Summary
Atherosclerosis and heart disease are the main cause of death in United States. The development of atherosclerosis includes lipid deposition and foam cell formation in the artery wall. Scavenger Receptors A-I and II (SRA-I/II) have an important role of in foam cell formation and atherogenesis. Most of the SRA-I/II studies had been performed using Iodine-125 radiolabeled modified LDL. This report attempts to validate the use of fluorescence microscopy techniques as an alternative to obtain qualitative and quantitative information of the uptake of fluorescence labeled AcLDL in adherent CHO cells expressing SRA-I/II. After verifying the protein expression of SRA-I and II, uptake was quantified using a Laser Scan Cytometer, and images of cells containing fluorescent AcLDL were obtained. A significant increase in fluorescence was found in the cells transfected with SRA-I/II versus those with empty vector. When SRA-I/II competitive ligands were used, the uptake of AcLDL was significantly decreased. In conclusion, the use of fluorescence microscopy techniques in obtaining qualitative and quantitative information of the uptake of fluorescence labeled AcLDL by adherent cells, such as CHO cells, is an alternative to the traditional use of radiolabeled iodine.
Keywords: Lipids, Low density lipoproteins, Scavenger Receptors, Macrophage, endocytosis
Introduction
Atherosclerosis and heart disease are the main cause of death in the United States. The development of atherosclerosis includes lipid deposition and foam cell formation in the artery wall. Scavenger receptors (SRs) are members of a family of membrane receptors, initially described as a binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein (AcLDL) (Goldstein et al. 1979). Today it is well recognized that SRs are a growing family of glycoproteins that not only have a broad ligand binding ability, but also are expressed in different cell types (Peiser and Gordon 2001). The scavenger receptors class A (SRA) are expressed in macrophages and have the ability to bind different kind of ligands such as modified LDL, negative charged particles, LPS, etc (Platt and Gordon 2001). SRAs also have an important role mediating cell adhesion (Fraser et al. 1993). Numerous studies had shown the important role of SRA I and II, two splice variants, in foam cell formation and atherogenesis (Babaev et al. 2000; de Winther et al. 1999a; de Winther et al. 1999b; Kunjathoor et al. 2002; Suzuki et al. 1997). Modified low density lipoproteins (mLDL) such as AcLDL or oxidized LDL (OxLDL) are taken through the macrophage scavenger receptor A I/II (SRA-I/II). Most of the SRA-I/II studies, where uptake of mLDL was quantified, had been performed using Iodine-125 (125I) radiolabeled AcLDL or OxLDL (Andersson and Freeman 1998; Ashkenas et al. 1993; Doi et al. 1993; Doi et al. 1994; Fortin et al. 2000; Freeman et al. 1991; Morimoto et al. 1999). It is known that 125I emits gamma radiation, and due to its ability to volatilize special precautions are required to prevent its inhalation or absorption through skin. 125I after reaching the bloodstream accumulates in thyroid gland increasing the risk of developing thyroid malignancy.
Currently the use of fluorescence labeled ligands such as antibodies or lipids are a better alternative, and flow cytometry is commonly used as a method for detection and quantification. However, it is require for flow cytometry analysis that cells be in suspension, which is a limitation for adherent cells. Moreover, some adherent cells will require to be treated with trypsin and/or EDTA for detachment and be in suspension. This treatment with trypsin and/or EDTA could interfere with surface receptors and concentration of free calcium necessary for endocytosis. Once adherent cells are detached, re-attachment must be prevented. The use of a rotator during incubation for a short time could prevent cell adhesion to the vial wall and keep cells in suspension, but when performing functional assays requiring long time exposure, adherent cells kept in suspension will experience changes in viability compromising the final results of the assay. In such case, the use of fluorescence microscopy for the detection of fluorescence labeled ligands in adherent cells when performing functional assays requiring long time exposure is a better alternative, because cells will be left attached to the surface, no requiring trypsin and /or EDTA treatment that could interfere with the assay, and the use of a rotator will not be required to prevent cell re-attachment.
