Abstract
This is a substudy of the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) Comparison of Nevirapine and Efavirenz for the Treatment of HIV-TB Co-infected Patients (ANRS 12146-CARINEMO) trial, which assessed the pharmacokinetics of rifampin or isoniazid with or without the coadministration of nonnucleoside reverse transcriptase inhibitor-based HIV antiretroviral therapy in HIV-tuberculosis-coinfected patients in Mozambique. Thirty-eight patients on antituberculosis therapy based on rifampin and isoniazid participated in the substudy (57.9% males; median age, 33 years; median weight, 51.9 kg; median CD4+ T cell count, 104 cells/μl; median HIV-1 RNA load, 5.5 log copies/ml). The daily doses of rifampin and isoniazid were 10 and 5 mg/kg of body weight, respectively. Twenty-one patients received 200 mg of nevirapine twice a day (b.i.d.), and 17 patients received 600 mg of efavirenz once a day (q.d.) in combination with lamivudine and stavudine from day 1 until the end of the study. Blood samples were collected at regular time-dosing intervals after morning administration of a fixed-dose combination of rifampin and isoniazid. When rifampin was administered alone, the median maximum concentration of drug in serum (Cmax) and the area under the concentration-time curve (AUC) at steady state were 6.59 mg/liter (range, 2.70 to 14.07 mg/liter) and 27.69 mg · h/liter (range, 11.41 to 109.75 mg · h/liter), respectively. Concentrations remained unchanged when rifampin was coadministered with nevirapine or efavirenz. When isoniazid was administered alone, the median isoniazid Cmax and AUC at steady state were 5.08 mg/liter (range, 1.26 to 11.51 mg/liter) and 20.92 mg · h/liter (range, 7.73 to 56.95 mg · h/liter), respectively. Concentrations remained unchanged when isoniazid was coadministered with nevirapine; however, a 29% decrease in the isoniazid AUC was observed when isoniazid was combined with efavirenz. The pharmacokinetic parameters of rifampin and isoniazid when coadministered with nevirapine or efavirenz were not altered to a clinically significant extent in these severely immunosuppressed HIV-infected patients. Patients experienced favorable clinical outcomes. (This study has been registered at ClinicalTrials.gov under registration no. NCT00495326.)
INTRODUCTION
Tuberculosis (TB) is a leading cause of death among human immunodeficiency virus (HIV)-infected individuals, and it is especially common in sub-Saharan Africa, where the high burden of HIV infection further increases the tuberculosis incidence. In 2011, there were 8.7 million incident cases of tuberculosis worldwide, of which 1.1 million were among HIV-positive individuals (1). Based on evidence that concomitant therapy reduces mortality associated with tuberculosis among HIV-infected patients, the coadministration of antituberculosis drugs and antiretroviral therapy is common practice in high-burden tuberculosis and HIV countries (2).
The World Health Organization (WHO) recommends a 6-month rifampin-based regimen as a first-line antituberculosis treatment (3). The efficacy of this treatment regimen for curing tuberculosis in adults is >95% (4–6), but it can be as low as 53.4% in untreated HIV-positive individuals (7, 8). The effectiveness of antituberculosis treatment is dependent on drug exposure, and several factors such as patients′ male gender, low weight, severe illness, malnutrition, drug formulation, drug-drug interaction, comorbid disease, and HIV infection itself have been shown to affect antituberculosis drug pharmacokinetics (9–13). Low exposure to TB drugs can lead to prolonged infectiousness, poor treatment outcome with increased risk of relapse, development of multidrug-resistant strains of Mycobacterium tuberculosis, and death.
In many resource-limited countries, first-line HIV antiretroviral treatment consists of a nonnucleoside reverse transcriptase inhibitor (NNRTI) (nevirapine or efavirenz) combined with two nucleoside reverse transcriptase inhibitors. Due to the risk of subtherapeutic plasma nevirapine concentrations in HIV/tuberculosis-coinfected patients receiving rifampin, concomitant, efavirenz-based antiretroviral treatment is the preferred NNRTI during the course of tuberculosis treatment (14). However, nevirapine is a common alternative to efavirenz in patients who have contraindications to efavirenz or who do not tolerate it. Nevirapine and efavirenz are substrates of cytochromes P-450 (CYPs), mainly CYP2B6 for efavirenz and CYP2B and CYP3A for nevirapine, and have drug metabolizing enzymes or transporter inducing and inhibiting properties (15, 16). To our knowledge, data of their effects on rifampin and isoniazid are scarce and have only recently been documented for efavirenz in the African population (13).
Rifampin is part of the 6-month tuberculosis therapy regimen recommended by the WHO and exhibits concentration-dependent bactericidal activity (17). Rifampin has a short half-life (around 2 h) and autoinduces its liver and presystemic metabolism, lowering its plasma concentrations over the course of treatment compared to those reached after a single oral dose (18). Additionally, rifampin is a substrate and a potent blocker of the influx transporter organic anion-transporting polypeptide 1B1 (OATP1B1), which regulates the uptake of endogenous compounds and drugs into hepatocytes (19). Isoniazid, another drug used in the 6-month tuberculosis therapy regimen, has excellent early bactericidal activity (20). Isoniazid is metabolized mainly by hepatic type 2 N-acetyltransferase (NAT2) (21). Whether transporters could be involved in isoniazid disposition is currently unknown. Although the pharmacokinetics of antituberculosis drugs have been reported to be affected by HIV infection (13, 22–24), whether efavirenz or nevirapine differentially impairs rifampin or isoniazid pharmacokinetics has not been documented.
In this study we aimed to document the effects of nevirapine and efavirenz on the disposition of both rifampin and isoniazid by comparing the pharmacokinetic parameters of rifampin and isoniazid with and without coadministration of nevirapine- or efavirenz-based antiretroviral therapy. This is a substudy of the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) Comparison of Nevirapine and Efavirenz for the Treatment of HIV-TB Co-infected Patients (ANRS 12146 CARINEMO) trial (registered at ClinicalTrials.gov under registration no. NCT00495326), which compared the efficacy and safety of nevirapine- and efavirenz-based antiretroviral regimens in HIV/tuberculosis-coinfected patients (25).
(Part of the study results were presented orally in the 43rd Conference of the International Union Against Tuberculosis and Lung Diseases [IUATLD], Kuala Lampur, Malaysia, 2012 [26]).
MATERIALS AND METHODS
Patients.
