Abstract
The objective of the present study was to assess the safety and tolerability of cefazolin therapy among patients with methicillin-sensitive Gram-positive bacterial infections who develop non-IgE-mediated hypersensitivity reactions (HSRs) to nafcillin. In this retrospective cohort analysis of the Outpatient Parenteral Antimicrobial Therapy program at the Massachusetts General Hospital from 2007 through 2013, we identified patients switched from nafcillin to cefazolin after an immune-mediated HSR. We reviewed patient demographics, details about the original HSR, and outcomes after the switch to cefazolin therapy. HSRs were classified by reaction type and likely mechanism. There were 467 patients treated with nafcillin, of which 60 (12.8%) were switched to cefazolin during their prescribed course. Of the 60 patients who transitioned to cefazolin, 17 (28.3%) were switched because of non-IgE-mediated HSRs. HSRs included maculopapular rash (n = 10), immune-mediated nephritis (n = 3), isolated eosinophilia (n = 2), immune-mediated hepatitis (n = 1), and a serum sickness-like reaction (n = 1). All but one patient (94.1%) who switched to cefazolin tolerated the drug with resolution of the HSR and completed their therapy with cefazolin. No patient experienced worsening of their rash or progressive organ dysfunction. With appropriate monitoring, therapy with cefazolin after non-IgE-mediated HSRs to nafcillin appears to be safe.
INTRODUCTION
Penicillins are the most common cause of drug allergy (1, 2). As a class, penicillins can cause any of the four types of Gell and Coombs (2) immunologic hypersensitivity reactions (HSRs) (Table 1), although IgE-mediated reactions (type I) and maculopapular rashes (type IV) are most commonly encountered (1–4). Less frequent penicillin-related HSRs include serum sickness-like reactions; organ-specific reactions, such as an immune-mediated nephritis or hepatitis; and severe cutaneous adverse reactions (SCARs), such as Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), and drug rash eosinophilia and systemic symptoms (DRESS) syndrome (non-IgE-mediated HSRs or type II to IV HSRs) (1, 5–10).
TABLE 1.
Types and classifications of HSRsb
| HSR classification | Reaction type | Immunologic mechanism | HSR name/symptom |
|---|---|---|---|
| I | IgE-mediated, immediate-type hypersensitivity | Antigen exposure causes IgE-mediated activation of mast cells and basophils with release of allergic mediators (histamine, prostaglandins, and leukotrienes). | Anaphylaxis, angioedema, bronchospasm, urticaria (hives) |
| II | Antibody dependent | An antigen or hapten on the cell binds to antibody (IgG), leading to cell or tissue injury. | Hemolytic anemia, thrombocytopenia, neutropenia |
| III | Immune complex disease | Antigen-antibody (IgG) complexes cause damage by complement activation and/or recruitment of neutrophils. | Serum sickness |
| IV | Cell-mediated, delayed-type hypersensitivity | Antigen exposure activates T cells, which then mediates tissue injury. | Maculopapular rash, organ-specific reactions,a SJS/TEN, DRESS syndrome |
Organ-specific reactions include acute interstitial nephritis, an immune-mediated nephritis, and immune-mediated hepatitis.
For most infections with methicillin-sensitive Gram-positive organisms, a penicillin or a cephalosporin, such as cefazolin, is considered first-line therapy (11–13). β-Lactam antibiotics are more efficacious in vitro, particularly when used in settings of high bacterial burden (11, 14). In vivo, for methicillin-sensitive Staphylococcus aureus (MSSA) bacteremia, treatment with oxacillin, nafcillin, or cefazolin is associated with lower mortality and decreased rates of treatment failure compared to those associated with vancomycin (15–19). However, β-lactam antibiotics, including nafcillin, have been implicated in non-IgE-mediated HSRs, including maculopapular rash, acute interstitial nephritis (AIN), and immune-mediated hepatitis (20–22). Because nafcillin and cefazolin are first-line agents for the treatment of infections due to methicillin-susceptible Gram-positive organisms, an important clinical question is whether patients who develop non-IgE-mediated HSRs to nafcillin can be successfully treated with cefazolin.
The antigenic determinants of type I, IgE-mediated penicillin hypersensitivity are known. Cross-reactivity with cephalosporins occurs when allergic individuals create drug-specific IgE that cross-reacts with cephalosporin antigens (1, 23). Allergy to cephalosporins can be directed at the β-lactam ring or a side chain, known as an R group (Fig. 1) (23–25). The rate of IgE-mediated cross-reactivity between penicillin and cephalosporins is approximately 2 to 4%, and the result of such cross-reactivity has been reported to include anaphylaxis (1, 23–25). Cefazolin has a β-lactam ring similar to that of nafcillin, but its R group is dissimilar to the R groups of the penicillins and other commonly used cephalosporins (23–25).
FIG 1.
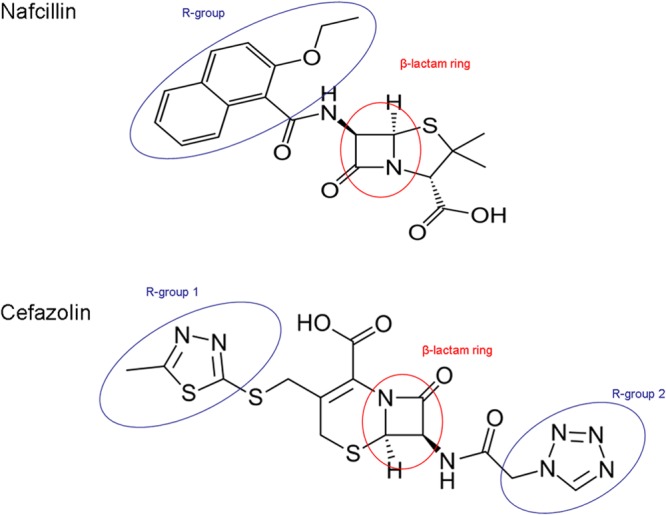
Chemical structures of nafcillin and cefazolin demonstrating the chemical structure similarity of their β-lactam rings and the chemical structure dissimilarity of their side chains (R groups).
