Abstract
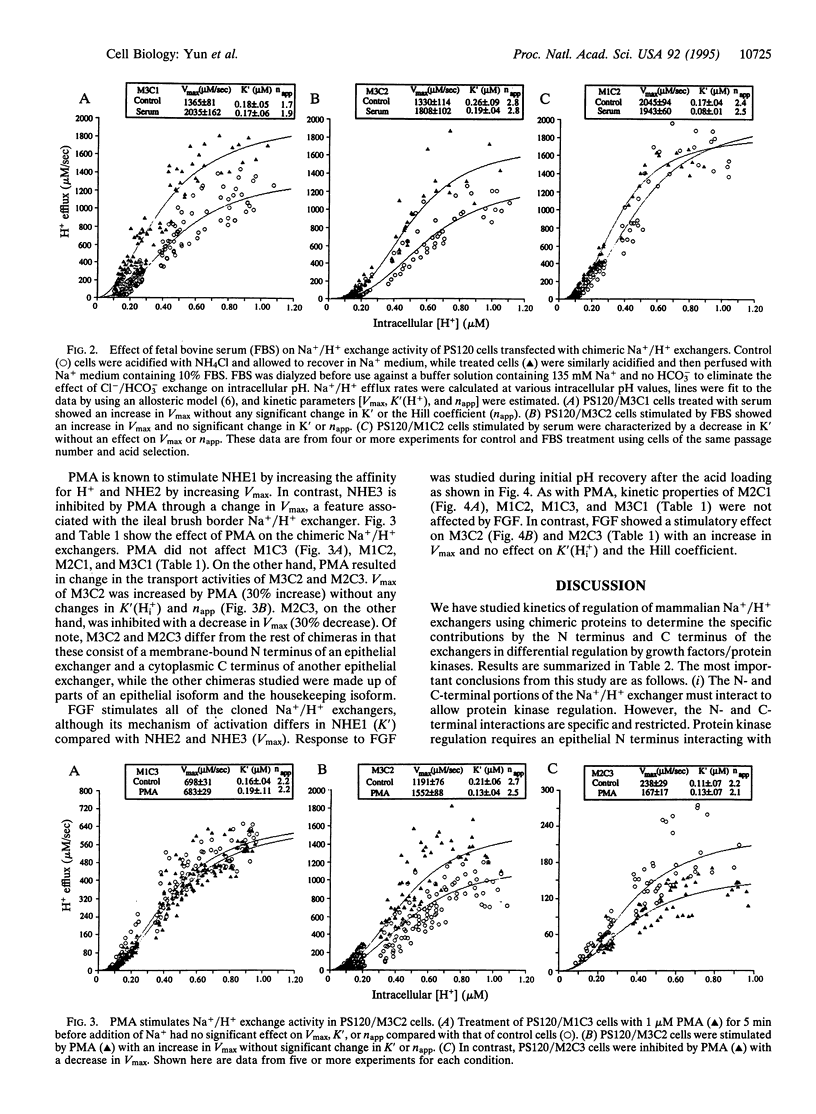
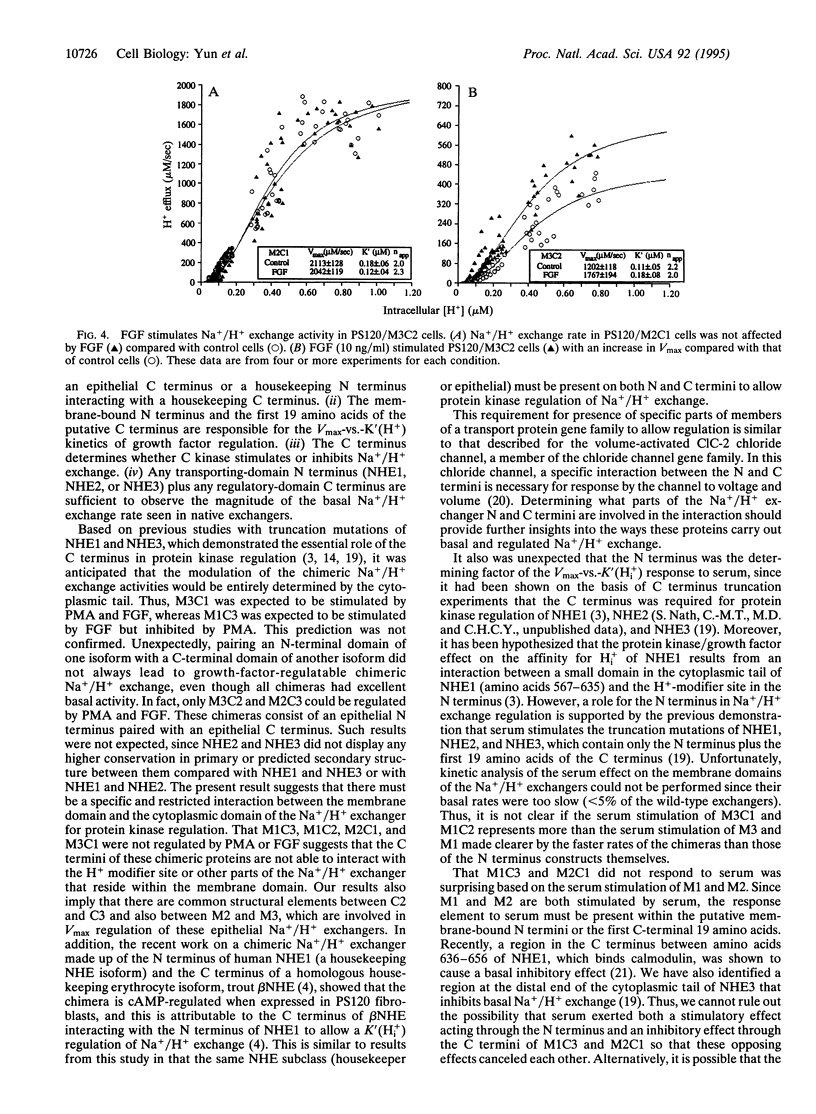
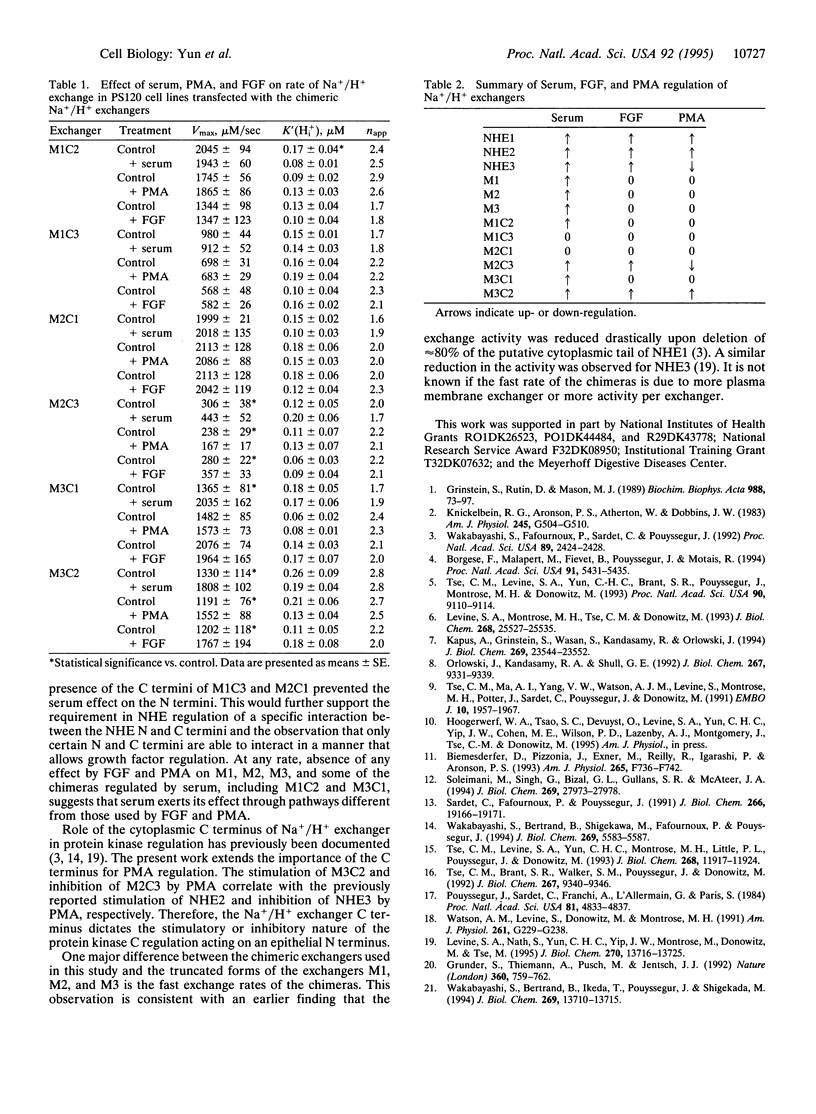
All cloned members of the mammalian Na+/H+ exchanger gene family encode proteins that consist of two functionally distinct domains: a membrane-bound N terminus and a cytoplasmic C terminus, which are required for ion transport and regulation of transport, respectively. Despite their similarity in structure, three members of this family, designated NHE1, NHE2, and NHE3, exhibit different kinetic mechanisms in response to growth factors and protein kinases. For instance, growth factors stimulate NHE1 by a change in the affinity constant for intracellular H+, K'(Hi+), and regulate NHE2 and NHE3 by a change in Vmax. We have constructed chimeric Na+/H+ exchangers by exchanging the N and C termini among three cloned rabbit Na+/H+ exchangers (NHE1 to NHE3) to determine which domain is responsible for the above Vmax-vs.-K'(H(i)+) effect of the Na+/H+ isoforms. All of the chimeras had functional exchange activity and basal kinetic properties similar to those of wild-type exchangers. Studies with serum showed that the N terminus is responsible for the Vmax-vs.-K'(H(i)+) stimulation of the Na+/H+ exchanger isoforms. Moreover, phorbol 12-myristate 13-acetate and fibroblast growth factor altered Na+/H+ exchange only in chimeras that had an epithelial N-terminal domain matched with an epithelial C-terminal domain. Therefore, the protein kinase-induced regulation of Na+/H+ exchangers is mediated through a specific interaction between the N- and C-termini, whcih is restricted so that epithelial N- and epithelial N-and C-terminal portions of the exchangers are required for regulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biemesderfer D., Pizzonia J., Abu-Alfa A., Exner M., Reilly R., Igarashi P., Aronson P. S. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am J Physiol. 1993 Nov;265(5 Pt 2):F736–F742. doi: 10.1152/ajprenal.1993.265.5.F736. [DOI] [PubMed] [Google Scholar]