Studying functional assays using fluorescence microscopy techniques requires the use of an appropriate method. Moreover when lipids are used as ligands, and membrane integrity needs to be preserved in order to prevent losing or misplacing the fluorescence labeled lipid. Only after choosing the appropriate method, the image analysis will be more accurate. This report attempts to validate the use of fluorescence microscopy techniques in obtaining qualitative and quantitative information of the uptake of fluorescent labeled AcLDL using stable transfected adherent CHO cells.
Materials and methods
Cell and Lipoproteins
Chinese Hamster Ovarian cells CHO-K1 cells (ATCC:CCL-61) were grown in Ham’s F12K medium with 2 mM L-glutamine and 10% fetal bovine serum at 37°C in a humidified 5% CO2 / 95% air incubator. Alexa Fluor 488 conjugated AcLDL (Invitrogen, Carlsbad, CA).
Plasmid Constructs
The murine SRA-I cDNA used was obtained from pXmSRI-28 donated Dr. Monty Krieger (Biology Department, MIT, MA). The murine SRA-II cDNA used was obtained from Invitrogen NIH Mammalian Gene Collection full-length (IRAV), clone ID 3594142 Mus musculus macrophage scavenger receptor 1 cDNA (SRA-II), which was inserted into a pCMV-SPORT6 vector. The following procedures were performed in order to subclone the SRA-I and SRA-II cDNA into the vector pcDNA3.1/myc-His-A and/or pcDNA3.1(+) Hygro (Invitrogen). The restriction sites KpnI and XbaI were inserted in the SRA-I and SRA-II sequences at the 5’ and 3’ terminus respectively by polymerase chain reaction (PCR) using Pfu DNA polymerase (Stratagene, La Jolla, CA). In order to introduce the SRA-I and SRA-II PCR products into the vectors, both were previously digested with KpnI and XbaI and then ligated with T4 DNA Ligase (Promega, Madison, WI). DH5α cells (Invitrogen) were transformed with each construct. After verifying the DNA sequence of the constructs, CHO cells were transfected.
Transfection
Five constructs were transfected in CHO cells separately: SRA-II-pcDNA3.1/myc-His-A (SRAII), SRA-I-pcDNA3.1(+)-Hygro (SRA-Ih), SRA-II-pcDNA3.1(+)-Hygro (SRA-IIh), and their respective empty vector. Lipofectamine 2000 Transfection Reagent (Invitrogen) was used for CHO cells transfection following manufacturer’s instructions. Briefly, 24 hours before transfection CHO cells were plated in 24-well plate at 1.2 × 105 cell per well in 0.5 ml of antibiotic free medium. Then 0.8 μg of vector DNA and 2.5 μl of transfection reagent were diluted in separate tubes with 50 μl of Opti-mem I reduced serum medium (Invitrogen). After a five-minute incubation at room temperature both solutions were combined and re-incubated at room temperature for 20 additional minutes. The combined solution was then added directly to the well containing CHO cells and mixed. Cells were incubated at the conditions previously mentioned and growth medium was changed after 4 hours. Forty-eight hours after transfection Geneticin or Hygromycin antibiotic (Invitrogen) was added at a final concentration of 500 and 700 μg/ml respectively for antibiotic selection of transfected cells. Successful clones were isolated and characterized.
