Abstract
Various techniques have been utilized historically to generate acute pulmonary inflammation in the murine system. Crystalline silica exposure results in acute inflammation followed by pulmonary fibrosis. Methods of exposure are varied in their techniques, as well as types of anesthesia. Therefore, the current study sought to compare the effects of two major anesthesia (isoflurane and ketamine) and three routes of instillation, intranasal (IN), intratracheal (IT), and trans-oral (TO), on markers of inflammation. Mice were anesthetized with isoflurane or ketamine and instilled IN with silica or phosphate buffered saline (PBS). Mice were sacrificed and lavaged after three days. To assess inflammation, alveolar cells were assessed by cytospin and lavage fluid was analyzed for inflammatory cytokines and total protein. While all parameters were increased in silica-exposed groups, regardless of anesthesia type, there was significant increase in neutrophils and total protein in mice anesthetized with ketamine, compared to isoflurane. In comparing instillation techniques, mice were anesthetized with isoflurane and instilled IN, IT, or TO with silica. Increases were observed in all parameters, except tumor necrosis factor-α, following IT silica instillation as compared to the IN and TO instillation groups. In addition, fluorescent microsphere uptake by alveolar macrophages supported the notion that all methods of instillation were uniform, but IT had significantly greater dispersion. Taken together, these data show that each method of exposure tested, generated significant inflammation among the silica groups, and any differences in parameters or techniques should be taken into consideration when developing an animal model to study pulmonary diseases.
Keywords: Silica, instillation, ketamine, isoflurane, inflammation, mouse
Introduction
Animal models of human disease are vital to biomedical research. Because of inherent physiological differences between human and mouse, the successful model is determined by a defined set of parameters and not the ability to exactly replicate the human condition. Both human and mouse exposures to silica can result in pulmonary fibrosis, referred to as silicosis. In the mouse model, successful exposure to crystalline silica results in acute inflammation followed by fibrotic lesions and decreased lung capacity (Huaux, F. 2007, Migliaccio, C.T., et al. 2008). These models have utilized different modes of exposure, including intranasal (IN) and intratracheal (IT), as well as inhalation techniques for the introduction of particles to murine lungs (Beamer, C.A. and Holian, A. 2005, Choi, M., et al. 2008, Migliaccio, C.T., et al. 2005, Paul, B.N., et al. 2002, Thakur, S.A., et al. 2009). A similar technique, trans-oral (TO) instillation, is a relative compromise between IN and IT for training and ease of use. While inhalation would most closely resemble human exposure, a large number of studies utilize instillations for its relative ease. A direct comparison of these techniques in the silicosis model has not been done.
The anesthesia method employed by individual laboratories can be based on such reasoning as availability, anecdotal evidence (i.e. pulmonary inflammation), or ease of use. Isoflurane is a volatile anesthesia commonly utilized in animal models, and is introduced by inhalation. It is well documented that volatile anesthetics have anti-inflammatory effects in certain organs, including the lungs (Fuentes, J.M., et al. 2006, Imai, T., et al. 1998, Lee, H.T., et al. 2007, Reutershan, J., et al. 2006). They have also been found to have similar effects in vitro and in human studies (Brand, J.M., et al. 1997, Xu, X., et al. 2008). However, others have found that with certain disease models volatile anesthetics have not attenuated acute inflammation following injury, but rather increased the inflammatory response in the mouse lungs (Nader-Djalal, N., et al. 1998). Another commonly used anesthesia, ketamine, is injected intraperitoneally (i.p.), and has been reported to lead to significant decreases in cardiovascular parameters compared to volatile anesthetics (Janssen, B.J., et al. 2004). While no known inflammatory response has been observed at the anesthetic level, Chang et al. found that macrophage function was suppressed at a concentration 10 times the clinically relevant dose of ketamine (Chang, Y., et al. 2005). To date, a direct comparison of these anesthesia methods has not been done in either the silicosis or acute inflammation mouse models.
