Abstract
The objective of this study was to determine if prior measurement of the minimum alveolar concentration (MAC) of isoflurane influences the effect of ketamine on the MAC of isoflurane in dogs. Eight mixed-breed dogs were studied on 2 occasions. Anesthesia was induced and maintained using isoflurane. In group 1 the effect of ketamine on isoflurane MAC was determined after initially finding the baseline isoflurane MAC. In group 2, the effect of ketamine on isoflurane MAC was determined without previous measure of the baseline isoflurane MAC. In both groups, MAC was determined again 30 min after stopping the CRI of ketamine. Plasma ketamine concentrations were measured during MAC determinations.
In group 1, baseline MAC (mean ± SD: 1.18 ± 0.14%) was decreased by ketamine (0.88 ± 0.14%; P < 0.05). The MAC after stopping ketamine was similar (1.09 ± 0.16%) to baseline MAC and higher than with ketamine (P < 0.05). In group 2, the MAC with ketamine (0.79 ± 0.11%) was also increased after stopping ketamine (1.10 ± 0.17%; P < 0.05). The MAC values with ketamine were different between groups (P < 0.05). Ketamine plasma concentrations were similar between groups during the events of MAC determination.
The MAC of isoflurane during the CRI of ketamine yielded different results when methods of same day (group-1) versus separate days (group-2) are used, despite similar plasma ketamine concentrations with both methods. However, because the magnitude of this difference was less than 10%, either method of determining MAC is deemed acceptable for research purposes.
Résumé
L’objectif de la présente étude était de déterminer si une mesure antérieure de la concentration alvéolaire minimum (MAC) d’isoflurane influence l’effet de la kétamine sur la MAC d’isoflurane chez les chiens. Huit chiens de race croisée ont été examinés à deux occasions. L’anesthésie fut induite et maintenue à l’aide d’isoflurane. Dans le groupe 1, l’effet de la kétamine sur la MAC d’isoflurane fut déterminé après avoir initialement trouvé la MAC de base de l’isoflurane. Dans le groupe 2, l’effet de la kétamine sur la MAC d’isoflurane fut déterminé sans mesure préalable de la MAC de base de l’isoflurane. Dans les deux groupes la MAC fut déterminée de nouveau 30 min après l’arrêt de la CRI de kétamine. Les concentrations de kétamine plasmatiques furent mesurées durant les déterminations de MAC.
Dans le groupe 1, la MAC de base (moyenne ± SD : 1,18 ± 0,14 %) fut diminuée par la kétamine (0,88 ± 0,14 %; P < 0,05). La MAC après l’arrêt de la kétamine était similaire (1,09 ± 0,16 %) à la MAC de base et plus élevée qu’avec la kétamine (P < 0,05). Dans le groupe 2, la MAC avec kétamine (0,79 ± 0,11 %) était également augmentée après l’arrêt de la kétamine (1,10 ± 0,17 %; P < 0,05). Les valeurs de MAC avec la kétamine étaient différentes entre les groupes (P < 0,05). Les concentrations plasmatiques de kétamine étaient similaires durant la détermination des MAC.
La MAC d’isoflurane durant la CRI de kétamine a donné des résultats différents lorsque les méthodes d’un jour unique (le groupe 1) versus des jours séparés (le groupe 2) étaient utilisées, malgré des concentrations plasmatiques de kétamine similaires avec les deux méthodes. Toutefois, étant donné que l’ampleur de cette différence était de moins de 10 %, chacune des deux méthodes pour déterminer la MAC est considérée comme acceptable à des fins de recherche.
(Traduit par Docteur Serge Messier)
Introduction
Minimum alveolar concentration (MAC) is defined as the end-tidal concentration of an inhalant anesthetic that prevents purposeful movement in 50% of the study population in response to a supramaximal noxious stimulus. The MAC is considered the standard method to compare potency of inhalant anesthetics since its introduction in the 1960s (1).
Studies on MAC that investigate the sparing effect of injectable anesthetic drugs are designed using one of 2 distinct methods. One method uses same day determination of the MAC for the inhalant anesthetic alone, followed immediately by administration of the injectable anesthetic or analgesic drug of interest to determine its sparing effect (2–5). The other method uses separate day determination, which consists of obtaining the MAC value for the inhalant on a day separate from the determination of the MAC value for the inhalant/injectable anesthetic combination (6,7).
