of photostimulation. The stage was moved using a custom plug-in for ImageJ (NIH) to interface with ESP300 (Newport) via SerialPort (SerialIO). Drugs used were NBQX (10μM; Tocris 1044) and CPP (20μM; Ascent Asc-159).
Retrograde labeling
Green RetroBeads IX were injected as received from Lumafluor into 4 week old mice. Mice were anesthetized with isofluorane (1-2.5%) and a small craniotomy was made with a dremmel. To label cortico-thalamic neurons, Green Retrobeads were injected into the dLGN (coordinates: 2mm posterior from bregma, 2mm lateral from midline, at a depth of 2.9-3mm) of 4 week old mice using a stereotactic apparatus. Beads (350nl) were injected bilaterally at a rate of 50nl/min.
Viral injections and photostimulation
Adeno-associated viruses (AAVs) for ChR2 were acquired from the University of Pennsylvania Viral Vector Core: AAV2/1.CAGGS.flex.ChR2.tdTomato.SV40. ChR2 virus was injected into newborn pups (between postnatal days 0 and 2) that were anesthetized on ice. Each animal was virally injected at three locations in V1 along the medio-lateral axis. At each location the virus was injected at two depths (550μm and 650μm; 13.2nl/depth). For extracellular recordings the photostimulus consisted of a 0.5s 20mW pulse delivered by an LED coupled to a fiber optic (470nm, 1mm diameter, Doric Lenses). Photostimulation of L6 during whole-cell and loose patch recordings consisted of either 7.5mW/cm2 pulses of 2ms duration or of ramps of increasing intensity (in vitro: 0 to 0.54mW/cm2; in vivo: 0 to 7.5mW/cm2; ramp duration: 1.5s except in Figure 1B where a shorter protocol was given (0.5s) to avoid excessive spike accommodation) using a 470nm wavelength LED (LEDC5 Thor Labs) through a GFP filter cube (GFP-3035BOMF-ZERO, Semrock BrightLine) and a 40× water-immersion objective.
Data analysis
Whole-cell recordings
Inhibitory and excitatory charge were computed as the time integral of the IPSC or EPSC, respectively. The integral began at the start of L6CT photoactivation and lasted for 1s. Adaptation was calculated as 1-FreqAVE/FreqINIT, where FreqAVE was the average instantaneous frequency over the entire current injection and FreqINIT was the average instantaneous frequency for the first 100ms of the current step injection. The current injection used for the measurement of adaptation and firing rate (Figure 6A) was the lowest current injection able to induce firing for the entire duration of the 1s step. Afterhyperpolarization was calculated on sweeps where the current injection was enough to elicit spikes but not for the entire duration of the current injection so a baseline could be determined. Spike waveforms used were an average of the first 5 recorded spikes. The peak to trough ratio was obtained by dividing the absolute value at the peak (second deflection) by the absolute value of the trough (first deflection).
Extracellular recordings
Data were analyzed with custom written software using Matlab. Single units were isolated using software provided by D.N.Hill, S.B.Mehta and D. Kleinfeld (Fee et al., 1996). Signals were high-pass filtered at 500Hz and waveforms were extracted from 4 adjacent electrode sites. Spikes were defined as events exceeding 4-5 s.d. of the noise. Waveforms were clustered using a k-means algorithm and further aligned using a graphical user interface. Fisher linear discriminant analysis and refractory period violations were used to assess unit isolation quality. Units were assigned a depth based on the channel in which they showed the strongest signal.
RetroBeads
For quantification of beads in cortico-thalmic neurons a confocal stack of images was made of layer 6. A stereotactic plane was drawn the centermost image (200μm medial lateral, L6 dorsal ventral). Included cells were those whose somas touched this plane without touching the bottom and left boundaries. The entire soma (as shown by either the reporter expression or the absence of labeling) was required to be in the stack of images to be included in counting. Cells whose soma included beads were designated cortico-thalamic.
Statistics
Error bars in all figures represent SEM. Statistical analysis was done using VassarStats (www.VassarStats.net). Mann Whitney Test was used for Figure 1B, 3D, 6A-C. Paired-T test was used for Figure S3B,C and for Arch/Halo extracellular recordings mentioned in the text. Fisher Test was used for Figure 2B Linear correlation and regression used for Figure S3D.