All patients included in this study gave written informed consent to participate in this ANRS 12214 pharmacokinetic study, a substudy of the ANRS 12146-CARINEMO clinical trial. The ANRS 12214 pharmacokinetic study was conducted in Mozambique and approved by the Mozambican National Bioethical Committee and Ethical Review Board of Médecins Sans Frontières-Switzerland. Patients with HIV-tuberculosis coinfection were randomized to receive nevirapine- or efavirenz-based antiretroviral therapy in addition to their antituberculosis treatment. Detailed participants' characteristics and study procedures are described elsewhere (25). Patients' eligibility criteria for the trial were new case of active tuberculosis (bacteriologically confirmed or not pulmonary tuberculosis cases and extrapulmonary tuberculosis cases), CD4+ T cell counts of <250 cells/μl, treatment-naive HIV-infected patients, Karnofsky score of ≥60, no significant hepatic dysfunction (levels of transaminase and total bilirubin <5× the upper normal limit [UNL]), absence of severe grade 4 clinical or laboratory signs, and willingness to provide informed consent. After 4 to 6 weeks of antituberculosis treatment, the trial participants were randomized to receive the fixed-dose combination (FDC) of 200 mg of nevirapine, 150 mg of lamivudine, and 30 mg of stavudine twice daily (Cipla, India) or 600 mg of efavirenz (Aurobindo, India) once daily combined with the FDC of 150 mg of lamivudine and 30 mg of stavudine (Cipla, India) twice daily. The antituberculosis drugs were administered orally and the daily dose calculated per unit of body weight (rifampin [10 mg/kg of body weight], isoniazid [5 mg/kg], pyrazinamide [25 mg/kg], and ethambutol [15 mg/kg]). The four FDC antituberculosis drugs (300 mg of rifampin, 150 mg of isoniazid, 400 mg of pyrazinamide, and 275 mg of ethambutol) were administered for 2 months, followed by the FDC containing 300 mg of rifampin and 150 mg of isoniazid for the remaining 4 months (Lupin, India). All antiretroviral therapy and antituberculosis drugs used in the trial were prequalified by the WHO. Patients' adherence to antituberculosis drug and antiretroviral therapy was monitored by study staff by pill counting, monitoring of the regularity and promptness of attendance at scheduled clinical visits, and detection of isoniazid metabolites in urine while on tuberculosis treatment with BBL Taxo isoniazid test strips (Becton, Dickinson, and Company, USA). Patients had regular visits during the trial to monitor clinical evolution, liver function tests, and treatment adherence and to detect potential adverse events. In addition, HIV-1 RNA viral load was monitored at antiretroviral treatment initiation and every 12 weeks until 48 weeks. CD4+ T cell counts were measured at antiretroviral treatment initiation and after 24 and 48 weeks (25). The last 172 patients enrolled in the ANRS 12146-CARINEMO trial had extended follow-up until 96 weeks to identify potential recurrent tuberculosis cases. Thirty-nine patients enrolled consecutively in the ANRS 12146-CARINEMO trial met the criteria and were included in the pharmacokinetic substudy (ANRS 12214) if they met the following additional criteria: to be on rifampin and isoniazid as part of a tuberculosis treatment regimen, to have had no antiretroviral therapy at the time of enrollment, and to be willing to participate in the additional blood sampling by providing signed informed consent.
Study design.
Each patient was requested to come fasting to the clinic in the morning, having had the last meal on the previous evening. At the clinic, patients were asked to recall the timing of the last antituberculosis drugs and any other medications taken concomitantly on the previous day. Blood samples were collected at steady state on two occasions, within a week before antiretroviral therapy initiation to allow estimation of pharmacokinetic parameters of rifampin and isoniazid alone and 4 weeks after antiretroviral therapy initiation when patients were also receiving nevirapine or efavirenz. During these two occasions, blood samples were drawn before morning antituberculosis drug intake (time zero) and 0.5 h, 1 h, 1.5 h, 2 h, 4 h, 6 h, 8 h, 10 h, and 12 h after the intake of antituberculosis drugs. The antituberculosis drugs were administered with 100 ml of water to each patient under the supervision of a study nurse just after the predose blood sampling. Within 30 min of each collection, blood samples were centrifuged (800 × g for 20 min) at room temperature. To improve stability of rifampin, each plasma sample of exactly 0.5 ml was stored in a polypropylene tube containing 0.5 ml of ascorbic acid solution (200 mg/liter). An extra blood sample was also drawn to measure predose nevirapine or middose plasma efavirenz concentrations when receiving antituberculosis drugs simultaneously with the antiretroviral treatment. All plasma samples were kept at −80°C in the study site until shipment for analysis to the pharmacology laboratory at Bicêtre Hospital (Paris, France).
Drug assays.
Plasma concentrations of rifampin and isoniazid were measured using validated reverse-phase high-performance liquid chromatography methods with UV detection according to validated assays (27, 28). In brief, rifampin was assayed in plasma after protein precipitation by acetonitrile and injection onto a LiChrospher 100 RP18 column and detection at 342 nm. The mobile phase consisted of a 10/40/50 (vol/vol) mixture of methanol-acetonitrile-citrate buffer (pH 4.3). Isoniazid was assayed in plasma after protein precipitation by 10% trichloroacetic acid in water and injection onto an Atlantis T3 column and detection at 265 nm. The mobile phase consisted of a mixture of 93.5% phosphate buffer (30 mM [pH 7.1]), 5% methanol, and 1.5% acetonitrile. The lower limit of quantification (LLOQ) of plasma rifampin and isoniazid concentrations was 0.1 mg/liter. Three quality control concentrations were included in each analytical run. Day-to-day variability of the quality controls was <8% for rifampin and <12% for isoniazid. Predose nevirapine and middose efavirenz concentrations were assayed in plasma by HPLC with limits of quantification of 0.00025 mg/liter and 0.00050 mg/liter, respectively (29).
Pharmacokinetic analysis.
The pharmacokinetic parameters for rifampin and isoniazid were estimated by the noncompartmental method (WinNonlin software; Pharsight Corporation, Mountain View, CA, USA). As the half-lives of rifampin and isoniazid are short, the areas under the concentration-time curves (AUCs) were calculated until the 12-hour time point using the linear up/log down trapezoidal method, up to the time of the last measurable concentration, and extrapolated to 12 h whenever the half-life could be calculated. Drug concentrations below the lower limit of quantification (LLOQ) occurring before the maximum concentration of drug in serum (Cmax) were assigned a value of LLOQ/2. Concentrations at 2 h postdosing (C2), Cmax, and time to Cmax (Tmax) were obtained visually from the plasma concentration-time curve. Rifampin and isoniazid pharmacokinetic parameters were estimated in the absence and presence of nevirapine or efavirenz. Plasma drug concentrations (including concentrations measured 2 h postdosing) were compared to the previously reported therapeutic concentration ranges, which were between 8 and 24 mg/liter for rifampin and between 3 and 5 mg/liter for isoniazid (30).
Statistical analysis.
This observational pilot pharmacokinetic study assessed plasma concentrations of rifampin and isoniazid off and on nevirapine- or efavirenz-based antiretroviral therapy. This study started after initiation of the ANRS12146-CARINEMO trial and was proposed to all included patients. Twenty patients per arm were expected to be enrolled to detect major differences in rifampin and isoniazid disposition.