The present study compared parameters of acute inflammation following silica exposure in mice anesthetized with isoflurane or ketamine, as well as a comparison of IN, IT and TO instillation techniques. The cellular composition of the alveolar space was compared, as were inflammatory cytokines (tumor necrosis factor (TNF)-α and interleukin (IL)-1β), total protein, and cell population changes in lung lavage. The goal of these studies was to determine if there is a significant difference between silica exposure techniques and the resulting acute inflammatory response, a precursor to the silica-induced pulmonary fibrosis observed in both the human condition and murine model. These results will provide guidance for the design of future protocols in an effort to fine-tune a model of a human disease.
Methods
Mice
Balb/c mice (Jackson Laboratories, Bar Harbor, ME) 6-8 weeks of age were used for all animal experiments. Animals were kept on a 12-h light/dark cycle and housed in microisolators. The mice were maintained on a standard diet and given deionized water ad libitum. The University of Montana Institutional Animal Care and use Committee (IACUC) approved all animal procedures.
Silica preparation
Acid-washed crystalline silica (Min-U-Sil-5, average particle size 1.5-2 μm) obtained from Pennsylvania Glass Sand Corporation (Pittsburgh, PA) was used for these studies. Silica was determined endotoxin-free by Limulus Amoebocyte Lysate assay (Cambrex, Walkersville, MD). Immediately prior to use in experiments, particles were resuspended in phosphate buffered saline (PBS) at a concentration of 1 mg/25 μl and sonicated for 1 min by using a cup-horn sonicator in a circulating water bath.
Silica Exposure
For silica and PBS exposures, mice were anesthetized intraperitoneally using 0.1 cc ketamine (1:4 in sterile PBS) or by a constant flow of 2% isoflurane. Mice were then instilled with 25 μl of specified treatment. Treatments included PBS alone or 1 mg crystalline silica in PBS. Mice were instilled with silica particles using one of the following methods: non-surgical intratracheal (IT), trans-oral (TO), or intranasal (IN). The IT instillation technique is as follows: mice are anesthetized, suspended on an angled board by hooking the front teeth on a suspended rubber band, the tongues are gently extended out of the oral cavity, and the suspension is instilled in the trachea using a blunt-ended 25-gauge needle that has been curved to accommodate the trachea. The TO aspiration technique follows the same procedure as discussed above, except the inoculum is instilled by using a pipette to instill the treatment directly behind the extended tongue. Mice instilled IN utilized the same anesthesia system as previously described. The anesthetized mice are suspended nose up by grasping behind the neck and shoulders and a pipette is used to drop the inoculum directly in the nose.
Isolation of alveolar cell populations
Cells from the alveoli were isolated by whole lung lavage. Each mouse was lavaged with 3-4 ml of cold PBS and collected fluid was stored on ice. The first draw (1 mL) was centrifuged (500g for 2 min) and this supernatant was set aside (on ice or at -20°C) for later analyses on protein and cytokine levels. The cell pellet was then added to the remaining 2-3 mL of lavage and centrifuged (500 × g for 10 min) to collect all cells. The cell pellet was resuspended in 500 μl PBS, and enumerated using a Coulter Counter.
Cytospin analysis
Cells obtained from lung lavage were resuspended in PBS+10% Fetal bovine serum (FBS) at a concentration of ∼3×104 cells per 30 μl. Cells were then added to 300 μl of PBS for cytocentrifugation (Cytospin III; Shandon Instruments, Pittsburgh, PA) onto positively charged microscope slides (Fisher Scientific, Pittsburgh, PA) at 1500 rpm for 5 min. Following 30 min of air-drying, slides were stained with Hema 3 (Fisher Scientific) then air-dried in laminar flow hoods prior to analysis. For differential analysis, 3-4 random fields were assessed per slide and >200 cells were enumerated with alveolar macrophage (AM) and polymorphonuclear leukocyte (PMN) percentages calculated.
