Abstract
Evidence for the association of diet and gastric cancer is equivocal, and the majority of previous studies have not evaluated the interaction of diet and infection with Helicobacter pylori, the leading risk factor for gastric cancer. We examined these associations among 226 cases and 451 controls nested within a prospective cohort. Dietary intakes were calculated from validated food frequency questionnaires. Blood levels of 15 antibodies to Helicobacter pylori proteins were assessed using multiplex serology. Odds ratios were calculated using logistic regression. Among individuals infected with high-risk Helicobacter pylori (sero-positivity to 5–6 virulent H. pylori proteins), increasing intake of red meat, heme iron, and sodium increased risk (comparing highest tertile to lowest: [ORs (95% CI): 1.85 (1.01–3.40); 1.95 (1.06–3.57); and 1.76 (0.91–3.43), respectively] while increasing intake of fruit decreased gastric cancer risk [comparing highest tertile of intake to lowest: OR (95% CI): 0.52 (0.28–0.94)]. No associations of diet with risk were found among individuals infected with low-risk H. pylori (P for interaction for red meat and sodium: 0.02 and 0.01, respectively). In this population with over 90% prevalence of CagA-positive H. pylori infection, categorizing individuals utilizing H. pylori multiplex serology may identify individuals for whom a diet intervention may be effective.
Keywords: epidemiological, gastric cancer, diet, Helicobacter pylori, red meat
INTRODUCTION
Once the most common cancer in the world, gastric cancer incidence has been on the decline for over half a century, but with consistently low survival rates, it remains the second leading cause of death from cancer worldwide (1). The bacterium Helicobacter pylori is the strongest known risk factor for gastric cancer, but the vast majority of individuals infected with H. pylori will never develop cancer (2). Diet has long been thought to be related to gastric cancer risk, but the most recent report on diet and cancer from the World Cancer Research Fund and the American Institute for Cancer Research resulted in equivocal findings, with a downgrading of the protective effects of fruits and vegetables for gastric cancer risk from “convincing” to “probable,” salt remaining as a “probable” risk factor, and the evidence for animal foods of any variety to be associated with gastric cancer risk to be deemed even weaker, at “limited” (3). However, the vast majority of published studies on the association of diet and gastric cancer did not take into consideration the role of H. pylori infection, and its interplay with diet in relation to gastric cancer risk. While a synergistic interaction between the two has been suggested (4), only 3 previous prospective cohort studies (5–7) have examined the potential for effect modification. While none of these studies found significant interaction terms, two of the three studies found evidence supporting the hypothesis that diet plays a greater role in the etiology of gastric cancer for individuals who are infected with H. pylori compared to uninfected persons.
In all of the studies that investigated the potential interaction of H. pylori with diet in relation to risk of gastric cancer, H. pylori status was categorized in a dichotomous manner (H. pylori− vs. H. pylori+), or, at best, in three categories, incorporating Cytotoxin-associated antigen (CagA) status (H. pylori−; H. pylori+, CagA−; and H. pylori+, CagA+). CagA, the most well-known marker of H. pylori strains associated with disease, is a component of the cag pathogenecity island, and cagA-positive strains of H. pylori inject CagA into host cells, triggering signaling pathways that ultimately lower the threshold for carcinogenesis (8, 9). In China and other parts of East Asia where rates of gastric cancer incidence and mortality are highest in the world (10), the majority of the population is infected with CagA-positive H. pylori strains, and yet do not develop gastric cancer; thus, sero-positivity to CagA is not an ideal risk marker in this high-risk area (11). To investigate the potential of an H. pylori biomarker that would better represent the heterogeneity of the bacteria, we recently evaluated antibodies to 15 H. pylori proteins in a nested case-control study of the Shanghai Men’s Health Study, and identified several additional markers of risk. Overall, we determined that sero-positive results to 5–6 H. pylori proteins was a better marker of gastric cancer risk in this population than prevalence of CagA-positive infection alone (12). Thus, we sought to assess the potential effect modification of the diet-gastric cancer association by H. pylori infection among members of a prospective cohort, with specific attention to virulent H. pylori sub-types in this population.
MATERIALS AND METHODS
Study population
The Shanghai Men’s Health Study (SMHS), a population-based prospective cohort study, recruited 61,491 permanent male residents of urban Shanghai aged 40–74 years from 2002 to 2006, as detailed in previous publications (13, 14). Briefly, at baseline participants were administered detailed in-person interviews, by trained interviewers, using a structured questionnaire to collect information on demographic characteristics and other lifestyle factors, disease and surgery history, and family cancer history. Additionally, at baseline participants were measured for height, weight, and waist and hip circumference according to a standard protocol. Using a previously described protocol (15), a 10-ml blood sample was drawn from 75% of participants, which were stored in insulated boxes with blue ice packs and then processed within 6 hours for long-term storage at −80°C. Plasma samples were used for H. pylori biomarker analyses. At the time of blood donation, information was gathered from each participant on date and time of sample collection and time of last meal, as well as intake of selected foods, smoking, and use of any medications over the past 24 hours and during the past week.