Immunoprecipitation and Western Blot
Ten million cells were incubated on ice for 30 min in 200 μl of lysis buffer (150 mM NaCl, 10 mM EDTA, 10 mM NaN3, 10 mM Tris [pH 8.0], 1 mM PMSF, 5 mM iodacetamide and 1% Nonidet P-40 [NP-40]). Rat anti mouse CD204 antibody (2F8) (Serotec, Oxford, UK) was added to reach a final dilution of 1:40, and incubated overnight at 4°C in an end-over-end rotator. Thereafter 30 μl of Protein G-agarose (Roche Applied Science, IN) was added and incubated under the same conditions overnight. The lysate containing the antibodies and protein G-agarose was washed three times and 30 μl of Laemmli sample buffer containing 5% beta mercaptoethanol was added. CD204 immunoprecipitated cell lysate from transfected and untransfected cells were incubated at 70°C for ten minutes, loaded in a 4-15% linear gradient Ready Gel Tris-HCl gel (Bio-Rad, Hercules, CA), and electrophoresed for 1 hour at 100 volts; then proteins were electrophoretically transferred to Trans-Blot Transfer medium nitrocellulose membrane (Bio-Rad) for 1.5 hrs at 100 volts. After blocking for 1 hour at room temperature, primary monoclonal anti-6X His tag antibody (Abcam, Cambridge, MA) was added to reach a final dilution of 1:5000, and incubated overnight at 4°C. Membranes were washed 3 × 5 minutes and exposed to horseradish peroxidase conjugated anti-rabbit IgG antibody (Cell Signaling Technology, Danvers, MA) for 1 h at room temperature. Enhanced chemiluminescence (ECL) development was performed with ECL western blotting detection reagents (Amersham Biosciences, Piscataway, NJ) according to manufacturer’s instructions. Images were taken using a Versadoc Imagining System 3000 (Bio-Rad).
AcLDL Uptake
Cells were plated in a Lab-Tek II eight-well chamber slide (Nalge Nunc International, Rochester, NY) at 5×104 cells per well 24 hours before exposure. When SRs ligands were used, cells were pre-treated with Polyinosinic acid (PolyI) (Sigma-Aldrich, Saint Louis, MO) at 100 μg/ml or anti CD204 blocking antibody (2F8) (Serotec) at 1 μg/ml for 30 minutes. Alexa Fluor 488 conjugated AcLDL was then added to the cells at a final concentration of 10 μg/ml and incubated for 90 minutes at 37°C. After incubation medium was removed, cells were fixed with 3% paraformaldehyde in PBS, incubated for 20 minutes at room temperature, and washed four times with PBS as reported by DiDonato et al., for the study of lipid droplets (DiDonato and Brasaemle 2003). Nuclei staining with propidium iodide (PI) was performed for cell detection. PI was added at a final concentration of 500 ng/ml, incubated for 5 min at room temperature and washed three times with PBS. Images were taken at 400x magnification with a Zeiss Axioskop Fluorescence Microscope using a FITC/GFP filter (blue light) for Alexa Fluor 488 and Rhodamine/Texas Red filter (green light) for PI. Confocal microscopy pictures were taken at 1000x magnification with a Radiance 2000 Laser Scanning System (BioRad) attached to a Nikon Eclipse TE300 microscope. Images were processed with Adobe Photoshop version 7.0.1 and ImageJ version 1.35q. For fluorescence quantification, slides were scanned using a Laser Scan Cytometer (LSC) with a 20x objective. Cells were detected by contouring around nucleic acid PI stain and data was analyzed with WinCyte LSC V3.6 from CompuCyte Corporation.
Statistical Analysis
Experiments were repeated at least three times. Results were presented as the media unless indicated. Data was analyzed using nonparametric statistics. Mann-Whitney U test was used when comparing two groups, and Kruskal-Wallis test when comparing three groups or more. Statistics analyses were performed using GB-Stat version 6.5.6. Alpha error was set at P<0.05.
Results
SRA-II Expression in CHO Cells
The protein expression of SRA-II in transfected CHO cells was confirmed by immunoprecipitation (IP) using anti CD204 antibody followed by Western Blot (WB) with the anti-6X His Tag antibody as described in methods (Figure 1). The SRA-II monomer has a molecular weight of approximately 75 kD, which is seen in the WB. Also a band of approximately 60 kD could be seen in each of the different groups. This band could be the result of some unspecific binding by the heavy chain of the anti-CD204 IgG used for IP. The presence of the 75kD band not only confirm the expression of SRA-II, but also that the full length of the SRA-II including the 6X His tag was expressed.