The pharmacokinetic parameters for rifampin and isoniazid were summarized by using descriptive statistics and unless otherwise indicated are presented as median and range. The values of Cmax and AUCs were log transformed. Two-sided 90% confidence intervals (CIs) were constructed for the ratios of the geometric mean values (with nonnucleoside reverse transcriptase inhibitors versus alone) of Cmax and AUCs for rifampin and isoniazid, which were compared to the bioequivalence range of 0.80 to 1.25. The Wilcoxon signed-rank test for paired samples was used to compare rifampin and isoniazid pharmacokinetic parameters without and with NNRTIs. The trough concentration (Ctrough) of nevirapine and middose of efavirenz at 4 weeks, which corresponded to the period of the second pharmacokinetic sampling for rifampin and isoniazid, were also determined. The antituberculosis treatment outcomes at the end of treatment (24 weeks), the proportion of recurrent tuberculosis cases, and the proportion of patients with hepatic toxicity were described. Tuberculosis treatment outcome definitions were based on WHO guidelines (3). All statistical analyses were conducted with StataSE software (release 10.0; StataCorp, College Station, TX, USA).
RESULTS
Patient characteristics.
Out of 570 patients randomized in the main trial, 39 were enrolled in the pharmacokinetic substudy, from which 22 patients were randomized in the nevirapine arm and 17 in the efavirenz arm. One patient was nonadherent to antiretroviral therapy in which nevirapine concentrations were below the limit of quantification at weeks 2, 3, and 4, and thus he was excluded from the pharmacokinetic analysis (Fig. 1). The baseline characteristics of the 38 patients are summarized in Table 1. The median age was 33 years and the median body weight was 51.9 kg. Among 38 patients, 28 (73.7%) patients had pulmonary tuberculosis and 10 (26.3%) patients had extrapulmonary tuberculosis (eight patients with pleural tuberculosis and two patients with disseminated tuberculosis). Patients were severely immunosuppressed, with a median CD4+ T cell count of 104 cells/μl (range, 2 to 214 cells/μl) and a median plasma HIV-1 RNA load of 5.5 log copies/ml (range, 4.2 to 7.0 log copies/ml). The median time between tuberculosis treatment initiation and first pharmacokinetic sampling was 30 days.
FIG 1.
Study profile. #, one patient with undetectable rifampin levels at the second pharmacokinetic sampling was excluded from the analysis.
TABLE 1.
Demographic, clinical, and laboratory characteristics of enrolled adult patients at baseline
| Characteristica | Datab for patients (n) in: |
||
|---|---|---|---|
| Nevirapine arm (21) | Efavirenz arm (17) | Total (38) | |
| Age (yr) | 34 (24–48) | 33 (21–50) | 33 (21–50) |
| Male | 12 (57.1) | 10 (58.8) | 22 (57.9) |
| Wt (kg) | 52.6 (40.5–68.5) | 51.3 (39.1–72.3) | 51.9 (39.1–72.3) |
| BMI (kg/m2) | 19.1 (14.4–24.3) | 18.7 (16.7–20.0) | 18.9 (14.4–30.1) |
| Hemoglobin (g/dl) | 8.9 (7.0–10.5) | 9.3 (7.5–11.7) | 9.1 (7.0–11.7) |
| ALT (IU/liter) | 25.3 (7.7–124.2) | 32.3 (13.3–123.6) | 29.9 (7.7–124.2) |
| Total bilirubin (mg/dl) | 0.4 (0.2–2.2) | 0.5 (0.1–1.3) | 0.4 (0.1–2.2) |
| CD4 count (cells/mm3) | 108 (2–206) | 93 (18–214) | 104 (2–214) |
| Plasma HIV-1 RNA (log10 copies/ml) | 5.7 (4.2–7.0) | 5.4 (4.3–6.6) | 5.5 (4.2–7.0) |
| Active hepatitis B infection | 1/17 (5.9) | 4/17 (23.5) | 5/34 (14.7) |
| HCV infection | 0 (0.0) | 1 (5.9) | 1 (2.6) |
| Gastrointestinal symptoms | 1 (4.8) | 1 (5.9) | 2 (5.3) |
| Time between start of tuberculosis therapy and start of antiretroviral therapy (days) | 33 (28–40) | 33 (28–41) | 33 (28–41) |
| Pulmonary tuberculosis | 18 (85.7) | 10 (58.8) | 28 (73.7) |
| Pulmonary tuberculosis smear results | |||
| Positive | 7 (33.3) | 8 (47.0) | 15 (39.5) |
| Negative or missing results | 14 (66.7) | 9 (53.0) | 23 (60.5) |
| Cavitary disease | 1/18 (5.6) | 0/16 (0) | 1/34 (2.9) |
BMI, body mass index; ALT, alanine aminotransferase; HBV, hepatitis B virus; HCV, hepatitis C virus.
Data are no. (%), median (range), or no. affected/no. tested (%).
At the first pharmacokinetic sampling, all patients were receiving the four antituberculosis drugs in fixed-dose combinations. At the second sampling, 73.7% (28/38) of patients were receiving rifampin-isoniazid in fixed-drug combinations and 26.3% (10/38) were still receiving a combination of the four antituberculosis drugs. The patients' body weights remained unchanged throughout the study.
Nonnucleoside reverse transcriptase inhibitor concentrations.
Only plasma samples to measure 12-h postdose concentrations were available. At 4 weeks of antiretroviral therapy, the median Ctrough of nevirapine was 4.11 mg/liter (range, 1.98 to 12. 72 mg/liter) and the median middose concentration of efavirenz was 3.31 mg/liter (range, 1.51 to 24.67 mg/liter), with a median sampling time of 13 h (range, 10.9 to 14.4 h) postdosing.
Rifampin pharmacokinetics.
