Abstract
Species of Verruconis and species of Ochroconis are dematiaceous fungi generally found in the environment but having the ability to infect humans, dogs, cats, poultry, and fish. This study presents the antifungal susceptibility patterns of these fungi at the species level. Forty strains originating from clinical and environmental sources were phylogenetically identified at the species level by using sequences of the ribosomal DNA internal transcribed spacer (rDNA ITS). In vitro antifungal susceptibility testing was performed against eight antifungals, using the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method. The geometric mean MICs for amphotericin B (AMB), flucytosine (5FC), fluconazole (FLC), itraconazole (ITC), voriconazole (VRC), and posaconazole (POS) and minimum effective concentrations (MECs) for caspofungin (CAS) and anidulafungin (AFG) across the Ochroconis and Verruconis species were as follows, in increasing order. For Verruconis species, the values (μg/ml) were as follows: AFG, 0.04; POS, 0.25; ITC, 0.37; AMB, 0.50; CAS, 0.65; VRC, 0.96; 5FC, 10.45; and FLC, 47.25. For Ochroconis species, the values (μg/ml) were as follows: AFG, 0.06; POS, 0.11; CAS, 0.67; VRC, 2.76; ITC, 3.94; AMB, 5.68; 5FC, 34.48; and FLC, 61.33. Antifungal susceptibility of Ochroconis and Verruconis was linked with phylogenetic distance and thermotolerance. Echinocandins and POS showed the greatest in vitro activity, providing possible treatment options for Ochroconis and Verruconis infections.
INTRODUCTION
Recently, by combined molecular phylogeny, morphology, and ecology, the taxonomy of the Ochroconis lineage was revised (1). Two genera were recognized: Ochroconis and Verruconis. Within melanized filamentous fungi, members of Ochroconis and Verruconis are morphologically exceptional by having sympodial conidiogenesis with rhexolytic conidial dehiscence (2). However, both genera are melanized, oligotrophic, and regularly encountered in indoor environments, in soil, or in heated habitats, and some species have the ability to cause superficial, cutaneous, and systemic infections in immunocompromised patients (3–6).
Verruconis species are thermophilic, with Verruconis gallopava occurring in hot environments, such as thermal soils, broiler house litter, hot springs, and self-heated waste (1). Pathology in Verruconis is restricted to V. gallopava, which is the main agent of human brain infections and is responsible for encephalitis in poultry and wild birds (7–15), dogs (16), and cats (17). In contrast, Ochroconis species are mesophilic saprobes, with an optimum growth temperature between 15 and 30°C and an inability to grow at 37°C, which occasionally infect cold-blooded vertebrates (1, 18). Only a single infection was noted in a warm-blooded animal, i.e., a subcutaneous lesion in a cat (19), while the first subcutaneous human infection due to Ochroconis tshawytschae was recently reported (20).
Despite significant medical and veterinary importance, little is known regarding the species-specific antifungal susceptibility profiles of Verruconis and Ochroconis species. The polyene agents exert their antifungal activity via binding to ergosterol in the fungal cell membrane. This disrupts cell permeability and results in rapid cell death. Flucytosine exerts antifungal activity via inhibition of both DNA synthesis and protein synthesis in the fungal cell. Azole agents exert their antifungal activity by blocking the demethylation of lanosterol, thereby inhibiting ergosterol synthesis. The mechanism of activity of the echinocandins is inhibition of the production of (1,3)-β-d-glucan, an essential component in the fungal cell wall (21). We therefore investigated the in vitro susceptibilities of a large collection of clinical and environmental isolates of thermophilic and mesophilic species to eight antifungal drugs.
(Some of these results were presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, 10 to 13 September 2013.)
MATERIALS AND METHODS
Fungal strains.
Strains used in this study are listed in Table 1, with origin, identification number, and clinical data for each isolate. In total, 40 strains from clinical and environmental sources were used. Lyophilized fungal strains were obtained from the reference collection of the CBS-KNAW Fungal Biodiversity Centre (CBS, Utrecht, The Netherlands) and selected according to their historical pathogenicity. In addition, the representative type species of saprophytic strains were used for environmental isolates of both genera (Table 1). All isolates were cultured on malt extract agar (MEA) at 24°C for 14 days. Morphological identifications were confirmed by sequence-based analysis of the internal transcribed spacer (ITS) of the ribosomal DNA (rDNA) region, as described previously (1). Briefly, sequences were edited using the SeqMan tool of Lasergene software (DNAStar Inc., Madison, WI) and then aligned interactively using Ward's averaging in the BioNumerics package v. 4.61 (Applied Maths, Kortrijk, Belgium). The ITS sequences were finally aligned with the program MUSCLE (www.ebi.ac.uk/Tools/msa/muscle), and the aligned sequences were adjusted using BioEdit v. 7.0.5.2. The ITS data set was then analyzed by use of MEGA5 software (22), in which the Tamura three-parameter model with gamma distribution (T92+G) was searched as the best model. The maximum likelihood (ML) heuristic method with 1,000-replicate bootstrapping and the maximum parsimony (MP) method with 1,000-replicate bootstrapping were performed for tree reconstructions and phylogeny tests. To strongly confirm the analyses, the ML method with the approximate likelihood ratio test (aLRT) was also performed with PhyML (23). Trees were viewed and edited with TreeView v. 1.6.6, FigTree v. 1.1.2, and MEGA5.
TABLE 1.