The MAC values determined by different laboratories may vary due to different methodology used, as well as differences between species and the type or intensity of noxious stimulation used during the experiments. For example, MAC values for isoflurane in dogs and rabbits determined by the same laboratory using electrical stimulation were 51% higher in rabbits (mean ± SEM: 2.04 ± 0.02%) than in dogs (1.35 ± 0.04%) (9). Conversely, a submaximal noxious stimulus (skin incision) resulted in only a 12% difference in MAC values between the 2 species, and this MAC value was higher in dogs (1.01 ± 0.07%) than in rabbits (0.90 ± 0.02%) (8). It is possible that despite eliciting purposeful movement during submaximal and supramaximal noxious stimulation in those species, different mechanisms are involved in activating pain and motor actions that facilitate movement in each species, since the MAC values in response to supramaximal and submaximal stimuli varied by 127% in rabbits and by only 34% in dogs (8).
In a previous study on rabbits, a constant rate infusion (CRI) of ketamine had a sparing effect on isoflurane requirements, which was independent of using the same day method for MAC determination or using the separate day method (9). Similar studies have not been carried out on dogs to demonstrate if both methods of MAC determination are equivalent in this species.
Several studies have demonstrated dose-dependent effects between plasma concentrations of a given injectable anesthetic drug and its influence on MAC for different inhalant anesthetics. Administration of the injectable drug at a CRI, after elevating plasma concentrations with a loading dose, also facilitates a steady concentration of the drug in plasma and less fluctuation in the MAC sparing effect over time. The MAC sparing effect of a CRI of ketamine has been reported in several animal species, including dogs (10–12), cats (4), horses (13), and rabbits (9).
The objective of this study was 2-fold. First, to determine and compare the sparing effect of ketamine in dogs by using the 2 MAC determination methods, same day and separate day. The second objective was to measure plasma concentrations of ketamine during the determination of MAC and after discontinuation of the CRI to determine if there is a dose-dependent effect.
Materials and methods
Animals
Using a prospective randomized crossover study, 8 mixed-breed dogs (5 females and 3 males) weighing 17.3 to 27.0 kg and 1 to 4 y of age were used in this study. All animals had a normal physical examination, as well as a complete blood (cell) count (CBC) and serum biochemical analysis. Each dog was used on 2 occasions using a crossover design, with at least a 5 d washout period between treatments, in a randomized order of treatment. Food was withheld 12 h prior to anesthesia, but water was available ad libitum. The Institutional Animal Care and Use Committee of the University of Guelph approved the study.
Anesthesia and instrumentation
Anesthesia was induced with isoflurane (Isoflo; Baxter Corporation, Mississauga, Ontario) in oxygen using a face mask attached to a coaxial rebreathing system (Universal F-circuit) with an oxygen flow rate of 3 to 4 L/min. Once the dogs were unconscious and endotracheally intubated with a low pressure high volume cuffed endotracheal tube, they were positioned in right lateral recumbency and the oxygen flow rate decreased to 2 L/min. Intermittent positive pressure ventilation was immediately established using an electronically controlled, time-cycled, pressure limited ventilator (EMC 2000; Hallowell, Massachusetts, USA), with a rate of 8 to 12 breaths/min and a tidal volume of 10 to 15 mL/kg in order to maintain end-tidal CO2 between 30 and 40 mmHg. Dogs were monitored during the first 30 min of anesthesia while at approximately 1.6% end-tidal isoflurane. Monitoring included end-tidal isoflurane and CO2 concentrations using a side-stream infrared gas analyzer (S/5; Datex-Ohmeda, Anesthesia monitor; GE Healthcare Finland, Helsinki, Finland) with the sampling port attached between the endotracheal tube and the breathing system. The anesthesia monitor was calibrated each morning using a calibration gas specifically designed for this purpose (DOT-34 NRC 300/375M1014; Datex-Ohmeda Division, Helsinki, Finland). Rectal temperature was monitored using a digital thermometer and was maintained between 37.0°C and 38.5°C using a hot air heating device (Lancaster; Trileaf Distribution, Toronto, Ontario). Cardiovascular measures, including direct systolic arterial blood pressure (SAP), mean arterial blood pressure (MAP), diastolic arterial blood pressure (DAP), electrocardiogram, and heart rate (HR), were monitored (S/5; Datex-Ohmeda, Anesthesia monitor; GE Healthcare Finland) throughout the duration of the experiment and recorded before and after each MAC determination.
The left cephalic vein and the right dorsal pedal artery were catheterized with 20-SWG (1.88 IN) IV catheters (BD Insyte-W; BD Infusion Therapy Systems Inc., Sandy, Utah, USA). The venous access was used to administer the treatment drugs, with the use of a syringe pump (Graseby 3500 Anesthesia Pump; Smiths Medical International Ltd, Wartford, Herts, UK), and a balanced electrolyte solution (Plasmalyte A; Baxter Corporation, Mississauga, Ontario) at 3 mL/kg per hour. The arterial access was used to measure SAP, MAP, and DAP with the use of an electronic pressure transducer (Becton Dickinson Critical Care Systems, Singapore) zeroed at the level of the sternum and interfaced with the anesthesia monitor. The arterial catheter was also used for periodic sampling for blood gas determination (CCX; Nova Biomedical, Waltham, Massachusetts, USA), which was collected into heparinized syringes (Gastlyte; Marquest Medical Products, Englewood, Colorado, USA), to verify the end-tidal CO2 readings. Blood was also collected from the arterial catheter for determination of plasma ketamine concentrations at the time of each MAC determination.