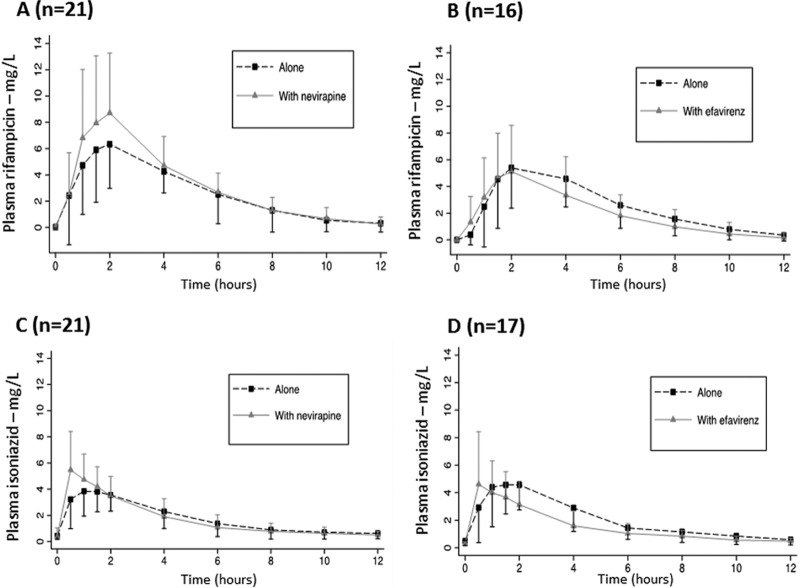
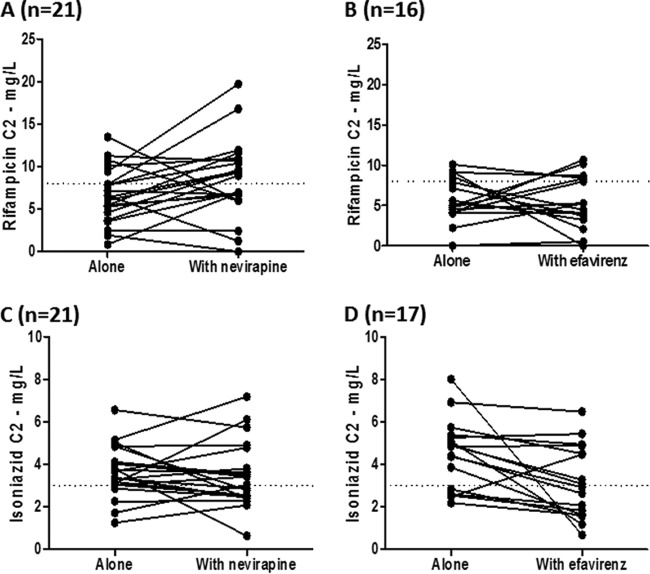
Plots of rifampin concentrations versus time for the nevirapine and efavirenz groups are shown in Fig. 2A and B, respectively. A trend for higher rifampin peak concentrations when combined with nevirapine, but not efavirenz, was noted. One patient had undetectable levels of rifampin when rifampin was coadministered with efavirenz and thus no pharmacokinetic parameters were estimated. In this patient, rifampin Cmax when administered alone was 3.92 mg/liter. The pharmacokinetic parameters of rifampin are listed in Table 2. Concentrations of rifampin before dosing were undetectable in all patients, except for three patients who had low rifampin concentrations (<1 mg/liter). A small nonsignificant increase in Cmax and AUC levels was observed when rifampin was administered with nevirapine but not with efavirenz. Geometric mean ratios for AUCs were close to 1, and the 90% confidence intervals remained within the 0.80 to 1.25 bioequivalence range. It should be pointed out that the interindividual plasma drug concentrations were highly variable in this population. Figure 3 shows rifampin C2 before and after the introduction of antiretroviral therapy. Of note, the rifampin C2 was <8 mg/liter in 16 patients (76.2%) before starting nevirapine but in only 10 patients (47.6%) after the addition of nevirapine. In contrast, the numbers of patients presenting a rifampin C2 of <8 mg/liter were comparable before (13 patients) and after (12 patients) addition of efavirenz.
FIG 2.
Mean ± standard deviation of plasma rifampin (A and B) and isoniazid (C and D) concentration-time profiles during the first 12 h of the 24-h dosing interval in the absence (closed squares and dotted lines) or the presence (closed triangles and solid lines) of nevirapine (A and C) or efavirenz (B and D).
TABLE 2.
Pharmacokinetic parameters of rifampin administered alone and with addition of nevirapine or efavirenz
| Treatment arm and data type | Value of pharmacokinetic parametera |
|||
|---|---|---|---|---|
| C2 (mg/liter) | Cmax (mg/liter) | Tmax (h) | AUC (mg · h/liter) | |
| Nevirapine arm | ||||
| No. of patients | 21 | 21 | 21 | 17 |
| Rifampin alone (median [range]) | 6.40 (0.87–13.49) | 6.59 (3.49–14.07) | 2 (0.7–4.0) | 29.71 (12.45–109.75) |
| Rifampin with nevirapine (median [range]) | 8.94 (0.05–19.74) | 8.94 (1.49–19.74) | 2 (1.0–6.0) | 36.10 (13.96–73.72) |
| GMRb (90% CI) | 1.18 (0.95–1.47) | 1.07 (0.97–1.17) | ||
| P | 0.06 | 0.06 | 0.11 | |
| Efavirenz arm | ||||
| No. of patients | 16 | 16 | 16 | 12 |
| Rifampin alone (median [range]) | 4.99 (0.05–10.09) | 6.69 (2.85–12.29) | 2 (1.0–4.0) | 31.15 (13.99–57.71) |
| Rifampin with efavirenz (median [range]) | 4.47 (0.05–10.67) | 6.27 (2.07–12.43) | 2 (1.0–8.0) | 23.63 (6.25–55.85) |
| GMR (90% CI) | 0.94 (0.71–1.24) | 0.96 (0.87–1.05) | ||
| P | 0.98 | 0.90 | 0.68 | |
C2, concentration 2 h postdosing; Cmax, maximum concentration; Tmax, time to achieve maximum concentration; AUC, area under the concentration-time curve during a dosing interval.
GMR, geometric mean ratio for the parameters with NNRTI compared to those without NNRTI.
FIG 3.
Individual plasma concentrations of rifampin (top) and isoniazid (bottom) drawn 2 h (C2) postdosing before and after starting antiretroviral treatment with nevirapine (left) or efavirenz (right).
Isoniazid pharmacokinetics.
Plots of isoniazid concentrations versus time for the nevirapine and efavirenz patient groups are shown in Fig. 2C and D, respectively. The time for Cmax of isoniazid occurred sooner when it was combined antiretroviral therapy, indicating a more rapid absorption. Concentration declines versus time remained unchanged when isoniazid was combined with nevirapine or efavirenz, indicating that the rates of elimination were unchanged. Pharmacokinetic parameters of isoniazid are listed in Table 3. A 21% higher isoniazid Cmax was observed when isoniazid was combined with nevirapine, in keeping with the shorter Tmax as an indication of likely faster absorption (geometric mean ratio [GMR] and 90% CI, 1.21 [1.01 to 1.45]). Of note, a 29% lower AUC as indicated by the GMR and 90% confidence interval of 0.71(0.55 to 0.92) when isoniazid was combined with efavirenz, with unchanged Cmax and half-lives (4.71 h and 4.53 h), although with high variability. Interestingly, the patient who had an undetectable concentration of rifampin when on efavirenz had detectable concentrations of isoniazid, although in the lower range (Ctrough 0.54 mg/liter, Cmax 1.05 mg/liter, and AUC 5.05 mg · h/liter). Before antiretroviral treatment initiation, an isoniazid C2 of <3 mg/liter was found in 4 patients (19.0%) of the nevirapine group and 5 patients (29.4%) of the efavirenz group. After starting antiretroviral therapy, an isoniazid C2 of <3 mg/liter was found in 10 patients (47.6%) of the nevirapine group and 9 patients (52.9%) of the efavirenz group, as shown in Fig. 3. None of the included patients had an isoniazid C2 below the MIC (MIC, 0.05 mg/liter).
TABLE 3.