Isolation data for examined strains of Ochroconis and Verruconis spp.
| CBS ID | Species | Other collection no. | GenBank accession no. | Source or origin | Geography (city, state, country) | Yr of isolationa | Reference(s) |
|---|---|---|---|---|---|---|---|
| CBS 125817 | V. calidifluminalis | IFM 54739 | AB385699 | Hot spring effluent | Kanakawa, Hakone, Japan | 2004 | 47 |
| CBS 125818 (type strain) | V. calidifluminalis | IFM 54738 | AB385698 | Hot spring effluent | Kanakawa, Hakone, Japan | 2004 | 47 |
| CBS 118.91 | V. gallopava | CDC B-4954 | HQ667551 | Human | Atlanta, GA, USA | 1991 | 48 |
| CBS 166.85 | V. gallopava | dH 14821 | HQ667554 | Environment | France | 1985 | 27 |
| CBS 265.97 | V. gallopava | dH 14836 | HQ667555 | Australorp chick | Brisbane, Queensland, Australia | 1990 | 14 |
| CBS 437.64 (type strain) | V. gallopava | ATCC 16027, CDC 45-492-62, MUCL 6683 | HQ667553 | Turkey (Meleagris gallopavo) | Bishopville, SC, USA | 1964 | 7 |
| CBS 547.81 | V. gallopava | HQ667560 | Environment | Christchurch, New Zealand | 1981 | 27 | |
| CBS 862.95 | V. gallopava | ATCC 60633, CDC B-4224 | Human | South Carolina | 1990 | 49 | |
| CBS 863.95 | V. gallopava | CDC B-5637 | HQ667548 | Human, bronchial aspirate | USA | 1996 | 27 |
| CBS 865.95 | V. gallopava | CDC B-4872 | HQ667549 | Human, mine worker, sputum | Johannesburg, South Africa | 1989 | 27 |
| CBS 866.95 | V. gallopava | CDC B-4767 | Human | Mobile, AL, USA | 1996 | 27 | |
| CBS 867.95 | V. gallopava | CDC B-4682 | HQ667561 | Human, sputum | Salisbury, MD, USA | 1996 | 27 |
| CBS 100437 | V. gallopava | ATCC 48169, IMI 241149 | HQ667556 | Broiler chicken | Scotland, UK | 1998 | 27 |
| CBS 116660 | V. gallopava | CDC B-5813 | HQ667557 | Human, bronchoalveolar lavage fluid | USA | ND | 27 |
| CBS 116646 | V. gallopava | IMI 308437 | HQ667559 | Human, sputum | Western Australia | 27 | |
| CBS 119640 | V. gallopava | dH 14079, NCPF 7122 | Human | Australia | ND | 27 | |
| CBS 119641 | V. gallopava | NCPF 2923, dH 4078 | HQ667547 | Human, sputum | UK | ND | 27 |
| CBS 119642 | V. gallopava | dH 14077, NCPF 2221 | HQ667550 | Chick | ND | ND | 27 |
| CBS 119922 | V. gallopava | dH 14073 | Human, L3 puncture | The Netherlands | |||
| CBS 120153 | V. gallopava | dH13131, RKI 579/00 | Human | Germany | |||
| CBS 729.95 (type strain) | O. mirabilis | dH 14850 | KF156029 | Regulator of diver | Haarlem, The Netherlands | 1995 | 50 |
| CBS 124.65 | O. mirabilis | MUCL 6479 | HQ667532 | Human | India | 1965 | |
| CBS 102468 | O. mirabilis | HQ667533 | Human | Nijmegen, The Netherlands | 2000 | ||
| CBS 113948 | O. mirabilis | dH 13215 | HQ667530 | Human | Haarlem, The Netherlands | ||
| CBS 118685 | O. mirabilis | HQ667529 | Human, 8-year-old girl | Sweden | |||
| CBS 123268 | O. mirabilis | dH 17473 | HQ667526 | Human | Denmark | ||
| CBS 124210 | O. mirabilis | dH 17059 | KF156028 | Human | Denmark | ||
| CBS 135920 | O. mirabilis | dH 22275 | KF156033 | Human | Thailand | 2011 | |
| CBS 100438 (type strain) | O. tshawytschae | dH 10758, dH 14814, ATCC 9915 | HQ667562 | Fish (Chinook salmon) | USA | 1946 | 51 |
| CBS 129970 | O. tshawytschae | CMCC(f)D.31a | JN974456 | Human | Nanjing, China | 2011 | 52 |
| CBS 100486 | O. constricta | NJM 9471 | KF156026 | Fish (devil stinger) | Kagoshima, Japan | 1995 | 53 |
| CBS 131913 | O. constricta | dH22432 | KF156025 | Human | Thailand | 2011 | |
| CBS 211.53 (type strain) | O. constricta | ATCC 11419, DAOM 28282, IMI 051380, MUCL 9896 | HQ667519 | Soil | Ancaster, Ontario, Canada | 1952 | |
| CBS 135766 | Ochroconis sp. | UIIII09 | Fish | Sweden | 2012 | ||
| CBS 475.80 (type strain) | O. cordanae | dH 14825 | KF156022 | Dead leaf (Palmae) | Colombia | 1979 | |
| CBS 116655 (type strain) | O. humicola | dH 13739, IMI 110131, UAMH 10241 | HQ667521 | Peat soil | Ontario, Canada | 1962 | 54 |
| CBS 510.71 (type strain) | O. minima | dH 14792, ATCC 22631, IMI 082933 | HQ667522 | Rhizosphere | Samaru, Zaria, Nigeria | 1967 | 55 |
| CBS 239.78 (type strain) | O. gamsii | dH 14835, CBS H-7440 | KF156019 | Plant leaf | Sri Lanka | 1973 | 56 |
| CBS 383.81 (type strain) | O. verrucosa | IMI 211655 | KF156015 | Soil | Kerala, Kolkata, India | 1981 | 57 |
| CBS 284.64 (type strain) | O. anellii | IHEM 4516, IMI 089069, MUCL 9473 | FR832477 | Stalactites | Bari, Italy | 1962 | 58 |
| CBS 131815 (type strain) | O. lascauxensis | CMFISB 1862, LX A1 | FR832474 | Black stains | Montignac, Lascaux Cave, France | 2008 | 59 |
ND, not determined.
In vitro antifungal susceptibility testing.
In vitro antifungal susceptibility testing was performed using a broth microdilution format against eight antifungal compounds according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (24). The final concentrations of the antifungal agents ranged from 0.016 to 16 μg/ml for amphotericin B (AMB), fluconazole (FLC), itraconazole (ITC), voriconazole (VRC), posaconazole (POS), caspofungin (CAS), and anidulafungin (AFG). Flucytosine (5FC) was assayed over a 2-fold concentration range from 0.064 to 64 μg/ml. Reading of results was performed using a reading mirror and a microtitration plate spectrophotometric reader (Anthos htIII; Anthos Labtec Instruments, Salzburg, Austria).