Cytokine and protein analysis
The first 1 mL of lung lavage fluid collected from the samples were assayed for cytokines with commercially available kits according to the manufacturer's protocol. IL-1β and TNF-α measurements were determined using Duo-set kits (R&D Systems). Samples were diluted 1:1 in both assays with reagent diluent. Colormetric analysis was used by analyzing the samples with the Spectra Max 340 plate reader (GE healthcare) at 450 nm. Data are shown as pg/ml of collected lavage fluid. Sample lavage fluid was also assayed using a BCA Protein kit (Pierce). Protein data is reported as mg/ml of collected lavage fluid.
Flow cytometry
Fluorophorex ™ Microspheres were purchased from Phosphorex, Inc, (Fall River, MA). Microspheres were suspended in PBS for a final 10 mg/ml stock solution. Mice were instilled IN, IT, or TO with 25 μl of this suspension. Four hours post instillation mice were sacrificed and lung lavage fluid was collected. Analysis of fluorescent microsphere uptake by alveolar macrophages was performed using the FACSAria (BD Biosciences, San Jose, CA) system. Briefly, cells isolated by lavage following microsphere instillation were resuspended in FACS buffer (0.1% BSA + 0.5% sodium azide). Cells were transferred to filter-top polypropylene tubes (BD Labware, Franklin Lakes, NJ) for analysis. Data analysis was performed using FACSDiva software (BD Biosciences).
Statistical analysis
All one-factor experimental designs were analyzed by one-way analysis of variance followed by Newman-Keuls's comparison of all mean pairs. Two-factor designs were analyzed by two-way ANOVA followed by Bonferroni's test. Sample size varied from 4 and 6 experimental replications per group, depending on the experiment and desired statistical power (> 0.80). Statistical significance was established as a two-tailed probability of type I error occurring at less than 5% (P < 0.05). All data expressed as mean ± SEM. Analysis and graphics was performed on Prism 4.0 software (GraphPad, San Diego, CA).
Results
Cellular analysis of inflammation following isoflurane and ketamine anesthesia
To assess the impact of anesthesia on acute inflammation following silica exposure, mice were anesthetized with isoflurane or ketamine and instilled with silica or phosphate buffered saline (PBS) using the IN procedure. At three days post-treatment, cells in the alveolar spaces were collected by lung lavage, enumerated, and characterized by cytospin analysis. All mice treated with silica showed a significant increase in total cell count compared to PBS controls, with no significant difference between types of anesthesia (Fig. 1A). Because neutrophil (PMN) influx is a classic marker of inflammation, differentials of the total cell counts were also compared (Fig. 1B). While both isoflurane and ketamine anesthesia groups showed a significant increase in PMN after silica exposure, mice receiving ketamine anesthesia had significantly higher numbers. The influx of PMN to the alveolar space following silica exposure explains the majority of the difference in total cell count where the AM counts were not significantly increased in either isoflurane of ketamine groups (data not shown). These results suggest a nuanced difference in the level of acute inflammation depending on the method of anesthesia.
FIG 1.

Comparison of anesthesia methods by total cell and PMN assessments following silica instillation. Following IN instillation with silica or PBS, mice were lavaged and cells analyzed by cytospin. Solid bars represent silica exposure. Open bars represent PBS exposure. (A) Total cells numbers from silica-treated mice were significantly increased (*, indicates p<0.05; and **, indicates p<0.01) over PBS controls. (B) PMN levels from silica-treated mice were significantly (***, indicates p<0.001) increased over corresponding PBS controls, and PMN levels in the silica-ketamine mice was significantly (*, indicates p<0.05) higher than that observed in the silica-isoflurane mice. Bars in the graphs represent means (n=4-8) ± S.E.
Protein analysis following isoflurane and ketamine anesthesia
To further characterize the difference in acute inflammation observed in mice anesthetized with isoflurane and ketamine, the levels of total protein and inflammatory cytokines (tumor necrosis factor (TNF)-α and interleukin (IL)-1β) were measured in lung lavage fluid. While the total protein in mice treated with silica and isoflurane was increased, it did not achieve statistical significance compared to the PBS control group; however, silica treatment with ketamine significantly increased total protein when compared with the PBS control, as well as the isoflurane silica treatment group (Fig. 2A). Silica increased the level of both cytokines, regardless of route of anesthesia, however, ketamine also increased the level of IL-1β in the PBS-treated mice (Fig. 2B), and TNFα levels in the isoflurane mice (PBS and silica) were higher than that observed in the corresponding ketamine groups (Fig. 2C). These results suggest that with minor differences, silica instillation generates classic inflammation regardless of anesthesia.
