Abstract
Mycoplasma genitalium has become well established as an etiological agent of sexually transmitted infections, but due to its fastidious growth requirements, only a few M. genitalium strains are available to determine the MICs of currently used and new antimicrobial agents. Recent clinical trials have suggested that treatment with azithromycin has decreasing efficacy due to an increasing prevalence of macrolide resistance, and alternative treatment with moxifloxacin is similarly under pressure from emerging resistance. Thus, there is an urgent need for new antimicrobials. The in vitro activity of the newly developed fluoroketolide solithromycin (CEM-101) was evaluated against a collection of 40 M. genitalium strains, including 15 with high-level macrolide resistance and 5 multidrug-resistant strains with resistance to both macrolides and quinolones. Furthermore, the MIC of solithromycin was correlated with mutations in the 23S rRNA gene and in the genes encoding ribosomal proteins L4 and L22. The in vitro results showed that solithromycin has activity against M. genitalium superior to that of other macrolides, doxycycline, and fluoroquinolones. Accordingly, this new fluoroketolide might be an effective option for treatment of M. genitalium infections. However, the efficacy of solithromycin in clinical trials with follow-up for test of cure and detection of genotypic and phenotypic resistance needs to be evaluated prior to widespread use. In a phase 2 clinical trial, solithromycin was highly effective as a single oral dose against C. trachomatis and Neisseria gonorrhoeae, suggesting that solithromycin could be a treatment option for several sexually transmitted infections, including in syndromic treatment of urethral and vaginal discharge.
INTRODUCTION
Mycoplasma genitalium, first isolated in 1980 (1), has become well established as an etiological agent of sexually transmitted infections (reviewed in reference 2). Several studies have demonstrated the association between M. genitalium and urethritis in men and urethritis, cervicitis, endometritis, and pelvic inflammatory disease in women (3–7). The prevalence of M. genitalium in men with nonchlamydial nongonococcal urethritis (NCNGU) ranges from 10% to 35% (2), and in men and women in the general population, it ranges from 1% to 3.3% (8–10).
Persistence of M. genitalium is associated with recurrent or persistent nongonococcal urethritis (NGU), as illustrated by the findings of Bradshaw et al. (11) showing that 91% of patients with persistent M. genitalium infection experienced persistent symptoms compared to 17% of patients in whom M. genitalium was eradicated. In men with persistent NCNGU after doxycycline therapy, as many as 41% were found to be M. genitalium positive (12).
M. genitalium, like other mycoplasmas, lacks a rigid peptidoglycan-containing cell wall (13). Hence, β-lactam antibiotics and other antibiotics targeting the cell wall are not active. Early in vitro studies showed that M. genitalium was highly susceptible to macrolides, particularly to azithromycin, but that it had reduced susceptibility to tetracyclines and older quinolones, such as norfloxacin and ciprofloxacin (14). Ketolides (15), which are related to macrolides such as azithromycin, and some of the newer fluoroquinolones, such as moxifloxacin, have sufficiently low MICs in vitro to be clinically useful (14).
Currently, no evidence-based guidelines specifically for the treatment of M. genitalium infection have been developed. Most early studies have shown insufficient microbiological and clinical cure rates with tetracyclines, whereas azithromycin (1-g single dose) appeared to be superior but far from perfect, with cure rates rarely exceeding 85% (16–18). However, more recent randomized clinical trials from the United States have shown a decreasing cure rate after azithromycin 1-g single-dose therapy, with a microbiological cure rate of 67% among patients included between 2006 and 2009 (19) reduced to 40% among patients included between 2007 and 2011 (20). Moxifloxacin is currently the most commonly used second-line antibiotic in patients failing azithromycin treatment (11, 21). However, the side effects, cost, and risk of selection for antimicrobial resistance limit the use of moxifloxacin.
Macrolide resistance in M. genitalium is primarily caused by mutations in nucleotide 2058 or 2059 (Escherichia coli numbering) in region V of the 23S rRNA gene and is commonly selected during treatment with macrolides (22, 23). The increasing level of macrolide resistance challenges the use of azithromycin as the first-line treatment for NGU, and new treatment options are needed, in particular in light of emerging resistance to moxifloxacin, as well (24).
In this study, we evaluated the in vitro activity of the newly developed fluoroketolide solithromycin (CEM-101) against a large collection of M. genitalium strains, including some with high-level macrolide resistance, and compared it to that of other antimicrobials currently or previously used for treatment of M. genitalium infection. Furthermore, we correlated the MIC of solithromycin with macrolide resistance, i.e., mutations in the 23S rRNA gene and in the genes encoding ribosomal proteins L4 and L22.