Usual diet was collected by the in-person administration of a validated food frequency questionnaire. For each individual food item or group of foods, participants were asked: 1) how frequently they consumed the item (daily, weekly, monthly, annually, or never); 2) the amount of that item typically consumed at one time (in liang; 1 liang = 50 g); and 3) how many months of the year that food item was eaten. Using the Chinese Food Composition Tables (16), individual nutrient and mineral intakes were calculated by multiplying the amount of each food item by the nutrient content per gram of the food. The food frequency questionnaire was validated in a subset of the participants utilizing twelve 24-hour dietary recalls and a second food frequency questionnaire at the end of the study (17). Average daily intake of red meat, heme iron, preserved meat, sodium, fruits, and vegetables were chosen as the dietary exposures of interest for the present study because of previous suggestions of an association of these food groups, minerals, or nutrients and gastric cancer risk in this population or similar populations (14, 18, 19). To classify consumption of each food group as well as heme iron and sodium, baseline dietary information was used to create tertiles of intake based on the distribution among controls. Heme iron was calculated by summing the products of the amounts of nondairy animal foods by the iron content of each food using Chinese food composition tables. Information collected on preserved meat intake in the FFQ, comprised of intake of smoked meat, salted meat, and Chinese sausage, included frequency of intake only (and not amount), and thus tertiles for preserved meat intake in this study are based on times per month of consumption, rather than average daily amount. Daily sodium intake was derived from foods only, not including preserved foods, and this definition, excluding seasoning (which is generally adding during cooking and not at the table in this population) and beverages, results in a lower median intake than would be observed if all factors related to sodium intake could be measured. The category of fruits includes all fruit except for watermelon, as watermelon accounts for half of total fruit intake, and the quantification of fruit eaten in such large quantities can be difficult to assess, and potentially leads to significant exposure measurement error. Furthermore, watermelon intake alone was not associated with gastric cancer risk in this population, and our previous study found a significant association between the category of all fruit except watermelon and distal gastric cancer risk.
Case identification
Incident distal gastric cancer cases were defined as those individuals with diagnoses that were coded by the International Classification of Diseases (ICD) as malignant neoplasm of the stomach (ICD-9 code 151) and with an ICD-Oncology code of 161–166, 168, or 169, to capture distal gastric cases and exclude gastric cardia cases (ICD code 160). Cases were identified through a combination of annual linkage with the Shanghai Cancer Registry and the Shanghai Vital Statistics database, and active follow-up evaluations. Study employees confirm cancer diagnoses (and deaths from cancer) for subjects in the cohort by manually checking all possible matches, performing follow-up home visits, and reviewing medical charts to collect pathology characteristics of the tumor. Additionally, every two to three years, a study interviewer visits the last known address of every living cohort member. These efforts have resulted in response rates of 97.6% and 93.2% for the first (2004–2008) and second (2008–2011) in-person follow-ups, respectively.
Control selection
For each of the 226 cases included in this study, comprised of distal gastric cancer cases that were identified between 2002 and 2009 who donated a blood sample and did not have cancer at baseline or within one month of blood sample collection, two controls were chosen. Controls were matched on: age (within 2 years), date (within 30 days) and time (morning or afternoon) of sample collection; time interval since last meal (within 2 hours); antibiotic use in the last week (yes/no); and availability of plasma.
Helicobacter pylori multiplex serology
H. pylori multiplex serology, a method based on a glutathione S-transferase (GST) capture immunosorbent assay combined with fluorescent-bead technology (20, 21), has been described in detail previously (22). For the 677 individuals in the present study, we utilized H. pylori multiplex serology to assess the quantity of antibodies, calculated as the median reporter fluorescence intensity (MFI), to fifteen H. pylori specific antigens (UreA, Catalase, GroEL, NapA, CagA, CagM, Cagδ, HP0231, VacA, HpaA, Cad, HyuA, Omp, HcpC, HP0305). Antigen-specific cut-point values for each of the 15 antigens are based on 46 sera defined as H. pylori seronegative in a previous study (23) and run within this assay. They were calculated as arithmetic mean of the median reporter fluorescence intensity (MFI) plus three standard deviations and excluding positive outliers. Among the twelve quality-control samples from one pooled sample analyzed on 4 separate places (3 per plate), the determination of sero-positivity for all 15 H. pylori proteins was highly consistent, with only 5 (2.8%) of the total 180 tests not identical with the others. In a previous study, we included 5 replicates for 2 individual controls, and the results of these quality control samples were very similar, whereby only 1 (3.3%) replicate of 30 was not identical with the others in determination of sero-positivity for all H. pylori proteins (23).
Statistical analysis
The association of distal gastric cancer risk with dietary intake was initially assessed using conditional logistic regression to determine odds ratios (ORs) and 95% confidence intervals (CIs). Factors related to the exposure (diet) and outcome (gastric cancer) and not in the causal pathway were adjusted for in the full models, and included age, smoking status, history of gastritis, regular aspirin use, total energy intake, and high-risk H. pylori infection. In this population, markers of socio-economic status (education, income, and occupation) were not associated with gastric cancer incidence, and thus were not included as confounders in the full models. Indicator variables were entered into the models to represent tertiles of intake, using the lowest tertile as the reference category. To test for a linear trend across levels of each food group, mineral, or nutrient, a variable was created and assigned the median value of consumption for each tertile of food group, mineral, or nutrient.
To examine the potential interaction of H. pylori protein-specific infection in relation to the association of dietary intake and risk of distal gastric cancer, separate models were created to stratify the study population into individuals with high-risk H. pylori infection compared to those without. Previously, we determined that number of sero-positive results to 6 H. pylori proteins (Omp, HP0305, HyuA, HpaA, CagA, and VacA) may be a risk marker for individuals at high risk for gastric cancer in a population with overwhelming prevalence of CagA-positive H. pylori infection (12). For the present study, individuals were classified as high-risk if they were sero-positive to more than four of the six virulent H. pylori proteins identified above. This divided the population into two groups: 326 with high-risk H. pylori infection (140 cases and 211 controls); and 351 without (86 cases and 240 controls). Within each group, the association of the dietary factors with risk of distal gastric cancer was assessed with unconditional logistic regression (as limiting the analyses to matched sets concordant on H. pylori status would have greatly reduced the power for these analyses), adjusting for all potential confounders as described above. To assess effect modification by H. pylori status for each of the dietary variables examined, all individuals were included in a conditional logistic regression model, along with an interaction term of H. pylori status and dietary intake of the specific food group or nutrient as a trend variable.