Despite the large body of knowledge regarding IgE-mediated hypersensitivity to penicillins and cross-reactivity with cephalosporins, much less is known about the cross-reactivity between penicillins and cephalosporins in non-IgE-mediated HSRs (1, 26). When a patient develops a non-IgE-mediated HSR, clinicians may, on the basis of clinical experience and consideration of the severity of the reaction, avoid all possibly cross-reactive antibiotics (1, 9). Allergy practice guidelines provide little guidance on this topic, due to the hypothetical cross-reactivity (1, 23–25, 27).
We present 17 patients who developed non-IgE-mediated (type III and IV) HSRs to nafcillin who were subsequently switched to cefazolin. To the best of our knowledge, this is the first published study addressing β-lactam cross-reactivity in non-IgE-mediated HSRs.
MATERIALS AND METHODS
OPAT program database.
Inpatients discharged from the Massachusetts General Hospital (MGH) who had at least 2 weeks of remaining parenteral therapy and who were seen in consultation with the infectious disease service during their admission were prospectively enrolled in the Outpatient Parenteral Antibiotic Therapy (OPAT) program. The OPAT program database is a clinically driven database maintained by a single administrative assistant. The following data elements are collected prospectively: the patient's name, medical record number, dates of treatment, site and/or type of infection, culture results, and antimicrobials administered, including subsequent medications, if treatment was changed during therapy. At the start and end of a course of therapy for each patient, the OPAT program medical director (S.B.N.), a board-certified practicing infectious diseases specialist, reviews all medical charts and laboratory reports, verifies database entries, and notes potential adverse drug events.
Research methods and analysis.
Patients switched from nafcillin to cefazolin after their index hospitalization from January 2007 through October 2013 were identified from the OPAT program database. Comprehensive electronic chart review was performed for all patients to identify the clinical circumstances leading to the therapy change. If the switch from nafcillin to cefazolin was in response to a possible immune-mediated HSR, paper-based OPAT program charts were additionally reviewed. HSRs were classified by type and likely mechanism (2) independently by two specialists from the Division of Rheumatology, Allergy, and Immunology (A.B., K.G.B.). Diagnoses were made on the basis of internal criteria that are consistent with literature definitions and previously reported cases (Table 2) (1, 9, 20–22, 28–34). For patients who were not switched directly from nafcillin to cefazolin, their infectious disease physician was contacted to discuss the antimicrobial management decision. In order to assess whether patients who were switched to cefazolin were different from those who were switched to vancomycin, we also reviewed patients in the OPAT program database who were switched from nafcillin to vancomycin. We determined demographic data and the rate of hospital readmission for patients who were switched from nafcillin to vancomycin because of possible immune-mediated HSRs. The research protocol was approved by the Partners Human Research Committee and the institutional review boards of the Brigham and Women's/Faulkner Hospital, Massachusetts General Hospital, McLean Hospital, and North Shore Medical Center.
TABLE 2.
Definitions used for diagnosing non-IgE-mediated HSRsa
| HSR | Diagnostic criteria |
|---|---|
| Maculopapular rash | Delayed onset of rash while on nafcillin treatment with physical exam findings of lesion morphology specifying macules, papules, macular, papular, maculopapular, and morbilliform, with renal and liver function remaining unchanged |
| Immune-mediated nephritis | Acute change in renal function defined as either a 50% change in creatinine or a rise in creatinine of >0.5 mg/dl coinciding temporally with nafcillin treatment with either one of the following: (i) rash or (ii) AEC of >500/ml |
| Serum sickness-like reaction | At least three of the following classic features, beginning after use of nafcillin and subsiding when the drug is eliminated from the body: (i) rash, (ii) fever, (iii) malaise, (iv) polyarthralgias or polyarthritis, (v) lymphadenopathy, or (vi) low complement |
| Eosinophilia | AEC of >1,000/ml in the absence of other symptoms coinciding temporally with nafcillin treatment, with renal and kidney function remaining unchanged |
| Immune-mediated hepatitis | Increased liver function tests with an AEC of >500/ml coinciding temporally with nafcillin treatment and with renal function remaining unchanged |
Descriptive data are displayed as numbers and frequencies. Medians with interquartile ranges (IQRs) describe the number of days of nafcillin treatment prior to the onset of the HSR and the duration of hospitalization for patients hospitalized for their HSRs. For HSRs present for more than one patient, we report either means with standard deviations or medians with interquartile ranges to describe laboratory data, including absolute eosinophil count (AEC), the change in the glomerular filtration rate (GFR), and creatinine clearance (CLCR). Fisher's exact test was used to compare hospital readmission frequency between patients switched to vancomycin and patients switched to cefazolin after a possible immune-mediated HSR to nafcillin.