FIG 2.
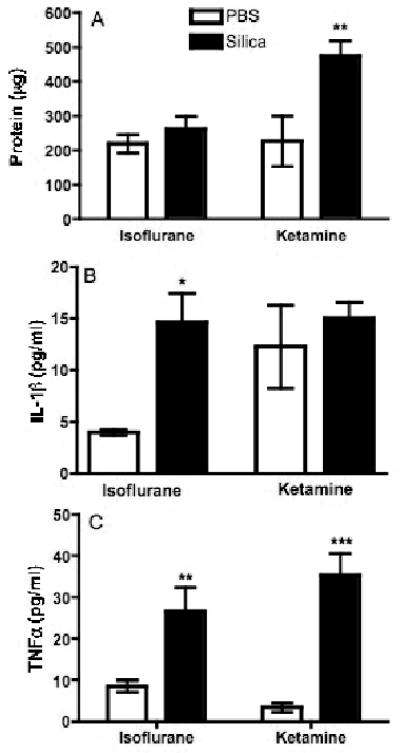
Comparison of anesthesia methods by assessment of soluble markers of inflammation in silica-exposed mice. Following IN instillation, mice were lavaged and the first 1 ml of fluid was analyzed for inflammatory cytokines and total protein. Solid bars represent silica exposure and open bars represent PBS exposure. (A) While total protein was increased in silica-treated mice for both anesthesia groups, significance was achieved in the silica-ketamine mice (*, indicates p<0.05) compared with corresponding PBS controls, as well as the isoflurane treatment groups. (B) IL-1β levels were increased in the silica-treated mice as compared to the corresponding PBS controls. (C) TNFα levels were significantly (*, indicates p<0.05; and ***, indicates p<0.001) increased in silica-treated mice over the corresponding PBS controls. In addition, TNF-α concentrations in the silica-isoflurane were significantly (**, indicates p<0.01) higher than the silica-ketamine mice. Bars in the graphs represent means (n=4-8) ± S.E.
Comparison of multiple instillation techniques
Since isoflurane is the easiest to use and results in the least number of anesthesia-induced deaths (data not shown), it was utilized to compare routes of instillation. At three days post-treatment, mice instilled by IT showed a significant increase in total cell count in lavage fluid compared to IN and TO instillation groups (Fig. 3A). Additionally, there was a trend in increased PMN in the IT treatment group compared with the IN and TO treatment groups, however this increase was not statistically significant (Fig. 3B). Assays for total protein, IL-1β, and TNF-α concentrations were conducted as described above. The total protein content in the alveolar spaces of mice instilled IT was significantly higher than that of the IN and TO treatment groups (Fig. 4A), while IL-1β was significantly elevated in both IT- and TO-instilled mice, as compared with the IN group (Fig. 4B). There was no significant difference observed in the TNFα concentrations when comparing instillation groups, however there was a slightly higher level in the IN treatment group (Fig. 4C). These results suggest that mice instilled IT with silica have an increased level of inflammation when compared with IN and TO instillation groups.
FIG 3.