Determination of MAC
The MAC was determined using a previous published technique (8). The noxious stimulation consisted of 50 volts at 50 cycles/s for 10 ms (S48 Stimulator; Astro-Medical Inc., West Warwick, Rhode Island, USA) applied subcutaneously by inserting two 25-gauge hypodermic polypropylene hub; 0.75-inch needles (Tyco Healthcare Group LP, Mansfield, Massachusetts, USA) 5 cm apart on the lateral aspect of the left ulna. In brief, the sequence of noxious stimulation consisted of administering 2 single stimuli followed by 2 continuous stimuli applied for 2 to 3 s, with 5-s intervals between all 4 stimuli. If gross purposeful movement, defined as jerking or twisting motion of the head or running motion of the extremities, was elicited at any time during the cycle the stimulation was immediately stopped, and the event was recorded as a positive response. If the response was positive, the end-tidal isoflurane concentration was increased by 0.1%; conversely, if the response was negative the end-tidal isoflurane concentration was decreased by 0.1%. The noxious stimulus was repeated after 15 to 20 min at the new end-tidal concentration, and if the opposite response to the previous stimulation was obtained then the end-tidal concentration was returned to the initial value using the 15 to 20 min equilibration process to verify the first response (duplicate MAC measurement). This procedure was repeated until opposite responses between contiguous end-tidal isoflurane concentrations were obtained in duplicate. The MAC was defined as the value midway between the lowest end-tidal isoflurane concentration that prevented purposeful movement and the highest end-tidal isoflurane concentration that allowed it.
Experimental design
After the instrumentation phase, dogs assigned to group 1 (same day MAC determination) had the end-tidal isoflurane concentration decreased to 1.2%, which corresponds to the approximate MAC value reported for dogs (8). This was maintained for at least 30 min to establish equilibration before starting MAC determinations, while receiving a bolus and CRI of a balanced electrolyte solution at the same volume rate as the ketamine infusion to be used later. Dogs assigned to group 2 (separate day MAC determination) had the end-tidal isoflurane concentration decreased to approximately 0.9% for the same time duration (30 min) after receiving a bolus of ketamine (Vetalar; Bioniche Animal Health Canada Inc., Belleville, Ontario) of 1 mg/kg, IV, followed immediately by a CRI at 40 μg/kg/min using a syringe pump. The CRI was maintained until the MAC determination was completed.
After completing the first MAC determinations, dogs in group 1 were administered a bolus and a CRI of ketamine (as described). End-tidal isoflurane concentrations were decreased to 0.9% and, after a 30-minute period of ketamine infusion, the MAC was determined again. Dogs in group 2 had the CRI of ketamine stopped, the end-tidal isoflurane concentration was increased to 1.2% during this time, and the MAC determinations started once again 30 min later while receiving an infusion of a balanced electrolyte solution at the same volume and rate as the previous ketamine infusion. Dogs in group 2 were allowed to recover from anesthesia once the second MAC determination was completed. Dogs in group 1 had the CRI of ketamine stopped and replaced with an infusion of a balanced electrolyte solution at the same volume and rate as the previous ketamine infusion after the second MAC determination. The end-tidal isoflurane concentration was increased to 1.2% during this time and the MAC determinations started once again 30 min later for a third time, the dogs were then allowed to recover from anesthesia. Two arterial blood samples (5 mL) were collected into sodium heparin tubes (BD Vacutainer, sodium heparin (NH) 68 USP units; BD, Franklin Lakes, New Jersey, USA) at the time of MAC determination for analysis of ketamine concentrations. One sample corresponded to the end-tidal isoflurane concentration at which the animal responded to the noxious stimulus and the other to the end-tidal isoflurane concentration at which it did not respond. The arterial blood was immediately centrifuged at 716.8 × g for 10 min (IEC MB Centrifuges; Damon/IEC Division, Needhan Heights, Massachusetts, USA) and the plasma stored at −80°C until further analysis. Meloxicam (0.1 mg/kg, IV; Metacam, Boehringer Ingelheim, Burlington, Ontario) was administered to all dogs before recovery.