MATERIALS AND METHODS
M. genitalium strains.
A collection of 40 M. genitalium isolates originating from 38 patients were tested. They included the M. genitalium G37 type strain and an early passage of the M30 strain isolated by David Taylor-Robinson in 1980 from two men from the United Kingdom (25). The latter was obtained from the Mollicutes Collection of Cultures and Antisera, Gainesville, FL, USA, and is distinctly different from the M30 strain deposited in the ATCC, which is genetically indistinguishable from the G37 type strain (26). Twelve isolates (M6257, M6280, M6281, M6285, M6286, M6302, M6311, M6315, M6328, M6375, M6475, and M6489) were obtained from patient samples collected at various Swedish sexually transmitted disease (STD) clinics. Seven strains (M2282, M2288, M2300, M2321, M2341, M6327, and M6604) were isolated from patient specimens obtained at a Danish STD clinic. Seven isolates (M6270, M6271, M6320, M6321, M6711, M6712, and M6713) were from samples collected at an STD clinic in Melbourne, Australia. Four strains (M6282, M6283, M6284, and M6287) were isolated from samples from patients attending private Japanese urology clinics in Miyazaki Prefecture. Four strains (M6303, M6593, M6714, and M6735) were isolated from samples from patients attending Norwegian STD clinics. Three strains (M6151, M6090, and M6312) were isolated from consecutive samples from the same patient attending a French STD clinic on days 0, 49, and 79, respectively. They had identical MgPa types (27), but sequence variation was observed in hypervariable domains of the MG191 and MG192 genes (28). One isolate (M6848) was cultured from a patient in the United States and kindly provided by Patricia Totten, Seattle, WA. A total of 15 strains from 15 patients had high-level resistance to macrolides with an erythromycin MIC of >16 μg/ml (Table 1).
TABLE 1.
Distribution of M. genitalium strains according to country of origin and high-level resistance to macrolidesa
| Country of origin | No. of strains | No. of macrolide-resistant strains |
|---|---|---|
| Sweden | 12 | 3 |
| Denmark | 7 | 1 |
| Australia | 7 | 6 |
| Norway | 4 | 4 |
| Japan | 4 | 0 |
| France | 3 | 0 |
| United Kingdom | 2 | 0 |
| United States | 1 | 1 |
An erythromycin MIC of >16 μg/ml.
Determination of the MICs of solithromycin and five additional antimicrobials.
The MICs of solithromycin (CEM-101; Cempra, Inc., Chapel Hill, NC, USA), azithromycin (Groton Laboratories, Pfizer Inc., Groton, CT, USA), erythromycin (Sigma-Aldrich Denmark, Vallensbaek Strand, Denmark), doxycycline (Sigma-Aldrich Denmark), and ciprofloxacin and moxifloxacin (Bayer Health Care, Lyngby, Denmark) were determined using a cell culture method where a defined inoculum of approximately 2,500 genome equivalents (geq), determined by quantitative PCR (29), was added to a Vero cell culture containing 2-fold dilutions of test antibiotic. The cells were grown at 37°C in 5% CO2 in 24-well microwell plates, as previously described (30). After a 3-week incubation period, cells and supernatant were harvested and the growth of M. genitalium was determined by quantitative PCR (29). MICs are expressed as the minimal concentration of the test antibiotic causing 99% inhibition of growth compared to the mean of three control cultures grown in wells without antibiotic (30).
Characterization of macrolide resistance mutations.
Fragments of the 23S rRNA gene spanning nucleotide positions 616 to 905 and 1986 to 2682 were PCR amplified and sequenced using the primers and cycling conditions described in Table 2. The complete ribosomal protein L4 and L22 genes (rpl4 and rpl22, respectively) were PCR amplified as previously described (22). All PCR amplicons were sequenced using conventional Sanger sequencing. Sequence editing and comparisons were performed in BioNumerics version 6.6 (Applied Maths NV, Sint-Martens-Latem, Belgium).
TABLE 2.
Primers and annealing temperatures for PCR amplification of genes associated with macrolide resistance in M. genitalium
| Target gene/segment | Primer (sequence) |
Amplicon length (bp) | Annealing temp (°C) | |
|---|---|---|---|---|
| Forward | Reverse | |||
| 23S rRNA domain II, 752 | Mg 23S-616f (CGAGTTATGATAGCAAGCGTTA) | Mg 23S-905r (CAGTAGCTTTACCTCCACTTATT) | 290 | 50 |
| 23S rRNA domain V, 2058/2059 | Mg 23S-1986f (GTGTAACCATCTCTTGACTGTCTCGG) | Mg 23S-2177r (TTCACATCAACAAATCCTTGCGA) | 192 | 60 |
| 23S rRNA domain V, 2609 | Mg 23S-2024f (TGAAATCCAGGTACGGGTGAAGAC) | Mg 23S-2682r (CGGTCCTCTCGTACTAGAAGCAAAG) | 659 | 60 |
| rpl4 | Mg L4–88f (GCCCCACAACTAAAAGCACC) | Mg L4+32r (TAAGAGTATGTTGGTTACATCCATAGCCTA) | 752 | 50 |
| rpl22 | Mg L22–70f (ATGGTAGGTCATAAGTTGGGTGAGTTT) | Mg L22+55f (AGTTCTTATTAATGCCAAACCTTAAGCC) | 560 | 60 |
Statistical methods.
Statistical analysis of the MIC data was performed using StatsDirect version 2.7.9. For pairwise comparisons between groups, the Mann-Whitney test was used. For comparisons across different antibiotics, the Kruskal-Wallis test was applied. For multiple comparisons, the results obtained by the Dwass-Steel-Chritchlow-Fligner method were used.
RESULTS
Antimicrobial susceptibility.
