Abstract
The objective of the present study was to evaluate polyclonal- and monoclonal-antibody-based immunohistochemical (IHC) tests for the detection of 2 genotypes of Porcine circovirus type 2 (PCV2), a and b, in formalin-fixed, paraffin-embedded lymph-node tissue from pigs with experimental or natural postweaning multisystemic wasting syndrome and to compare the IHC results with those of in-situ hybridization (ISH) assays. The ISH assays proved more sensitive than the IHC tests for the detection of PCV2a and PCV2b. According to these findings, polyclonal-antibody-based IHC testing is the most practical routine diagnostic method for the detection of PCV2 regardless of genotype because IHC testing is less technically complex than ISH testing. However, ISH assays are useful to differentiate between PCV2a and PCV2b in surveillance programs for the monitoring of PCV2 in swine herds.
Résumé
L’objectif de la présente étude était d’évaluer des épreuves immunohistochimiques (IHC) à base d’anticorps polyclonaux et monoclonaux pour la détection de deux génotypes de circovirus porcin de type 2 (PCV2), a et b, dans des nœuds lymphatiques fixés dans la formaline et enrobés de paraffine provenant de porcs atteints naturellement ou expérimentalement du syndrome de dépérissement multi-systémique en post-sevrage et de comparer les résultats d’IHC à ceux d’épreuves d’hybridation in situ (ISH). Les épreuves d’ISH se sont avérées plus sensibles que les épreuves d’IHC pour la détection de PCV2a et PCV2b. À la lumière de ces résultats, l’épreuve IHC à base d’anticorps polyclonaux s’avère la méthode diagnostique de routine la plus pratique pour la détection de PCV2 indépendamment du génotype étant donné que l’épreuve IHC est techniquement moins complexe que l’épreuve ISH. Toutefois, les épreuves ISH sont utiles pour distinguer entre PCV2a et PCV2b dans des programmes de surveillance pour PCV2 dans les troupeaux porcins.
(Traduit par Docteur Serge Messier)
Porcine circovirus type 2 (PCV2) is associated with a number of diseases and syndromes collectively referred to as porcine circovirus-associated disease (PCVAD) (1,2). Postweaning multisystemic wasting syndrome (PMWS), the main clinical manifestation of PCVAD, is characterized clinically by wasting, decreased weight gain, enlarged lymph nodes, and dyspnea (1). Phylogenetic analysis has categorized PCV2 into at least 2 major genotypes, PCV2a and PCV2b (3). Epidemiologic studies have strongly suggested a link between PCV2b, PMWS, and a genotype shift from PCV2a to PCV2b (4).
The diagnosis of PMWS is somewhat different from the diagnosis of other swine viral diseases. Virus isolation is not considered to be the gold standard of PMWS diagnosis because PCV2 has frequently been isolated and detected in lymph nodes from healthy pigs without a diagnosis of clinical PMWS (1,5). Hence, other confirmatory PMWS diagnostic methods should be used to detect the PCV2 in histopathological lesions such as depleted lymphoid tissue and granulomatous inflammation (1).
Immunohistochemical (IHC) and in-situ hybridization (ISH) tests are better than a polymerase chain reaction (PCR) assay for the detection of PCV2 within histopathological lesions (6). Both of the former methods provide cellular detail and histologic architecture, allowing the number of PCV2-infected cells and characteristic histopathological lesions to be observed simultaneously in the same section (6). High quality of PCV2 antibody is required for the IHC assay of PCV2 antigen in formalin-fixed, paraffin-embedded (FFPE) tissues. Polyclonal and monoclonal antibodies against PCV2 are now commercially available. The objective of the present study was to compare those antibodies in the IHC detection and differentiation of the 2 genotypes of PCV2 in FFPE tissues and to compare the results with those of ISH assay.
Experimental PMWS was reproduced in pigs by coinfection of PCV2b and Porcine parvovirus (PPV) as previously described (7). Tissue-culture-propagated PCV2 (strain SNUVR000463) and PPV (strain SNUVR000464) were the sources of the viral inocula. For inoculation, a PCV2 pool containing a median tissue culture infective dose (TCID50) of 1.2 × 105 per milliliter and a PPV pool containing 1.3 × 105 TCID50/mL were prepared as previously described (7). Twenty-five 1-day-old conventional pigs, all seronegative for PCV, PPV, and Porcine reproductive and respiratory syndrome virus, were randomly divided into 3 groups. The 10 pigs in group 1 were inoculated intranasally with a mixture of equal volumes of a 1:20 dilution of the PCV2a pool and a 1:20 dilution of the PPV pool. The 10 pigs in group 2 were inoculated intranasally with a mixture of equal volumes of a 1:20 dilution of the PCV2b pool and a 1:20 dilution of the PPV pool. The 5 negative-control pigs in group 3 were inoculated with PCV-free PK-15 cell lysates. The groups were housed in separate isolators, fed a commercial sterile milk substitute, and examined at regular intervals. At 32 d after inoculation, all the pigs were sedated by an intravenous injection of sodium pentobarbital and then euthanized by electrocution. Inguinal lymph node, which had been found to show a consistent and intense hybridization signal for PCV2, was selected for IHC and ISH analysis (7). The methods had been approved by the Seoul National University Institutional Animal Care and Use Committee.