To calculate odds ratios that incorporate both dietary intake and H. pylori protein-specific status, all study participants were classified into one of four categories for each food group of interest: low-intake and low-risk H. pylori; high intake and low-risk H. pylori; low intake and high-risk H. pylori; and high intake and high-risk H. pylori. Level of intake was based on the median value of intake among controls, so that individuals would be classified as low intake in separate models by food group if they consumed ≤49.7 g/day of red meat, ≤3.14 mg/day of heme iron, ≤0.63 times/month of preserved meat, ≤3506 mg/day of sodium, ≤59.2 g/day of fruit, and ≤325.7 g/day of vegetables. A multivariable-adjusted conditional logistic regression model for each food group was then conducted, using indicator variables for the combined characteristics of H. pylori infection and dietary intake, with the category assumed to be at the lowest risk of cancer (low intake and low-risk H. pylori for red meat, heme iron, preserved meat, and sodium, and high intake and low-risk H. pylori for fruits and vegetables) as the reference category.
All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
Compared to the controls, cases of distal gastric cancer were more likely to be ever smokers and to be have given a diagnosis of gastritis, and were less likely to be a regular aspirin user. Compared to individuals without high-risk H. pylori infection, study participants with high-risk H. pylori, both cases and controls, were slightly more likely to have a family history of gastric cancer, but all other baseline demographic characteristics were similar between those categorized by H. pylori protein-specific infection (Table 1).
Table 1.
Baseline demographic characteristics of the gastric cancer cases and matched controls in the Shanghai Men’s Health Study, 2002–2009
| Cases | Controls | |||||
|---|---|---|---|---|---|---|
| All (N=226) | Low-risk H. pylori (N=86) | High-risk H. pylori (N=140) | All (N=451) | Low-risk H. pylori (n=240) | High-risk H. pylori (n=211) | |
|
| ||||||
| Mean age, y ± SD | 62.6 ± 9.5 | 63.6 ± 9.4 | 62.0 ± 9.6 | 62.7 ± 9.3 | 63.3 ± 9.4 | 61.9 ± 9.2 |
| Family history of gastric cancer, n (%) † | 19 (8.4) | 4 (4.7) | 15 (10.7) | 35 (7.8) | 15 (6.3) | 20 (9.5) |
| Mean BMI, kg/m2 ± SD | 23.5 ± 3.1 | 23.6 ± 3.3 | 23.4 ± 3.0 | 24.0 ± 3.3 | 23.9 ± 3.1 | 24.1 ± 3.5 |
| Smoking status, n (%) * | ||||||
| Never smoker | 59 (26.1) | 25 (29.1) | 34 (24.3) | 152 (33.7) | 86 (35.8) | 66 (31.3) |
| Former smoker | 37 (16.4) | 18 (20.9) | 19 (13.6) | 81 (18.0) | 40 (16.7) | 41 (19.4) |
| Current smoker, <20 cigs/day | 63 (27.9) | 23 (26.7) | 40 (28.6) | 109 (24.2) | 55 (22.9) | 54 (25.6) |
| Current smoker, ≥20 cigs/day | 67 (29.7) | 20 (23.3) | 47 (33.6) | 109 (24.2) | 59 (24.6) | 50 (23.7) |
| Alcohol drinking status, n (%) | ||||||
| Never drinker | 137 (60.6) | 52 (60.5) | 85 (60.7) | 292 (64.8) | 150 (62.5) | 142 (67.3) |
| Ever drinker | 89 (39.4) | 34 (39.5) | 55 (39.3) | 159 (35.3) | 90 (37.5) | 69 (32.7) |
| Highest education level achieved, n (%) | ||||||
| Elementary school or less | 22 (9.8) | 12 (14.1) | 10 (7.2) | 57 (12.8) | 34 (14.5) | 23 (11.0) |
| Junior High School | 96 (42.9) | 34 (40.0) | 62 (44.6) | 153 (34.4) | 77 (32.8) | 76 (36.2) |
| High School | 62 (27.7) | 25 (29.4) | 37 (26.6) | 141 (31.7) | 73 (31.1) | 68 (32.4) |
| Professional high education or above | 44 (19.6) | 14 (16.5) | 30 (21.6) | 94 (21.1) | 51 (21.7) | 43 (20.5) |
| Shanghai native, n (%) | 129 (57.1) | 48 (55.8) | 81 (57.9) | 259 (57.4) | 130 (54.2) | 129 (61.1) |
| Regular aspirin use, n (%) * | 19 (8.4) | 8 (9.3) | 11 (7.9) | 63 (14.0) | 33 (13.8) | 30 (14.2) |
| Diagnosis of chronic gastritis, n (%) * | 57 (25.2) | 22 (25.6) | 35 (25.0) | 80 (17.7) | 50 (20.8) | 30 (14.2) |
| Diet | ||||||
| Mean red meat intake, g/day ± SD | 64.7 ± 45.5 | 57.2 ± 40.3 | 69.3 ± 47.9 | 60.6 ± 45.6 | 63.7 ± 49.9 | 57.1 ± 39.9 |
| Mean heme iron intake, mg/day ± SD | 3.3 ± 1.8 | 3.0 ± 1.5 | 3.5 ± 1.9 | 3.1 ± 2.0 | 3.3 ± 2.3 | 2.9 ± 1.8 |
| Mean preserved meat intake, times/month ± SD | 1.2 ± 1.7 | 0.9 ± 1.3 | 1.4 ± 1.9 | 1.5 ± 2.7 | 1.6 ± 3.2 | 1.4 ± 1.8 |