- Borgese F., Malapert M., Fievet B., Pouyssegur J., Motais R. The cytoplasmic domain of the Na+/H+ exchangers (NHEs) dictates the nature of the hormonal response: behavior of a chimeric human NHE1/trout beta NHE antiporter. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5431–5435. doi: 10.1073/pnas.91.12.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Rotin D., Mason M. J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta. 1989 Jan 18;988(1):73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Gründer S., Thiemann A., Pusch M., Jentsch T. J. Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature. 1992 Dec 24;360(6406):759–762. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- Kapus A., Grinstein S., Wasan S., Kandasamy R., Orlowski J. Functional characterization of three isoforms of the Na+/H+ exchanger stably expressed in Chinese hamster ovary cells. ATP dependence, osmotic sensitivity, and role in cell proliferation. J Biol Chem. 1994 Sep 23;269(38):23544–23552. [PubMed] [Google Scholar]
- Knickelbein R., Aronson P. S., Atherton W., Dobbins J. W. Sodium and chloride transport across rabbit ileal brush border. I. Evidence for Na-H exchange. Am J Physiol. 1983 Oct;245(4):G504–G510. doi: 10.1152/ajpgi.1983.245.4.G504. [DOI] [PubMed] [Google Scholar]
- Levine S. A., Montrose M. H., Tse C. M., Donowitz M. Kinetics and regulation of three cloned mammalian Na+/H+ exchangers stably expressed in a fibroblast cell line. J Biol Chem. 1993 Dec 5;268(34):25527–25535. [PubMed] [Google Scholar]