Ochroconis isolates were incubated at 25°C, and Verruconis isolates were incubated at 37°C. Agitation of plates was not used. The MICs of AMB, FLC, 5FC, ITC, VRC, and POS were determined visually with an inverted mirror by comparison of growth in the wells containing the drug and that of the drug-free control. The minimum effective concentrations (MECs) of CAS and AFG were read with a plate microscope (Olympus SZX9; Olympus Nederland, Zoeterwoude, The Netherlands) at a magnification of ×25 to ×50. The MEC was defined as the lowest concentration at which abnormal, short, and branched hyphal clusters were observed, in contrast to the long, unbranched hyphal elements that were seen in the growth control well.
Paecilomyces variotii (ATCC 22319), Candida parapsilosis (ATCC 22019) and Candida krusei (ATCC 6258) were used as quality controls in all experiments. The ranges and geometric means (GM) of the MICs and MECs were determined for each species and drug after 48 to 168 h of incubation. Furthermore, the MIC50s and MIC90s for the isolates were calculated by use of the criteria for MIC determinations described above. The MIC50 and MIC90 values were calculated for those species with 10 or more isolates. If the MIC values of the replicates were different, the GM values of the replicates were used for comparison with other isolates. All experiments were performed in three independent replicates with each strain on different days.
Statistical analysis.
Data analyses were performed by using GraphPad Prism, version 5.0, for Windows (GraphPad Software, San Diego, CA). MIC/MEC distributions between isolates were compared by using the Mann-Whitney-Wilcoxon test. Statistical significance was defined as having a P value of ≤0.05 (two-tailed).
RESULTS
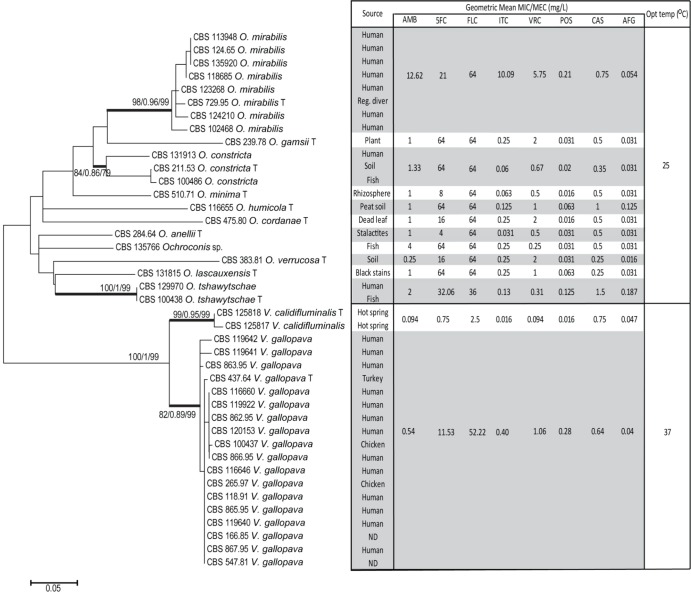
All strains were identified to the species level by sequence-based analysis and tested against eight antifungal compounds. Two genera have been recognized based on molecular phylogeny, viz., Verruconis and Ochroconis. In Fig. 1, data are summarized for relevant species, displaying their mutual phylogenetic distances, interspecies variability according to temperature tolerance, and antifungal susceptibility profiles per species. GM MICs, MIC ranges, and MIC50 and MIC90 distributions for eight antifungal agents are summarized in Table 2.
FIG 1.
MEGA5 maximum likelihood tree created from ITS sequences of Ochroconis and Verruconis isolates. The geometric mean susceptibility profiles of eight antifungals against each species have been incorporated into the figure. Numbers on branches are percent bootstrap values obtained from ML, aLRT, and MP analyses. Type strains are highlighted by a “T.” ND, not determined.
TABLE 2.
Geometric mean MICs, MIC ranges, MIC50s, and MIC90s obtained by susceptibility testing of antimycotic agents against Ochroconis and Verruconis spp.
| Organism (n) and drug | MIC or MEC (mg/liter)a |
|||
|---|---|---|---|---|
| Range | 50% | 90% | Geometric mean | |
| All thermotolerant strains (Verruconis spp.) (n = 20) | ||||
| Amphotericin B | 0.125–4 | 0.25 | 0.5 | 0.50 |
| Flucytosine | 0.5–64 | 4 | 32 | 10.45 |
| Fluconazole | 1–>64 | 64 | >64 | 47.25 |
| Itraconazole | <0.016–4 | 0.125 | 0.5 | 0.37 |
| Voriconazole | 0.063–2 | 1 | 2 | 0.96 |
| Posaconazole | <0.016–4 | 0.031 | 0.125 | 0.25 |
| Caspofungin | 0.25–1 | 0.5 | 1 | 0.65 |
| Anidulafungin | 0.016–0.125 | 0.031 | 0.063 | 0.04 |
| All mesophilic strains (Ochroconis spp.) (n = 20) | ||||
| Amphotericin B | 0.25->16 | 2 | >16 | 5.68 |
| Flucytosine | 0.125->64 | 16 | >64 | 34.48 |
| Fluconazole | 8->64 | 64 | >64 | 61.33 |
| Itraconazole | <0.016–>16 | 0.25 | >16 | 3.94 |
| Voriconazole | 0.125–8 | 2 | 8 | 2.76 |
| Posaconazole | <0.016–0.25 | 0.063 | 0.25 | 0.11 |
| Caspofungin | 0.063–2 | 0.5 | 1 | 0.67 |
| Anidulafungin | 0.016–0.25 | 0.031 | 0.125 | 0.06 |
| V. gallopava (n = 18) | ||||
| Amphotericin B | 0.125–4 | 0.25 | 0.5 | 0.54 |
| Flucytosine | 0.5–64 | 4 | 32 | 11.53 |
| Fluconazole | 4->64 | 64 | >64 | 52.22 |
| Itraconazole | 0.016–4 | 0.125 | 0.5 | 0.40 |
| Voriconazole | 0.5–2 | 1 | 2 | 1.06 |
| Posaconazole | <0.016–4 | 0.031 | 0.125 | 0.28 |
| Caspofungin | 0.25–1 | 0.5 | 1 | 0.64 |
| Anidulafungin | 0.016–0.125 | 0.031 | 0.063 | 0.04 |
| V. calidifluminalis (n = 2) | ||||