| Mean sodium intake, mg/day ± SD | 393.1 ± 175.9 | 384.7 ± 187.0 | 398.4 ± 169.2 | 381.5 ± 179.2 | 397.9 ± 187.4 | 362.8 ± 167.9 |
| Mean fruit intake, g/day ± SD * | 65.4 ± 68.5 | 70.0 ± 63.6 | 62.6 ± 71.4 | 77.5 ± 72.1 | 81.0 ± 76.9 | 73.6 ± 66.1 |
| Mean vegetable intake, g/day ± SD † | 335.3 ± 190.9 | 357.0 ± 216.5 | 321.9 ± 172.9 | 354.5 ± 198.8 | 387.2 ± 206.0 | 317.2 ± 183.8 |
Abbreviation: SD, standard deviation
P < 0.05 comparing cases and controls;
P < 0.05 comparing individuals with low-risk H. pylori infection to individuals with high-risk H. pylori infection
Among all participants, there were suggestions that increasing intake of red meat and heme iron and decreasing intake of fruit were associated with an increased risk of distal gastric cancer, but the trends by level of consumption reached statistical significance for heme iron only (P for trend=0.02) (Table 2). Among only those individuals determined to have high-risk H. pylori (defined as sero-positivity to >4 of the 6 virulent H. pylori proteins of Omp, HP0305, HyuA, HpaA, CagA, and VacA), there was a significant direct association between distal gastric cancer risk and red meat intake (the highest tertile compared to the lowest: OR=1.85; 95% CI=1.01–3.40; P for trend=0.05) and heme iron intake (the highest tertile compared to the lowest: OR=1.95; 95% CI=1.06–3.57; P for trend=0.03), and a suggestion of an increase in risk for increasing sodium intake (the highest tertile compared to the lowest: OR=1.76; 95% CI=0.91–3.43; P for trend=0.08). Among these individuals only there was also a significant inverse association of increasing fruit intake and gastric cancer risk (the highest tertile compared to the lowest: OR=0.52; 95% CI=0.28–0.94; P for trend=0.03). No associations between any of the dietary variables and gastric cancer risk were found among individuals determined to be at low risk by their H. pylori status (defined as 0–4 sero-positive results). These divergent trends produced significant interaction terms for red meat intake (P for interaction=0.02) and sodium intake (P for interaction=0.01), and a suggestion of an interaction by H. pylori status for intake of heme iron (P for interaction=0.098) and preserved meat (P for interaction=0.09). In this population, intake of these dietary factors were all significantly correlated with one another, even after adjustment for total energy intake and age, particularly red meat and heme iron (Pearson correlation coefficient=0.70, p<0.0001) and sodium and heme iron (Pearson correlation coefficient=0.57, p<0.0001), and, on a smaller scale, red meat and sodium (Pearson correlation coefficient=0.24, p<0.0001), and intake of preserved meat and heme iron, red meat, and sodium (Pearson correlation coefficients of 0.16, 0.12, and 0.12, p-values of <0.0001, 0.001, and 0.003, respectively).
Table 2.
Odds ratios of distal gastric cancer by dietary intake in a nested case-control study in the Shanghai Men’s Health Study (226 cases and 451 controls), stratified by number of sero-positive results to 6 H. pylori proteins (Omp, HP0305, HyuA, HpaA, CagA, VacA)
| T1 (referent) | T2 | T3 | Ptrend | Pinteractiona | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. cases | Range | OR | No. cases | Range | OR | 95% CI | No. cases | Range | OR | 95% CI | |||
| Red meat (g/day) | ≤36.0 | 36.1–66.5 | >66.5 | ||||||||||
| Alla | 70 | 1.00 | 64 | 1.02 | 0.66, 1.56 | 92 | 1.45 | 0.93, 2.28 | 0.10 | ||||
| Low-risk (0–4 sero-positive results)b | 34 | 1.00 | 19 | 0.56 | 0.29, 1.09 | 33 | 1.19 | 0.60, 2.36 | 0.68 | ||||
| High-risk (5–6 sero-positive results)b | 36 | 1.00 | 45 | 1.68 | 0.94, 3.01 | 59 | 1.85 | 1.01, 3.40 | 0.05 | 0.02 | |||
| Heme iron (mg/day) | ≤2.2 | 2.3–3.3 | >3.3 | ||||||||||
| Alla | 66 | 1.00 | 63 | 1.01 | 0.65, 1.58 | 97 | 1.66 | 1.05, 2.62 | 0.02 | ||||
| Low-risk (0–4 sero-positive results)b | 28 | 1.00 | 22 | 0.88 | 0.45, 1.72 | 36 | 1.69 | 0.84, 3.38 | 0.13 | ||||
| High-risk (5–6 sero-positive results)b | 38 | 1.00 | 41 | 1.27 | 0.72, 2.24 | 61 | 1.95 | 1.06, 3.57 | 0.03 | 0.098 | |||
| Preserved meat intake (times/month) | |||||||||||||