RESULTS
Between January 2007 and October 2013, 3,011 patients were enrolled in MGH's OPAT program. Of these, 467 (15.5%) were discharged from the hospital on nafcillin. A total of 157 (33.6%) patients prescribed nafcillin were switched to a different antibiotic prior to the anticipated completion of parenteral therapy. One hundred fifty (95.5%) were switched to other parenteral agents, including vancomycin (n = 77, 50.6%), cefazolin (n = 60, 39.5%), clindamycin (n = 7, 4.6%), and daptomycin (n = 6, 3.9%). Seven (4.5%) were switched to oral linezolid.
Among the 60 patients identified who switched from nafcillin to cefazolin, the treatment for 39 patients (65.0%) was changed for reasons other than immune-mediated HSRs (neutropenia, volume overload, renal dysfunction without other features of hypersensitivity, patient preference, and/or logistics). Twenty-one patients (35%) were switched to cefazolin for possible immune-mediated HSRs. Of these, 17 (28.3%) met our study criteria (Table 2). These included the delayed, type IV HSRs maculopapular rash (n = 10), immune-mediated nephritis (n = 3), isolated eosinophilia (n = 2), and immune-mediated hepatitis (n = 1) and the type III HSR serum sickness (n = 1) (Table 3).
TABLE 3.
Patients with non-IgE-mediated HSRs to nafcillin switched to cefazolina
| Patient no. | Age (yr)/gender | Infectious diagnosis | Pathogen | HSR | Duration of therapy prior to HSR onset (days) | Level of care required for HSR management | Therapy outcome |
|---|---|---|---|---|---|---|---|
| 1 | 40/F | Prosthetic joint infection | MSSA | Maculopapular rash | 9 | Hospitalization | Completed cefazolin |
| 2 | 25/M | Epidural abscess | MSSA | Maculopapular rash | 22 | Hospitalization | Completed cefazolin |
| 3 | 50/M | Epidural abscess | MSSA | Maculopapular rash | 26 | Office visit | Completed cefazolin |
| 4 | 50/F | Septic arthritis | Group A streptococcus | Maculopapular rash | 8 | Hospitalization | Completed cefazolin |
| 5 | 80/F | Hardware infection | MSSA | Maculopapular rash | 13 | Office visit | Completed cefazolin |
| 6 | 41/M | Septic arthritis | MSSA | Maculopapular rash | 24 | Hospitalization | Completed cefazolin |
| 7 | 69/F | Endocarditis | CoNS | Maculopapular rash | 9 | Office visit | Completed cefazolin |
| 8 | 64/F | Osteomyelitis | MSSA | Maculopapular rash | 21 | Office visit | Completed cefazolin |
| 9 | 26/M | Osteomyelitis | MSSA | Maculopapular rash | 9 | Hospitalization | Completed vancomycin |
| 10 | 25/M | Osteomyelitis | CoNS | Maculopapular rash | 7 | ED visit | Completed cefazolin |
| 11 | 72/M | Endocarditis | MSSA | Immune-mediated nephritis | 19 | Hospitalization | Completed cefazolin |
| 12 | 48/F | Septic thrombophlebitis | MSSA | Immune-mediated nephritis | 13 | Hospitalization | Completed cefazolin |
| 13 | 73/M | Hardware infection | MSSA | Immune-mediated nephritis | 15 | ED visit | Completed cefazolin |
| 14 | 54/M | Bacteremia | MSSA | Eosinophilia | 20 | Hospitalization | Completed cefazolin |
| 15 | 82/F | Osteomyelitis | MSSA | Eosinophilia | 36 | Office visit | Completed cefazolin |
| 16 | 79/F | Hardware infection | MSSA | Serum sickness | 19 | SNF visit | Completed cefazolin |
| 17 | 74/F | Osteomyelitis | MSSA | Immune-mediated hepatitis | 20 | Hospitalization | Completed cefazolin |
Data are for 17 patients. Abbreviations: M, male; F, female; MSSA, methicillin-sensitive Staphylococcus aureus; CoNS, coagulase-negative Staphylococcus; ED, emergency department; SNF, skilled nursing facility.
Patients were most commonly being treated for orthopedic infections (n = 11, 64.7%), including osteomyelitis, hardware infections, septic arthritis, and prosthetic joint infections. Other infections included endocarditis (n = 2, 11.8%), epidural abscess (n = 2, 11.8%), septic thrombophlebitis (n = 1, 5.9%), and bacteremia (n = 1, 5.9%). The most commonly treated organism was MSSA (n = 14, 82.4%). The median duration of therapy prior to the onset of HSR was 20 days (IQR, 9 to 21 days). The average time until HSR onset trended toward later in the treatment course for patients with eosinophilia (28 days) and earlier for patients with maculopapular rash (15 days).
Clinical characteristics of HSRs.
Of the 10 patients with nafcillin-induced maculopapular rash, the median peak AEC was 360/ml (IQR, 280 to 613/ml; range, 7 to 1,470/ml). Three of these patients also had fever, and one patient had transaminase levels that increased from a baseline aspartate aminotransferase (AST) level of 14 mg/ml and a baseline alanine aminotransferase (ALT) level of 9 mg/ml to a peak AST level of 125 mg/ml and a peak ALT level of 228 mg/ml. Of the three patients with immune-mediated nephritis, two presented with rash. The median peak AEC was 550/ml (IQR, 500 to 880/ml; range, 450 to 990/ml). The average change in GFR was 41%, and the mean peak CLCR was 1.88 mg/dl. For the two patients with isolated eosinophilia, the mean AEC was 1,165/ml. The patient with immune-mediated hepatitis had an AEC of 1,356/ml as well as transaminase levels that increased from a baseline AST level of 12 mg/ml and a baseline ALT level of 12 mg/ml to a peak AST level of 362 mg/ml and a peak ALT level of 90 mg/ml. The patient with serum sickness had complained of itching, rash, joint pain, and joint swelling, with laboratory evaluation revealing an AEC of 559/ml, a C3 level of 55 mg/dl (normal range, 86 to 184 mg/dl), and a C4 level of 15 mg/dl (normal range, 16 to 38 mg/dl).