| Amphotericin B | 0.063–0.125 | NC | NC | 0.094 |
| Flucytosine | 0.5–1 | NC | NC | 0.75 |
| Fluconazole | 1–4 | NC | NC | 2.5 |
| Itraconazole | ≤0.16 | NC | NC | 0.016 |
| Voriconazole | 0.063–0.125 | NC | NC | 0,.094 |
| Posaconazole | ≤0.16 | NC | NC | 0.016 |
| Caspofungin | 0.5–1 | NC | NC | 0.75 |
| Anidulafungin | 0.031–0.063 | NC | NC | 0.047 |
| O. mirabilis (n = 8) | ||||
| Amphotericin B | 1->16 | NC | NC | 12.625 |
| Flucytosine | 8–64 | NC | NC | 21 |
| Fluconazole | ≥64 | NC | NC | 64 |
| Itraconazole | 0.25–>64 | NC | NC | 10.09375 |
| Voriconazole | 2–8 | NC | NC | 5.75 |
| Posaconazole | 0.063–0.25 | NC | NC | 0.211 |
| Caspofungin | 0.5–1 | NC | NC | 0.75 |
| Anidulafungin | 0.031–0.125 | NC | NC | 0.05475 |
| O. tshawytschae (n = 2) | ||||
| Amphotericin B | 0.125–>64 | NC | NC | 2 |
| Flucytosine | 8–>64 | NC | NC | 32.0625 |
| Fluconazole | 0.016–0.25 | NC | NC | 36 |
| Itraconazole | 0.125–0.5 | NC | NC | 0.133 |
| Voriconazole | <0.016–0.125 | NC | NC | 0.3125 |
| Posaconazole | 1–2 | NC | NC | 0.125 |
| Caspofungin | 0.125–0.25 | NC | NC | 1.5 |
| Anidulafungin | 0.031–0.125 | NC | NC | 0.1875 |
| O. constricta (n = 3) | ||||
| Amphotericin B | 1–2 | NC | NC | 1.33 |
| Flucytosine | >64 | NC | NC | 64.00 |
| Fluconazole | 64–> 64 | NC | NC | 64.00 |
| Itraconazole | 0.063 | NC | NC | 0.06 |
| Voriconazole | 0.5–1 | NC | NC | 0.67 |
| Posaconazole | 0.016 | NC | NC | 0.02 |
| Caspofungin | 0.063–0.5 | NC | NC | 0.35 |
| Anidulafungin | 0.031 | NC | NC | 0.03 |
| Ochroconis sp. (n = 1) | ||||
| Amphotericin B | 4 | NC | NC | NC |
| Flucytosine | 64 | NC | NC | NC |
| Fluconazole | 64 | NC | NC | NC |
| Itraconazole | 0.25 | NC | NC | NC |
| Voriconazole | 0.25 | NC | NC | NC |
| Posaconazole | 0.031 | NC | NC | NC |
| Caspofungin | 0.5 | NC | NC | NC |
| Anidulafungin | 0.016 | NC | NC | NC |
| O. cordanae (n = 1) | ||||
| Amphotericin B | 1 | NC | NC | NC |
| Flucytosine | 16 | NC | NC | NC |
| Fluconazole | 64 | NC | NC | NC |
| Itraconazole | 0.25 | NC | NC | NC |
| Voriconazole | 2 | NC | NC | NC |
| Posaconazole | 0.016 | NC | NC | NC |
| Caspofungin | 0.5 | NC | NC | NC |
| Anidulafungin | 0.031 | NC | NC | NC |
| O. humicola (n = 1) | ||||
| Amphotericin B | 1 | NC | NC | NC |
| Flucytosine | 64 | NC | NC | NC |
| Fluconazole | 64 | NC | NC | NC |
| Itraconazole | 0.125 | NC | NC | NC |
| Voriconazole | 1 | NC | NC | NC |
| Posaconazole | 0.063 | NC | NC | NC |
| Caspofungin | 1 | NC | NC | NC |
| Anidulafungin | 0.125 | NC | NC | NC |
| O. minima (n = 1) | ||||
| Amphotericin B | 1 | NC | NC | NC |
| Flucytosine | 8 | NC | NC | NC |
| Fluconazole | 64 | NC | NC | NC |
| Itraconazole | 0.063 | NC | NC | NC |
| Voriconazole | 0.5 | NC | NC | NC |
| Posaconazole | 0.016 | NC | NC | NC |
| Caspofungin | 0.5 | NC | NC | NC |
| Anidulafungin | 0.031 | NC | NC | NC |
| O. gamsii (n = 1) | ||||
| Amphotericin B | 1 | NC | NC | NC |
| Flucytosine | 64 | NC | NC | NC |
| Fluconazole | 64 | NC | NC | NC |
| Itraconazole | 0.25 | NC | NC | NC |
| Voriconazole | 2 | NC | NC | NC |
| Posaconazole | 0.031 | NC | NC | NC |
| Caspofungin | 0.5 | NC | NC | NC |
| Anidulafungin | 0.031 | NC | NC | NC |
| O. verrucosa (n = 1) | ||||
| Amphotericin B | 0.25 | NC | NC | NC |
| Flucytosine | 16 | NC | NC | NC |
| Fluconazole | 64 | NC | NC | NC |
| Itraconazole | 0.25 | NC | NC | NC |
| Voriconazole | 2 | NC | NC | NC |
| Posaconazole | 0.031 | NC | NC | NC |
| Caspofungin | 0.25 | NC | NC | NC |
| Anidulafungin | 0.016 | NC | NC | NC |
| O. anellii (n = 1) | ||||
| Amphotericin B | 1 | NC | NC | NC |
| Flucytosine | 4 | NC | NC | NC |
| Fluconazole | 64 | NC | NC | NC |
| Itraconazole | 0.031 | NC | NC | NC |
| Voriconazole | 0.5 | NC | NC | NC |
| Posaconazole | 0.031 | NC | NC | NC |
| Caspofungin | 0.5 | NC | NC | NC |
| Anidulafungin | 0.031 | NC | NC | NC |
| O. lascauxensis (n = 1) | ||||
| Amphotericin B | 1 | NC | NC | NC |
| Flucytosine | 64 | NC | NC | NC |
| Fluconazole | 64 | NC | NC | NC |
| Itraconazole | 0.25 | NC | NC | NC |
| Voriconazole | 1 | NC | NC | NC |
| Posaconazole | 0.063 | NC | NC | NC |
| Caspofungin | 0.25 | NC | NC | NC |
| Anidulafungin | 0.031 | NC | NC | NC |
The MIC50 and MIC90 values were calculated for those species with 10 or more isolates. NC, not calculated, because <10 strains per species were available for testing.