Figure 1.
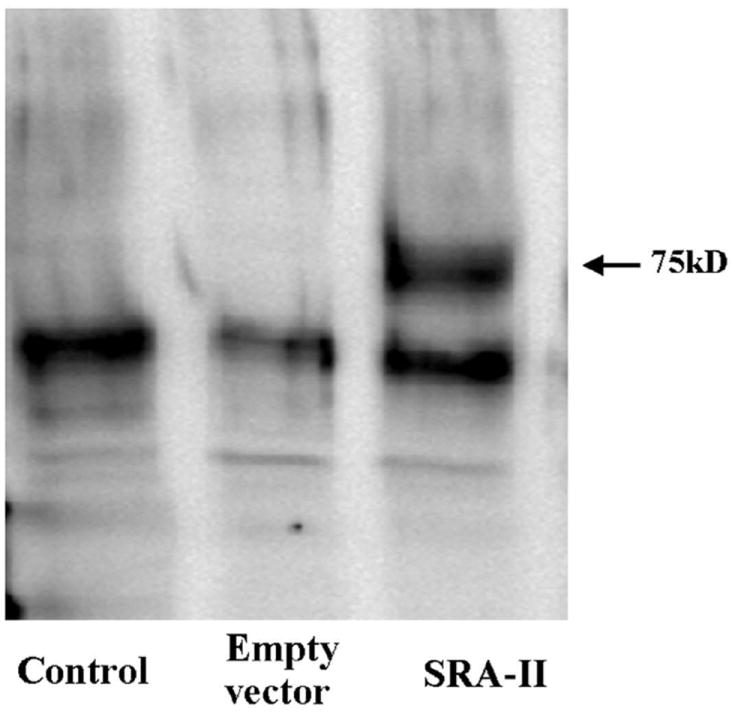
Protein expression of SRA-II was confirmed by immunoprecipitation followed by western blot in CHO transfected cells (arrowhead at 75 kD). Untransfected and Empty Vector transfected cells showed no band. Results shown are representative of three experiments.
Receptors Functionality: AcLDL Uptake
The uptake of AcLDL was determined to evaluate functionality of the constructs in CHO cells. Transfected cells were incubated with Alexa Fluor 488 labeled AcLDL as described in methods. Cells transfected with SRA-II showed, under the fluorescence microscope, uptake of fluorescence AcLDL while cells transfected with empty vector (EV) did not (data not shown). Then, AcLDL uptake was quantified using the LSC and the increase in fluorescence was calculated as shown in Figure 2. After analyzing each experiment with its respective control, an increase in fluorescence of more than two times was found in the cells transfected with SRA-II than with EV. This increase was statistically significant.
Figure 2.
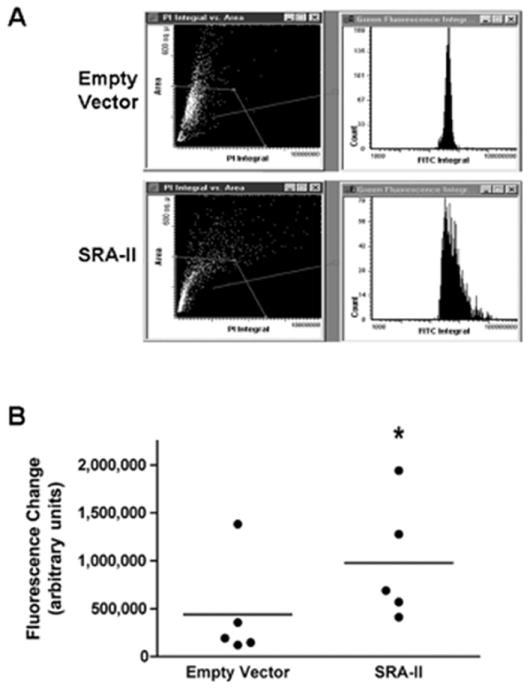
Uptake of AcLDL by CHO cells transfected with Empty vector and SRA-II. Cells were exposed to fluorescence labeled AcLDL for 90 minutes. Quantification was performed using a Laser Scan Cytometer. (A) Representative results of one experiment comparing empty vector and SRA-II transfected CHO cells. (B) All experiments analyzed data presented as Median *P<0.05; n=5.