| Alla | 71 | ≤0.2 | 1.00 | 81 | 0.21–1.42 | 1.13 | 0.74, 1.72 | 74 | >1.42 | 1.01 | 0.66, 1.55 | 0.99 | |
| Low-risk (0–4 sero-positive results)b | 37 | 1.00 | 29 | 0.96 | 0.53, 1.72 | 20 | 0.79 | 0.41, 1.51 | 0.49 | ||||
| High-risk (5–6 sero-positive results)b | 34 | 1.00 | 52 | 1.42 | 0.80, 2.52 | 54 | 1.34 | 0.76, 2.36 | 0.35 | 0.09 | |||
| Sodium (mg/day) | ≤293 | 294–424 | >4199 | ||||||||||
| Alla | 68 | 1.00 | 78 | 1.29 | 0.82, 2.05 | 80 | 1.39 | 0.83, 2.33 | 0.22 | ||||
| Low-risk (0–4 sero-positive results)b | 31 | 1.00 | 27 | 0.85 | 0.45, 1.62 | 28 | 1.04 | 0.50, 2.15 | 0.92 | ||||
| High-risk (5–6 sero-positive results)b | 37 | 1.00 | 51 | 1.77 | 1.00, 3.12 | 52 | 1.76 | 0.91, 3.43 | 0.08 | 0.01 | |||
| Fruit (no watermelon) (g/day) | ≤31.9 | 32.0–94.0 | >94.0 | ||||||||||
| Alla | 90 | 1.00 | 82 | 0.98 | 0.66, 1.47 | 54 | 0.64 | 0.41, 1.00 | 0.06 | ||||
| Low-risk (0–4 sero-positive results)b | 32 | 1.00 | 31 | 1.00 | 0.54, 1.84 | 23 | 0.87 | 0.45, 1.70 | 0.69 | ||||
| High-risk (5–6 sero-positive results)b | 58 | 1.00 | 51 | 0.93 | 0.55, 1.59 | 31 | 0.52 | 0.28, 0.94 | 0.03 | 0.83 | |||
| Vegetables (g/day) | ≤238.5 | 238.6–397.3 | >397.3 | ||||||||||
| Alla | 82 | 1.00 | 71 | 0.99 | 0.65, 1.52 | 73 | 0.99 | 0.63, 1.56 | 0.96 | ||||
| Low-risk (0–4 sero-positive results)b | 25 | 1.00 | 35 | 1.58 | 0.83, 3.00 | 26 | 1.14 | 0.55, 2.38 | 0.72 | ||||
| High-risk (5–6 sero-positive results)b | 57 | 1.00 | 36 | 0.68 | 0.39, 1.19 | 47 | 1.12 | 0.63, 2.00 | 0.76 | 0.14 | |||
conditional logistic regression, additionally adjusted for age, smoking, history of gastritis, regular aspirin use, total energy intake, and high-risk H. pylori infection
unconditional logistic regression adjusted for age, smoking, history of gastritis, regular aspirin use, average and total energy intake
When categorizing individuals by joint H. pylori status and dietary intake level, individuals with a high intake of red meat and high-risk H. pylori infection had a 2.77-fold increased odds of developing gastric cancer compared to those with a low red meat intake and a low-risk H. pylori status (95% CI=1.65–4.67), a similar increase in odds as for individuals with high heme iron intake and high-risk H. pylori infection as compared to those with low heme iron intake and low-risk H. pylori infection (OR=2.90; 95% CI=1.72–4.89) (Figure 1). Among individuals with high-risk H. pylori infection, amount of preserved meat intake did not alter relative odds for gastric cancer occurrence (OR=1.56; 95% CI=1.00–2.43 and OR=1.60; 95% CI=1.00–2.56 for individuals with high-risk H. pylori and low or high preserved meat intake, respectively) compared to individuals with low intake of preserved meat and low-risk H. pylori. However, the only individuals for whom high sodium intake increased risk of gastric cancer were those who were also infected with high-risk H. pylori (compared to individuals with low intake of sodium and low-risk H. pylori, OR=2.36; 95% CI: 1.39–3.99).
Figure 1.
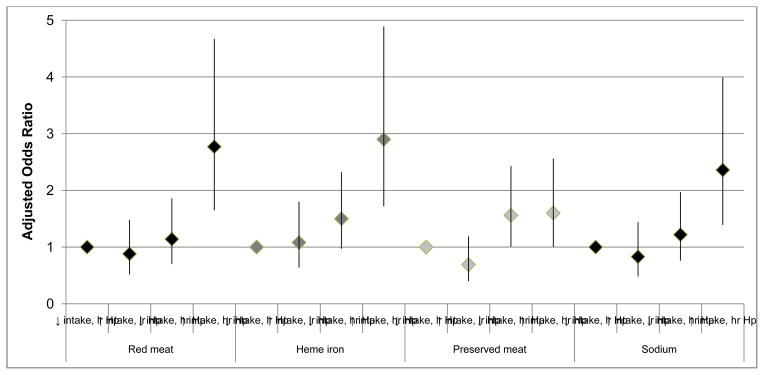
Odds ratiosa of distal gastric cancer by the combined status of dietary intake levelb and number of sero-positive resultsc to 6 H. pylori proteins in a nested case-control study in the Shanghai Men’s Health Study (226 cases and 451 controls)
a conditional logistic regression, additionally adjusted for adjusted for age, smoking, history of gastritis, regular aspirin use, and total energy intake.
b based on ≤ median vs. >median values among controls: 49.7 g/day for red meat; 3.14 mg/day for heme iron; 0.63 times per month for preserved meat; and 350.6 mg/day for sodium.
c low-risk (lr) and high-risk (hr) H. pylori (Hp) is defined based on a cut-off of ≤ 4 vs. 5–6 sero-positive results to 6 H. pylori proteins (Omp, HP0305, HyuA, HpaA, CagA, VacA)
DISCUSSION
The present study found that only among individuals infected with a high-risk H. pylori sub-type is diet associated with risk of distal gastric cancer. Specifically, for these individuals, increasing red meat, heme iron, and sodium consumption increase risk of gastric cancer, and increasing fruit intake decreases risk of gastric cancer.