Management of HSRs.
Nafcillin was the only likely causative agent in 16/17 (94.1%) patients, with discontinuation of the nafcillin alone leading to resolution of the HSR. Because clinicians attributed the HSRs to nafcillin, other home medications and prescribed antimicrobial agents thought less likely to have caused the HSR were continued. In one patient with maculopapular rash, the HSR was attributed to either nafcillin or nonsteroidal anti-inflammatory agents (NSAIDs), and both medications were simultaneously discontinued with resolution of the HSR.
HSR treatment required hospitalization in 9 (52.9%) patients, office evaluation in 5 (29.4%), and emergency department evaluation in 2 (11.8%) patients (Table 3). One patient was managed by phone call only but was a resident in a skilled nursing facility and was seen by that facility's medical staff. Among patients readmitted, the median duration of the hospitalization was 6 days (IQR, 2 to 6 days). Management for all patients included supportive care and discontinuation of nafcillin.
Sixteen (94.1%) of the patients who were switched to cefazolin after immune-mediated HSR from nafcillin tolerated cefazolin with resolution of the HSR and completed their therapy with cefazolin (Table 3). One patient (59%) did not complete the predetermined cefazolin course. This patient had a maculopapular rash after beginning both nafcillin and NSAIDs that was resolving, though he developed a urticarial eruption while on cefazolin. When he was readmitted for management, dermatology and allergy/immunology consultations thought that his urticaria was more likely from new intravenous opiates rather than the cefazolin, but his antimicrobial therapy was switched to vancomycin for the completion of his treatment. No patients had worsening of their rash, organ dysfunction, or progression into SJS/TEN or DRESS syndrome with cefazolin treatment.
Fourteen patients (82.4%) were immediately switched from nafcillin to cefazolin. Three patients (17.6%), all with maculopapular rash, were given an alternative antibiotic (either vancomycin or daptomycin) for 7 to 9 days prior to changing to cefazolin. The infectious disease physician caring for these three patients did this in order to give the rash time to improve while the patient was receiving a structurally dissimilar drug prior to starting a potentially cross-reactive drug.
Patients with possible immune-mediated HSRs to nafcillin switched to cefazolin compared to those switched to vancomycin.
Among patients whose treatment was changed to cefazolin (n = 60), 21 patients (mean age, 55 years; 50% male) were switched for possible immune-mediated HSRs. Of these patients with HSRs, 11 (52.4%) were readmitted for management of the HSR and treatment change. Among the 17 patients with HSRs meeting our criteria, 9 (52.9%) were readmitted for management of their HSR. Among patients who were switched to vancomycin (n = 77), 26 patients (mean age, 58 years; 48% male) were switched for possible immune-mediated HSRs. Of these, 15 (57.7%) were readmitted for management of their HSR and treatment change. The rate of readmission for HSR management was not different between those switched to cefazolin and those switched to vancomycin (P > 0.5).
DISCUSSION
Our findings are novel and support the suggestion that cefazolin may be safe for use in patients who develop non-IgE-mediated HSRs to nafcillin. An overwhelming majority (94.1%) of patients with immune-mediated HSRs to nafcillin tolerated cefazolin with resolution of the HSR. None of the patients experienced any worsening of their rash, organ dysfunction, SJS/TEN, or DRESS syndrome.
Given the limited published data and absence of informative guidelines, clinicians confronted with non-IgE-mediated HSRs may choose to avoid all possibly cross-reactive antibiotics, potentially contributing to suboptimal infectious outcomes (1, 9). While maculopapular rash is unlikely to be dangerous, in practice, clinicians switch therapy because of patient comfort and limited information on the risks of worsening rash, organ involvement, or progression to DRESS syndrome or SJS/TEN (1, 7, 35, 36). Because immune-mediated nephritis, including AIN, can have serious sequelae, including hemodialysis-dependent renal failure, clinicians who suspect AIN may choose to continue therapy with an alternative, unrelated agent. However, there are no documented cases of cross-reactivity in patients with AIN (9). In isolated cases of eosinophilia, while the culprit agent does not necessarily need to be changed, an AEC greater than 1,000/ml may prompt a medication change, given that eosinophilia can be a marker of organ-specific HSRs and SCARs (1, 22, 28, 36, 37). Nafcillin-induced immune-mediated hepatitis has been reported, though there are no prior reports of subsequent treatment with an alternative β-lactam drug (21). Lastly, though a patient with a serum sickness-like reaction could be treated through this type of HSR, if necessary, the symptoms are uncomfortable and patients may not want to be switched to a drug that has the potential to cause similar discomfort.
With delayed, type IV HSRs, despite removal of the offending drug, the HSR can temporarily worsen (1, 36). Clinically, this would result in a patient with worsening of the rash, eosinophilia, or organ injury, despite removal of the offending drug. For that reason, three patients with maculopapular rash were not switched directly from nafcillin to cefazolin but, rather, were given an unrelated alternative, such as vancomycin, for a period of 7 to 9 days prior to initiating treatment with cefazolin. In these cases, the patient's infectious disease physician chose to start a drug that is completely structurally dissimilar to nafcillin to allow improvement of the HSR prior to starting cefazolin. To the best of our knowledge, this strategy has not been previously described but may be the optimal method for treating these patients and evaluating cefazolin and nafcillin prospectively in non-IgE-mediated HSRs.