In order to compare functionality of SRA-I and SRA-II without the presence of the 6X-His tag, constructs expressing SRA-I and II without the tag were stably transfected and exposed to AcLDL. As reported above, cells transfected with SRA-Ih and IIh showed AcLDL uptake (Figure 3), and increase in fluorescence (Figure 4) statistically significant.
Figure 3.
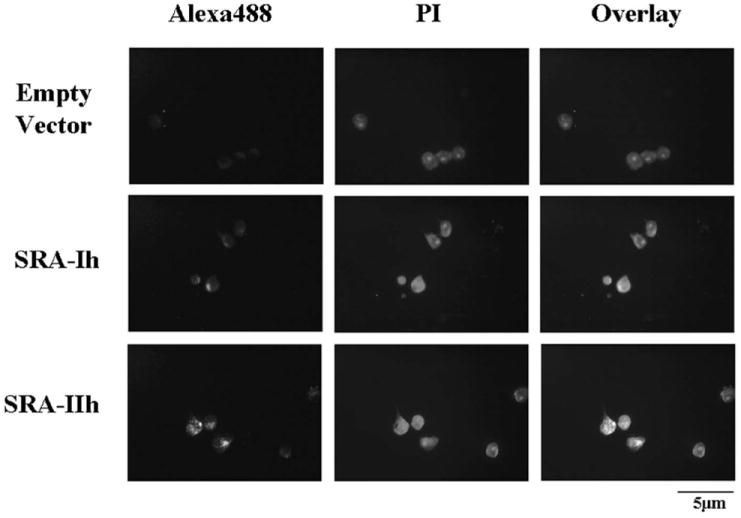
Uptake of AcLDL by CHO cells transfected with EV, SRA-Ih, and SRA-IIh. Cells were exposed to fluorescence labeled AcLDL for 90 minutes. Pictures were taken at 400x magnification using a blue filter for Alexa Fluor 488 (first column) and green filter for propidium iodide (second column). The overlay at the third column shows co-localization between AcLDL and cells.
Figure 4.
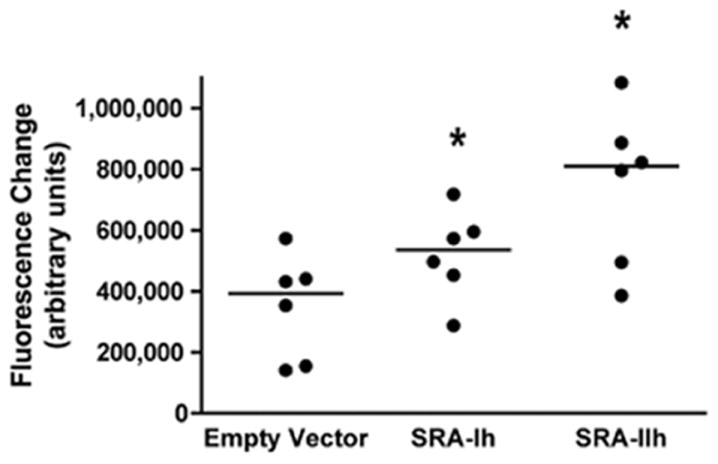
Uptake of AcLDL by CHO cells transfected with SRA-Ih and SRA-IIh. Cells were exposed to fluorescence labeled AcLDL for 90 minutes. Quantification was performed using a Laser Scan Cytometer. Data shown are presented as Median *P<0.05; n=6.