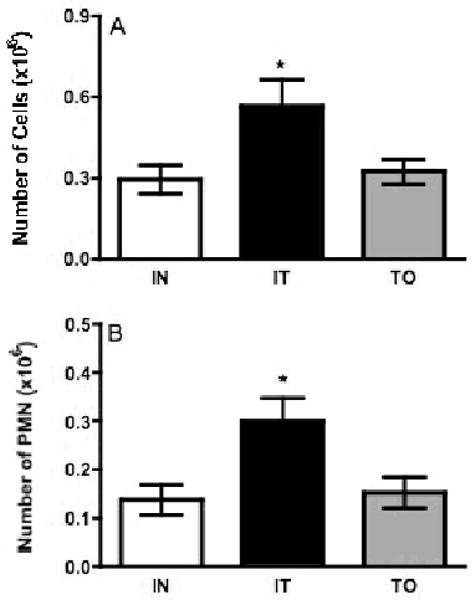
Comparison of instillation techniques by total cell and PMN assessments following silica instillation. All mice were anesthetized by isoflurane and instilled by one of the following techniques: IN, IT, or TO. Following instillation with silica, mice were lavaged and cells analyzed by cytospin. Open bars represent intranasal (IN) silica instillation, black bars represent intratracheal (IT) instillation, and gray bars represent trans-oral (TO) instillation. (A) Mice instilled by IT had significantly (*, indicates p<0.05) higher levels of total cells as compared to TO and IN. (B) Mice from each group had large increases in PMN numbers following silica instillation, and while higher levels were observed in the IT group it did not reach significance. Bars in the graphs represent means (n=4-8) ± S.E.
FIG 4.

Comparison of instillation techniques by assessment of soluble markers of inflammation in silica-exposed mice. All mice were anesthetized by isoflurane and instilled by one of the following techniques: IN, IT, or TO. Following instillation with silica, mice were lavaged and cells analyzed by cytospin. Open bars represent intranasal (IN) silica instillation, black bars represent intratracheal (IT) instillation, and gray bars represent trans-oral (TO) instillation. (A) Total protein levels were significantly (**, indicates p<0.01 compared with TO treatment group; and ***, indicates p<0.001 compared with IN treatment) higher in the IT instilled mice. (B) IL-1β levels were significantly (*, indicates p<0.05) elevated in both the IT and TO mice as compared to the IN group. (C) All groups had detectable TNF-α with no significant differences between them. Bars in the graphs represent means (n=4-8) ± S.E.
Fluorescent microsphere uptake by alveolar macrophages
To further compare techniques, fluorescent microspheres were instilled to indirectly assess particle dispersion. Mice were anesthetized with isoflurane and instilled IN, IT, and TO with fluorescent microspheres. At four hours post treatment, mice were euthanized and AM were collected by lavage and analyzed for particle uptake by flow cytometry. The percentage of cells positive for fluorescent particle was assessed using a 2-parameter dotblot (Fig. 5A). While all groups showed particle uptake by AM, there were significantly more positive macrophages in the IT and TO groups as compared to the IN animals (Fig. 5B). These data show a strong correlation between initial particle dispersion and the relative intensity of acute inflammation induction.
FIG 5.
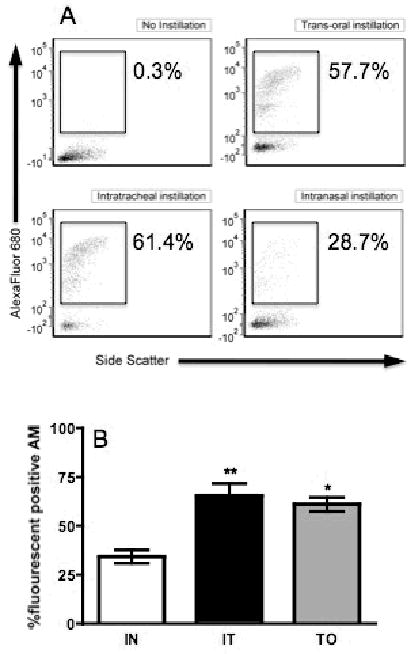
Comparison of instillation techniques by assessment of fluorescent microsphere particle uptake by alveolar macrophages. All mice were anesthetized by isoflurane and instilled by one of the following techniques: IN, IT, or TO. Following instillation, mice were lavaged and cells analyzed by flow cytometry. In order to assess the AM uptake of fluorescent microspheres cells were assessed on a 2-parameter dotblot (side scatter vs. fluorescence). (A) These are representative blots of the three types of instillation, in addition to a “no instillation” control. Each blot has the percentage of AM that had phagocytosed the microspheres. (B) Open bars represent intranasal (IN) silica instillation, black bars represent intratracheal (IT) instillation, and gray bars represent trans-oral (TO) instillation. Uptake of microspheres was observed for all instillation methods, but was significantly higher in the TO and IT mice (*, indicates p<0.05; **, indicates p<0.01) compared with the IN instillation mice. Bars in the graphs represent means (n=4-8) ± S.E.