Overall, visual and spectrophotometric readings gave similar results for the MIC and MEC endpoints. The GM MICs for AMB, 5FC, FLC, ITC, VRC, and POS and the MEC values for CAS and AFG across the genera in this study are shown below, in increasing order. For Verruconis, the values (μg/ml) were as follows: AFG, 0.04; POS, 0.25; ITC, 0.37; AMB, 0.50; CAS, 0.65; VRC, 0.96; 5FC, 10.45; and FLC, 47.25. For Ochroconis, the values (μg/ml) were as follows: AFG, 0.06; POS, 0.11; CAS, 0.67; VRC, 2.76; ITC, 3.94; AMB, 5.68; 5FC, 34.48; and FLC, 61.33. The widest ranges were seen for FLC (range, 1 to ≥64 μg/ml) and 5FC (range, 0.5 to 64 μg/ml). The highest GM MICs were 47.25 μg/ml, for FLC, followed by 10.45 μg/ml, for 5FC. AMB MICs ranged from 0.125 to >16 μg/ml, and ITC had a MIC range of <0.016 to >16 μg/ml. POS exhibited potent activity against all strains, with MICs ranging from <0.0016 to 4 μg/ml, while the GM MIC of VRC (0.96 μg/ml) was 2 log2 dilution steps less potent than that of POS (0.25 μg/ml) against thermotolerant strains and 6 log2 dilution steps less active than in the Ochroconis species (2.76 μg/ml VRC versus 0.11 μg/ml POS). Notably, Ochroconis isolates had higher MICs of AMB, 5FC, FLC, ITC, and VRC than those for Verruconis strains. The two echinocandins showed susceptible profiles in their MECs. In most cases, AFG had a higher activity than that of CAS (AFG MEC of 0.04 μg/ml versus 0.65 μg/ml CAS against Verruconis strains, and AFG MEC of 0.06 μg/ml versus 0.67 μg/ml CAS against Ochroconis species). In addition, various susceptibility profiles were demonstrated within the genera. For Ochroconis, the triazole derivatives ITC and VRC and AMB offered significantly (P ≤ 0.05) higher susceptible profiles for O. mirabilis than for the other species. However, 5FC and FLC were found to be less active against V. gallopava than against V. calidifluminalis (P ≤ 0.05).
DISCUSSION
The genus Ochroconis was recently revised and currently contains 13 species (1). Species accepted within the lineage, within the order Venturiales, were keyed out on the basis of molecular phylogeny and phenotypic and physiologic characteristics. A new genus, Verruconis, was proposed for the neurotropic opportunist Ochroconis gallopava and its close relatives.
Notably, thermotolerance has a significant impact on the virulence potential of Ochroconis and Verruconis species, as shown previously in other melanized fungi. Species able to grow at temperatures of 37°C or above (e.g., Cladophialophora bantiana, Exophiala dermatitidis, and Exophiala jeanselmei) (25) may cause systemic or disseminated infections in mammals. The black yeast Exophiala dermatitidis has a maximum growth temperature of 42 to 45°C and has a natural habitat in association with birds and bats, which have a body temperature well above that of humans (26, 27). Mesophilic species with maximum growth temperatures of 27 to 33°C are restricted to cold-blooded vertebrates (25) or, occasionally, invertebrates (28, 29).
The availability of in vitro susceptibility profiles according to the latest taxonomic studies of Ochroconis and Verruconis species (1) is scant. Our study provides the first antifungal susceptibility data on a large set of clinical and environmental strains from a wide range of sources and origins. Our results indicate that thermotolerance has a significant impact on the antifungal susceptibility of Ochroconis and Verruconis species. Both thermotolerant and mesophilic species had susceptibility profiles with a uniform pattern of low MICs for POS, AFG, and CAS. VRC, AMB, and ITC showed efficacy against Verruconis species, with 1-, 3-, and 4-log2 less susceptibility, respectively, than Ochroconis species. The majority of strains demonstrated high MICs for 5FC and FLC, indicating poor activity of these drugs against the pathogens. Both echinocandins were found to have potent in vitro activity against Ochroconis species. This matches previously reported data for CAS, with a MEC of 0.25 μg/ml against Ochroconis tshawytschae (20) and 0.03 to 1 μg/ml against Verruconis gallopava (30, 31). This is in contrast with previously published data on most melanized fungi, which appear to be tolerant to echinocandins, probably due to the presence of melanin, which prevents penetration of antifungals into fungal cells (32). Nevertheless, O. mirabilis demonstrated less susceptibility to ITC, VRC, and AMB (P ≤ 0.05) than the other Ochroconis species, and V. calidifluminalis was more susceptible to 5FC and FLC than V. gallopava (P ≤ 0.05).
Given that the echinocandins and the triazole POS showed the highest in vitro activity against thermotolerant and mesophilic species, a possible treatment option for Ochroconis and Verruconis infections in both warm-blooded (human) and cold-blooded animals may be provided. The triazole POS is an expanded-spectrum triazole with fungicidal activity against a wide spectrum of molds, including Aspergillus species and members of the Mucorales, as well as enhanced activity against Candida and other yeasts (33). The echinocandins represent the newest class of antifungals that exhibit fungicidal activity against many Candida species, making this drug class a desirable alternative to the azole agents, which exhibit only static activity against yeasts. Because mammalian cells have no cell wall, the echinocandins have very few adverse effects in humans (33).
In general, the divergent antifungal profiles of the Verruconis and Ochroconis genera and the interspecies variability observed for O. mirabilis and V. calidifluminalis clearly suggest that routine in vitro susceptibility testing can be useful for obtaining reliable information on treatment options. Until now, there have been no guidelines for optimal antifungal regimens for Ochroconis and Verruconis species. Although various efficacies have been documented (34), several studies suggest that POS and ITC may provide optimal therapies for Ochroconis infection, followed by AMB and VRC, and that 5FC and FLC are the least effective drugs (6, 30, 31, 35, 36), which is in agreement with the in vitro results of the present study.