Ketamine and norketamine analysis
Determination of ketamine and norketamine concentrations in plasma was done (Waters 2996 photodiode array detector, Waters Alliance 2695 HPLC separation system; Waters, Mississauga, Ontario). The analytical column used was an XBridge C18 column (100 mm × 2.1 mm ID, 3.5 μm; Waters, Ireland), connected with an XBridge C18 guard column (2.1 mm × 10.0 mm ID, 3.5 μm). Canine plasma samples were purified (Oasis MCX cartridges; Waters, Milford, Massachusetts, USA). An isocratic chromatographic separation was done using a mobile phase containing acetonitrile-methanol-10 mM ammonium acetate (15:30:55 v/v/v; Sigma-Aldrich-Fluka, Oakville, Ontario; and Caledon Laboratories Ltd., Georgetown, Ontario), at a flow rate of 0.25 mL/min. A 50 μL sample injection volume was used and the eluent was monitored at 222.0 nm. Under these conditions the retention times observed for ketamine, norketamine, and MK-801 were 8.9, 5.8, and 11.3 min, respectively (14–16).
Stock solutions of ketamine and norketamine (Cerilliant Corp, Round Rock, Texas, USA) and MK-801 (Sigma-Aldrich-Fluka) were prepared in methanol (Caledon Laboratories Ltd.) at a concentration of 500 μg/mL. Calibration curve (reference) standards were prepared by spiking 10 μL of norketamine and ketamine working solutions (10, 20, 50, 100, and 250 μg/mL) and 10 μL of MK-801 internal standard solution (50 μg/mL) into 500 μL blank (control) canine plasma samples.
Ketamine and norketamine were extracted and purified using cartridges (Oasis MCX; Mixed-mode cation exchange). Ten microliters of internal standard solution (50 μg/mL MK 801 in methanol) were added to 500 μL aliquots of canine test plasma samples. The plasma samples were acidified with 500 μL of 4% phosphoric acid, solutions vortexed for 30 s, and centrifuged at 1612.8 × g for 3 min. The supernatants were used for solid-phase extraction purification. Cartridges were washed and conditioned by passing 1 mL of methanol and 1 mL of water. The 1 mL acidified plasma samples were loaded, the cartridges were washed with 1 mL of 0.1 N hydrochloric acid and 1 mL of methanol, then eluted with 1 mL of 5% ammonium in methanol. The elutes containing ketamine, norketamine, and the internal standard MK 801 were evaporated under a constant flow of nitrogen. The residues were reconstituted with 100 μL of the mobile phase (acetonitrile-methanol-10 mM ammonium acetate 15:30:55, v/v/v) and 50 μL of this was injected into the analytical column (17,18).
For validation of the assay method, canine test plasma samples were quantified using the ratios of the peak area of ketamine and norketamine to that of the MK 801 internal standard as the assay measure. Peak area ratios were plotted against ketamine and norketamine concentrations and standard curves, in the form of y = A + Bx, calculated using weighted (1 x−2) least squares linear regression. A calibration curve of 5 points was plotted and showed to be linear and reproducible in the concentration range from 200 to 5000 ng/mL for norketamine and ketamine, with the correlation coefficient (r2) > 0.99 for all calibration curves. For assay specificity to ketamine and norketamine, no interfering peaks were observed in blank plasma samples at retention times corresponding to the drug and internal standard.
Statistical analysis
The MAC values were calculated by mathematical averaging of 2 subsequent concentrations of inhalant anesthetic at which gross purposeful movement and no gross purposeful movement were observed. Statistical comparison between the 2 groups and within groups was carried out for MAC values induced by ketamine administration, MAC values after stopping ketamine administration, and time of MAC determinations using an ANOVA for repeated measures with Bonferroni correction, after verification of normality of the data by use of the D’Agostino-Pearson and Kolmogorov-Smirnov test (MedCalc Software, version 11.2.1; Mariakerke, Belgium). The MAC values and time of determination are reported as mean ± SD. The SAP, MAP, DAP, end-tidal CO2 concentrations, HR, and rectal temperature throughout the study were compared between groups using an independent samples t-test. The correlation between the MAC values and plasma ketamine concentrations were assessed using linear regression analysis (MedCalc Software, version 11.2.1). Values of P < 0.05 were considered significant.
Results
All dogs had cardiopulmonary measures within acceptable limits for MAC determinations and recovered uneventfully from the experiments. End-tidal CO2, HR, SAP, MAP, DAP, and rectal temperature values were 37.0 ± 2.1 mmHg, 87 ± 15 beats/min, 120.0 ± 13.0 mmHg, 74.0 ± 10.0 mmHg, 60.0 ± 8.0 mmHg, and 37.4 ± 0.6°C, respectively for group 1, and 36.0 ± 2.9 mmHg, 98 ± 27 beats/min, 124.0 ± 24.0 mmHg, 78.0 ± 17.0 mmHg, 65.0 ± 14.0 mmHg, and 37.6 ± 0.5°C, respectively for group 2, throughout the experiment.