Discussion
Mice are utilized for modeling a variety of human lung diseases. While no animal model is an exact mimic of the human condition, steps are taken and adjustments made in an attempt to be as close as possible. The adequacy of a specific model is based on the attainment of pre-determined, measurable parameters. Significant exposure to silica results in pulmonary fibrosis in both the human and murine conditions. The goal of this work was to assess different instillation protocols using the silica model of acute inflammation. These studies compared types of anesthesia, as well as instillation techniques. The anesthesia methods compared were intraperitoneally (i.p.) injected ketamine or inhaled isoflurane, while the three methods of instillation were intratracheal (IT), trans-oral (TO), or intranasal (IN).
Silicosis is one of many pulmonary diseases that uses animals exposed to particulates for researching the human disease. This pulmonary model utilizes the same particles as the human condition to induce inflammation and fibrosis. Previous work in our laboratory has described aspects of the acute inflammation, as well as the later development of fibrosis (Beamer, C.A. and Holian, A. 2005, Migliaccio, C.T., et al. 2008, Migliaccio, C.T., et al. 2005, Thakur, S.A., et al. 2009). In the present study we showed that instillation of silica particles resulted in an increase in various markers of inflammation. Where different methods of anesthesia have been employed in mouse models, one of the aims of the present study was to assess potential effects of anesthesia on the silica-induced pulmonary inflammation model. Silica instillations were performed using one of the methods described above. The IT technique directly instills the suspended particles in the trachea, while the TO technique instills the suspension behind the tongue, and the IN technique delivers the suspension drop-wise into the nose. Isoflurane is an inhaled volatile anesthetic while ketamine is injected intraperitoneally. It has been documented that volatile anesthetics have anti-inflammatory effects in certain organs (Fuentes, J.M., et al. 2006, Imai, T., et al. 1998, Lee, H.T., et al. 2007, Lee, H.T., et al. 2007, Reutershan, J., et al. 2006), including the lungs. However, other research groups have shown in certain disease models volatile anesthetics increased the inflammatory response in murine lungs (Nader-Djalal, N., et al. 1998). While ketamine had not yet been examined in terms of the resulting inflammation, studies have shown that ketamine can suppress macrophage function (Chang, Y., et al. 2005).
The comparative anesthesia studies demonstrated that when utilizing anesthesia in a pulmonary disease model, different agents cause varying inflammatory responses. While the markers of inflammation all increased with silica instillation, regardless of anesthesia, there were differences in intensity for certain assays. Total cells, total protein, and PMN levels were higher in the ketamine mice, as compared to the isoflurane groups. The differences seen in the PMN cell counts (Fig. 1B) and the protein concentrations (Fig. 2A) were significantly higher in the silica instilled ketamine group than the corresponding isoflurane group. Although not all assays showed a significant difference between anesthesia groups, and one (Fig. 2C) actually presented with the opposite trend, there was a general trend of a decreased inflammatory response in the isoflurane group. It should also be noted that there is a risk of occasional death when injecting ketamine due to many various error factors. The constant flow of isoflurane presents relative ease of use when compared with the risk of ketamine as no accidental deaths have been observed, to date.
The comparative analysis of particle instillation techniques suggests that there is a difference between the IN, IT, and TO instillation techniques. When comparing cell populations, there is an association between silica instillation and an increased recruitment of cells, specifically PMN, to the lung, indicating an increased inflammatory response. The difference in total cell number was significantly higher with IT instillation (Fig. 3A), while PMN counts demonstrated a similar trend; the difference was not significant (Fig. 3B). When examining inflammatory parameters in collected lavage fluid, a similar trend is present. The IT instillation group showed a significant increase in total protein concentration when compared with both the IN and TO instillation groups (Fig. 4A). In addition, both the IT and TO instillation groups had significantly higher IL-1β concentrations when compared to the IN instillation group (Fig. 4B). These data taken together suggest that while IT generates the highest relative levels of inflammation and IN the lowest, all three techniques result in significant pulmonary inflammation.