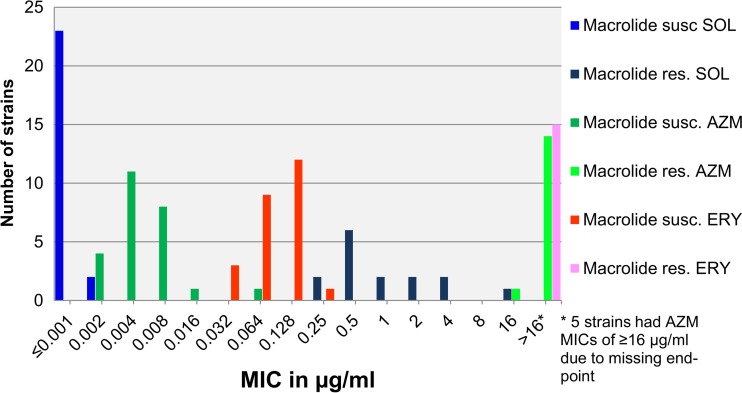
The MIC range for solithromycin was ≤0.001 to 16 μg/ml (MIC50, 0.001 μg/ml, and MIC90, 2 μg/ml). For the 25 macrolide-susceptible strains, the MICs of solithromycin ranged from ≤0.001 to 0.002 μg/ml (MIC90, ≤0.001 μg/ml). For the 15 macrolide-resistant strains, the solithromycin MICs ranged from 0.25 to 16 μg/ml (MIC90, 4 μg/ml) (Table 3 and Fig. 1). The antimicrobial activity of solithromycin was significantly superior to that of azithromycin (P = 0.0001), with a median difference of 2 dilution steps for the macrolide-susceptible strains and a median difference of 6 dilution steps for the macrolide-resistant strains (P < 0.0001). For erythromycin, the difference was even more pronounced, with a median difference of 6 dilution steps for the susceptible strains (P < 0.0001) (Table 3 and Fig. 1).
TABLE 3.
MICs for 40 M. genitalium strains with isolation histories and presence of mutations
| Strain ID (reference)a | Sample collection yr | Country | MIC (μg/ml)b |
Mutationc |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AZM | ERY | SOL | CIP | MXF | DOX | 23S rRNA | rpl4 | rpl22 | |||
| G37 (1) | 1980 | United Kingdom | 0.008 | 0.125 | 0.002 | 8 | 0.125 | 0.5 | WT | WT | WT |
| M30 (1, 29) | 1980 | United Kingdom | 0.008 | 0.125 | ≤0.001 | 8 | 0.125 | 0.5 | WT | WT | WT |
| M2288 (46) | 1991 | Denmark | 0.002 | 0.125 | ≤0.001 | 0.5 | 0.125 | 0.063 | WT | N172S, A114V, A116V | WT |
| M2282 (46) | 1991 | Denmark | 0.004 | 0.063 | ≤0.001 | 4 | 0.125 | 0.25 | WT | WT | WT |
| M2300 (46) | 1991 | Denmark | 0.008 | 0.125 | ≤0.001 | 16 | 0.125 | 0.25 | WT | WT | WT |
| M2321 (46) | 1991 | Denmark | 0.004 | 0.063 | ≤0.001 | 8 | 0.25 | 0.5 | WT | WT | WT |
| M2341 (46) | 1991 | Denmark | 0.008 | 0.25 | ≤0.001 | 2 | 0.25 | 1 | WT | WT | WT |
| M6090d (29) | 1994 | France | 0.004 | 0.063 | ≤0.001 | 2 | 0.063 | 0.125 | WT | WT | WT |
| M6151d (29) | 1994 | France | 0.008 | 0.125 | ≤0.001 | 4 | 0.125 | 0.25 | WT | WT | WT |
| M6312d (30) | 1994 | France | 0.004 | 0.063 | ≤0.001 | 8 | 0.125 | 0.25 | WT | WT | WT |
| M6285 (47) | 1997 | Sweden | 0.004 | 0.125 | ≤0.001 | 1 | 0.125 | 0.5 | WT | WT | WT |
| M6280 (47) | 1997 | Sweden | 0.004 | 0.063 | ≤0.001 | 1 | 0.063 | 0.125 | WT | N172S | WT |
| M6328 (47) | 1998 | Sweden | 0.004 | 0.125 | ≤0.001 | 2 | 0.125 | 0.25 | WT | N21K | A43V |
| M6281 (47) | 2001 | Sweden | 0.008 | 0.063 | ≤0.001 | 0.5 | 0.125 | 0.25 | WT | WT | WT |
| M6286 (47) | 2001 | Sweden | 0.002 | 0.03 | ≤0.001 | 2 | 0.03 | 0.25 | WT | A114S, R45K | WT |
| M6287 (47) | 2003 | Japan | 0.002 | 0.03 | ≤0.001 | 4 | 2 | 0.125 | WT | P81S | WT |
| M6284 (47) | 2003 | Japan | 0.004 | 0.125 | ≤0.001 | 4 | 0.125 | 0.25 | WT | WT | WT |
| M6283 (47) | 2003 | Japan | 0.004 | 0.063 | ≤0.001 | 2 | 0.125 | 0.5 | WT | WT | WT |
| M6282 (47) | 2003 | Japan | 0.008 | 0.125 | ≤0.001 | 1 | 0.125 | 0.25 | WT | WT | WT |
| M6311 | 2004 | Sweden | 0.004 | 0.125 | ≤0.001 | 1 | 0.063 | 0.5 | WT | WT | WT |
| M6327 (22) | 2005 | Denmark | 0.016 | 0.125 | 0.002 | 8 | 0.25 | 0.25 | WT | WT | WT |
| M6375 | 2005 | Sweden | 0.004 | 0.063 | ≤0.001 | 1 | 0.03 | 0.125 | WT | WT | WT |
| M6315 | 2005 | Sweden | 0.002 | 0.063 | ≤0.001 | 1 | 0.125 | 0.5 | WT | WT | WT |
| M6475 | 2006 | Sweden | 0.008 | 0.125 | ≤0.001 | 1 | 0.5 | 0.5 | WT | WT | WT |
| M6713 | 2010 | Australia | 0.063 | 0.03 | ≤0.001 | 0.5 | 0.125 | 0.5 | WT | P81S | WT |
| M6303 (22) | 2003 | Norway | >8 | >16 | 0.5 | 8 | 0.25 | 2 | A2059G | H69R | WT |
| M6270 (22) | 2004 | Australia | >8 | >16 | 0.5 | 4 | 0.125 | 0.25 | A2059G | WT | E123K |
| M6320 (22) | 2004 | Australia | ≥64 | ≥64 | 0.25 | 0.5 | 0.063 | 0.25 | A2059G | WT | WT |
| M6489 | 2007 | Sweden | >16 | >16 | 1 | >16 | >16 | 1 | A2059G | H69R | WT |
| M6593 | 2008 | Norway | 16 | >16 | 0.5 | 2 | 0.125 | 0.063 | A2059G | WT | WT |