The MAC for isoflurane was 1.18 ± 0.14% (group 1). In both groups, ketamine administration resulted in a significantly lower MAC value than the baseline MAC value for isoflurane obtained in group 1; however, the MAC value for ketamine-isoflurane was significantly lower in group 2 than in group 1 (Table I). In both groups the MAC value after discontinuation of ketamine was similar between them and significantly higher within each group than the MAC value obtained during the ketamine CRI. Although the MAC values after discontinuation of ketamine were lower than the MAC for isoflurane obtained in group 1, it was not significant (Table I). There were no significant differences in times required for MAC determinations between the 2 groups (Table I).
Table I.
The minimum alveolar concentration (MAC) values and time after ketamine administration (1 mg/kg bolus, IV, followed by 40 μg/kg per minute) in 8 dogs with same day MAC determination (group 1) or separate day MAC determination (group 2)
| Isoflurane baseline | Isoflurane + ketamine | After stopping ketamine | Total anesthesia time (min) | |
|---|---|---|---|---|
| Group 1, same day | ||||
| MAC, % | 1.18 ± 0.14 | 0.88 ± 0.14 (P = 0.0001)a | 1.09 ± 0.16 (P = 0.0079)b | |
| MAC reduction, % | ND | 25 | 8 | |
| Time, min | 48.0 ± 5.1 | 75.0 ± 16.4 | 65.0 ± 10.4 | 202.0 ± 22.6 |
| Group 2, separate days | ||||
| MAC (%) | ND | 0.79 ± 0.11 (P = 0.0001)a (P = 0.028)c | 1.10 ± 0.17 (P = 0.0003)b | |
| MAC reduction, % | ND | 33 | 8 | |
| Time, min | ND | 74.0 ± 13.2 | 76.0 ± 20.2 | 170.0 ± 30.1 |
Values expressed as mean ± SD.
Significant difference from isoflurane baseline.
Significant difference from isoflurane + ketamine within groups.
Significant difference from isoflurane + ketamine from group 1.
ND — not determined.
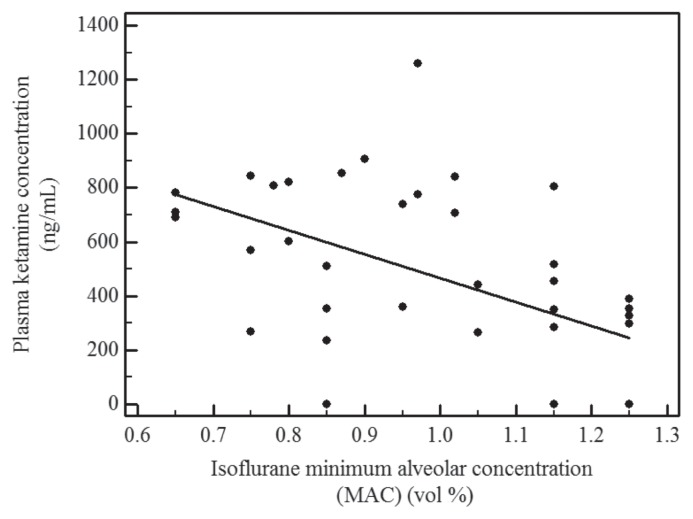
Ketamine concentrations (ng/mL; mean ± SD) during the CRI and after discontinuation of the CRI were similar at the time of determination of MAC values between groups (Table II). Ketamine concentrations decreased in both groups after discontinuation of the CRI and the correlation between MAC values and ketamine plasma concentrations during the CRI and after discontinuation of the CRI was r = 0.55 (Figure 1). The norketamine plasma concentrations could only be determined for 9/32 samples collected after ketamine administration for group 1 and 2/32 for group 2, because the concentrations during MAC determinations were below the limit of quantification (LOQ) of the assay (200 ng/mL). Norketamine concentrations between 205 to 294 ng/mL were measured in 5 of the 8 dogs in group 1 and of 228 and 259 ng/mL in 1 dog each of the 8 dogs in group 2. The time of ketamine CRI administration corresponds to the same time as for MAC determination of the isoflurane and ketamine combination (Table I).
Table II.