Axonal and dendritic density
Heat maps of reconstructed interneurons were done by normalizing the size of each neuron by the total cortical depth and converting Neuroleucida recontructions into bitmap images using Adobe Illustrator. Then the following bitmap manipulations were done using ImageJ (NIH): To allow mapping of layers onto one another the neurites in each layer were stretched or shrunk along the dorsal ventral axis to the match same dorsal ventral dimension across different slices. Cells bodies were aligned in the medial lateral axis. Bitmap images were Gaussian filtered to a radius of 50 pixels (approximately 35μm). The contrast of each cell's Gaussian filtered image was adjusted to make the highest pixel intensity for the image the maximum value possible before averaging the images for each cell type. These group average images were again adjusted for contrast to make the highest pixel intensity for the image the maximum value possible. The color look-up table used was ImageJ's “Fire”.
Supplementary Material
Acknowledgements
We wish to thank J. Isaacson, J. Reynolds, our reviewers and the members of the Scanziani and Isaacson laboratories for helpful discussions of this project; J. Evora for DAB biocytin processing, mouse colony maintenance and administrative support; M.Chan and A.Linder for neonatal viral injections; the Gene Expression Nervous System Atlas (GENSAT) Project, NINDS Contracts N01NS02331 & HHSN271200723701C to The Rockefeller University (New York, NY); the UCSD Neuroscience Microscopy Facility (P30 NS047101) for the use of their imaging equipment; and R. Lowry and Vassar College for the statistical analysis tools.. D.S.B. is supported by Ruth L. Kirschstein NRSA (1F32NS076185-01A1). M.S. is an investigator of the Howard Hughes Medical Institute. This work was also supported National Institutes of Health grant RO1 NS069010 and by the Gatsby Charitable Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464:1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo R, D'Urso G, Dal Maschio M, Farisello P, Bovetti S, Clovis Y, Lassi G, Tucci V, De Pietri Tonelli D, Fellin T. Layer-specific excitatory circuits differentially control recurrent network dynamics in the neocortex. Nat Neurosci. 2013;16:227–234. doi: 10.1038/nn.3306. [DOI] [PubMed] [Google Scholar]
- Binzegger T, Douglas RJ, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz J, Gilbert CD. Generation of end-inhibition in the visual cortex via interlaminar connections. Nature. 1986;320:362–365. doi: 10.1038/320362a0. [DOI] [PubMed] [Google Scholar]
- Bourassa J, Deschenes M. Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an anterograde tracer. Neuroscience. 1995;66:253–263. doi: 10.1016/0306-4522(95)00009-8. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Emerging views of corticothalamic function. Current opinion in neurobiology. 2008;18:403–407. doi: 10.1016/j.conb.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan KA, Blackman AV, Moreau AW, Elgar D, Costa RP, Lalanne T, Tudor Jones AA, Oyrer J, Sjostrom PJ. Target-specific expression of presynaptic NMDA receptors in neocortical microcircuits. Neuron. 2012;75:451–466. doi: 10.1016/j.neuron.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM. Local circuits in primary visual cortex of the macaque monkey. Annu Rev Neurosci. 1998;21:47–74. doi: 10.1146/annurev.neuro.21.1.47. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM. Deep cortical layers are activated directly by thalamus. Science. 2013;340:1591–1594. doi: 10.1126/science.1236425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzker JL, Callaway EM. Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat Neurosci. 2000;3:701–707. doi: 10.1038/76656. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Fee MS, Mitra PP, Kleinfeld D. Automatic sorting of multiple unit neuronal signals in the presence of anisotropic and non-Gaussian variability. J Neurosci Methods. 1996;69:175–188. doi: 10.1016/S0165-0270(96)00050-7. [DOI] [PubMed] [Google Scholar]
- Ferster D, Lindstrom S. Synaptic excitation of neurones in area 17 of the cat by intracortical axon collaterals of cortico-geniculate cells. J Physiol. 1985;367:233–252. doi: 10.1113/jphysiol.1985.sp015822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979;280:120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve KL, Sillito AM. A re-appraisal of the role of layer VI of the visual cortex in the generation of cortical end inhibition. Exp Brain Res. 1991;87:521–529. doi: 10.1007/BF00227077. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Sakmann B, Feldmeyer D. Neuronal correlates of local, lateral, and translaminar inhibition with reference to cortical columns. Cereb Cortex. 2009;19:926–937. doi: 10.1093/cercor/bhn141. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nat Neurosci. 2013;16:210–218. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. second edn Cambridge University Press; 2007. [Google Scholar]
- Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci. 1996;16:2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisvarday ZF, Martin KA, Friedlander MJ, Somogyi P. Evidence for interlaminar inhibitory circuits in the striate cortex of the cat. J Comp Neurol. 1987;260:1–19. doi: 10.1002/cne.902600102. [DOI] [PubMed] [Google Scholar]
- Kumar P, Ohana O. Inter- and intralaminar subcircuits of excitatory and inhibitory neurons in layer 6a of the rat barrel cortex. J Neurophysiol. 2008;100:1909–1922. doi: 10.1152/jn.90684.2008. [DOI] [PubMed] [Google Scholar]
- Lefort S, Tomm C, Floyd Sarria JC, Petersen CC. The excitatory neuronal network of the C2 barrel column in mouse primary somatosensory cortex. Neuron. 2009;61:301–316. doi: 10.1016/j.neuron.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. La corteza cerebral de raton. Trabajos del Laboratorio de Investigaciones Biologicas de la Universidad de Madrid. 1922;20:41–78. [Google Scholar]
- Lund JS. Anatomical organization of macaque monkey striate visual cortex. Annu Rev Neurosci. 1988;11:253–288. doi: 10.1146/annurev.ne.11.030188.001345. [DOI] [PubMed] [Google Scholar]
- Lund JS, Hawken MJ, Parker AJ. Local circuit neurons of macaque monkey striate cortex: II. Neurons of laminae 5B and 6. J Comp Neurol. 1988;276:1–29. doi: 10.1002/cne.902760102. [DOI] [PubMed] [Google Scholar]
- Lund JS, Henry GH, MacQueen CL, Harvey AR. Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. J Comp Neurol. 1979;184:599–618. doi: 10.1002/cne.901840402. [DOI] [PubMed] [Google Scholar]
- Malpeli JG. Activity of cells in area 17 of the cat in absence of input from layer a of lateral geniculate nucleus. J Neurophysiol. 1983;49:595–610. doi: 10.1152/jn.1983.49.3.595. [DOI] [PubMed] [Google Scholar]
- Margrie TW, Brecht M, Sakmann B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Archiv : European journal of physiology. 2002;444:491–498. doi: 10.1007/s00424-002-0831-z. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Murphy PC, Sillito AM. Corticofugal feedback influences the generation of length tuning in the visual pathway. Nature. 1987;329:727–729. doi: 10.1038/329727a0. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature. 2012;483:47–52. doi: 10.1038/nature10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwark HD, Malpeli JG, Weyand TG, Lee C. Cat area 17. II. Response properties of infragranular layer neurons in the absence of supragranular layer activity. J Neurophysiol. 1986;56:1074–1087. doi: 10.1152/jn.1986.56.4.1074. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Jones HE. Corticothalamic interactions in the transfer of visual information. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2002;357:1739–1752. doi: 10.1098/rstb.2002.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Cowey A. Combined Golgi and electron microscopic study on the synapses formed by double bouquet cells in the visual cortex of the cat and monkey. J Comp Neurol. 1981;195:547–566. doi: 10.1002/cne.901950402. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Cowey A, Halasz N, Freund TF. Vertical organization of neurones accumulating 3H-GABA in visual cortex of rhesus monkey. Nature. 1981;294:761–763. doi: 10.1038/294761a0. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Inhibitory neurons in the anterior piriform cortex of the mouse: classification using molecular markers. J Comp Neurol. 2010;518:1670–1687. doi: 10.1002/cne.22295. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Neocortical layer 6, a review. Front Neuroanat. 4:13. doi: 10.3389/fnana.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP. Interlaminar connections in the neocortex. Cereb Cortex. 2003;13:5–14. doi: 10.1093/cercor/13.1.5. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Wang Y, Bannister AP. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2-5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cereb Cortex. 2002;12:936–953. doi: 10.1093/cercor/12.9.936. [DOI] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DC, Mercer A, Kirchhecker S, Morris OT, Thomson AM. Layer 6 corticothalamic pyramidal cells preferentially innervate interneurons and generate facilitating EPSPs. Cereb Cortex. 2006;16:200–211. doi: 10.1093/cercor/bhi098. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Deschenes M. Intracortical axonal projections of lamina VI cells of the primary somatosensory cortex in the rat: a single-cell labeling study. J Neurosci. 1997;17:6365–6379. doi: 10.1523/JNEUROSCI.17-16-06365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.