| M6735 | 2010 | Norway | >16 | ≥64 | 0.5 | 16 | 8 | 1 | A2059G | H69R | WT |
| M6712 | 2010 | Australia | >16 | ≥64 | 0.5 | >16 | 8 | 1 | A2059G | H69R | S81N |
| M6711 | 2010 | Australia | >16 | ≥64 | 0.25 | >16 | 8 | 1 | A2059G | WT | WT |
| M6271 (22) | 2004 | Australia | >8 | >32 | 0.5 | 4 | 0.125 | 0.25 | A2058G | WT | WT |
| M6257 (22) | 2004 | Sweden | >8 | > 16 | 2 | 1 | 0.25 | 0.5 | A2058G | WT | WT |
| M6321 (22) | 2004 | Australia | ≥64 | ≥64 | 1 | 2 | 0.03 | 0.125 | A2058G | WT | WT |
| M6604 | 2009 | Denmark | 64 | >16 | 2 | 4 | 0.25 | 0.5 | A2058G | WT | WT |
| M6714 | 2010 | Norway | >16 | ≥64 | 4 | 16 | 4 | 1 | A2058G | WT | WT |
| M6302 (22) | 2004 | Sweden | >8 | >16 | 16 | 8 | 0.25 | 0.125 | A2058C | WT | WT |
| M6848 | 2008 | United States | >16 | ≥64 | 4 | ND | ND | ND | A2058C | WT | WT |
Strains without a reference have not been described previously.
AZM, azithromycin; ERY, erythromycin; SOL, solithromycin; CIP, ciprofloxacin; MXF, moxifloxacin; DOX, doxycycline; ND, not determined.
Macrolide resistance-mediating mutations in the 23S rRNA gene (E. coli numbering) and mutations in the genes encoding the ribosomal proteins L4 and L22 (M. genitalium numbering). WT, wild-type sequence of the gene.
M6090, M6151, and M6312 are consecutive isolates from the same patient.
FIG 1.
Distributions of solithromycin (SOL), azithromycin (AZM), and erythromycin (ERY) MICs for 40 M. genitalium strains. Susc., susceptible; res., resistant.
The MIC range for doxycycline was 0.06 to 2 μg/ml (MIC50, 0.25 μg/ml, and MIC90, 1 μg/ml), and no associations with solithromycin MICs were observed.
The MIC ranges for ciprofloxacin and moxifloxacin were 0.5 to >16 μg/ml (MIC50, 2 μg/ml, and MIC90, 16 μg/ml) and 0.032 to >16 μg/ml (MIC50, 0,125 μg/ml, and MIC90, 4 μg/ml), respectively. No associations with solithromycin MICs were observed. Interestingly, for the five strains from patients failing both azithromycin and moxifloxacin treatment, the MICs of solithromycin were 0.25, 0.5, 0.5, 1, and 4 μg/ml (Table 3).
Analysis of resistance mutations.
For eight strains with the 23S rRNA gene A2059G mutation, the median MIC of solithromycin was 2 dilution steps lower than that of the five strains with the A2058G mutation (P = 0.02) and also lower than that of the two strains with the rare A2058C mutation (P = 0.04). All strains, regardless of macrolide susceptibility, carried a 23S rRNA gene with a C in position 752 in contrast to the A752 in wild-type E. coli strains. No other mutations were found in the region. In the 2609 position known to interact with position 752 (31), all strains had T2609, as found in wild-type E. coli.
Ribosomal protein L22 was remarkably conserved, with 37 strains (93%) having identical amino acid sequences and the remaining 3 having single amino acid substitutions outside the L22 loop; one strain was macrolide susceptible, and two were macrolide resistant due to 23S rRNA A2059G mutations and had solithromycin MICs of 0.5 μg/ml (Table 3).
In ribosomal protein L4, the vast majority (30 strains; 75%) were 100% identical to the G37 type strain. Four strains had a His69 (corresponding to the E. coli Gly64) amino acid replaced with Arg in the loop structure, but these strains also had A2059G 23S rRNA gene mutations. Three of these strains had solithromycin MICs of 0.5 μg/ml, and one had an MIC of 1 μg/ml; thus, these mutations did not appear to increase the MIC over that of other strains with similar 23S rRNA gene mutations (P = 0.3). No other amino acid changes were found in the L4 loop structure. Of the remaining six strains, four had one amino acid change compared to the type strain, one had two amino acid changes, and one had three changes. All six of these strains were macrolide susceptible (Table 3).