Effect of SR ligands in AcLDL Uptake
To verify that SRA-I and II expression was responsible for the increase of AcLDL uptake, a competition assay was performed. Cells were pretreated with SRs ligands as competitors for AcLDL binding. The specific SRA-I/II anti CD204 antibody and the nonspecific SR ligand PolyI were used for this assay. The use of anti CD204 antibody and PolyI produced a decrease in AcLDL uptake in all the SRA-I and II transfected cells (Figure 5). As expected greater decreased was found with PolyI than with anti CD204 antibody because PolyI has the ability to bind different SRs members.
Figure 5.
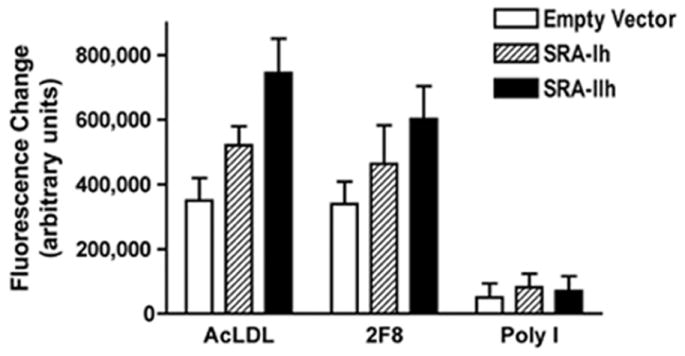
Uptake of AcLDL by CHO cells transfected with SRA-Ih and SRA-IIh when exposed to the SRAs ligands in a competition assay. CD204 a specific SRA-I/II ligand (2F8), and poly I a nonspecific ligand were used. Cells were pre-incubated 30 minutes with SRAs ligands before being exposed to fluorescence labeled AcLDL for 90 minutes. Quantification was performed using a Laser Scan Cytometer. Data shown are presented as Mean ± SEM. n=6.
Internalization of AcLDL
Cells SRA-II transfected were imaged using confocal microscopy to determine if the presence of fluorescence was due to AcLDL internalization and not simply binding. As shown in Figure 6, fluorescence labeled AcLDL could be localized on the surface and inside the cells. This finding confirms that CHO transfected cells internalize AcLDL.
Figure 6.
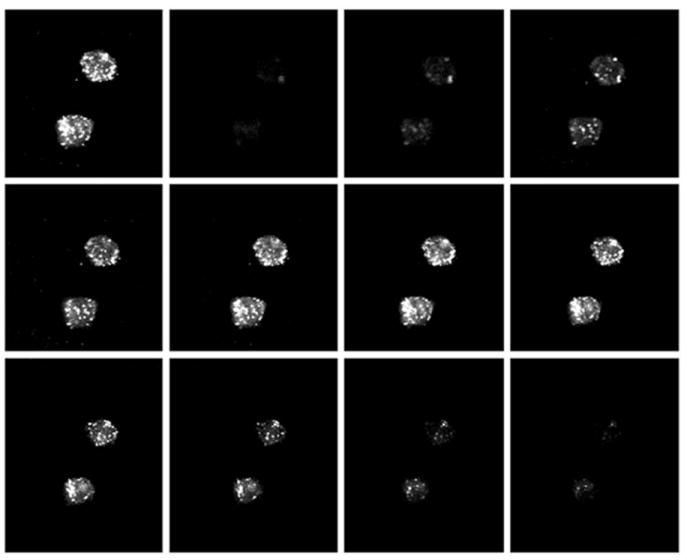
Montage of confocal images at 1000x magnification of fluorescence labeled AcLDL incorporated by CHO cells transfected with SRA-II. Upper left picture is a z projection of a stack of 11 optical slices. Fluorescence AcLDL could be localized at the surface and inside the cytosol.