Treatment of Verruconis infections with VRC is supported by in vitro results, and it proved to be active in a chronic granulomatous disease (CGD) patient (34). VRC has an optimal oral bioavailability and penetration to the blood-brain barrier, indicating its use for cerebral infections. In some cases of V. gallopava infections, AMB was also used successfully in empirical antifungal therapy (37). However, further studies are required to establish the optimal treatment. In addition, as recommended for other fungal infections, supportive management strategies, such as surgical excision of lesions, are recommended whenever feasible (34). Early diagnosis and treatment are also mandatory in order to avoid dissemination to the brain, which carries a very poor prognosis (20).
In conclusion, although there are no clinically defined breakpoints for Verruconis and Ochroconis species and the lack of interpretative breakpoints makes MICs difficult to interpret, antifungal susceptibility testing can be helpful in guiding clinical management of patients with these infections. Based on the data presented in the current study, POS and echinocandins were the antimycotics with the best overall activity, having broad-spectrum activity against both thermotolerant and mesophilic species. The apparently good penetration of POS into the central nervous system (CNS), with the MIC falling well below the serum levels achievable with standard dosing regimens (38), combined with excellent in vitro data (39) and activity in animal models (40–43), supports the use of POS for difficult-to-treat disseminated brain infections. In the clinical setting, POS has been used successfully in cases of cerebral and disseminated phaeohyphomycosis (44, 45). In addition, POS and VRC are routinely recommended for treatment, prophylaxis, and salvage therapy of life-threatening fungal infections, such as Aspergillus diseases. POS also has a label indication for the treatment of less common infections, including chromoblastomycosis, mycetoma, and coccidioidomycosis. Therefore, standard dosing regimens and provisional target concentrations used for the prevention or treatment of invasive fungal infections (46) might be optimal tentative suggestions for Verruconis and Ochroconis infections.
ACKNOWLEDGMENTS
This publication was prepared as a collaborative study between the CBS-Fungal Biodiversity Centre, Utrecht, The Netherlands, the Veterinary Mycology and Black Yeast Working Groups of the International Society for Human and Animal Mycology (ISHAM), and the Department of Medical Microbiology, Radboudumc, Nijmegen, The Netherlands.
S.S., K.S., W.J.G.M. and G.S.D.H. have no conflicts of interest. J.W.M. and P.E.V. have served as consultants to and have received research grants from Astellas, Basilea, Gilead Sciences, Merck, and Pfizer.
Footnotes
Published ahead of print 31 March 2014
REFERENCES
- 1.Samerpitak K, Van der Linde E, Choi HJ, Gerrits van den Ende AHG, Machouart M, Gueidan C, de Hoog GS. 2014. Taxonomy of Ochroconis, genus including opportunistic pathogens on humans and animals. Fungal Divers. 65:89–126. 10.1007/s13225-013-0253-6 [DOI] [Google Scholar]
- 2.de Hoog GS, Guarro J, Gene J, Figueras MJ. 2009. Atlas of clinical fungi: the ultimate benchtool for diagnostics. A pilot version of the 3rd ed. Centraalbureau voor Schimmelcultures, KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands [Google Scholar]
- 3.Silveira F, Nucci M. 2001. Emergence of black moulds in fungal disease: epidemiology and therapy. Curr. Opin. Infect. Dis. 14:679–684. 10.1097/00001432-200112000-00003 [DOI] [PubMed] [Google Scholar]
- 4.Qureshi ZA, Kwak EJ, Nguyen MH, Silveira FP. 2012. Ochroconis gallopava: a dematiaceous mold causing infections in transplant recipients. Clin. Transplant. 26:E17–E23. 10.1111/j.1399-0012.2011.01528.x [DOI] [PubMed] [Google Scholar]
- 5.Cardeau-Desangles I, Fabre A, Cointault O, Guitard J, Esposito L, Iriart X, Berry A, Valentin A, Cassaing S, Kamar N. 2013. Disseminated Ochroconis gallopava infection in a heart transplant patient. Transpl. Infect. Dis. 15:E115–E118. 10.1111/tid.12084 [DOI] [PubMed] [Google Scholar]
- 6.Shoham S, Pic-Aluas L, Taylor J, Cortez K, Rinaldi MG, Shea Y, Walsh TJ. 2008. Transplant-associated Ochroconis gallopava infections. Transpl. Infect. Dis. 10:442–448. 10.1111/j.1399-3062.2008.00327.x [DOI] [PubMed] [Google Scholar]
- 7.Georg LK, Bierer BW, Cooke WB. 1964. Encephalitis in turkey poults due to a new fungus species. Sabouraudia 3:239–244. 10.1080/00362176485190401 [DOI] [PubMed] [Google Scholar]
- 8.Blalock HG, Georg LK, Derieux WT. 1973. Encephalitis in turkey poults due to Dactylaria (Diplorhinotrichum) gallopava—a case report and its experimental reproduction. Avian Dis. 17:197–204. 10.2307/1588939 [DOI] [PubMed] [Google Scholar]
- 9.Ranck FM, Jr, Georg LK, Wallace DH. 1974. Dactylariosis—a newly recognized fungus disease of chickens. Avian Dis. 18:4–20. 10.2307/1589237 [DOI] [PubMed] [Google Scholar]
- 10.Randall CJ, Owen DM, Kirkpatrick KS. 1981. Encephalitis in broiler chickens caused by a hyphomycete resembling Dactylaria gallopava. Avian Pathol. 10:31–41. 10.1080/03079458108418456 [DOI] [PubMed] [Google Scholar]
- 11.Shane SM, Markovits J, Snider TG, 3rd, Harrington KS. 1985. Encephalitis attributed to dactylariosis in Japanese quail chicks (Coturnix coturnix japonica). Avian Dis. 29:822–828. 10.2307/1590673 [DOI] [PubMed] [Google Scholar]