Infections with MSSA are common, with approximately 20% of OPAT program patients and an estimated 400,000 patients in the United States in 2007 alone (38–41). First-line treatment for MSSA infections includes nafcillin, oxacillin, or cefazolin, with the final decision to use a specific treatment based on provider preference and individual patient-specific factors. While cefazolin has efficacy similar to that of nafcillin in treating patients with MSSA bacteremia (13, 15–19, 42, 43) and orthopedic infections (44, 45), nafcillin is still preferred by some clinicians for treatment of deep infections or those with a high bacterial load because of MSSA's potential for producing a β-lactamase that degrades cefazolin faster than semisynthetic penicillins (43, 46, 47). Additionally, cefazolin is not used for central nervous system infections, since it does not easily cross the blood-brain barrier (11, 48). For these reasons, nafcillin may be more commonly used than cefazolin at MGH, despite cefazolin's lower cost and more convenient dosing schedule (13, 49, 50). There are many infections caused by organisms other than methicillin-sensitive staphylococci for which a penicillin is the drug of choice, including those due to streptococci, ampicillin-sensitive Enterococcus, Peptostreptococcus, Actinomyces, Propionibacterium acnes, Listeria monocytogenes, and Treponema pallidum (51–56). Therefore, many patients experience non-IgE-mediated HSRs to nafcillin and other penicillins, and the understanding of the cross-reactivity between penicillins and cephalosporins is necessary for the optimal treatment of these patients.
Our OPAT program patients whose therapy was changed from nafcillin for any reason most commonly received vancomycin, although for methicillin-sensitive infections, cefazolin is associated with superior outcomes (13, 15, 16, 45). In addition to efficacy concerns, the use of vancomycin is associated with higher toxicity than the use of β-lactam antibiotics, (45, 49, 57, 58), and its overuse can lead to greater antimicrobial resistance (59, 60). Our experience suggests that with appropriate monitoring, these patients could have been safely switched to cefazolin.
This study has several limitations, including its small size, its retrospective design, inclusion of patients healthy enough for outpatient therapy, and a lack of clinical data or diagnostic testing to make a firm diagnosis of an HSR. While our sample size was small, there are no prior published studies documenting any patient tolerance of cefazolin after any non-IgE-mediated HSRs to nafcillin. The study is retrospective and subject to selection bias. It is possible that patients with more severe type II to IV HSRs with high mortality rates were not prescribed cefazolin (8, 36, 61). While we showed that patients switched to vancomycin after possible immune-mediated HSRs to nafcillin were not different from those switched to cefazolin with respect to hospital readmission rates for their HSR, without a grading scale for HSR, we could not otherwise confirm that these individuals were not different in other ways. Our patients with immune-mediated nephritis did have relatively mild changes in GFR, so our findings may not be applicable to patients with severe HSRs. At the onset, these patients were healthy enough to be treated for their infections as outpatients, so we cannot know if the results would be similar for those being treated for their infections as inpatients. Lastly, to make a definitive diagnosis of HSRs, additional data, such as data from evaluation of skin biopsy specimens (maculopapular rash), renal biopsy specimen, urine eosinophils, urinary sediment (immune-mediated nephritis/AIN) (22, 28), or liver biopsy specimens (immune-mediated hepatitis), would be necessary. Because these data were unavailable, we relied on a clinical diagnosis supported by patient history, physical exam, and available laboratory data. However, these data are akin to what would be available to most clinicians in practice, and two allergists reviewed all clinical data and were in complete agreement with the definitions of the patient reactions. Finally, studying delayed HSRs is challenging since there are no diagnostic tests that can be used to precisely attribute the HSR to nafcillin. Although validated skin testing exists for type I, IgE-mediated reactions to penicillin (1, 4, 62, 63), there is currently no standardized testing for non-IgE-mediated HSRs. Emerging data support the use of patch testing or delayed readings of intradermal immediate hypersensitivity skin testing in the diagnostic evaluations of some cutaneous reactions, including maculopapular rash, though the predictive value of these tests is unknown (64–67). However, despite the lack of confirmatory testing for nafcillin hypersensitivity, removal of nafcillin alone in all but one patient led to HSR resolution, which supports the presumption that these were, indeed, nafcillin-induced HSRs.
On the basis of our analysis of a large OPAT program database, cefazolin treatment appears to be well tolerated in patients with a history of non-IgE-mediated HSRs to nafcillin. Future research is needed to determine the actual clinical cross-reactivity between nafcillin and cefazolin in non-IgE-mediated HSRs prospectively and by type of HSR. This will impact care not only for patients on extended antimicrobial therapy who develop an HSR and require additional therapy but also for all patients with histories of a non-IgE-mediated HSR who require new antibiotic therapy.