Plasma ketamine concentration values and time at minimum alveolar concentration (MAC) determinations before, during, and after stopping ketamine administration (1 mg/kg bolus, IV, followed by 40 μg/kg per minute) in 8 dogs with same day MAC determination (group 1) or separate day MAC determination (group 2)
| Isoflurane | Isoflurane + ketamine | After stopping ketamine | |
|---|---|---|---|
| Group 1, same day | |||
| Ketamine plasma concentration, ng/mL | 0 ± 0 | 824 ± 196 | 407 ± 176a |
| Time, min | 48 ± 5 | 75 ± 16 | 65 ± 10 |
| Group 2, separate days | |||
| Ketamine plasma concentration, ng/mL | 0 ± 0 | 729 ± 133 | 347 ± 81a |
| Time, min | ND | 74 ± 13 | 76 ± 20 |
Plasma concentrations are based on the average of 2 arterial blood samples obtained from each dog during the corresponding bracketing of MAC (positive and negative response to noxious stimulation). Values expressed as mean ± SD.
Significant difference from isoflurane + ketamine value.
ND — not determined.
Figure 1.
Minimum alveolar concentration (MAC) of isoflurane versus plasma concentration of ketamine (ng/mL); r = 0.55; P < 0.001) in dogs.
Discussion
The results of this study are in agreement with previous studies (10–12,17,18) that report ketamine decreased the MAC of isoflurane in dogs. The decrease in the isoflurane MAC (group 1) with the ketamine CRI corresponded to a 25% reduction, similar to values reported by other authors in dogs using a CRI of 10 μg/kg per minute (10). In another study, the reduction in sevoflurane MAC of a CRI of 50 and 100 μg/kg per minute was 40% and 45%, respectively (19). In a previous study with the same study design as the present study, the reduction induced by ketamine in the MAC of isoflurane in rabbits was also similar (24%) (9).
The MAC values obtained during the CRI of ketamine in dogs were statistically different for the 2 methods used. During separate day determination (group 2) the MAC of isoflurane with ketamine was 10% lower than that one obtained during the same day. This is in contrast with the results obtained on rabbits in a previous study, in which MAC values were statistically similar despite a 6% lower value obtained during the separate day experiment with respect to the same day value (9). Variations in MAC values of up to 10% within the same animal and of up to 20% between species are accepted in MAC studies (20), although data from different laboratories report larger variations between them due to differences in the type and intensity of the noxious stimulus applied during MAC studies and the subjectivity of the assessment of purposeful movement (8). We controlled for the type and intensity of stimulus in this study and despite the likelihood of subjectivity in assessing movement, the assessments were uniform and conducted by the same investigators, therefore it is likely that the 10% variation in the MAC values between the 2 methods used in this study can be attributed to the power of the study and that increasing the number of subjects for a study such as this could potentially yield similar results between both species (dogs and rabbits).
Plasma ketamine concentrations measured at the time of MAC determinations are consistent with those reported in other studies for the same degree of MAC reduction. In this study, the corresponding plasma ketamine concentration at which the MAC value was obtained was approximately 824 ng/mL for group 1 and 729 ng/mL for group 2. In other studies, at target plasma ketamine concentrations of 1000 ng/mL after administration of a CRI based on the individual dogs pharmacokinetic rate constants to achieve a pseudo-steady-state at the desired concentration, the MAC of isoflurane was reduced by approximately 11% to 39% (11). In another study using sevoflurane and infusing ketamine at 50 or 100 μg/kg per minute, plasma ketamine concentrations of 1057 ng/mL and 2191 ng/mL were obtained, respectively, which resulted in higher MAC reductions of approximately 40% to 45% (19). It has been estimated that the MAC of isoflurane can be reduced by 50% at plasma ketamine concentrations of approximately 3000 ng/mL (18).
Plasma ketamine concentrations have been correlated with the degree of sparing effect on the MAC of isoflurane (18). The relationship is described by an inverse sigmoid curve with the steepest section of the curve between approximately 1000 ng/mL and 8000 ng/mL where it appears to be linear and in inverse relationship, the higher the plasma concentration the lower the MAC value. The maximal MAC reduction has been estimated at 92% (18). Below 1000 ng/mL, the contribution to the MAC sparing effect is less and above 8000 ng/mL it adds little effect to the MAC sparing effect already attained. Consequently the correlation was lower in our study (r = 0.55). Dogs administered a loading dose of 26 mg/kg over 20 min and a CRI of 300 μg/kg per minute during enflurane anesthesia achieved plasma concentrations of 22 000 ng/mL and only a 73% MAC reduction (21). It is also recognized that there is high variability in the disposition of ketamine in dogs under anesthesia with isoflurane (17). The elimination half-life for dogs under isoflurane anesthesia is reported as 94 ± 37 min (17). In conscious dogs administered 15 mg/kg IV of ketamine, the half-life was 61 min (range: 44–77 min) (22).