Forty natural PMWS cases were selected on the basis of clinical signs, histopathological lesions, detection of PCV2 by IHC testing, and PCV2 isolation (1). The main clinical signs in all 40 cases were wasting or failure to gain weight and pallor of the skin. In 18 cases PCV2a was isolated, and in the other 22 cases PCV2b was isolated. The PCV2 genotype was identified by sequence analysis. All 40 cases fell into same diagnostic category (1), and inguinal lymph nodes were used for the IHC and ISH analyses.
Four serial sections (4 μm thick) of each node sample were placed on positively charged slides and stored at room temperature. Two sections were processed for polyclonal- and monoclonal-antibody-based IHC testing (1 slide each), and the other 2 sections were processed for PCV2a- and PCV2b-based ISH assay (1 slide each). The order of the serial slides was the same for each test. To determine the optimal concentration of antibody for the IHC testing, polyclonal and monoclonal antibodies prediluted to 1:200, 1:500, and 1:1000 were used as primary antibody. No significantly different numbers of positive cells were detected at any dilution, but the best signal intensity was obtained with a dilution of 1:500. Therefore, the polyclonal antibodies (Veterinary Diagnostic Laboratory, Iowa State University, Ames, Iowa, USA) and the monoclonal antibodies (Rural Technologies, Brookings, South Dakota, USA) were diluted 1:500 in phosphate-buffered saline (0.01 M, pH 7.4) containing 0.1% Tween 20. The IHC testing was done as previously described (6), and PCV2a- and 2b-specific probes were prepared and used as previously described for the ISH assays (8).
For quantitative data, the slides were analyzed with the US National Institutes of Health ImageJ image-processing program, version 1.43 m. For each slide, 10 fields were randomly selected, and the number of positive cells per 0.25 mm2 was counted, as previously described (7). The mean values were also calculated. The number of PCV2-positive cells was compared between the 3 groups by 1-way nonparametric analysis of variance (Kruskal–Wallis test) followed by individual Mann–Whitney U-tests. Statistical significance was accepted at P < 0.05.
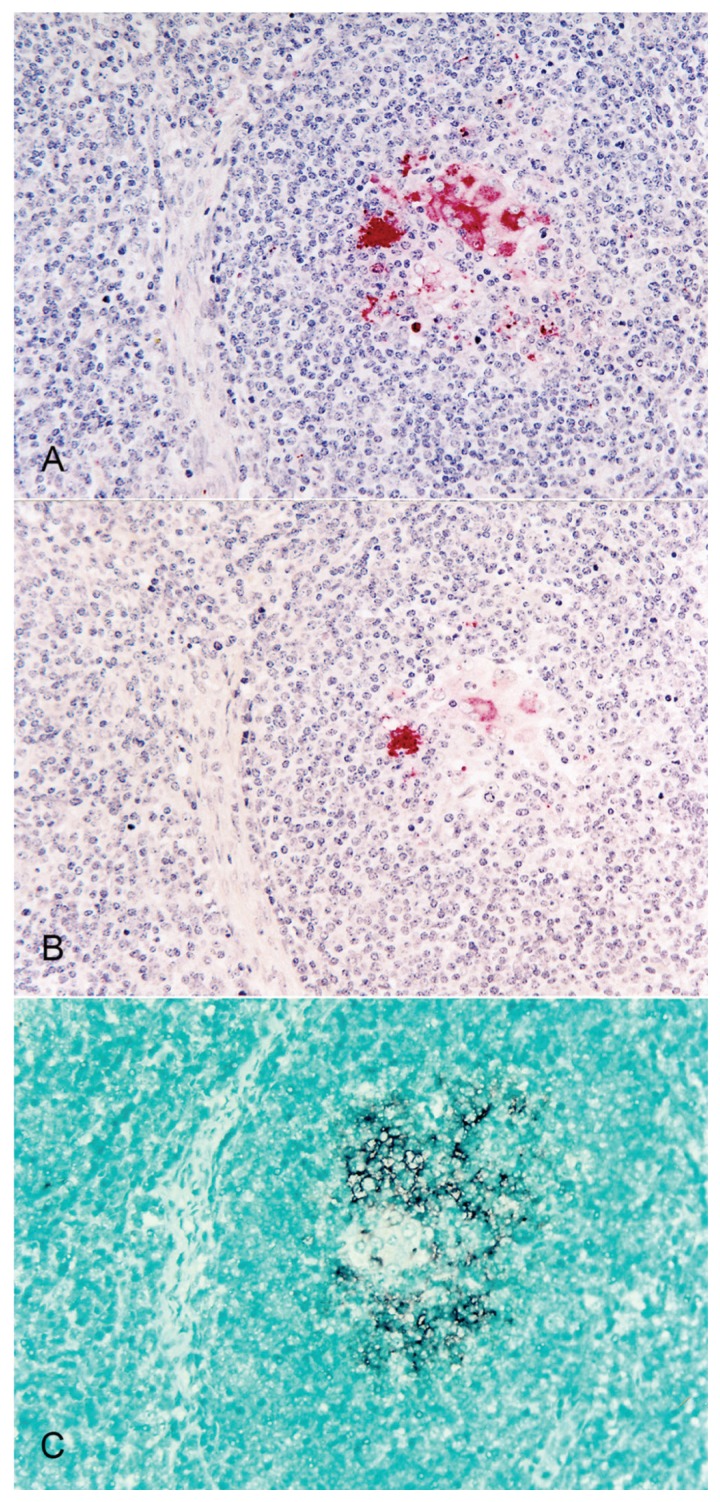