DISCUSSION
This is the first evaluation of the antimicrobial activity of the new extended-spectrum fluoroketolide solithromycin (CEM-101) relative to other antimicrobials currently or previously used for treatment of M. genitalium infections against a large M. genitalium strain collection (one of the largest available). A previous study (15) investigated the solithromycin susceptibilities of five macrolide-susceptible isolates, but three of the isolates obtained from the ATCC are genetically indistinguishable (32, 33). The strain collection examined in the present study contained geographically, temporally, and genetically diverse M. genitalium isolates, including 15 strains with high-level macrolide resistance, of which five strains were from patients failing both azithromycin and moxifloxacin treatment. Such multidrug-resistant strains have proven to be extremely difficult to treat despite their apparent susceptibility to doxycycline in vitro; the reason for this discrepancy between the in vitro MIC and treatment efficacy remains unclear. With the emergence of these multidrug-resistant strains, development of new treatment options is urgently needed.
Macrolide resistance in M. genitalium is mainly caused by single-nucleotide polymorphisms in the 23S rRNA gene (22), which is present in only one copy in M. genitalium, and there is strong evidence that the resistance is selected, in particular during treatment with the commonly used 1-g single dose of azithromycin (22, 23). This is illustrated, for example, by a prevalence of azithromycin resistance-mediating mutations in M. genitalium-positive samples approaching 100% in Greenland, where Chlamydia trachomatis infections are extremely common and where single-dose 1-g azithromycin is commonly used for treatment of chlamydial infection (34).
Solithromycin has good activity against intracellular bacteria due to high intracellular accumulation (35), and as M. genitalium may survive intracellularly, at least in vitro (36), this property may be an important advantage of the drug. Furthermore, solithromycin is well absorbed orally, with high levels in plasma and good tissue distribution; has a long postantimicrobial effect and anti-inflammatory properties; and has been demonstrated to be safe and well tolerated even at high doses (1.6-g single dose), according to phase 1 dose escalation studies in healthy subjects (37). Furthermore, in a recent phase 2 clinical trial comparing levofloxacin and solithromycin for treatment of community-acquired bacterial pneumonia, solithromycin had a very favorable safety profile (38). Based on the pharmacokinetic findings (37) and MIC testing, the recommended tentative breakpoints for solithromycin against pneumococci and Streptococcus pyogenes have been set at a MIC of ≤1 μg/ml for susceptible bacterial isolates and a MIC of ≥4 μg/ml for resistant bacterial isolates (39).
The in vitro results of the present study show that the activity of solithromycin is superior to that of azithromycin and erythromycin and also to that of fluoroquinolones and tetracyclines. Most importantly, solithromycin was shown to be much more active against high-level macrolide-resistant M. genitalium strains than azithromycin, although cross-resistance, i.e., significantly higher solithromycin MICs, was observed. No information is available regarding the correlation between the in vitro MIC of solithromycin and clinical cure rates, but if strains with MICs of ≥4 μg/ml are considered resistant (as for Streptococcus pneumoniae), 37 (93%) of all the examined strains and 12 (80%) of the 15 macrolide-resistant strains would be treatable with solithromycin. If only strains with MICs of ≤1 μg/ml are considered treatable as a conservative estimate, 35 (88%) of all the examined strains and 10 (67%) of the 15 macrolide-resistant strains would be fully susceptible to solithromycin. However, it should be noted that the strain collection tested was enriched for resistant isolates, so clinical studies regarding the treatment efficacy of M. genitalium are needed.
Solithromycin has three binding sites on the bacterial ribosome compared to one for current macrolides, such as azithromycin, leading to substantially higher activity (40). Although 12 of the 15 macrolide-resistant M. genitalium strains were inhibited by solithromycin at a MIC of ≤2 μg/ml, two strains with solithromycin MICs of 4 μg/ml and one with a MIC of 16 μg/ml were apparently resistant. The strain with a MIC of 16 μg/ml and one of the strains with a MIC of 4 μg/ml had the unusual A2058C 23S rRNA mutation, and it was suspected that this would be the explanation for the high MIC. The finding that mutations in position 2058 apparently lead to higher solithromycin MICs than the more common ones in position 2059 is surprising, as solithromycin has been shown to interact strongly with both residues by protecting from modifications with dimethyl sulfate (40). We therefore also examined other putative mechanisms for the increased solithromycin MICs in these strains, such as mutations at nucleotide positions 752 and 2609 of the 23S rRNA gene, which are known to be involved in the binding of other ketolides (31), but all M. genitalium strains, regardless of their macrolide susceptibility status, carried a 23S rRNA gene with a C at position 752 in contrast to the A752 in wild-type E. coli strains, and no other mutations were found in the region. In the 2609 position, all strains had T2609, as found in wild-type E. coli.