Discussion
The SRA-I and SRA-II are expressed in macrophages and have the ability to bind different kind of ligands such as mLDL, negatively charged particles, asbestos (Resnick et al. 1993), and LPS (Hampton et al. 1991). In this paper the murine SRA-I and II ability to bind fluorescence labeled AcLDL had been studied when expressed in adherent CHO cells in an attempt to validate the use of fluorescence microscopy techniques to obtain qualitative and quantitative information of the uptake as an alternative to the traditional use of radiolabeled iodide and/or flow cytometry with less risks and costs related to their use.
After transfection, protein expression was verified by IP using a specific rat anti mouse CD204 antibody. This antibody recognizes only the native trimeric structure of the SRA-I and SRA-II. CD204 antibody has been previously used as a specific inhibitor and for IP, however it has low affinity for immunohistochemistry, flow cytometry and WB studies. In this study the anti CD204 antibody was used for IP and as SRA-I/II inhibitor verifying the expression and validating functionality of the SRA-I and II in the AcLDL uptake. In order to visualize the immunoprecipitated SRA-II, WB was performed using an antibody for the 6X-His tag incorporated to the C terminus of the SRA-II. In further experiments, the SRA-Ih and IIh constructs were used. These constructs, which do not express the 6X-tag, were used to prevent any interference of the tag with AcLDL binding.
Images of cells containing AcLDL were taken using a fluorescence microscope and a confocal one. Fluorescent vesicles were seen in cells transfected with SRA-I or SRA-II. These fluorescence vesicles could not be seen in the cells transfected with EV. Further quantification studies showed a statistically significant increase in fluorescence, and the competition assay with SR ligands showed that the presence of SRA-I or II in CHO cells was required for the increase in fluorescence. It was noticed that SRA-I transfected cells had lower increase in fluorescence than its SRA-II counterpart. This observation was previously reported by Freeman et al. when looking for radiolabeled AcLDL and OxLDL degradation in adherent CHO cells transfected with bovine SRA-I and SRA-II (Freeman et al. 1991). It was reported that bovine SRA-II activity was higher than bovine SRA-I, and that this difference could be related to clonal or intrinsic differences between receptors. Our study found this same difference in activity using a murine version of the SRA I and II, which favors the possibility of intrinsic differences between both splice variants.
Conclusions
In conclusion, this report validates the use of fluorescence microscopy techniques in obtaining qualitative and quantitative information of the uptake of fluorescence labeled AcLDL by adherent cells such as CHO cells as an alternative to the use of radiolabeled iodide and/or flow cytometry.
Acknowledgments
The authors thank the Molecular Histology and Fluorescence Imaging Core, the Florescence Cytometry Core and Dr. Andrij Holian from the Center for Environmental Health Sciences, The University of Montana – Missoula for their support in carrying out this project. This publication was made possible by Grant Number P20-RR-017670 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Contributor Information
Francisco J. Leyva, Division of Lung Diseases, National Heart, Lung, and Blood Institute, National Institutes of Health, Two Rockledge Center Suite 10169, 6701 Rockledge Drive, Bethesda, MD 20892, leyvaf@mail.nih.gov, Phone: (301) 435-0213, Fax: (301) 480-3451
Mark A. Pershouse, Center for Environmental Health Sciences, The University of Montana, 32 Campus Dr, Missoula, MT 59812
References