Distinctly positive IHC and ISH signals were detected in the lymph nodes from the pigs with experimental and natural PMWS. Positive cells typically exhibited red reaction products and dark-brown reaction products in the IHC and ISH assays, respectively, without background staining (Figure 1). The polyclonal- and monoclonal-antibody-based IHC assays detected both PCV2a and PCV2b. The PCV2a-based ISH assay detected PCV2a in pigs with PCV2a-associated PMWS, and the PCV2b-based ISH assay detected PCV-2b in pigs with PCV2b-associated PMWS. The polyclonal- and monoclonal-antibody-based IHC assays and the PCV2a (or PCV2b)-based ISH assays of the serial sections of the lymph nodes indicated that cells that stained positive for PCV2a (or PCV2b) antigen were also positive for PCV2a (or PCV2b) DNA.
Figure 1.
Serial sections of a lymph node from a pig with experimental postweaning multisystemic wasting syndrome. Porcine circovirus type 2b (PCV2b) antigens were detected in 16 macrophages per 0.25 mm2 with polyclonal-antibody-based immunohistochemical (IHC) testing (A) and in 5 macrophages per 0.25 mm2 with monoclonal-antibody-based IHC testing (B). With PCV2b-based in-situ hybridization, PCV2b DNA was detected in 25 macrophages per 0.25 mm2 (C).
The polyclonal-antibody-based IHC assay detected significantly more PCV2a- or PCV2b-positive cells than did the monoclonal-antibody-based IHC assay in the lymph nodes from pigs with experimental PMWS (P = 0.012 for PCV2a and P = 0.039 for PCV2b) and natural PMWS (P = 0.029 for PCV2a and P = 0.047 for PCV2b). The PCV2a (or PCV2b)-based ISH assay detected significantly more PCV2a (or PCV2b)-positive cells than did the polyclonal- and monoclonal-antibody-based IHC assays in the lymph nodes from pigs with experimental PMWS (P < 0.01) and natural PMWS (P < 0.05 for the polyclonal-antibody-based IHC assay and P < 0.01 for the monoclonal-antibody-based IHC assay) (Table I).
Table I.
Numbers of cells in slides of lymph-node tissue from pigs with postweaning multisystemic wasting syndrome (PMWS) found to be positive for Porcine circovirus type 2 (PCV2) by immunohistochemical (IHC) testing with polyclonal or monoclonal antibody (Ab) and by in-situ hybridization (ISH) with PCV2a- and PCV2b-specific probes
| Type of PMWS and PCV2 genotype | Mean number of positive cells ± standard deviation per 0.25 mm2 | |||
|---|---|---|---|---|
|
| ||||
| IHC assay | ISH assay | |||
|
|
|
|||
| Polyclonal Ab | Monoclonal Ab | PCV2a probe | PCV2b probe | |
| Experimental | ||||
| PCV2a | 78 ± 21.55a | 45 ± 14.31 | 121 ± 34.17b | 0 |
| PCV2b | 77 ± 29.12a | 52 ± 11.09 | 0 | 129 ± 46.41b |
| Natural | ||||
| PCV2a | 83 ± 25.24a | 51 ± 19.35 | 139 ± 43.21c | 0 |
| PCV2b | 89 ± 35.53a | 63 ± 21.52 | 0 | 135 ± 29.62c |
Significantly greater (P < 0.05) than with monoclonal-antibody-based IHC testing.