- 12.Karesh WB, Russell R, Gribble D. 1987. Dactylaria gallopava encephalitis in two grey-winged trumpeters (Psophia crepitans). Avian Dis. 31:685–688. 10.2307/1590762 [DOI] [PubMed] [Google Scholar]
- 13.Salkin IF, Dixon DM, Kemna ME, Danneman PJ, Griffith JW. 1990. Fatal encephalitis caused by Dactylaria constricta var. gallopava in a snowy owl chick (Nyctea scandiaca). J. Clin. Microbiol. 28:2845–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connole MD. 1990. Review of animal mycoses in Australia. Mycopathologia 111:133–164. 10.1007/BF02282798 [DOI] [PubMed] [Google Scholar]
- 15.Mohapatra N. 1993. Fungal encephalitis in poultry. Poult. Advis. 26:61–62 [Google Scholar]
- 16.Singh K, Flood J, Welsh RD, Wyckoff JH, Snider TA, Sutton DA. 2006. Fatal systemic phaeohyphomycosis caused by Ochroconis gallopavum in a dog (Canis familaris). Vet. Pathol. 43:988–992. 10.1354/vp.43-6-988 [DOI] [PubMed] [Google Scholar]
- 17.Padhye AA, Amster RL, Browning M, Ewing EP. 1994. Fatal encephalitis caused by Ochroconis gallopava in a domestic cat. J. Med. Vet. Mycol. Res. 32:141–145. 10.1080/02681219480000191 [DOI] [PubMed] [Google Scholar]
- 18.Hatai K, Kubota SS. 1989. A visceral mycosis in cultured masu salmon (Oncorhynchus masou) caused by a species of Ochroconis. J. Wildl. Dis. 25:83–88. 10.7589/0090-3558-25.1.83 [DOI] [PubMed] [Google Scholar]
- 19.VanSteenhouse JL, Padhye AA, Ajello L. 1988. Subcutaneous phaeohyphomycosis caused by Scolecobasidium humicola in a cat. Mycopathologia 102:123–127. 10.1007/BF00437449 [DOI] [PubMed] [Google Scholar]
- 20.Ge YP, Lv GX, Shen YN, Li M, Deng SW, De Hoog S, Samerpitak K, Liu WD. 2012. First report of subcutaneous phaeohyphomycosis caused by Ochroconis tshawytschae in an immunocompetent patient. Med. Mycol. 50:637–640. 10.3109/13693786.2011.653834 [DOI] [PubMed] [Google Scholar]
- 21.Lewis RE. 2011. Current concepts in antifungal pharmacology. Mayo Clin. Proc. 86:805–817. 10.4065/mcp.2011.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M, Claverie JM, Gascuel O. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36:W465–W469. 10.1093/nar/gkn180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed. CLSI document M38–A2, vol 28, no 16 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 25.de Hoog GS, Vicente VA, Najafzadeh MJ, Harrak MJ, Badali H, Seyedmousavi S. 2011. Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia 27:46–72. 10.3767/003158511X614258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudhadham M, Prakitsin S, Sivichai S, Chaiyarat R, Dorrestein GM, Menken SB, de Hoog GS. 2008. The neurotropic black yeast Exophiala dermatitidis has a possible origin in the tropical rain forest. Stud. Mycol. 61:145–155. 10.3114/sim.2008.61.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horré R, de Hoog GS. 1999. Primary cerebral infections by melanized fungi: a review. Stud. Mycol. 43:176–193 [Google Scholar]
- 28.Vicente VA, Orelis-Ribeiro R, Najafzadeh MJ, Sun J, Guerra RS, Miesch S, Ostrensky A, Meis JF, Klaassen CH, de Hoog GS, Boeger WA. 2012. Black yeast-like fungi associated with lethargic crab disease (LCD) in the mangrove-land crab, Ucides cordatus (Ocypodidae). Vet. Microbiol. 158:109–122. 10.1016/j.vetmic.2012.01.031 [DOI] [PubMed] [Google Scholar]
- 29.Seyedmousavi S, Guillot J, de Hoog GS. 2013. Phaeohyphomycoses, emerging opportunistic diseases in animals. Clin. Microbiol. Rev. 26:19–35. 10.1128/CMR.00065-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer N, Bastani B. 2009. A case of pulmonary cavitary lesion due to Dactylaria constricta var. gallopava in a renal transplant patient. Nephrology 14:262. 10.1111/j.1440-1797.2009.01107.x [DOI] [PubMed] [Google Scholar]
- 31.Wong JS, Schousboe MI, Metcalf SS, Endre ZH, Hegarty JM, Maze MJ, Keith ER, Seaward LM, Podmore RG. 2010. Ochroconis gallopava peritonitis in a cardiac transplant patient on continuous ambulatory peritoneal dialysis. Transpl. Infect. Dis. 12:455–458. 10.1111/j.1399-3062.2010.00523.x [DOI] [PubMed] [Google Scholar]
- 32.Odabasi Z, Paetznick VL, Rodriguez JR, Chen E, Ostrosky-Zeichner L. 2004. In vitro activity of anidulafungin against selected clinically important mold isolates. Antimicrob. Agents Chemother. 48:1912–1915. 10.1128/AAC.48.5.1912-1915.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodds Ashley ES, Varkey JB, Krishna G, Vickery D, Ma L, Yu X, Malavade D, Goodwin M, Perfect JR, Power E. 2009. Pharmacokinetics of posaconazole administered orally or by nasogastric tube in healthy volunteers. Antimicrob. Agents Chemother. 53:2960–2964. 10.1128/AAC.01178-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meriden Z, Marr KA, Lederman HM, Illei PB, Villa K, Riedel S, Carroll KC, Zhang SX. 2012. Ochroconis gallopava infection in a patient with chronic granulomatous disease: case report and review of the literature. Med. Mycol. 50:883–889. 10.3109/13693786.2012.681075 [DOI] [PubMed] [Google Scholar]
- 35.Jenney A, Maslen M, Bergin P, Tang SK, Esmore D, Fuller A. 1998. Pulmonary infection due to Ochroconis gallopavum treated successfully after orthotopic heart transplantation. Clin. Infect. Dis. 26:236–237. 10.1086/517075 [DOI] [PubMed] [Google Scholar]