Footnotes
Published ahead of print 17 March 2014
REFERENCES
- 1.Solensky R, Khan D. 2010. Drug allergy: an updated practice parameter. Ann. Allergy Asthma Immunol. 105:259–273. 10.1016/j.anai.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 2.Gell PGH, Coombs R. (ed) 1963. The classification of allergic reactions underlying disease, p 317–37 In Clinical aspects of immunology, 1st ed Blackwell, Oxford, England [Google Scholar]
- 3.Pichler WJ. 2013. Drug allergy: classification and clinical features. In Basow DS. (ed), UpToDate. Wolters Kluwer Health, Philadelphia, PA [Google Scholar]
- 4.Weiss ME, Adkinson NF. 1988. Immediate hypersensitivity reactions to penicillin and related antibiotics. Clin. Allergy 18:515–540. 10.1111/j.1365-2222.1988.tb02904.x [DOI] [PubMed] [Google Scholar]
- 5.Maraqa NF, Gomez MM, Rathore MH, Alvarez AM. 2002. Higher occurrence of hepatotoxicity and rash in patients treated with oxacillin, compared with those treated with nafcillin and other commonly used antimicrobials. Clin. Infect. Dis. 34:50–54. 10.1086/338047 [DOI] [PubMed] [Google Scholar]
- 6.Orbak Z, Sepetcigil O, Karakelleoglu C, Gulses S. 2010. Penicillin V-induced drug rash with eosinophilia and systemic symptoms. West Indian Med. J. 59:722–725 [PubMed] [Google Scholar]
- 7.Harr T, French LE. 2010. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet. J. Rare Dis. 5:39. 10.1186/1750-1172-5-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrandiz-Pulido C, Garcia-Patos V. 2013. A review of causes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Arch. Dis. Child. 98:998–1003. 10.1136/archdischild-2013-303718 [DOI] [PubMed] [Google Scholar]
- 9.Whitman CB, Wike MJ. 2012. Possible case of nafcillin-induced acute interstitial nephritis. Am. J. Health Syst. Pharm. 69:1049–1053. 10.2146/ajhp110357 [DOI] [PubMed] [Google Scholar]
- 10.Presti ME, Janney CG, Neuschwander-Tetri BA. 1996. Nafcillin-associated hepatotoxicity. Report of a case and review of the literature. Dig. Dis. Sci. 41:180–184 [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:285–292. 10.1093/cid/cir034 [DOI] [PubMed] [Google Scholar]
- 12.Federman DD, Nabel EG. (ed). 2014. Infectious diseases: the clinician's guide to diagnosis, treatment, and prevention. Decker Publishing Inc., Hamilton, Ontario, Canada [Google Scholar]
- 13.Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Török ME, Walker S, Wertheim HF, Wilson P, Llewelyn MJ. 2011. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect. Dis. 11:208–222. 10.1016/S1473-3099(10)70285-1 [DOI] [PubMed] [Google Scholar]
- 14.LaPlante KL, Rybak MJ. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665–4672. 10.1128/AAC.48.12.4665-4672.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stryjewski ME, Szcech LA, Benjamin DK, Inrig JK, JKanafani ZA, Engemann JJ, Chu VH, Joyce MJ, Reller B, Corey R, Fowler VG. 2007. Use of vancomycin of first generation cephalosporins for the treatment of hemodialysis dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin. Infect. Dis. 44:190–196. 10.1086/510386 [DOI] [PubMed] [Google Scholar]
- 16.Schweizer ML, Furuno J, Harris AD, Johnson K, Shardell MD, McGregor JC, Thom KA, Cosgrove SE, Sakoulas G, Perencevich EN. 2011. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect. Dis. 11:279. 10.1186/1471-2334-11-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González C, Rubio M, Romero-Vivas J, González M, Picazo JJ. 1999. Bacteremic pneumonia due to Staphylococcus aureus: a comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms. Clin. Infect. Dis. 29:1171–1177. 10.1086/313440 [DOI] [PubMed] [Google Scholar]
- 18.Chan KE, Warren HS, Thadhani RI, Steele D, Hymes JL, Maddux FW, Hakim RM. 2012. Prevalence and outcomes of antimicrobial treatment for Staphylococcus aureus bacteremia in outpatients with ESRD. J. Am. Soc. Nephrol. 23:1551–1559. 10.1681/ASN.2012010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang FY, Peacock JEJ, Musher DM, Triplett P, MacDonald BB, Mylotte JM, O'Donnell A, Wagener MM, Yu VL. 2003. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine 82:333–339. 10.1097/01.md.0000091184.93122.09 [DOI] [PubMed] [Google Scholar]
- 20.Hoppes T, Prikis M, Segal A. 2007. Four cases of nafcillin-associated acute interstitial nephritis in one institution. Nat. Clin. Pract. Nephrol. 3:456–461. 10.1038/ncpneph0561 [DOI] [PubMed] [Google Scholar]
- 21.Alam MB, Kadoura A, Sathaiah M. 2012. A fatal case of nafcillin-induced hepatotoxicity: a case report and the literature review. Case Rep. Med. 2012:953714. 10.1155/2012/953714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker RJ, Pusey CD. 2004. The changing profile of acute tubulointerstitial nephritis. Nephrol. Dial. Transplant. 19:8–11. 10.1093/ndt/gfg464 [DOI] [PubMed] [Google Scholar]
- 23.Pichichero ME. 2005. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 115:1048–1057. 10.1542/peds.2004-1276 [DOI] [PubMed] [Google Scholar]