Significantly greater (P < 0.01) than with IHC testing.
Significantly greater than with polyclonal (P < 0.05) — or monoclonal (P < 0.01) — antibody-based IHC testing.
The specificity of IHC and ISH testing was confirmed by the rigorous observance of 2 controls: i) treatment of normal serum in place of primary PCV2 antibody for the IHC assay and predigestion of a tissue section with a solution of DNase before ISH testing, which precluded development of the signals; and ii) use of PCV2 antibody and a DNA probe for IHC and ISH testing, respectively, on tissues of control pigs that were consistently positive and negative.
The results of this study demonstrate that IHC and ISH assays are of potential value in confirming the diagnosis of PMWS. However, there are clear differences between IHC and ISH testing in the detection and differentiation of the 2 genotypes of PCV2 in FFPE lymph-node tissue from pigs with experimental and natural PMWS. Although monoclonal- and polyclonal-antibody-based IHC assays specific for the detection of PCV2 viral proteins are available, none have been shown to differentiate PCV2a from PCV2b. In addition, ISH testing was found to be more sensitive than IHC testing for the detection of PCV2a and PCV2b, as in a previous study (9). There may be 2 reasons for the significantly higher sensitivity of ISH testing compared with IHC testing in this study. First, PCV2 infection may be nonproductive. In this study, the ISH assay detected PCV2 DNA mainly in the cytoplasm of macrophage-lineage cells. These types of cells have been shown to be unable to support PCV2 replication (10,11), and most of the cytoplasmic ISH signals were identified as being from nonreplicative DNA forms (12). Hence, there was not a sufficient level of viral antigen in these cells, and thus these cells could not be detected by IHC testing. Second, formalin fixation may have cross-linked the viral proteins (antigens). Such cross-linking can affect the immunoreactive epitopes of the PCV2 viral proteins, inhibiting detection by IHC testing. Formalin fixation may not affect ISH testing as much as IHC testing because ISH detects PCV2 DNA and not PCV2 viral protein.
In the present study, polyclonal-antibody-based IHC was more sensitive than monoclonal-antibody-based IHC testing in detecting 2 genotypes of PCV2 in FFPE lymph nodes. These results are in contrast with those from a previous study in which significant differences were not detected between monoclonal- and polyclonal-antibody-based IHC (9). We have no clear explanation for this discrepancy, but it may be due to different reactivity against an epitope of PCV2 by different monoclonal antibodies (13), such that the commercial monoclonal antibody used in this study had less reactivity against an epitope of PCV2 than the monoclonal antibody used in the previous study. Although the epitope(s) targeted by the monoclonal and polyclonal antibodies used in this study are unknown, the monoclonal antibody targets a single epitope, whereas the polyclonal antibody targets more epitopes. This difference could explain the significantly higher sensitivity of the polyclonal antibody relative to that of the monoclonal antibody in this study.
The IHC procedure could be made more sensitive by using avidin–biotin complex. However, we have not found a significant difference between avidin–biotin complex and the alkaline phosphatase method (data not shown). In addition, nonspecific signals were frequently detected by the avidin–biotin complex method because endogenous biotin is distributed widely in many tissues of pigs (14). From the present results it is recommended that either IHC or ISH testing be used depending on the purpose of detection or differentiation between the 2 genotypes of PCV2. Polyclonal-antibody-based IHC testing is a more practical routine diagnostic method for the detection of PCV2 regardless of the genotype because IHC testing is less technically complex than ISH testing. Furthermore, the differentiation between PCV2a and PCV2b is not clinically meaningful when one is selecting the proper PCV2 vaccine because PCV2a-based vaccines effectively control PCV2b infection in pigs (15,16). On the other hand, ISH testing is useful to differentiate between PCV2a and PCV2b in surveillance programs for monitoring PCV2 in swine herds.
Acknowledgments
This research was supported by the Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, by contract research funds of the Research Institute for Veterinary Science of the College of Veterinary Medicine, and by the Brain Korea 21 Plus Program for Creative Veterinary Science Research in the Republic of Korea.