A search for mutations in the ribosomal proteins L4 and L22, known to be involved in macrolide resistance from in vitro selection in Mycoplasma pneumoniae (41), was also unsuccessful in explaining the higher solithromycin MICs. Ribosomal protein L22 was remarkably conserved, with 37 (93%) strains having 100% identical amino acid sequences and the remaining 3 having single amino acid substitutions outside the L22 β-hairpin tip; one strain was macrolide susceptible, and two were macrolide resistant due to A2059G 23S rRNA mutations and had solithromycin MICs of 0.5 μg/ml. The L22 β-hairpin tip around R87 and A88, corresponding to E. coli L22 R109 and A110, is involved in stabilizing the ketolide binding to the ribosomal complex (42), and by in vitro selection of resistance mutations in M. pneumoniae by increasing concentrations of various antimicrobials, the corresponding positions R113 and A114 in M. pneumoniae were mutated by selection only by telithromycin, leading to high-level resistance (41). In ribosomal protein L4, 30 (75%) strains had an amino acid sequence identical to that of the G37 type strain. Four strains with A2059G 23S rRNA gene mutations had an H69R mutation (the position corresponding to E. coli Gly64) in the loop structure of L4. Three of these strains had solithromycin MICs of 0.5 μg/ml, and one had an MIC of 1 μg/ml; thus, these mutations did not appear to increase the MIC over that of other strains with similar 23S rRNA gene mutations. In M. pneumoniae, the corresponding H70R mutation was induced by passage in subinhibitory concentrations of clindamycin, leading to a 4-fold increase in the MIC of the antibiotic but with no changes in the erythromycin and azithromycin MICs and only a 2-fold increase in the telithromycin MIC (41).
Macrolide resistance mutation detection has been performed routinely as part of the diagnostic procedures at Statens Serum Institut, Copenhagen, Denmark, since December 2007. In a survey ending in December 2010, mutation detection could be completed for 1,085 (97%) of the M. genitalium-positive specimens. Resistance was detected in 420 specimens (39%), and the most common mutations were 23S rRNA A2058G (61%) and A2059G (35%), while the A2058C mutation resulting in higher solithromycin MICs was exceedingly rare (0.5%) (48). If 4 out of 5 A2058G and all A2059G strains had MICs of <4 μg/ml, as in the present study, this would suggest that approximately 85% of the azithromycin-resistant strains, or 94% of all M. genitalium infections, could be cured by solithromycin treatment. Using the conservative estimate considering only strains with MICs of ≤1 μg/ml treatable, approximately 65% of the resistant strains, or 85% of all M. genitalium infections, could be cured. However, as no correlates between the in vitro MIC of solithromycin and treatment outcome have been established, these figures remain speculative, but any improvement over azithromycin would be a significant step toward limiting the use of moxifloxacin, an antimicrobial agent with several serious side effects, both for the patient and ecologically, in promoting the development of resistance.
In conclusion, the in vitro results of the present study show that solithromycin has activity superior to that of azithromycin and other macrolides, but also many other classes of antimicrobials. Accordingly, this new fluoroketolide might be an effective option for treatment of M. genitalium infections. However, the efficacy of solithromycin in appropriately conducted clinical trials with follow-up for test of cure and detection of genotypic and phenotypic resistance needs to be evaluated prior to widespread use. Solithromycin appears also to be highly effective against C. trachomatis (43) and Neisseria gonorrhoeae (44, 45), suggesting that solithromycin could be a treatment option for several sexually transmitted infections, including in syndromic treatment of urethral and vaginal discharge.
ACKNOWLEDGMENTS
The present work was supported by funding from the Statens Serum Institut and a grant from Cempra Inc., Chapel Hill, NC, USA.
Technicians at Statens Serum Institut provided excellent technical assistance.
Footnotes
Published ahead of print 17 March 2014
REFERENCES
- 1.Tully JG, Taylor-Robinson D, Cole RM, Rose DL. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet i:1288–1291 [DOI] [PubMed] [Google Scholar]
- 2.Taylor-Robinson D, Jensen JS. 2011. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin. Microbiol. Rev. 24:498–514. 10.1128/CMR.00006-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen CR, Manhart LE, Bukusi EA, Astete S, Brunham RC, Holmes KK, Sinei SK, Bwayo JJ, Totten PA. 2002. Association between Mycoplasma genitalium and acute endometritis. Lancet 359:765–766. 10.1016/S0140-6736(02)07848-0 [DOI] [PubMed] [Google Scholar]
- 4.Manhart LE, Critchlow CW, Holmes KK, Dutro SM, Eschenbach DA, Stevens CE, Totten PA. 2003. Mucopurulent Cervicitis and Mycoplasma genitalium. J. Infect. Dis. 187:650–657. 10.1086/367992 [DOI] [PubMed] [Google Scholar]