While the present study focused on different methods of instillation, the inhalation of silica particles is another viable technique. There are two variations of inhalation methods: whole body and nose-only. The argument in favor of inhalation techniques is the obvious relevance to the human exposure. However, there are complicating factors that can discourage use of these for relative ease of instillation methods. The major point of discouragement is the requirement of a special apparatus for either inhalation. In addition, whole body has the added complication of particle deposition on the surface of the mice and the potential for added, unregulated exposures. As well the nose-only apparatus has the potential for stress-induced complications. However, because mice are nasal breathers, the use of IN instillations could, arguably, be considered the closest approximation to inhalation techniques.
In an effort to explain the higher levels of inflammation using the IT technique, mice were comparatively instilled with fluorescent microspheres to assess the potential role of particle dispersion. It was reasoned that with all things being equal (mice, anesthesia, particles), particle uptake would be a direct measure of availability (i.e. dispersion), and because the particles have a net negative charge, they are a good indicator of macrophage phagocytosis/uptake. Four hours following instillations alveolar macrophages (AM) were collected and the relative distribution of the microspheres was assessed by flow cytometry (Fig. 5). When comparing the IN, IT, and TO instillation techniques, the percent of fluorescent-positive AM was assessed, and a similar trend was observed. The IT and TO particle instillation groups had a significantly higher percentage of AM with fluorescent particles when compared with the IN instillation group, and, although not significant, the IT instillation group resulted in the highest mean percent of particle uptake by AM. One explanation for these results would be that there is a delay, or decrease, in particle delivery to the lungs following IN instillation, where particles may be caught in the nasal passages.
These studies determined that regardless of anesthesia or route of instillation, silica induces acute inflammation in mice. Taken together, these results suggest that the most effective method would employ ketamine anesthesia coupled with IT instillations. While this protocol would likely achieve the greatest level of acute inflammation by the parameters measured, there are other considerations when determining which method is best. For example, although it requires a special apparatus, isoflurane is much easier to administer and regulate than ketamine, while ketamine has the relatively common observation of overdose deaths (unpublished). Likewise, mice anesthetized with isoflurane have a much quicker recovery time than ketamine-treated animals. In addition, there are technical considerations when evaluating instillation methods, where the IN instillation technique is the least challenging and requires minimal training, and more closely resembles an inhalation exposure. Finally, because previous studies in our laboratory have utilized the IN technique, it is known that, despite its apparent deficit in initial particle dispersion, the hallmark of pulmonary fibrosis is still achieved in long-term studies. The present study did not assess long-term implications of any of the differences in the techniques being compared. All these differences should be taken into account when designing a protocol for lung exposure models in mice to determine the best fit for the experimental parameters to be tested.
Acknowledgments
We extend our appreciation to Pam Shaw and the Flow Cytometry Core for assistance in assays and data analysis, and Raymond Hamilton for statistical guidance.