Pharmacokinetic parameters of isoniazid administered alone and with addition of nevirapine or efavirenz
| Treatment arm and data type | Value of pharmacokinetic parametera |
|||
|---|---|---|---|---|
| C2 (mg/liter) | Cmax (mg/liter) | Tmax (h) | AUC (mg · h/liter) | |
| Nevirapine arm | ||||
| No. of patients | 21 | 21 | 21 | 19 |
| Isoniazid alone (median [range]) | 3.39 (1.25–6.56) | 4.83 (1.26–7.75) | 1.0 (0.5–4.0) | 19.70 (8.32–39.53) |
| Isoniazid with nevirapine (median [range]) | 3.05 (0.64–7.19) | 5.96 (1.88–9.80) | 0.5 (0.4–2.2) | 17.41 (12.56–43.90) |
| GMRb (90% CI) | 1.21 (1.01–1.45) | 1.03 (0.95–1.11) | ||
| P | 0.53 | 0.06 | 0.43 | |
| Efavirenz arm | ||||
| No. of patients (median [range]) | 17 | 17 | 17 | 17 |
| Isoniazid alone (median [range]) | 4.84 (2.19–8.00) | 5.20 (2.19–11.51) | 1.5 (1.0–4.0) | 23.56 (7.73–56.95) |
| Isoniazid with efavirenz (median [range]) | 2.93 (0.68–6.48) | 4.91 (1.05–13.47) | 0.5 (0.5–2.0) | 14.06 (4.94–34.51) |
| GMR (90% CI) | 0.89 (0.68–1.15) | 0.71 (0.55–0.92) | ||
| P | 0.0052 | 0.62 | 0.0106 | |
C2, concentration 2 h postdosing; Cmax, maximum concentration; Tmax, time to achieve maximum concentration; AUC, area under the concentration-time curve during a dosing interval.
GMR, geometric mean ratio for the parameters with NNRTI compared to those without NNRTI.
Clinical outcome and safety.
Thirty-seven patients (97.4%) successfully completed the tuberculosis treatment. One patient (2.6%), in whom plasma rifampin concentrations were below the lower limit of quantification at all time points during the second pharmacokinetic period, died 10 weeks after starting antiretroviral treatment due to wasting syndrome associated with advanced HIV infection. Defaulter or treatment failure was not observed in the study. From 38 patients initially enrolled in the pharmacokinetic study, 33 (86.8%) were followed for 96 weeks. Of these 33 patients, one developed tuberculosis recurrence at week 92, with lymph node involvement. In this patient, rifampin Cmax levels before and after antiretroviral therapy initiation were 8.86 mg/liter and 11.63 mg/liter, respectively, and isoniazid Cmax levels before and after antiretroviral therapy initiation were 6.07 mg/liter and 8.92 mg/liter, respectively.
Five weeks after antiretroviral initiation, two patients had an increase in ALT of grade ≥2 with levels of 163.9 IU/liter (grade 3) and 588.0 IU/liter (grade 4) and one patient had total bilirubin of grade ≥2 with a level of 8.1 mg/dl (grade 3). Of note, ALT grade 4 and total bilirubin grade 3 were found in a hepatitis B virus (HBV) carrier patient who had rifampin and isoniazid Cmax levels close to the median (6.91 mg/liter and 6.11 mg/liter, respectively). Both patients with increased ALT levels had Cmax levels of rifampin (3.23 mg/liter and 6.91 mg/liter) and isoniazid (3.29 mg/liter and 6.11 mg/liter) within the ranges described for the whole studied population. The decrease in plasma HIV-1 RNA from baseline after 12 weeks of antiretroviral therapy was at least 1 log in all enrolled patients. The proportions of patients with HIV-1 RNA levels of <50 copies/ml at weeks 24 and 48 were 86.1% and 85.7%, respectively.
DISCUSSION
Efavirenz is currently the antiretroviral backbone recommended for HIV-tuberculosis-coinfected patients, but in the absence of an alternative to efavirenz in patients who cannot receive it, nevirapine is still prescribed for some HIV-tuberculosis-coinfected patients. This is the first study comparing pharmacokinetic parameters of rifampin and isoniazid when prescribed alone and with nevirapine when prescribed without a lead-in dose in Mozambican HIV-tuberculosis-coinfected patients. To our knowledge, our study contributes to the limited data on the pharmacokinetics of antituberculosis drugs in HIV-infected patients treated with efavirenz. Our main finding is that rifampin exposure was not altered to a clinically significant extent when combined with either nevirapine or efavirenz. A 29% significant decrease in isoniazid exposure (AUC) was demonstrated when coadministered with efavirenz but not nevirapine. The consequence of such a reduction is unknown. However, it has been suggested from an in vitro pharmacodynamic model that the AUC which corresponds to drug exposure that achieves 50% of the maximal effect is 1 mg · h/liter, below the AUCs estimated in our population (31, 32). The mechanism of this interaction is unclear. Induction of the isoniazid CYP-mediated pathway is unlikely, as during the duration of the study isoniazid was combined with rifampin, a very potent inducer of drug-metabolizing enzymes. Concomitant decreases in Cmax suggest that isoniazid absorption could be decreased. Whether this interaction could be efflux or uptake transporter mediated remains to be demonstrated.
The WHO recommends the systematic initiation of antiretroviral treatment in any HIV-tuberculosis-coinfected patients, regardless of CD4+ T cell levels. For this reason, it is of utmost importance to know that the introduction of nonnucleoside reverse transcriptase inhibitor-based antiretroviral treatment will not impair antituberculosis drug exposure. Concentrations of rifampin and isoniazid measured in our population of HIV-tuberculosis-coinfected patients are similar to those reported in previous studies (9, 13, 33–36). Tuberculosis and HIV infection were found to alter absorption and, consequently, decrease antituberculosis drug concentrations (11, 22, 23, 37). Interestingly, Barroso et al. (38) compared rifampin and isoniazid Cmax levels in healthy volunteers and patients with susceptible tuberculosis. Cmax levels of rifampin were higher in healthy controls (5.7 mg/liter) than in tuberculosis-infected patients (2.11 mg/liter), whereas Cmax levels of isoniazid were similar in healthy controls and tuberculosis patients (3.26 mg/liter and 2.85 mg/liter, respectively). In addition, the same study demonstrated that 82% of the tuberculosis patients had rifampin Cmax levels below the 8-mg/liter threshold compared to 50% of the healthy subjects, whereas there was no difference for isoniazid concentrations below the 3 mg/liter threshold (39.3% and 46.7% for tuberculosis patients and healthy subjects, respectively). Reduced intestinal permeability of patients compared with healthy subjects was found to be dependent on cofactors such as alcoholism, smoking, body mass index, and levels of hemoglobin and albumin. Such patients' characteristics can also be identified in severely immunosuppressed HIV-infected patients. In a study conducted in Botswana by Chideya et al., among 255 tuberculosis patients, 84% had rifampin Cmax levels of <8 mg/liter and 69% of them were HIV infected. Rifampin Cmax levels were significantly lower in patients with CD4+ T cells counts of <200 cells/μl than in those with CD4+ T cell counts of >200 cells/μl, pointing out the role of a functional immune system in drug absorption (9). Interestingly, despite the inclusion of patients with advanced immune suppression, concentrations measured 2 h postdose and after starting antiretroviral therapy compared favorably to those obtained in healthy volunteers. Of note, we found undetectable or low concentrations of rifampin and isoniazid in one of the included patients, who died from wasting syndrome shortly after sampling for drug assay. Such a discrepancy between rifampin and isoniazid exposure levels could be related to poor rifampin absorption in critically ill patients (39). Several factors could be associated with relatively good rifampin and isoniazid exposure. On the one hand, patients received support to enhance adherence to treatment, and bioavailability was optimized with prescription of WHO-prequalified FDC antituberculosis drugs using small body weight band ranges (8, 40–44). Indeed, we used a 5-kg body weight band range to adjust tuberculosis treatment; this diverged from current recommendations of a national tuberculosis program which use a 10-kg body weight band range. On the other hand, early initiation of potent and efficacious antiretroviral therapy according to recent guidelines (45) may have improved patients' health status.