- 5.Cohen CR, Mugo NR, Astete SG, Odondo R, Manhart LE, Kiehlbauch JA, Stamm WE, Waiyaki PG, Totten PA. 2005. Detection of Mycoplasma genitalium in women with laparoscopically diagnosed acute salpingitis. Sex. Transm. Infect. 81:463–466. 10.1136/sti.2005.015701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anagrius C, Loré B, Jensen JS. 2005. Mycoplasma genitalium: prevalence, clinical significance, and transmission. Sex. Transm. Infect. 81:458–462. 10.1136/sti.2004.012062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falk L, Fredlund H, Jensen JS. 2005. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex. Transm. Infect. 81:73–78. 10.1136/sti.2004.010439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen B, Sokolowski I, Østergaard L, Moller JK, Olesen F, Jensen JS. 2007. Mycoplasma genitalium: prevalence and behavioural risk factors in the general population. Sex. Transm. Infect. 83:237–241. 10.1136/sti.2006.022970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oakeshott P, Aghaizu A, Hay P, Reid F, Kerry S, Atherton H, Simms I, Taylor-Robinson D, Dohn B, Jensen JS. 2010. Is Mycoplasma genitalium in women the “new chlamydia?” A community-based prospective cohort study. Clin. Infect. Dis. 51:1160–1166. 10.1086/656739 [DOI] [PubMed] [Google Scholar]
- 10.Manhart LE, Holmes KK, Hughes JP, Houston LS, Totten PA. 2007. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am. J. Public Health 97:1118–1125. 10.2105/AJPH.2005.074062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradshaw CS, Chen MY, Fairley CK. 2008. Persistence of Mycoplasma genitalium following azithromycin therapy. PLoS One 3:e3618. 10.1371/journal.pone.0003618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikström A, Jensen JS. 2006. Mycoplasma genitalium: a common cause of persistent urethritis among men treated with doxycycline. Sex. Transm. Infect. 82:276–279. 10.1136/sti.2005.018598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor-Robinson D, Gilroy CB, Jensen JS. 2000. The biology of Mycoplasma genitalium. Venereology 13:119–127 [Google Scholar]
- 14.Hamasuna R, Jensen JS, Osada Y. 2009. Antimicrobial susceptibilities of Mycoplasma genitalium by broth dilution and quantitative PCR. Antimicrob. Agents Chemother. 53:4938–4939. 10.1128/AAC.00724-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waites KB, Crabb DM, Duffy LB. 2009. Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob. Agents Chemother. 53:2139–2141. 10.1128/AAC.00090-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falk L, Fredlund H, Jensen JS. 2003. Tetracycline treatment does not eradicate Mycoplasma genitalium. Sex. Transm. Infect. 79:318–319. 10.1136/sti.79.4.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Björnelius E, Anagrius C, Bojs G, Carlberg H, Johannisson G, Johansson E, Moi H, Jensen JS, Lidbrink P. 2008. Antibiotic treatment of symptomatic Mycoplasma genitalium infection in Scandinavia: a controlled clinical trial. Sex. Transm. Infect. 84:72–76. 10.1136/sti.2007.027375 [DOI] [PubMed] [Google Scholar]
- 18.Jensen JS. 2009. Single-dose azithromycin treatment for Mycoplasma genitalium-positive urethritis: best but not good enough. Clin. Infect. Dis. 48:1655–1656. 10.1086/599034 [DOI] [PubMed] [Google Scholar]
- 19.Schwebke JR, Rompalo A, Taylor S, Sena AC, Martin DH, Lopez LM, Lensing S, Lee JY. 2011. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens—a randomized clinical trial. Clin. Infect. Dis. 52:163–170. 10.1093/cid/ciq074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manhart LE, Gillespie CW, Lowens MS, Khosropour CM, Colombara DV, Golden MR, Hakhu NR, Thomas KK, Hughes JP, Jensen NL, Totten PA. 2013. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: a randomized controlled trial. Clin. Infect. Dis. 56:934–942. 10.1093/cid/cis1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jernberg E, Moghaddam A, Moi H. 2008. Azithromycin and moxifloxacin for microbiological cure of Mycoplasma genitalium infection: an open study. Int. J. STD AIDS 19:676–679. 10.1258/ijsa.2008.008038 [DOI] [PubMed] [Google Scholar]
- 22.Jensen JS, Bradshaw CS, Tabrizi SN, Fairley CK, Hamasuna R. 2008. Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin. Infect. Dis. 47:1546–1553. 10.1086/593188 [DOI] [PubMed] [Google Scholar]
- 23.Anagrius C, Lore B, Jensen JS. 2013. Treatment of Mycoplasma genitalium. Observations from a Swedish STD Clinic. PLoS One 8:e61481. 10.1371/journal.pone.0061481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. 2013. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int. J. STD AIDS 24:822–828. 10.1177/0956462413502008 [DOI] [PubMed] [Google Scholar]
- 25.Tully JG, Taylor-Robinson D, Rose DL, Cole RM, Bove JM. 1983. Mycoplasma genitalium, a new species from the human urogenital tract. Int. J. Syst. Bacteriol. 33:387–396. 10.1099/00207713-33-2-387 [DOI] [Google Scholar]
- 26.Kokotovic B, Friis NF, Jensen JS, Ahrens P. 1999. Amplified-fragment length polymorphism fingerprinting of Mycoplasma species. J. Clin. Microbiol. 37:3300–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hjorth SV, Björnelius E, Lidbrink P, Falk L, Dohn B, Berthelsen L, Ma L, Martin DH, Jensen JS. 2006. Sequence-based typing of Mycoplasma genitalium reveals sexual transmission. J. Clin. Microbiol. 44:2078–2083. 10.1128/JCM.00003-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jensen JS. 2006. Mycoplasma genitalium infections. Diagnosis, clinical aspects, and pathogenesis. Dan. Med. Bull. 53:1–27 [PubMed] [Google Scholar]