Norketamine (N-demethylketamine) is considered the most important product of hepatic metabolism of ketamine (23). Approximately 62% of ketamine is biotransformed to norketamine (22). Pharmacokinetic studies in dogs receiving ketamine, IV, have shown that norketamine appears very rapidly in plasma and maximum concentrations are achieved 6.5 ± 4.8 min after administration and have a terminal half-life of 64 ± 24 min (17). The decline in concentrations is parallel to that of ketamine (17,22). The plasma concentrations of norketamine in this study were below the LOQ of the assay (200 ng/mL) for the majority of dogs. This differs from previous reports where plasma norketamine concentrations after single IV boluses of ketamine (3 or 15 mg/kg) were above the LOQ of this study for at least 30 to 60 min (17,22). It is possible that the lower loading dose of 1 mg/kg used in this study was not enough to raise the initial ketamine concentration (Cmax) to levels that would result in significant amounts of norketamine. For example, using a loading dose of 3 mg/kg, a Cmax of approximately 10 000 ng/mL was measured (17), whereas a dose of 15 mg/kg increased the concentration to approximately 15 000 to 25 000 ng/mL (22). The low and inconsistent norketamine concentrations achieved in this study were unlikely to influence the results on MAC determinations since norketamine only has approximately 10% of the anesthetic, analgesic, and anti-inflammatory properties of ketamine (24).
The MAC values for isoflurane obtained in both groups after stopping the CRI of ketamine were lower, although not statistically, by approximately 7% than the baseline value obtained in group 1. The determinations for those MAC values were completed 65 min (group 1) and 76 min (group 2) after stopping the CRI of ketamine. Based on the half-life of ketamine and the ketamine concentrations measured during those MAC determinations, 407 ng/mL and 347 ng/mL, respectively, it is clear that not enough time was allowed for the clearance of ketamine to allow the return of MAC to baseline. In addition, this finding is consistent with the minimal effect on MAC (< 10%) at plasma concentrations significantly below 1000 ng/mL (18).
In conclusion, ketamine had a significant sparing effect on the MAC of isoflurane in dogs. The MAC of isoflurane during the CRI of ketamine yielded different results when methods of same day versus separate days are used, despite similar plasma ketamine concentrations with both methods. However, this difference in MAC values is within acceptable limits of variation (≤ 10%) for MAC determinations.
Acknowledgment
This work was funded by a grant from PetTrust-OVC.
Footnotes
Presented at the 16th International Veterinary Emergency and Critical Care Symposium and Annual Conference of the American College of Veterinary Anesthesiologists, 2010, San Antonio, Texas, USA.
References
- 1.Eger EI, Saidman L. Minimum alveolar anesthetic concentration: A standard of anesthetic potency. Anesthesiology. 1965;26:756–763. doi: 10.1097/00000542-196511000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Golder FJ, Pascoe PJ, Bailey CS, et al. The effect of epidural morphine on the minimum alveolar concentration of isoflurane in cats. J Vet Anesth. 1998;25:52–56. [Google Scholar]
- 3.Valverde A, Doherty TJ, Hernández J, et al. Effect of lidocaine on the minimum alveolar concentration of isoflurane in dogs. Vet Anaesth Analg. 2004;31:264–271. doi: 10.1111/j.1467-2995.2004.00165.x. [DOI] [PubMed] [Google Scholar]
- 4.Pascoe PJ, Ilkiw JE, Craig C, et al. The effects of ketamine on the minimum alveolar concentration of isoflurane in cats. Vet Anaesth Analg. 2007;34:31–39. doi: 10.1111/j.1467-2995.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- 5.Seddighi MR, Egger CM, Rohrbach BW, et al. Effects of tramadol on the minimum alveolar concentration of sevoflurane in dogs. Vet Anaesth Analg. 2009;36:334–340. doi: 10.1111/j.1467-2995.2009.00468.x. [DOI] [PubMed] [Google Scholar]
- 6.Pypendop BH, Pascoe PJ, Ilkiw JE. Effects of epidural administration of morphine and buprenorphine on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res. 2006;67:1471–1475. doi: 10.2460/ajvr.67.9.1471. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira TH, Aguiar AJA, Valverde A, et al. Effect of remifentanil hydrochloride administered via constant rate infusion on the minimum alveolar concentration of isoflurane in cats. Am J Vet Res. 2009;70:581–588. doi: 10.2460/ajvr.70.5.581. [DOI] [PubMed] [Google Scholar]