Our study has a number of limitations. First, the sequential design of the study did not allow us to discriminate between a drug-drug interaction and improvement of the patients with initiation of antiretroviral therapy. However, in the 4-week time period between the two sampling periods, the average patient weight remained unchanged, indicating that a sequence effect is unlikely. No sample was drawn 24 h after drug intake; therefore, the AUCs over the 12-h dosing interval (AUC0–12) were calculated assuming that the AUC0–12 will be very close to the AUCs over the 24-h dosing interval (AUC0–24), as the half-lives of both rifampin and isoniazid are very short (median half-lives, 1.59 h and 4.71 h, respectively). In addition, antituberculosis therapy was not superimposable in all patients, as for the second pharmacokinetic period, nine patients (seven on nevirapine and two on efavirenz) remained on the four-drug antituberculosis regimen while the others were on the rifampin-isoniazid dual regimen. Important changes in rifampin and isoniazid pharmacokinetics due to ethambutol discontinuation are unlikely, as this drug is eliminated mostly unchanged through the kidneys. However, a slight effect of pyrazinamide cannot be ruled out, given that a single-dose study found a 12% decrease in rifampin AUCs when rifampin was administered with pyrazinamide (46). More recently, studies have demonstrated higher rifampin AUCs during the continuation phase than during the initiation phase during tuberculosis and HIV therapy (20.6 versus 15.7 mg · h/liter, respectively), although half-lives remained unchanged (2.6 versus 2.9 h), suggesting that increased absorption is related to improvement in the general health condition rather than to drug-drug interactions (47). Second, the included patients were not genotyped for drug metabolism enzyme or transporter polymorphisms. The genetically polymorphic NAT2 gene is responsible for isoniazid metabolism (48), but disease progression in HIV infection and AIDS may alter expression of the NAT2 gene (49). Rifampin is a substrate and inhibitor of the uptake transporter OATP1B1 encoded by the SLCO1B1 gene, for which a genetic polymorphism has been demonstrated (50). Such genetic polymorphisms explain at least in part the large interindividual variability in the pharmacokinetic parameters of isoniazid and rifampin observed and as previously reported (20, 51) but are unlikely to interfere with drug-drug interactions. Third, only rifampin and isoniazid concentrations were measured, but they are the backbone of antituberculosis therapy of susceptible strains. Finally, no attempt was made to relate the measured concentrations to antituberculosis efficacy, which was defined only on the basis of the clinical response, especially for patients with smear-negative pulmonary and extrapulmonary tuberculosis. Indeed, this study was not designed to investigate drug exposure-effect relationships but sought to compare drug exposure on and off antiretroviral therapy; therefore, our sample size was limited. Even though rifampin and isoniazid concentrations were highly variable, peak serum drug concentrations remained well above the MICs for Mycobacterium tuberculosis (i.e., ≥0.25 mg/liter for rifampin and ≥0.05 mg/liter for isoniazid) in all patients, which could be the reason for good tuberculosis treatment outcomes despite advanced HIV infection.
In conclusion, our data show that neither the rifampin nor the isoniazid pharmacokinetic parameters were altered to a clinically significant extent when these drugs were combined with nevirapine or efavirenz, even though the lead-in dose of nevirapine was omitted. Although we were not able to relate drug exposure to clinical outcome, the high treatment success rate is reassuring.
ACKNOWLEDGMENTS
The ANRS 12146-CARINEMO clinical trial study group members were Ilesh V. Jani, Nádia Sitoe, Adolfo Vubil, Maria Nhadzombo, Fernando Sitoe, Delário Nhumaio, and Odete Bule (Instituto Nacional de Saúde, Mozambique), Rui Bastos and Elizabete Nunes (Hospital Central de Maputo, Mozambique), Paula Samo Gudo (National Tuberculosis Control Program, Mozambique), Josué Lima and Mie Okamura (International Center for AIDS Care and Treatment Programs, Mozambique), Laura Ciaffi, Agnès Sobry, Mariano Lugli, and Bruno Lab (Médecins Sans Frontières-Switzerland, Mozambique), Avertino Barreto (Mozambique National AIDS Service Organization, Mozambique), Christophe Michon (Regional Hospital, Annecy, France), Alexandra Calmy (Médecins Sans Frontières and Division of Infectious Diseases, Geneva University Hospital, Geneva, Switzerland), Alpha Diallo (French Research Agency for HIV/AIDS Pharmacovigilance Unit, Paris, France), and Christine Rouzioux (Paris-Descartes University, EA3620, Sorbonne Paris Cite, APHP, Necker Hospital, Paris, France).
We thank the patients who participated in this study, and we thank the staff of ANRS 12146-CARINEMO for supporting the study implementation, the Instituto Nacional de Saúde (INS), Mozambique, for testing biological samples, and Médecins Sans Frontières-Switzerland (MSF-CH) for providing unconditional logistical support. We also thank Claire Rekacewicz and Alpha Diallo from the ANRS, the Scientific Committee chaired by Alexandra Calmy, and the Independent Data Monitoring Committee chaired by Bernard Hirschel.
This work was supported by the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (project no. ANRS 12214).