- Levine S. A., Nath S. K., Yun C. H., Yip J. W., Montrose M., Donowitz M., Tse C. M. Separate C-terminal domains of the epithelial specific brush border Na+/H+ exchanger isoform NHE3 are involved in stimulation and inhibition by protein kinases/growth factors. J Biol Chem. 1995 Jun 9;270(23):13716–13725. doi: 10.1074/jbc.270.23.13716. [DOI] [PubMed] [Google Scholar]
- Orlowski J., Kandasamy R. A., Shull G. E. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem. 1992 May 5;267(13):9331–9339. [PubMed] [Google Scholar]
- Pouysségur J., Sardet C., Franchi A., L'Allemain G., Paris S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4833–4837. doi: 10.1073/pnas.81.15.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardet C., Fafournoux P., Pouysségur J. Alpha-thrombin, epidermal growth factor, and okadaic acid activate the Na+/H+ exchanger, NHE-1, by phosphorylating a set of common sites. J Biol Chem. 1991 Oct 15;266(29):19166–19171. [PubMed] [Google Scholar]
- Soleimani M., Singh G., Bizal G. L., Gullans S. R., McAteer J. A. Na+/H+ exchanger isoforms NHE-2 and NHE-1 in inner medullary collecting duct cells. Expression, functional localization, and differential regulation. J Biol Chem. 1994 Nov 11;269(45):27973–27978. [PubMed] [Google Scholar]
- Tse C. M., Brant S. R., Walker M. S., Pouyssegur J., Donowitz M. Cloning and sequencing of a rabbit cDNA encoding an intestinal and kidney-specific Na+/H+ exchanger isoform (NHE-3). J Biol Chem. 1992 May 5;267(13):9340–9346. [PubMed] [Google Scholar]
- Tse C. M., Levine S. A., Yun C. H., Brant S. R., Pouyssegur J., Montrose M. H., Donowitz M. Functional characteristics of a cloned epithelial Na+/H+ exchanger (NHE3): resistance to amiloride and inhibition by protein kinase C. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9110–9114. doi: 10.1073/pnas.90.19.9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse C. M., Levine S. A., Yun C. H., Montrose M. H., Little P. J., Pouyssegur J., Donowitz M. Cloning and expression of a rabbit cDNA encoding a serum-activated ethylisopropylamiloride-resistant epithelial Na+/H+ exchanger isoform (NHE-2). J Biol Chem. 1993 Jun 5;268(16):11917–11924. [PubMed] [Google Scholar]
- Tse C. M., Ma A. I., Yang V. W., Watson A. J., Levine S., Montrose M. H., Potter J., Sardet C., Pouyssegur J., Donowitz M. Molecular cloning and expression of a cDNA encoding the rabbit ileal villus cell basolateral membrane Na+/H+ exchanger. EMBO J. 1991 Aug;10(8):1957–1967. doi: 10.1002/j.1460-2075.1991.tb07725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi S., Bertrand B., Ikeda T., Pouysségur J., Shigekawa M. Mutation of calmodulin-binding site renders the Na+/H+ exchanger (NHE1) highly H(+)-sensitive and Ca2+ regulation-defective. J Biol Chem. 1994 May 6;269(18):13710–13715. [PubMed] [Google Scholar]
- Wakabayashi S., Bertrand B., Shigekawa M., Fafournoux P., Pouysségur J. Growth factor activation and "H(+)-sensing" of the Na+/H+ exchanger isoform 1 (NHE1). Evidence for an additional mechanism not requiring direct phosphorylation. J Biol Chem. 1994 Feb 25;269(8):5583–5588. [PubMed] [Google Scholar]
- Wakabayashi S., Fafournoux P., Sardet C., Pouysségur J. The Na+/H+ antiporter cytoplasmic domain mediates growth factor signals and controls "H(+)-sensing". Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2424–2428. doi: 10.1073/pnas.89.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A. J., Levine S., Donowitz M., Montrose M. H. Kinetics and regulation of a polarized Na(+)-H+ exchanger from Caco-2 cells, a human intestinal cell line. Am J Physiol. 1991 Aug;261(2 Pt 1):G229–G238. doi: 10.1152/ajpgi.1991.261.2.G229. [DOI] [PubMed] [Google Scholar]