Of the 14 published studies we are aware of that investigate the potential effect modification by H. pylori status in relation to the diet and gastric cancer association (5–7, 24–34), most found a suggestion of an association with diet for H. pylori-infected individuals only, but only 3 studies found significant interactions: for smoked-salted meat and fermented pork fat (31); for high-salt “preference” (27); and for sodium intake and salt taste preference (33). To note, all three of these were case-control studies, subject to recall bias for dietary exposure and misclassification of H. pylori status due to loss of evidence of the bacteria as cancer develops. Furthermore, none of the 13 previous studies categorized infection with H. pylori beyond positive versus negative, or in three categories based on the combination of antibodies to H. pylori as well as CagA, whereas in the present study we utilized a finer measurement of risk based on results of sero-positivity to antibodies to six H. pylori proteins. Our finding of a significant interaction for H. pylori infection in the association of sodium intake and gastric cancer risk, and a suggestion of an interaction with preserved meat intake, is still consistent with these studies, and the biological plausibility of a synergism between H. pylori infection and high salt intake with gastric cancer risk has been demonstrated in animal models (35, 36). It is hypothesized that in gastric cancer etiology, salt is not a primary cause but rather an adjuvant that increases expression of H. pylori CagA, secretion of pro-inflammatory cytokines, and rate of cell replication (37, 38). Recently, analysis of 36 H. pylori strains from individuals in two regions in Colombia with differing incidences of gastric cancer found that increased salt concentrations affect CagA expression differently in different strains, again supporting our findings of a difference in association by H. pylori sub-type (39).
While none of the previous studies found a significant interaction between H. pylori infection and red meat intake in relation to gastric cancer, as seen in the present study, analyses of the European Prospective Investigation Into Cancer and Nutrition (EPIC), comprising over 500,000 individuals from 10 countries, did find a suggestion of such an association. Specifically, they found significant associations between total, red, and processed meat intake and noncardia gastric cancer among H. pylori-positive individuals, but not among H. pylori-negative individuals, although they were limited in these analyses as only 12 of the noncardia gastric cancer cases were H. pylori-negative (5). It is possible that the mechanism whereby red meat increases risk of noncardia gastric cancer among H. pylori-positive individuals only is due to the contribution of iron to the growth of H. pylori, which would then increase risk of gastric cancer. Recent analyses of dietary intake of heme iron and gastric cancer risk in EPIC found that increasing heme iron intake increased risk of gastric cancer, particularly for individuals with below-average plasma vitamin C (40), similar to our finding of a direct association of heme iron intake and gastric cancer risk only among individuals infected with high-risk strains of H. pylori. However, these findings are not present in all populations as several studies have demonstrated that iron deficiency is a risk factor for gastric premalignant and malignant lesions in certain populations, and treatment of H. pylori results in a reversal of this disorder. Further, our group has recently demonstrated that iron depletion augments the function of the cag virulence locus (41). An explanation for these seemingly disparate findings could be that iron consumption does increase the risk of gastric cancer by enhancing the growth of H. pylori, but at the same time individuals harboring high-risk strains of H. pylori are more likely to be iron-deficient, whether they be high consumers of iron or not, both because the infection reduces the host’s ability to absorb iron and because the bacteria is able to scavenge the iron for itself (42).
Our results are also consistent with studies that have found diet as an important risk (or protective) factor only for high risk groups defined by other established gastric cancer risk factors, such as sex and/or smoking status. Specifically, a number of studies have found a stronger protective association of greater fruit intake and a stronger direct association of increased salt or meat intake among males, compared to females (14, 43, 44). Furthermore, two studies just among males found strong significant dietary associations (45, 46). Similarly, two studies that stratified by smoking status found stronger associations with dietary variables among ever or current smokers, as compared to non-smokers (14, 24).
No studies to our knowledge have examined the possibility of the preventive effects of reducing red meat, heme iron and/or sodium intake among individuals at high risk for gastric cancer. In relation to our findings for fruit intake, randomized trials assessing the potential benefit of vitamin supplements (specifically beta-carotene, vitamin A, vitamin C, and vitamin E) on gastric cancer risk have generally found null results, and a recent systematic review of 20 such trials found no evidence of such a protective effect overall (47). However, the majority of these trials were conducted in countries with sufficient levels of antioxidant vitamins, and a stronger effect might be seen among individuals who are vitamin deficient, and/or other populations that are at particularly high risk of gastric cancer. Our findings support the suggestion (14, 48) that diet as an etiologic factor may be most important in certain individuals at high risk for gastric cancer, and if this is true, targeted dietary intervention studies among high-risk individuals – such as those harboring the most virulent forms of H. pylori – may be the most effective study design for the reduction of the morbidity and mortality from this important disease.