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 24 March 2014
REFERENCES
- 1.World Health Organization. 2012. Global tuberculosis report 2012. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, Khan M, Pienaar J, El-Sadr W, Friedland G, Abdool Karim Q. 2010. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N. Engl. J. Med. 362:697–706. 10.1056/NEJMoa0905848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2010. Guidelines for treatment of tuberculosis, fourth edition World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/2010/9789241547833/en/index.html [Google Scholar]
- 4.Barry C, Waring J, Stapledon R, Konstantinos A, National Tuberculosis Advisory Committee, for the Communicable Diseases Network Australia 2012. Tuberculosis notifications in Australia, 2008 and 2009. Commun. Dis. Intell. Q. Rep. 36:82–94 [DOI] [PubMed] [Google Scholar]
- 5.Peloquin CA. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62:2169–2183. 10.2165/00003495-200262150-00001 [DOI] [PubMed] [Google Scholar]
- 6.Iseman MD. 2002. Tuberculosis therapy: past, present and future. Eur. Respir. J. Suppl. 36:87s–94s. 10.1183/09031936.02.00309102 [DOI] [PubMed] [Google Scholar]
- 7.Sanchez M, Bartholomay P, Arakaki-Sanchez D, Enarson D, Bissell K, Barreira D, Harries A, Kritski A. 2012. Outcomes of TB treatment by HIV status in national recording systems in Brazil, 2003–2008. PLoS One 7:e33129. 10.1371/journal.pone.0033129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ismail I, Bulgiba A. 2013. Determinants of unsuccessful tuberculosis treatment outcomes in Malaysian HIV-infected patients. Prev. Med. 57(Suppl):S27–S30. 10.1016/j.ypmed.2012.12.023 [DOI] [PubMed] [Google Scholar]
- 9.Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, Reingold AL, Kenyon TA, Moeti TL, Tappero JW. 2009. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin. Infect. Dis. 48:1685–1694. 10.1086/599040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. 2006. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob. Agents Chemother. 50:1170–1177. 10.1128/AAC.50.4.1170-1177.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurumurthy P, Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Padmapriyadarsini C, Swaminathan S, Bhagavathy S, Venkatesan P, Sekar L, Mahilmaran A, Ravichandran N, Paramesh P. 2004. Decreased bioavailability of rifampin and other antituberculosis drugs in patients with advanced human immunodeficiency virus disease. Antimicrob. Agents Chemother. 48:4473–4475. 10.1128/AAC.48.11.4473-4475.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahai J, Gallicano K, Swick L, Tailor S, Garber G, Seguin I, Oliveras L, Walker S, Rachlis A, Cameron DW. 1997. Reduced plasma concentrations of antituberculosis drugs in patients with HIV infection. Ann. Intern. Med. 127:289–293. 10.7326/0003-4819-127-4-199708150-00006 [DOI] [PubMed] [Google Scholar]
- 13.McIlleron H, Rustomjee R, Vahedi M, Mthiyane T, Denti P, Connolly C, Rida W, Pym A, Smith PJ, Onyebujoh PC. 2012. Reduced antituberculosis drug concentrations in HIV-infected patients who are men or have low weight: implications for international dosing guidelines. Antimicrob. Agents Chemother. 56:3232–3238. 10.1128/AAC.05526-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. 2010. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach: 2010 revision. World Health Organization, Geneva, Switzerland: http://www.who.int/hiv/pub/arv/adult2010/en/index.html [PubMed] [Google Scholar]
- 15.Rakhmanina NY, van den Anker JN. 2010. Efavirenz in the therapy of HIV infection. Expert Opin. Drug Metab. Toxicol. 6:95–103. 10.1517/17425250903483207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Back D, Gibbons S, Khoo S. 2003. Pharmacokinetic drug interactions with nevirapine. J. Acquir. Immune Defic. Syndr. 34(Suppl 1):S8–S14 [DOI] [PubMed] [Google Scholar]
- 17.Dickinson JM, Aber VR, Mitchison DA. 1977. Bactericidal activity of streptomycin, isoniazid, rifampin, ethambutol, and pyrazinamide alone and in combination against Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 116:627–635 [DOI] [PubMed] [Google Scholar]
- 18.Loos U, Musch E, Jensen JC, Mikus G, Schwabe HK, Eichelbaum M. 1985. Pharmacokinetics of oral and intravenous rifampicin during chronic administration. Klin. Wochenschr. 63:1205–1211. 10.1007/BF01733779 [DOI] [PubMed] [Google Scholar]
- 19.Tirona RG, Leake BF, Wolkoff AW, Kim RB. 2003. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J. Pharmacol. Exp. Ther. 304:223–228. 10.1124/jpet.102.043026 [DOI] [PubMed] [Google Scholar]
- 20.Wilkins JJ, Langdon G, McIlleron H, Pillai G, Smith PJ, Simonsson US. 2011. Variability in the population pharmacokinetics of isoniazid in South African tuberculosis patients. Br. J. Clin. Pharmacol. 72:51–62. 10.1111/j.1365-2125.2011.03940.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, Chiang CH, Chang FY, Lee SD. 2003. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology 37:924–930. 10.1053/jhep.2003.50144 [DOI] [PubMed] [Google Scholar]
- 22.Gurumurthy P, Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Padmapriyadarsini C, Swaminathan S, Venkatesan P, Sekar L, Kumar S, Krishnarajasekhar OR, Paramesh P. 2004. Malabsorption of rifampin and isoniazid in HIV-infected patients with and without tuberculosis. Clin. Infect. Dis. 38:280–283. 10.1086/380795 [DOI] [PubMed] [Google Scholar]
- 23.Holland DP, Hamilton CD, Weintrob AC, Engemann JJ, Fortenberry ER, Peloquin CA, Stout JE. 2009. Therapeutic drug monitoring of antimycobacterial drugs in patients with both tuberculosis and advanced human immunodeficiency virus infection. Pharmacotherapy 29:503–510. 10.1592/phco.29.5.503 [DOI] [PubMed] [Google Scholar]
- 24.van Crevel R, Alisjahbana B, de Lange WC, Borst F, Danusantoso H, van der Meer JW, Burger D, Nelwan RH. 2002. Low plasma concentrations of rifampicin in tuberculosis patients in Indonesia. Int. J. Tuberc. Lung Dis. 6:497–502. 10.0000/09640569513002 [DOI] [PubMed] [Google Scholar]
- 25.Bonnet M, Bhatt N, Baudin E, Silva C, Michon C, Taburet AM, Ciaffi L, Sobry A, Bastos R, Nunes E, Rouzioux C, Jani I, Calmy A. 2013. Nevirapine versus efavirenz for patients coinfected with HIV and tuberculosis: a randomised noninferiority trial. Lancet Infect. Dis. 13:303–312. 10.1016/S1473-3099(13)70007-0 [DOI] [PubMed] [Google Scholar]
- 26.Bhatt NB, Barau C, Amin A, Baudin E, Meggi B, Silva C, Furlan V, Grinzstejn B, Barrail-Tran A, Bonnet M, Taburet AM, ANRS 12146-CARINEMO Study Group 2012. Pharmacokinetics of rifampicin and isoniazid in TB-HIV co-infected patients on nevirapine- or efavirenz-based antiretroviral treatment (ANRS12214), p S82, abstr OP-119-15 Abstr. 43rd Conference of the International Union Against Tuberculosis and Lung Diseases (IUATLD) [Google Scholar]