- 29.Jensen JS, Björnelius E, Dohn B, Lidbrink P. 2004. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J. Clin. Microbiol. 42:683–692. 10.1128/JCM.42.2.683-692.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamasuna R, Osada Y, Jensen JS. 2005. Antibiotic susceptibility testing of Mycoplasma genitalium by TaqMan 5′ nuclease real-time PCR. Antimicrob. Agents Chemother. 49:4993–4998. 10.1128/AAC.49.12.4993-4998.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong L, Korkhin Y, Mankin AS. 2005. Binding site of the bridged macrolides in the Escherichia coli ribosome. Antimicrob. Agents Chemother. 49:281–288. 10.1128/AAC.49.1.281-288.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma L, Taylor S, Jensen JS, Myers L, Lillis R, Martin DH. 2008. Short tandem repeat sequences in the Mycoplasma genitalium genome and their use in a multilocus genotyping system. BMC Microbiol. 8:130. 10.1186/1471-2180-8-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma L, Jensen JS, Mancuso M, Hamasuna R, Jia Q, McGowin CL, Martin DH. 2010. Genetic variation in the complete MgPa operon and its repetitive chromosomal elements in clinical strains of Mycoplasma genitalium. PLoS One 5:e15660. 10.1371/journal.pone.0015660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gesink DC, Mulvad G, Montgomery-Andersen R, Poppel U, Montgomery-Andersen S, Binzer A, Vernich L, Frosst G, Stenz F, Rink E, Olsen OR, Koch A, Jensen JS. 2012. Mycoplasma genitalium presence, resistance and epidemiology in Greenland. Int. J. Circumpolar Health 71:1–8. 10.3402/ijch.v71i0.18203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemaire S, Van BF, Tulkens PM. 2009. Cellular accumulation and pharmacodynamic evaluation of the intracellular activity of CEM-101, a novel fluoroketolide, against Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila in human THP-1 macrophages. Antimicrob. Agents Chemother. 53:3734–3743. 10.1128/AAC.00203-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jensen JS, Blom J, Lind K. 1994. Intracellular location of Mycoplasma genitalium in cultured Vero cells as demonstrated by electron microscopy. Int. J. Exp. Pathol. 75:91–98 [PMC free article] [PubMed] [Google Scholar]
- 37.Still JG, Schranz J, Degenhardt TP, Scott D, Fernandes P, Gutierrez MJ, Clark K. 2011. Pharmacokinetics of solithromycin (CEM-101) after single or multiple oral doses and effects of food on single-dose bioavailability in healthy adult subjects. Antimicrob. Agents Chemother. 55:1997–2003. 10.1128/AAC.01429-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oldach D, Clark K, Schranz J, Das A, Craft JC, Scott D, Jamieson BD, Fernandes P. 2013. Randomized, double-blind, multicenter phase 2 study comparing the efficacy and safety of oral solithromycin (CEM-101) to those of oral levofloxacin in the treatment of patients with community-acquired bacterial pneumonia. Antimicrob. Agents Chemother. 57:2526–2534. 10.1128/AAC.00197-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGhee P, Clark C, Kosowska-Shick KM, Nagai K, Dewasse B, Beachel L, Appelbaum PC. 2010. In vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanisms. Antimicrob. Agents Chemother. 54:230–238. 10.1128/AAC.01123-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Llano-Sotelo B, Dunkle J, Klepacki D, Zhang W, Fernandes P, Cate JH, Mankin AS. 2010. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob. Agents Chemother. 54:4961–4970. 10.1128/AAC.00860-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereyre S, Guyot C, Renaudin H, Charron A, Bebear C, Bebear CM. 2004. In vitro selection and characterization of resistance to macrolides and related antibiotics in Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 48:460–465. 10.1128/AAC.48.2.460-465.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kostopoulou ON, Petropoulos AD, Dinos GP, Choli-Papadopoulou T, Kalpaxis DL. 2012. Investigating the entire course of telithromycin binding to Escherichia coli ribosomes. Nucleic Acids Res. 40:5078–5087. 10.1093/nar/gks174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roblin PM, Kohlhoff SA, Parker C, Hammerschlag MR. 2010. In vitro activity of CEM-101, a new fluoroketolide antibiotic, against Chlamydia trachomatis and Chlamydia (Chlamydophila) pneumoniae. Antimicrob. Agents Chemother. 54:1358–1359. 10.1128/AAC.01343-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golparian D, Fernandes P, Ohnishi M, Jensen JS, Unemo M. 2012. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against a large collection of clinical Neisseria gonorrhoeae isolates and international reference strains, including those with high-level antimicrobial resistance: potential treatment option for gonorrhea? Antimicrob. Agents Chemother. 56:2739–2742. 10.1128/AAC.00036-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hook EW, Jamieson BD, Oldach D, Harbison H, Whittington A, Fernandes P. 2013. A phase II, dose ranging study to evaluate the efficacy and safety of single-dose oral solithromycin (CEM-101) for treatment of patients with uncomplicated urogenital gonorrhoea. Sex. Transm. Infect. 89:A29–A30. 10.1136/sextrans-2013-051184.0092 [DOI] [Google Scholar]
- 46.Jensen JS, Hansen HT, Lind K. 1996. Isolation of Mycoplasma genitalium strains from the male urethra. J. Clin. Microbiol. 34:286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamasuna R, Osada Y, Jensen JS. 2007. Isolation of Mycoplasma genitalium from first-void urine specimens by coculture with Vero cells. J. Clin. Microbiol. 45:847–850. 10.1128/JCM.02056-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salado-Rasmussen K, Jensen JS. Clin. Infect. Dis., in press [DOI] [PMC free article] [PubMed] [Google Scholar]