- 36.Wang TK, Chiu W, Chim S, Chan TM, Wong SS, Ho PL. 2003. Disseminated Ochroconis gallopavum infection in a renal transplant recipient: the first reported case and a review of the literature. Clin. Nephrol. 60:415–423. 10.5414/CNP60415 [DOI] [PubMed] [Google Scholar]
- 37.Kralovic SM, Rhodes JC. 1995. Phaeohyphomycosis caused by Dactylaria (human dactylariosis): report of a case with review of the literature. J. Infect. 31:107–113. 10.1016/S0163-4453(95)92060-9 [DOI] [PubMed] [Google Scholar]
- 38.Ruping MJ, Albermann N, Ebinger F, Burckhardt I, Beisel C, Muller C, Vehreschild JJ, Kochanek M, Fatkenheuer G, Bangard C, Ullmann AJ, Herr W, Kolbe K, Hallek M, Cornely OA. 2008. Posaconazole concentrations in the central nervous system. J. Antimicrob. Chemother. 62:1468–1470. 10.1093/jac/dkn409 [DOI] [PubMed] [Google Scholar]
- 39.Fothergill AW, Rinaldi MG, Sutton DA. 2009. Antifungal susceptibility testing of Exophiala spp.: a head-to-head comparison of amphotericin B, itraconazole, posaconazole and voriconazole. Med. Mycol. 47:41–43. 10.1080/13693780802512451 [DOI] [PubMed] [Google Scholar]
- 40.Al-Abdely HM, Najvar LK, Bocanegra R, Graybill JR. 2005. Antifungal therapy of experimental cerebral phaeohyphomycosis due to Cladophialophora bantiana. Antimicrob. Agents Chemother. 49:1701–1707. 10.1128/AAC.49.5.1701-1707.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calvo E, Pastor FJ, Rodriguez MM, Mayayo E, Salas V, Guarro J. 2010. Murine model of a disseminated infection by the novel fungus Fonsecaea monophora and successful treatment with posaconazole. Antimicrob. Agents Chemother. 54:919–923. 10.1128/AAC.01284-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graybill JR, Najvar LK, Johnson E, Bocanegra R, Loebenberg D. 2004. Posaconazole therapy of disseminated phaeohyphomycosis in a murine model. Antimicrob. Agents Chemother. 48:2288–2291. 10.1128/AAC.48.6.2288-2291.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marine M, Pastor FJ, Guarro J. 2009. Combined antifungal therapy in a murine model of disseminated infection by Cladophialophora bantiana. Med. Mycol. 47:45–49. 10.1080/13693780802526840 [DOI] [PubMed] [Google Scholar]
- 44.Negroni R, Helou SH, Petri N, Robles AM, Arechavala A, Bianchi MH. 2004. Case study: posaconazole treatment of disseminated phaeohyphomycosis due to Exophiala spinifera. Clin. Infect. Dis. 38:e15–e20. 10.1086/380840 [DOI] [PubMed] [Google Scholar]
- 45.Al-Abdely HM, Alkhunaizi AM, Al-Tawfiq JA, Hassounah M, Rinaldi MG, Sutton DA. 2005. Successful therapy of cerebral phaeohyphomycosis due to Ramichloridium mackenziei with the new triazole posaconazole. Med. Mycol. 43:91–95. 10.1080/13693780400011104 [DOI] [PubMed] [Google Scholar]
- 46.Seyedmousavi S, Mouton JW, Verweij PE, Bruggemann RJ. 2013. Therapeutic drug monitoring of voriconazole and posaconazole for invasive aspergillosis. Expert Rev. Anti Infect. Ther. 11:931–941. 10.1586/14787210.2013.826989 [DOI] [PubMed] [Google Scholar]
- 47.Yarita K, Sano A, Samerpitak K, Kamei K, de Hoog GS, Nishimura K. 2010. Ochroconis calidifluminalis, a sibling of the neurotropic pathogen O. gallopava, isolated from hot spring. Mycopathologia 170:21–30. 10.1007/s11046-010-9292-7 [DOI] [PubMed] [Google Scholar]
- 48.Sides EH, 3rd, Benson JD, Padhye AA. 1991. Phaeohyphomycotic brain abscess due to Ochroconis gallopavum in a patient with malignant lymphoma of a large cell type. J. Med. Vet. Mycol. 29:317–322 [PubMed] [Google Scholar]
- 49.Terreni AA, DiSalvo AF, Baker AS, Jr, Crymes WB, Morris PR, Dowda H., Jr 1990. Disseminated Dactylaria gallopava infection in a diabetic patient with chronic lymphocytic leukemia of the T-cell type. Am. J. Clin. Pathol. 94:104–107 [DOI] [PubMed] [Google Scholar]
- 50.Horré R, de Hoog GS, Kluczny C, Marklein G, Schaal KP. 1999. rDNA diversity and physiology of Ochroconis and Scolecobasidium species reported from humans and other vertebrates. Stud. Mycol. 43:194–205 [Google Scholar]
- 51.Doty MS, Slater DW. 1946. A new species of Heterobasidium tshawytschae pathogenic on young chinook salmon. Amer. Midl. Naturalist 36:663–665 [Google Scholar]
- 52.Ge YP, Lv GX, Shen YN, Li M, Deng SW, de Hoog S, Samerpitak K, Liu WD. 2012. First report of subcutaneous phaeohyphomycosis caused by Ochroconis tshawytschae in an immunocompetent patient. Med. Mycol. 50:637–640. 10.3109/13693786.2011.653834 [DOI] [PubMed] [Google Scholar]
- 53.Wada S, Nakamura K, Hatai K. 1995. First case of Ochroconis humicola infection in marine cultured fish in Japan. Fish Pathol. 30:125–126 [Google Scholar]
- 54.de Hoog GS, von Arx JA. 1973. Revision of Scolecobasidium and Pleurophragmium. Kavaka 1:55–60 [Google Scholar]
- 55.Fassatiová O. 1967. Česká Mykol. 21:87 [Google Scholar]
- 56.de Hoog GS. 1985. Taxonomy of the Dactylaria complex, IV. Dactylaria, Neta, Subulispora and Scolecobasidium. Stud. Mycol. 26:1–60 [Google Scholar]
- 57.Zachariah S, Sankaran KV, Leelavathy KM. 1981. A new species of Septonema from Indian soil. Mycologia 73:208–210 [Google Scholar]
- 58.Graniti A. 1962. Scolecobasidium anellii n. sp., agenti di annerimenti superficiali di stalattiti. Giorn. Bot. Ital. 69:360–365 [Google Scholar]
- 59.Martin-Sanchez PM, Nováková A, Bastian F, Alabouvette C, Saiz-Jimenez C. 2012. Two new species of the genus Ochroconis, O. lascauxensis and O. anomala isolated from black stains in Lascaux Cave, France. Fungal Biol. 116:574–589. 10.1016/j.funbio.2012.02.006 [DOI] [PubMed] [Google Scholar]