Acknowledgments
This work was supported by the National Cancer Institute at the National Institutes of Health (K07 CA151782 to M.E., R01 CA82729 to X.-O.S., and R01 CA77955, R01 DK58587 and P01 CA116087 to R.P.).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–53. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR; 2007. [Google Scholar]
- 4.Yamaguchi N, Kakizoe T. Synergistic interaction between Helicobacter pylori gastritis and diet in gastric cancer. Lancet Oncology. 2001;2:88–94. doi: 10.1016/S1470-2045(00)00225-4. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez CA, Jakszyn P, Pera G, Agudo A, Bingham S, Palli D, et al. Meat intake and risk of stomach and esophageal adenocarcinoma within the European Prospective Investigation Into Cancer and Nutrition (EPIC) Journal of the National Cancer Institute. 2006;98:345–54. doi: 10.1093/jnci/djj071. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez CA, Pera G, Agudo A, Bueno-de-Mesquita HB, Ceroti M, Boeing H, et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) International Journal of Cancer. 2006;118:2559–66. doi: 10.1002/ijc.21678. [DOI] [PubMed] [Google Scholar]
- 7.Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. International Journal of Cancer. 2006;119:196–201. doi: 10.1002/ijc.21822. [DOI] [PubMed] [Google Scholar]
- 8.Peek RM., Jr Helicobacter pylori strain-specific modulation of gastric mucosal cellular turnover: implications for carcinogenesis. J Gastroenterol. 2002;37 (Suppl 13):10–6. doi: 10.1007/BF02990093. [DOI] [PubMed] [Google Scholar]
- 9.Pinto-Santini D, Salama NR. The biology of Helicobacter pylori infection, a major risk factor for gastric adenocarcinoma. Cancer Epi Biomarkers Prev. 2005;14:1853–58. doi: 10.1158/1055-9965.EPI-04-0784. [DOI] [PubMed] [Google Scholar]
- 10.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [internet] International Agency for Research on Cancer; Lyon, France: 2010. ( http://globocan.iarc.fr) [Google Scholar]
- 11.Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, et al. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351–65. doi: 10.1111/j.1440-1746.2008.05314.x. [DOI] [PubMed] [Google Scholar]
- 12.Epplein M, Zheng W, Xiang YB, Peek RM, Jr, Li H, Correa P, et al. Prospective study of Helicobacter pylori biomarkers for gastric cancer risk among Chinese men. Cancer Epi Biomarkers Prev. 2012 doi: 10.1158/1055-9965.EPI-12-0792-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai H, Zheng W, Xiang YB, Xu WH, Yang G, Li H, et al. Dietary patterns and their correlates among middle-aged and elderly Chinese men: a report from the Shanghai Men’s Health Study. Br J Nutr. 2007;98:1006–13. doi: 10.1017/S0007114507750900. [DOI] [PubMed] [Google Scholar]
- 14.Epplein M, Shu XO, Xiang YB, Chow WH, Yang G, Li HL, et al. Fruit and vegetable consumption and risk of distal gastric cancer in the Shanghai Women’s and Men’s Health studies. Am J Epidemiol. 2010;172:397–406. doi: 10.1093/aje/kwq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, et al. The Shanghai Women’s Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–31. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 16.Yang YX, Wang GY, Pan XC, editors. China Food Composition Tables 2002. Beijing: Beijing University Medical Press; 2002. [Google Scholar]
- 17.Villegas R, Yang G, Liu D, Xiang YB, Cai H, Zheng W, et al. Validity and reproducibility of the food-frequency questionnaire used in the Shanghai men’s health study. Br J Nutr. 2007;97:993–1000. doi: 10.1017/S0007114507669189. [DOI] [PubMed] [Google Scholar]
- 18.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 19.Nyren O, Adami H-O. Stomach cancer. In: Adami H-O, Hunter D, Trichopoulos D, editors. Textbook of Cancer Epidemiology. 2. New York: Oxford University Press; 2008. pp. 239–74. [Google Scholar]
- 20.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem. 2005;51:1845–53. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 21.Waterboer T, Sehr P, Pawlita M. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods. 2006;309:200–4. doi: 10.1016/j.jim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Michel A, Waterboer T, Kist M, Pawlita M. Helicobacter pylori multiplex serology. Helicobacter. 2009;14:525–35. doi: 10.1111/j.1523-5378.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- 23.Epplein M, Signorello LB, Zheng W, Peek RM, Jr, Michel A, Williams SM, et al. Race, African ancestry, and Helicobacter pylori infection in a low-income United States population. Cancer Epidemiol Biomarkers Prev. 2011;20:826–34. doi: 10.1158/1055-9965.EPI-10-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekstrom AM, Serafini M, Nyren O, Hansson L-E, Ye W, Wolk A. Dietary antioxident intake and the risk of cardia cancer and noncardia cancer of the intestinal and diffuse types: A population-based case-control study in Sweden. International Journal of Cancer. 2000;87:133–40. [PubMed] [Google Scholar]
- 25.Epplein M, Nomura AM, Hankin JH, Blaser MJ, Perez-Perez G, Stemmermann GN, et al. Association of Helicobacter pylori infection and diet on the risk of gastric cancer: a case-control study in Hawaii. Cancer Causes Control. 2008;19:869–77. doi: 10.1007/s10552-008-9149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HJ, Kim MK, Chang WK, Choi HS, Choi BY, Lee SS. Effect of nutrient intake and Helicobacter pylori infection on gastric cancer in Korea: a case-control study. Nutrition & Cancer. 2005;52:138–46. doi: 10.1207/s15327914nc5202_4. [DOI] [PubMed] [Google Scholar]