References
- 1.Chae C. Postweaning multisystemic wasting syndrome: A review of aetiology, diagnosis and pathology. Vet J. 2004;168:41–49. doi: 10.1016/j.tvjl.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Chae C. A review of porcine circovirus 2-associated syndromes and disease. Vet J. 2005;169:326–336. doi: 10.1016/j.tvjl.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Grau-Roma L, Crisci E, Sibila M, et al. A proposal on porcine circovirus type 2 (PCV2) genotype definition and their relation with postweaning multisystemic wasting syndrome (PMWS) occurrence. Vet Microbiol. 2008;128:23–35. doi: 10.1016/j.vetmic.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Gagnon CA, Tremblay D, Tijssen P, et al. The emergence of porcine circovirus 2b genotype (PCV-2b) in swine in Canada. Can Vet J. 2007;48:811–819. [PMC free article] [PubMed] [Google Scholar]
- 5.Calsamiglia M, Segalés J, Quintana J, Rosell C, Domingo M. Detection of porcine circovirus types 1 and 2 in serum and tissue samples of pigs with and without postweaning multisystemic wasting syndrome. J Clin Microbiol. 2002;40:1848–1850. doi: 10.1128/JCM.40.5.1848-1850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Chae C. A comparison of virus isolation, polymerase chain reaction, immunohistochemistry, and in situ hybridization for the detection of porcine circovirus 2 and porcine parvovirus in experimentally and naturally coinfected pigs. J Vet Diagn Invest. 2004;16:45–50. doi: 10.1177/104063870401600108. [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Choi C, Chae C. Pathogenesis of postweaning multisystemic wasting syndrome reproduced by co-infection with Korean isolates of porcine circovirus 2 and porcine parvovirus. J Comp Pathol. 2003;128:52–59. doi: 10.1053/jcpa.2002.0605. [DOI] [PubMed] [Google Scholar]
- 8.Kim D, Ha Y, Oh Y, et al. Development of in situ hybridization assay that differentiates between two genotypes of Porcine circovirus-2 in formalin-fixed, paraffin-embedded tissues. J Vet Diagn Invest. 2010;22:231–233. doi: 10.1177/104063871002200209. [DOI] [PubMed] [Google Scholar]
- 9.Kim D, Ha Y, Lee Y-H, et al. Comparative study of in situ hybridization and immunohistochemistry for the detection of porcine circovirus 2 in formalin-fixed, paraffin-embedded tissues. J Vet Med Sci. 2009;71:1001–1004. doi: 10.1292/jvms.71.1001. [DOI] [PubMed] [Google Scholar]
- 10.Gilpin DF, McCullough K, Meehan BM, et al. In vitro studies on the infection and replication of porcine circovirus type 2 in cells of the porcine immune system. Vet Immunol Immunopathol. 2003;94:149–161. doi: 10.1016/s0165-2427(03)00087-4. [DOI] [PubMed] [Google Scholar]
- 11.Vincent IE, Carrasco CP, Herrmann B, et al. Dendritic cells harbor infectious porcine circovirus type 2 in the absence of apparent cell modulation or replication of the virus. J Virol. 2003;77:13288–13300. doi: 10.1128/JVI.77.24.13288-13300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Martin E, Rovira A, Calsamiglia M, et al. A new method to identify cell types that support porcine circovirus type 2 replication in formalin-fixed, paraffin-embedded swine tissues. J Virol Methods. 2007;146:86–95. doi: 10.1016/j.jviromet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 13.McNeilly F, McNair I, Mackie DP, et al. Production, characterisation and application of monoclonal antibodies to porcine circovirus 2. Arch Virol. 2001;146:909–922. doi: 10.1007/s007050170124. [DOI] [PubMed] [Google Scholar]
- 14.Cooper KM, Kennedy S, McConnell S, Kennedy DG, Frigg M. An immunohistochemical study of the distribution of biotin in tissues of pigs and chickens. Res Vet Sci. 1997;63:219–225. doi: 10.1016/s0034-5288(97)90024-2. [DOI] [PubMed] [Google Scholar]
- 15.Fort M, Sibila M, Allepuz A, et al. Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine. 2008;26:1063–1071. doi: 10.1016/j.vaccine.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Opriessnig T, Patterson AR, Madson DM, Pal N, Halbur PG. Comparison of efficacy of commercial one dose and two dose PCV2 vaccines using a mixed PRRSV–PCV2–SIV clinical infection model 2–3-months post vaccination. Vaccine. 2009;27:1002–1007. doi: 10.1016/j.vaccine.2008.11.105. [DOI] [PubMed] [Google Scholar]