- 24.Pichichero ME. 2006. Cephalosporins can be prescribed safely for penicillin-allergic patients. J. Fam. Pract. 55:106–112 [PubMed] [Google Scholar]
- 25.Pichichero ME. 2007. Use of selected cephalosporins in penicillin-allergic patients: a paradigm shift. Diagn. Microbiol. Infect. Dis. 57:13S–18S. 10.1016/j.diagmicrobio.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 26.Patriarca G, D'Ambrosio C, Schiavino D, Larocca LM, Nucera E, Milani A. 1999. Clinical usefulness of patch and challenge tests in the diagnosis of cell-mediated allergy to betalactams. Ann. Allergy Asthma Immunol. 83:256–266 [DOI] [PubMed] [Google Scholar]
- 27.DePestel DD, Benninger MS, Danzinger L, LaPlante KL, May C, Luskin A, Pichichero M, Hadley JA. 2008. Cephalosporin use in treatment of patients with penicillin allergies. J. Am. Pharm. Assoc. 48:530–540. 10.1331/JAPhA.2008.07006 [DOI] [PubMed] [Google Scholar]
- 28.Baldwin DS, Levine BB, McCluskey RT, Gallo GR. 1968. Renal failure and interstitial nephritis due to penicillin and methicillin. N. Engl. J. Med. 279:1245–1252. 10.1056/NEJM196812052792302 [DOI] [PubMed] [Google Scholar]
- 29.Mazuryk H, Kastenberg D, Rubin R, Muñoz SJ. 1993. Cholestatic hepatitis associated with the use of nafcillin. Am. J. Gastroenterol. 88:1960–1962 [PubMed] [Google Scholar]
- 30.Bronnimann M, Yawalkar N. 2005. Histopathology of drug-induced exanthems: is there a role in diagnosis of drug allergy? Curr. Opin. Allergy Clin. Immunol. 5:317–321. 10.1097/01.all.0000173787.65777.77 [DOI] [PubMed] [Google Scholar]
- 31.Bircher AJ. 2013. Exanthematous (morbilliform) drug eruption. In Basow DS. (ed), UpToDate. Wolters Kluwer Health, Philadelphia, PA [Google Scholar]
- 32.Praga M, Appel GB. 2013. Clinical manifestations and diagnosis of acute interstitial nephritis. In Basow DS. (ed), UpToDate. Wolters Kluwer Health, Philadelphia, PA [Google Scholar]
- 33.Weller P. 2013. Approach to the patient with eosinophilia. In Basow DS. (ed), UpToDate. Wolters Kluwer Health, Philadelphia, PA [Google Scholar]
- 34.Wener MH. 2013. Serum sickness and serum sickness-like reactions. In Basow DS. (ed), UpToDate. Wolters Kluwer Health, Philadelphia, PA [Google Scholar]
- 35.Bouvresse S, Valeyrie-Allanore L, Ortonne N, Konstantinou MP, Kardaun SH, Bagot M, Wolkenstein P, Roujeau JC. 2012. Toxic epidermal necrolysis, DRESS, AGEP: do overlap cases exist? Orphanet. J. Rare Dis. 7:72. 10.1186/1750-1172-7-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bocquet H, Bagot M, Roujeau JC. 1996. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin. Cutan. Med. Surg. 15:250–257. 10.1016/S1085-5629(96)80038-1 [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Yang X, Li M. 2013. Peripheral blood eosinophil counts predict the prognosis of drug eruptions. J. Investig. Allergol. Clin. Immunol. 23:248–255 [PubMed] [Google Scholar]
- 38.Paladino J, Poretz D. 2010. Outpatient parenteral antimicrobial therapy today. Clin. Infect. Dis. 51(Suppl 2):S198–S208. 10.1086/653520 [DOI] [PubMed] [Google Scholar]
- 39.Chapman ALN, Dixon S, Andrews D, Lillie PJ, Bazaz R, Patchett JD. 2009. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. J. Antimicrob. Chemother. 64:1316–1324. 10.1093/jac/dkp343 [DOI] [PubMed] [Google Scholar]
- 40.Barr DA, Semple L, Seaton RA. 2012. Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital-based practice: a retrospective cohort study describing experience and evolution over 10 years. Int. J. Antimicrob. Agents 39:407–413. 10.1016/j.ijantimicag.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 41.Cox AM, Malani PN, Wiseman SW, Kauffman CA. 2007. Home intravenous antimicrobial infusion therapy: a viable option in older adults. J. Am. Geriatr. Soc. 55:645–650. 10.1111/j.1532-5415.2007.01133.x [DOI] [PubMed] [Google Scholar]
- 42.Madhavan T, Quinn EL, Freimer E, Fisher EJ, Cox F, Burch K, Pohlod D. 1973. Clinical studies of cefazolin and comparison with other cephalosporins. Antimicrob. Agents Chemother. 4:525–531. 10.1128/AAC.4.5.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinn EL, Pohlod D, Madhavan T, Burch K, Fisher E, Cox F. 1973. Clinical experiences with cefazolin and other cephalosporins in bacterial endocarditis. J. Infect. Dis. 128(Suppl):S386–S389. 10.1093/infdis/128.Supplement_2.S386 [DOI] [PubMed] [Google Scholar]
- 44.Shuford JA, Piper KE, Hein M, Trampuz A, Steckelberg JM, Patel R. 2006. Lack of association of Staphylococcus aureus type A beta-lactamase with cefazolin combined with antimicrobial spacer placement prosthetic joint infection treatment failure. Diagn. Microbiol. Infect. Dis. 54:189–192. 10.1016/j.diagmicrobio.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 45.Wynn M, Dalovisio JR, Tice AD, Jiang X. 2005. Evaluation of the efficacy and safety of outpatient parenteral antimicrobial therapy for infections with methicillin-sensitive Staphylococcus aureus. South. Med. J. 98:590–595. 10.1097/01.SMJ.0000145300.28736.BB [DOI] [PubMed] [Google Scholar]