- 27.Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J Epidemiol. 2003;13:162–8. doi: 10.2188/jea.13.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Carrillo L, Lopez-Cervantes M, Robles-Diaz G, Ramirez-Espitia A, Mohar-Betancourt A, Meneses-Garcia A, et al. Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico. International Journal of Cancer. 2003;106:277–82. doi: 10.1002/ijc.11195. [DOI] [PubMed] [Google Scholar]
- 29.Lunet N, Valbuena C, Carneiro F, Lopes C, Barros H. Antioxidant vitamins and risk of gastric cancer: a case-control study in Portugal. Nutrition & Cancer. 2006;55:71–7. doi: 10.1207/s15327914nc5501_9. [DOI] [PubMed] [Google Scholar]
- 30.Machida-Montani A, Sasazuki S, Inoue M, Natsukawa S, Shaura K, Koizumi Y, et al. Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer. 2004;7:46–53. doi: 10.1007/s10120-004-0268-5. [DOI] [PubMed] [Google Scholar]
- 31.Phukan RK, Narain K, Zomawia E, Hazarika NC, Mahanta J. Dietary habits and stomach cancer in Mizoram, India. Journal of Gastroenterology. 2006;41:418–24. doi: 10.1007/s00535-006-1761-x. [DOI] [PubMed] [Google Scholar]
- 32.Serafini M, Bellocco R, Wolk A, Ekstrom AM. Total antioxidant potential of fruit and vegetables and risk of gastric cancer [see comment] Gastroenterology. 2002;123:985–91. doi: 10.1053/gast.2002.35957. [DOI] [PubMed] [Google Scholar]
- 33.Zhong C, Li KN, Bi JW, Wang BC. Sodium intake, salt taste and gastric cancer risk according to helicobacter pylori infection, smoking, histological type and tumor site in china. Asian Pac J Cancer Prev. 2012;13:2481–4. doi: 10.7314/apjcp.2012.13.6.2481. [DOI] [PubMed] [Google Scholar]
- 34.Peleteiro B, Lopes C, Figueiredo C, Lunet N. Salt intake and gastric cancer risk according to Helicobacter pylori infection, smoking, tumour site and histological type. Br J Cancer. 2011;104:198–207. doi: 10.1038/sj.bjc.6605993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nozaki K, Shimizu N, Inada K, Tsukamoto T, Inoue M, Kumagai T, et al. Synergistic promoting effects of Helicobacter pylori infection and high-salt diet on gastric carcinogenesis in Mongolian gerbils. Jpn J Cancer Res. 2002;93:1083–9. doi: 10.1111/j.1349-7006.2002.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tatematsu M, Nozaki K, Tsukamoto T. Helicobacter pylori infection and gastric carcinogenesis in animal models. Gastric Cancer. 2003;6:1–7. doi: 10.1007/s101200300000. [DOI] [PubMed] [Google Scholar]
- 37.Charnley G, Tannenbaum SR. Flow cytometric analysis of the effect of sodium chloride on gastric cancer risk in the rat. Cancer Res. 1985;45:5608–16. [PubMed] [Google Scholar]
- 38.Loh JT, Torres VJ, Cover TL. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709–15. doi: 10.1158/0008-5472.CAN-06-4746. [DOI] [PubMed] [Google Scholar]
- 39.Loh JT, Friedman DB, Piazuelo MB, Bravo LE, Wilson KT, Peek RM, Jr, et al. Analysis of Helicobacter pylori cagA promoter elements required for salt-induced upregulation of CagA expression. Infect Immun. 2012;80:3094–106. doi: 10.1128/IAI.00232-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakszyn P, Agudo A, Lujan-Barroso L, Bueno-de-Mesquita HB, Jenab M, Navarro C, et al. Dietary intake of heme iron and risk of gastric cancer in the European prospective investigation into cancer and nutrition study. International journal of cancer Journal international du cancer. 2012;130:2654–63. doi: 10.1002/ijc.26263. [DOI] [PubMed] [Google Scholar]
- 41.Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest. 2012 doi: 10.1172/JCI64373. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell HM, Kaakoush NO, Sutton P. Extragastric manifestations of Helicobacter pylori infection. In: Sutton P, Mitchell HM, editors. Helicobacter pylori in the 21st Century. Oxfordshire: CAB International; 2010. pp. 69–93. [Google Scholar]
- 43.Kato I, Tominaga S, Ito Y, Kobayashi S, Yoshii Y, Matsuura A, et al. A comparative case-control analysis of stomach cancer and atrophic gastritis. Cancer Res. 1990;50:6559–64. [PubMed] [Google Scholar]
- 44.Nomura AM, Hankin JH, Kolonel LN, Wilkens LR, Goodman MT, Stemmermann GN. Case-control study of diet and other risk factors for gastric cancer in Hawaii (United States) Cancer Causes Control. 2003;14:547–58. doi: 10.1023/a:1024887411846. [DOI] [PubMed] [Google Scholar]
- 45.Wu-Williams AH, Yu MC, Mack TM. Life-style, workplace, and stomach cancer by subsite in young men of Los Angeles County. Cancer Res. 1990;50:2569–76. [PubMed] [Google Scholar]
- 46.Nouraie M, Pietinen P, Kamangar F, Dawsey SM, Abnet CC, Albanes D, et al. Fruits, vegetables, and antioxidants and risk of gastric cancer among male smokers. Cancer Epidemiol Biomarkers Prev. 2005;14:2087–92. doi: 10.1158/1055-9965.EPI-05-0038. [DOI] [PubMed] [Google Scholar]
- 47.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Systematic review: primary and secondary prevention of gastrointestinal cancers with antioxidant supplements. Alimentary pharmacology & therapeutics. 2008;28:689–703. doi: 10.1111/j.1365-2036.2008.03785.x. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez CA, Lujan-Barroso L, Bueno-de-Mesquita HB, Jenab M, Duell EJ, Agudo A, et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: a reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study after a longer follow-up. International journal of cancer Journal international du cancer. 2012;131:2910–9. doi: 10.1002/ijc.27565. [DOI] [PubMed] [Google Scholar]