Footnotes
This publication was made possible by grants 4743-RFA04-4/06-4 from HEI, and RR-017670 from the NCRR, a component of NIH, and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR, NIEHS, or NIH.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Beamer CA, Holian A. Scavenger receptor class A type I/II (CD204) null mice fail to develop fibrosis following silica exposure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L186–195. doi: 10.1152/ajplung.00474.2004. [DOI] [PubMed] [Google Scholar]
- Brand JM, Kirchner H, Poppe C, Schmucker P. The effects of general anesthesia on human peripheral immune cell distribution and cytokine production. Clin Immunol Immunopathol. 1997;83:190–194. doi: 10.1006/clin.1997.4351. [DOI] [PubMed] [Google Scholar]
- Chang Y, Chen TL, Sheu JR, Chen RM. Suppressive effects of ketamine on macrophage functions. Toxicol Appl Pharmacol. 2005;204:27–35. doi: 10.1016/j.taap.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Choi M, Cho WS, Han BS, Cho M, Kim SY, Yi JY, Ahn B, Kim SH, Jeong J. Transient pulmonary fibrogenic effect induced by intratracheal instillation of ultrafine amorphous silica in A/J mice. Toxicol Lett. 2008;182:97–101. doi: 10.1016/j.toxlet.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Fuentes JM, Talamini MA, Fulton WB, Hanly EJ, Aurora AR, De Maio A. General anesthesia delays the inflammatory response and increases survival for mice with endotoxic shock. Clin Vaccine Immunol. 2006;13:281–288. doi: 10.1128/CVI.13.2.281-288.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaux F. New developments in the understanding of immunology in silicosis. Curr Opin Allergy Clin Immunol. 2007;7:168–173. doi: 10.1097/ACI.0b013e32802bf8a5. [DOI] [PubMed] [Google Scholar]
- Imai T, Takahashi K, Masuo F, Goto F. Anaesthesia affects outcome of sepsis in mice. Can J Anaesth. 1998;45:360–366. doi: 10.1007/BF03012029. [DOI] [PubMed] [Google Scholar]
- Janssen BJ, De Celle T, Debets JJ, Brouns AE, Callahan MF, Smith TL. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol. 2004;287:H1618–1624. doi: 10.1152/ajpheart.01192.2003. [DOI] [PubMed] [Google Scholar]
- Lee HT, Emala CW, Joo JD, Kim M. Isoflurane improves survival and protects against renal and hepatic injury in murine septic peritonitis. Shock. 2007;27:373–379. doi: 10.1097/01.shk.0000248595.17130.24. [DOI] [PubMed] [Google Scholar]
- Lee HT, Kim M, Kim M, Kim N, Billings FTt, D'Agati VD, Emala CW., Sr Isoflurane protects against renal ischemia and reperfusion injury and modulates leukocyte infiltration in mice. Am J Physiol Renal Physiol. 2007;293:F713–722. doi: 10.1152/ajprenal.00161.2007. [DOI] [PubMed] [Google Scholar]
- Migliaccio CT, Buford MC, Jessop F, Holian A. The IL-4Ralpha pathway in macrophages and its potential role in silica-induced pulmonary fibrosis. J Leukoc Biol. 2008;83:630–639. doi: 10.1189/jlb.0807533. [DOI] [PubMed] [Google Scholar]
- Migliaccio CT, Hamilton RF, Jr, Holian A. Increase in a distinct pulmonary macrophage subset possessing an antigen-presenting cell phenotype and in vitro APC activity following silica exposure. Toxicol Appl Pharmacol. 2005;205:168–176. doi: 10.1016/j.taap.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Nader-Djalal N, Knight PR, Bacon MF, Tait AR, Kennedy TP, Johnson KJ. Alterations in the course of acid-induced lung injury in rats after general anesthesia: volatile anesthetics versus ketamine. Anesth Analg. 1998;86:141–146. doi: 10.1097/00000539-199801000-00029. [DOI] [PubMed] [Google Scholar]
- Paul BN, Prakash A, Kumar S, Yadav AK, Mani U, Saxena AK, Sahu AP, Lal K, Dutta KK. Silica induced early fibrogenic reaction in lung of mice ameliorated by Nyctanthes arbortristis extract. Biomed Environ Sci. 2002;15:215–222. [PubMed] [Google Scholar]
- Reutershan J, Chang D, Hayes JK, Ley K. Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology. 2006;104:511–517. doi: 10.1097/00000542-200603000-00019. [DOI] [PubMed] [Google Scholar]
- Thakur SA, Beamer CA, Migliaccio CT, Holian A. Critical Role of Marco in Crystalline Silica-Induced Pulmonary Inflammation. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Feng J, Zuo Z. Isoflurane preconditioning reduces the rat NR8383 macrophage injury induced by lipopolysaccharide and interferon gamma. Anesthesiology. 2008;108:643–650. doi: 10.1097/ALN.0b013e318167aeb4. [DOI] [PubMed] [Google Scholar]