- 46.Nannini EC, Stryjewski ME, Singh KV, Bourgogne A, Rude TH, Corey GR, Fowler VG, Jr, Murray BE. 2009. Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphylococcus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob. Agents Chemother. 53:3437–3441. 10.1128/AAC.00317-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nannini EC, Stryjewski ME, Singh KV, Rude TH, Corey GR, Fowler VG, Jr, Murray BE. 2010. Determination of an inoculum effect with various cephalosporins among clinical isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 54:2206–2208. 10.1128/AAC.01325-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tice AD, Rehm SJ, Dalovisio JR, Bradley JS, Martinelli LP, Graham DR, Gainer B, Kunkel MJ, Yancey RW, Williams DN. 2004. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin. Infect. Dis. 38:1651–1672. 10.1086/420939 [DOI] [PubMed] [Google Scholar]
- 49.Faden D, Faden HS. 2009. The high rate of adverse drug events in children receiving prolonged outpatient parenteral antibiotic therapy for osteomyelitis. Pediatr. Infect. Dis. J. 28:539–541. 10.1097/INF.0b013e318193ef38 [DOI] [PubMed] [Google Scholar]
- 50.Dahlgren AF. 1997. Adverse drug reactions in home care patients receiving nafcillin or oxacillin. Am. J. Health Syst. Pharm. 54:1176–1179 [DOI] [PubMed] [Google Scholar]
- 51.Anonymous. 2004. Drugs for sexually transmitted infections. Treat. Guidel. Med. Lett. 2:67–74 [PubMed] [Google Scholar]
- 52.Smego RA, Foglia G. 1998. Actinomyces. Clin. Infect. Dis. 26:1255–1261. 10.1086/516337 [DOI] [PubMed] [Google Scholar]
- 53.Dodge RA, Daly JS, Davaro R, Glew RH. 1997. High-dose ampicillin plus streptomycin for treatment of a patient with severe infection due to multiresistant enterococci. Clin. Infect. Dis. 25:1269–1270. 10.1086/516977 [DOI] [PubMed] [Google Scholar]
- 54.Tally FP, Gorback SL. 1985. Therapy of mixed anaerobic-aerobic infections. Lessons from intra-abdominal sepsis. Am. J. Med. 78:145–153 [DOI] [PubMed] [Google Scholar]
- 55.Murphy EC, Frick IM. 2013. Gram-positive anaerobic cocci—commensals and opportunistic pathogens. FEMS Microbiol. Rev. 37:520–553. 10.1111/1574-6976.12005 [DOI] [PubMed] [Google Scholar]
- 56.Cohen SE, Klausner JD, Engelman J, Philip S. 2013. Syphilis in the modern era: an update for physicians. Infect. Dis. Clin. North Am. 27:705–722. 10.1016/j.idc.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 57.Norton K, Ingram PR, Heath CH, Manning L. 2014. Risk factors for nephrotoxicity in patients receiving outpatient continuous infusions of vancomycin in an Australian tertiary hospital. J. Antimicrob. Chemother. 69:805–808. 10.1093/jac/dkt402 [DOI] [PubMed] [Google Scholar]
- 58.Rybak M, Lomaestro B, Rotschaefer JC, Moellering RJ, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 66:82–98. 10.2146/ajhp080434 [DOI] [PubMed] [Google Scholar]
- 59.Tacconelli E, Cataldo MA. 2008. Vancomycin-resistant enterococci (VRE): transmission and control. Int. J. Antimicrob. Agents 31:99–106. 10.1016/j.ijantimicag.2007.08.026 [DOI] [PubMed] [Google Scholar]
- 60.Hospital Infection Control Practices Advisory Committee (HICPAC). 1995. Recommendations for preventing the spread of vancomycin resistance. Infect. Control Hosp. Epidemiol. 16:105–113 [DOI] [PubMed] [Google Scholar]
- 61.Tan SK, Tay YK. 2012. Profile and pattern of Stevens-Johnson syndrome and toxic epidermal necrolysis in a general hospital in Singapore: treatment outcomes. Acta Derm. Venereol. 92:62–66. 10.2340/00015555-1169 [DOI] [PubMed] [Google Scholar]
- 62.Blanca M, Torres MJ, Garcia JJ, Romano A, Mayorga C, de Ramon E, Vega JM, Miranda A, Juarez C. 1999. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J. Allergy Clin. Immunol. 103(Pt 1):918–924. 10.1016/S0091-6749(99)70439-2 [DOI] [PubMed] [Google Scholar]
- 63.Macy E, Ngor E. 2013. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine. J. Allergy Clin. Immunol. Pract. 1:258–263. 10.1016/j.jaip.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 64.Barbaud A. 2009. Skin testing in delayed reactions to drugs. Immunol. Allergy Clin. North Am. 29:517–535. 10.1016/j.iac.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 65.Wolkenstein P, Chosidow O, Fléchet ML, Robbiola O, Paul M, Dumé L, Revuz J, Roujeau JC. 1996. Patch testing in severe cutaneous adverse drug reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis. Contact Dermatitis 35:234–236. 10.1111/j.1600-0536.1996.tb02364.x [DOI] [PubMed] [Google Scholar]
- 66.Romano A, Blanca M, Torres MJ, Bircher A, Aberer W, Brockow K, Pichler WJ, Demoly P, ENDA, EAACI 2004. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy 59:1153–1160. 10.1111/j.1398-9995.2004.00678.x [DOI] [PubMed] [Google Scholar]
- 67.Romano A, Quaratino D, Di Fonso M, Papa G, Venuti A, Gasbarrini G. 1999. A diagnostic protocol for evaluating nonimmediate reactions to aminopenicillins. J. Allergy Clin. Immunol. 103:1186–1190. 10.1016/S0091-6749(99)70197-1 [DOI] [PubMed] [Google Scholar]