- 8.Valverde A, Morey TE, Hernández J, et al. Validation of several types of noxious stimuli for use in determining the minimum alveolar concentration for inhalation anesthetics in dogs and rabbits. Am J Vet Res. 2003;64:957–962. doi: 10.2460/ajvr.2003.64.957. [DOI] [PubMed] [Google Scholar]
- 9.Gianotti G, Valverde A, Sinclair M, et al. Prior determination of baseline minimum alveolar concentration (MAC) of isoflurane does not influence the effect of ketamine on MAC in rabbits. Can J Vet Res. 2012;76:261–267. [PMC free article] [PubMed] [Google Scholar]
- 10.Muir WW, 3rd, Wiese AJ, March PA. Effects of morphine, lidocaine, ketamine, and morphine-lidocaine-ketamine drug combination on minimum alveolar concentration in dogs anesthetized with isoflurane. Am J Vet Res. 2003;64:1155–1160. doi: 10.2460/ajvr.2003.64.1155. [DOI] [PubMed] [Google Scholar]
- 11.Solano AM, Pypendop BH, Boscan PL, et al. Effect of intravenous administration of ketamine on the minimum alveolar concentration of isoflurane in anesthetized dogs. Am J Vet Res. 2006;67:21–25. doi: 10.2460/ajvr.67.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Love L, Egger C, Rohrbach B, et al. The effect of ketamine on the MAC(BAR) of sevoflurane in dogs. Vet Anaesth Analg. 2011;38:292–300. doi: 10.1111/j.1467-2995.2011.00616.x. [DOI] [PubMed] [Google Scholar]
- 13.Muir WW, 3rd, Sams R. Effects of ketamine infusion on halothane minimal alveolar concentration in horses. Am J Vet Res. 1992;53:1802–1806. [PubMed] [Google Scholar]
- 14.Bolze S, Boulieu S. HPLC determination of ketamine, norketamine, and dehydronorketamine in plasma with a high-purity reversed-phase sorbent. Clinical Chem. 1998;44:560–564. [PubMed] [Google Scholar]
- 15.Svensson J, Gustafsson LL. Determination of ketamine and norketamine enantiomers in plasma by solid-phase extraction and high–performance liquid chromatography. J Chromatogr B Biomed Appl. 1996;78:373–376. doi: 10.1016/0378-4347(95)00545-5. [DOI] [PubMed] [Google Scholar]
- 16.Parkin MC, Turfus SC, Kicman AT, et al. Detection of ketamine and its metabolites in urine by ultra high pressure liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876:137–142. doi: 10.1016/j.jchromb.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 17.Pypendop BH, Ilkiw JE. Pharmacokinetics of ketamine and its metabolite, norketamine after intravenous administration of a bolus of ketamine to isoflurane-anesthetized dogs. Am J Vet Res. 2005;66:2034–2038. doi: 10.2460/ajvr.2005.66.2034. [DOI] [PubMed] [Google Scholar]
- 18.Pypendop BH, Solano A, Boscan P, et al. Characteristics of the relationship between plasma ketamine concentration and its effect on the minimum alveolar concentration of isoflurane in dogs. Vet Anaesth Analg. 2007;34:209–212. doi: 10.1111/j.1467-2995.2006.00324.x. [DOI] [PubMed] [Google Scholar]
- 19.Wilson J, Doherty TJ, Egger CM, et al. Effects of intravenous lidocaine, ketamine, and the combination on the minimum alveolar concentration of sevoflurane in dogs. Vet Anaesth Analg. 2008;35:289–296. doi: 10.1111/j.1467-2995.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 20.Quasha AL, Eger EI, II, Tinker JH. Determination and applications of MAC. Anesthesiology. 1980;53:315–334. doi: 10.1097/00000542-198010000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Schwieger IM, Szlam F, Hugh CC., Jr The pharmacokinetics and pharmacodynamics of ketamine in dogs anesthetized with enflurane. J Pharmacokinet Biopharm. 1991;19:145–156. doi: 10.1007/BF01073866. [DOI] [PubMed] [Google Scholar]
- 22.Kaka JS, Hayton WL. Pharmacokinetics of ketamine and two metabolites in the dog. J Pharmacokinet Biopharm. 1980;8:193–202. doi: 10.1007/BF01065193. [DOI] [PubMed] [Google Scholar]
- 23.Woolf TF, Adams JD. Biotransformation of ketamine, (Z)-6-hydroxyketamine, and (E)-6-hydroxyketamine by rat, rabbit, and human liver microsomal preparations. Xenobiotica. 1987;17:839–847. doi: 10.3109/00498258709043993. [DOI] [PubMed] [Google Scholar]
- 24.Muir WW., 3rd NMDA receptor antagonists and pain: Ketamine. Vet Clin North Am Equine Pract. 2010;26:565–578. doi: 10.1016/j.cveq.2010.07.009. [DOI] [PubMed] [Google Scholar]