- Andersson L, Freeman MW. Functional changes in scavenger receptor binding conformation are induced by charge mutants spanning the entire collagen domain. J Biol Chem. 1998;273:19592–19601. doi: 10.1074/jbc.273.31.19592. [DOI] [PubMed] [Google Scholar]
- Ashkenas J, Penman M, Vasile E, Acton S, Freeman M, et al. Structures and high and low affinity ligand binding properties of murine type I and type II macrophage scavenger receptors. J Lipid Res. 1993;34:983–1000. [PubMed] [Google Scholar]
- Babaev VR, Gleaves LA, Carter KJ, Suzuki H, Kodama T, et al. Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arterioscler Thromb Vasc Biol. 2000;20:2593–2599. doi: 10.1161/01.atv.20.12.2593. [DOI] [PubMed] [Google Scholar]
- de Winther MP, Gijbels MJ, van Dijk KW, van Gorp PJ, Suzuki H, et al. Scavenger receptor deficiency leads to more complex atherosclerotic lesions in APOE3Leiden transgenic mice. Atherosclerosis. 1999a;144:315–321. doi: 10.1016/s0021-9150(98)00332-3. [DOI] [PubMed] [Google Scholar]
- de Winther MP, van Dijk KW, van Vlijmen BJ, Gijbels MJ, Heus JJ, et al. Macrophage specific overexpression of the human macrophage scavenger receptor in transgenic mice, using a 180-kb yeast artificial chromosome, leads to enhanced foam cell formation of isolated peritoneal macrophages. Atherosclerosis. 1999b;147:339–347. doi: 10.1016/s0021-9150(99)00204-x. [DOI] [PubMed] [Google Scholar]
- DiDonato D, Brasaemle DL. Fixation methods for the study of lipid droplets by immunofluorescence microscopy. J Histochem Cytochem. 2003;51:773–780. doi: 10.1177/002215540305100608. [DOI] [PubMed] [Google Scholar]
- Doi T, Higashino K, Kurihara Y, Wada Y, Miyazaki T, et al. Charged collagen structure mediates the recognition of negatively charged macromolecules by macrophage scavenger receptors. J Biol Chem. 1993;268:2126–2133. [PubMed] [Google Scholar]
- Doi T, Kurasawa M, Higashino K, Imanishi T, Mori T, et al. The histidine interruption of an alpha-helical coiled coil allosterically mediates a pH-dependent ligand dissociation from macrophage scavenger receptors. J Biol Chem. 1994;269:25598–25604. [PubMed] [Google Scholar]
- Fortin A, Penman M, Stevenson MM, Krieger M, Gros P. Identification and characterization of naturally occurring variants of the macrophage scavenger receptor (SR-A) Mamm Genome. 2000;11:779–785. doi: 10.1007/s003350010131. [DOI] [PubMed] [Google Scholar]
- Fraser I, Hughes D, Gordon S. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature. 1993;364:343–346. doi: 10.1038/364343a0. [DOI] [PubMed] [Google Scholar]
- Freeman M, Ekkel Y, Rohrer L, Penman M, Freedman NJ, et al. Expression of type I and type II bovine scavenger receptors in Chinese hamster ovary cells: lipid droplet accumulation and nonreciprocal cross competition by acetylated and oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1991;88:4931–4935. doi: 10.1073/pnas.88.11.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RY, Golenbock DT, Penman M, Krieger M, Raetz CR. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Wada Y, Hinagata J, Imanishi T, Kodama T, et al. VXFD in the cytoplasmic domain of macrophage scavenger receptors mediates their efficient internalization and cell-surface expression. Biol Pharm Bull. 1999;22:1022–1026. doi: 10.1248/bpb.22.1022. [DOI] [PubMed] [Google Scholar]
- Peiser L, Gordon S. The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect. 2001;3:149–159. doi: 10.1016/s1286-4579(00)01362-9. [DOI] [PubMed] [Google Scholar]
- Platt N, Gordon S. Is the class A macrophage scavenger receptor (SR-A) multifunctional? - The mouse’s tale. J Clin Invest. 2001;108:649–654. doi: 10.1172/JCI13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick D, Freedman NJ, Xu S, Krieger M. Secreted extracellular domains of macrophage scavenger receptors form elongated trimers which specifically bind crocidolite asbestos. J Biol Chem. 1993;268:3538–3545. [PubMed] [Google Scholar]
- Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]