- 27.Aparicio I, Bello MA, Callejon M, Guiraum A. 2006. Simultaneous determination of rifampicin and sulbactam in mouse plasma by high-performance liquid chromatography. Biomed. Chromatogr. 20:748–752. 10.1002/bmc.591 [DOI] [PubMed] [Google Scholar]
- 28.Miscoria G, Leneveu A, Walle C, Roux A. 1988. Application of a method of analysis using high performance liquid chromatography of isoniazid and acetylisoniazid to determine the phenotype of acetylation. Ann. Biol. Clin. 46:734–740 (In French.) [PubMed] [Google Scholar]
- 29.Titier K, Lagrange F, Pehourcq F, Edno-Mcheik L, Moore N, Molimard M. 2002. High-performance liquid chromatographic method for the simultaneous determination of the six HIV-protease inhibitors and two nonnucleoside reverse transcriptase inhibitors in human plasma. Ther. Drug Monit. 24:417–424. 10.1097/00007691-200206000-00015 [DOI] [PubMed] [Google Scholar]
- 30.Berning SE, Huitt GA, Iseman MD, Peloquin CA. 1992. Malabsorption of antituberculosis medications by a patient with AIDS. N. Engl. J. Med. 327:1817–1818. 10.1056/NEJM199212173272514 [DOI] [PubMed] [Google Scholar]
- 31.Gumbo T, Louie A, Deziel MR, Liu W, Parsons LM, Salfinger M, Drusano GL. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 51:3781–3788. 10.1128/AAC.01533-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gumbo T, Louie A, Liu W, Brown D, Ambrose PG, Bhavnani SM, Drusano GL. 2007. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob. Agents Chemother. 51:2329–2336. 10.1128/AAC.00185-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perlman DC, Segal Y, Rosenkranz S, Rainey PM, Remmel RP, Salomon N, Hafner R, Peloquin CA. 2005. The clinical pharmacokinetics of rifampin and ethambutol in HIV-infected persons with tuberculosis. Clin. Infect. Dis. 41:1638–1647. 10.1086/498024 [DOI] [PubMed] [Google Scholar]
- 34.Peloquin CA, Namdar R, Dodge AA, Nix DE. 1999. Pharmacokinetics of isoniazid under fasting conditions, with food, and with antacids. Int. J. Tuberc. Lung Dis. 3:703–710 [PubMed] [Google Scholar]
- 35.Peloquin CA, Namdar R, Singleton MD, Nix DE. 1999. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest 115:12–18. 10.1378/chest.115.1.12 [DOI] [PubMed] [Google Scholar]
- 36.Tostmann A, Mtabho CM, Semvua HH, van den Boogaard J, Kibiki GS, Boeree MJ, Aarnoutse RE. 2013. Pharmacokinetics of first-line tuberculosis drugs in Tanzanian patients. Antimicrob. Agents Chemother. 57:3208–3213. 10.1128/AAC.02599-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peloquin CA. 1998. Serum concentrations of the antimycobacterial drugs. Chest 113:1154–1155. 10.1378/chest.113.5.1154 [DOI] [PubMed] [Google Scholar]
- 38.Barroso EC, Pinheiro VG, Facanha MC, Carvalho MR, Moura ME, Campelo CL, Peloquin CA, Guerrant RL, Lima AA. 2009. Serum concentrations of rifampin, isoniazid, and intestinal absorption, permeability in patients with multidrug resistant tuberculosis. Am. J. Trop. Med. Hyg. 81:322–329 [PubMed] [Google Scholar]
- 39.Koegelenberg CF, Nortje A, Lalla U, Enslin A, Irusen EM, Rosenkranz B, Seifart HI, Bolliger CT. 2013. The pharmacokinetics of enteral antituberculosis drugs in patients requiring intensive care. S. Afr. Med. J. 103:394–398. 10.7196/samj.6344 [DOI] [PubMed] [Google Scholar]
- 40.Singla R, Srinath D, Gupta S, Visalakshi P, Khalid UK, Singla N, Gupta UA, Bharty SK, Behera D. 2009. Risk factors for new pulmonary tuberculosis patients failing treatment under the Revised National Tuberculosis Control Programme, India. Int. J. Tuberc. Lung Dis. 13:521–526 [PubMed] [Google Scholar]
- 41.Tabarsi P, Chitsaz E, Moradi A, Baghaei P, Farnia P, Marjani M, Shamai M, Amiri M, Nikaein S, Mansouri D, Masjedi M, Altice F. 2012. Treatment outcome, mortality and their predictors among HIV-associated tuberculosis patients. Int. J. STD AIDS 23:e1–e4. 10.1258/ijsa.2009.009093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaheen A, Najmi MH, Saeed W, Farooqi ZU. 2012. Pharmacokinetics of standard dose regimens of rifampicin in patients with pulmonary tuberculosis in Pakistan. Scand. J. Infect. Dis. 44:459–464. 10.3109/00365548.2011.647832 [DOI] [PubMed] [Google Scholar]
- 43.Hoa NB, Lauritsen JM, Rieder HL. 2013. Changes in body weight and tuberculosis treatment outcome in Viet Nam. Int. J. Tuberc. Lung Dis. 17:61–66. 10.5588/ijtld.12.0369 [DOI] [PubMed] [Google Scholar]
- 44.Lienhardt C, Cook SV, Burgos M, Yorke-Edwards V, Rigouts L, Anyo G, Kim SJ, Jindani A, Enarson DA, Nunn AJ. 2011. Efficacy and safety of a 4-drug fixed-dose combination regimen compared with separate drugs for treatment of pulmonary tuberculosis: the Study C randomized controlled trial. JAMA 305:1415–1423. 10.1001/jama.2011.436 [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization. 2013. World Health Organization Consolidated guidelines on the use of antiretriviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization, Geneva, Switzerland: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html [PubMed] [Google Scholar]
- 46.Jain A, Mehta VL, Kulshrestha S. 1993. Effect of pyrazinamide on rifampicin kinetics in patients with tuberculosis. Tuber. Lung Dis. 74:87–90. 10.1016/0962-8479(93)90032-S [DOI] [PubMed] [Google Scholar]
- 47.Saleri N, Dembele SM, Villani P, Carvalho AC, Cusato M, Bonkoungou V, Nacanabo R, Kouanda S, Comelli M, Regazzi M, Matteelli A. 2012. Systemic exposure to rifampicin in patients with tuberculosis and advanced HIV disease during highly active antiretroviral therapy in Burkina Faso. J. Antimicrob. Chemother. 67:469–472. 10.1093/jac/dkr445 [DOI] [PubMed] [Google Scholar]
- 48.Shenfield GM. 2004. Genetic polymorphisms, drug metabolism and drug concentrations. Clin. Biochem. Rev. 25:203–206 [PMC free article] [PubMed] [Google Scholar]
- 49.O'Neil WM, Gilfix BM, DiGirolamo A, Tsoukas CM, Wainer IW. 1997. N-acetylation among HIV-positive patients and patients with AIDS: when is fast, fast and slow, slow? Clin. Pharmacol. Ther. 62:261–271. 10.1016/S0009-9236(97)90028-X [DOI] [PubMed] [Google Scholar]
- 50.Weiner M, Peloquin C, Burman W, Luo CC, Engle M, Prihoda TJ, Mac Kenzie WR, Bliven-Sizemore E, Johnson JL, Vernon A. 2010. Effects of tuberculosis, race, and human gene SLCO1B1 polymorphisms on rifampin concentrations. Antimicrob. Agents Chemother. 54:4192–4200. 10.1128/AAC.00353-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilkins JJ, Savic RM, Karlsson MO, Langdon G, McIlleron H, Pillai G, Smith PJ, Simonsson US. 2008. Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption. Antimicrob. Agents Chemother. 52:2138–2148. 10.1128/AAC.00461-07 [DOI] [PMC free article] [PubMed] [Google Scholar]