Abstract
Cefquinome is a cephalosporin with broad-spectrum antibacterial activity, including activity against Staphylococcus aureus. The objective of our study was to examine the in vivo activity of cefquinome against S. aureus strains by using a neutropenic mouse thigh infection model. Cefquinome kinetics and protein binding in infected neutropenic mice were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS). In vivo postantibiotic effects (PAEs) were determined after a dose of 100 mg/kg of body weight in mice infected with S. aureus strain ATCC 29213. The animals were treated by subcutaneous injection of cefquinome at doses of 2.5 to 320 mg/kg of body weight per day divided into 1, 2, 3, 6, or 12 doses over 24 h. Cefquinome exhibited time-dependent killing and produced in vivo PAEs at 2.9 h. The percentage of time that serum concentrations were above the MIC (%T>MIC) was the pharmacokinetic-pharmacodynamic (PK-PD) index that best described the efficacy of cefquinome. Subsequently, we employed a similar dosing strategy by using increasing total cefquinome doses that increased 4-fold and were administered every 4 h to treat animals infected with six additional S. aureus isolates. A sigmoid maximum effect (Emax) model was used to estimate the magnitudes of the ratios of the %T that the free-drug serum concentration exceeded the MIC (%T>fMIC) associated with net bacterial stasis, a 0.5-log10 CFU reduction from baseline, and a 1-log10 CFU reduction from baseline; the respective values were 30.28 to 36.84%, 34.38 to 46.70%, and 43.50 to 54.01%. The clear PAEs and potent bactericidal activity make cefquinome an attractive option for the treatment of infections caused by S. aureus.
INTRODUCTION
Staphylococcus aureus, especially methicillin-resistant S. aureus (MRSA), is an important human pathogen that is also an emerging concern in veterinary medicine and animal agriculture. All known mammalian species, including common laboratory rodent and rabbit species, are susceptible to colonization with S. aureus. MRSA has been shown to have a prevalence of 39% and 24.9% in pigs in the Netherlands (1) and Canada (2), respectively, and 28% in veal calves in the Netherlands (3). A Canadian study identified MRSA in 6.3% of ground pork and 5.6% of ground beef but in 32% and 45% of positive pork and beef samples, respectively (4). Some samples of commercially sold meat products in Japan were also found to harbor MRSA strains (5). Because of its ability to colonize a wide range of species, S. aureus can be readily transmitted from one species to another, including from humans to animals and vice versa. Moreover, a majority of S. aureus isolates are resistant to various antimicrobial agents (6), and thus, there are limited antimicrobial options for treatment; accordingly, there is a growing need for more potent antimicrobials to attack these resistant pathogens. Cefquinome (formerly HR 111V) is a fourth-generation cephalosporin developed solely for veterinary use. It displays antimicrobial activities in vitro and in vivo against a broad spectrum of Gram-positive and Gram-negative bacterial species, including methicillin-resistant and methicillin-susceptible S. aureus, and it is considered to be highly stable against β-lactamases encoded by chromosomes and genes on plasmids (7–9).
Previous studies with β-lactams have established that the percentage of a 24-h dosing interval in which the free-drug concentration exceeds the MIC (%T>fMIC) is the pharmacokinetic-pharmacodynamic (PK-PD) parameter best linked with efficacy (10–16). The goal of our studies was to characterize the PK-PD parameter that is predictive of the efficacy of cefquinome against susceptible and resistant S. aureus strains in a neutropenic murine infection model. Furthermore, PK-PD studies were also conducted to elucidate the PK-PD index associated with cefquinome efficacy and to identify the magnitude of the PK-PD parameter that is most predictive of efficacy. It is proposed that these findings might be used with MIC90 data for specific species to provide a rational approach to designing dosage schedules that optimize efficacy with respect to bacteriological and clinical cures.
MATERIALS AND METHODS
Antimicrobial agents.
Cefquinome was obtained from commercial sources as a powder (purity, 83.097%). Test solutions of the antimicrobial agent were freshly prepared prior to use.
Bacterial strains.
Six S. aureus strains isolated from chicken carcasses and pork and S. aureus ATCC strain 29213 were evaluated in this study. The strains include two MRSA strains (CR30 and BKED) and five methicillin-susceptible S. aureus strains (ATCC 29213, SAP00, SAP01, AP02, and AP04). The organisms were grown, subcultured, and quantified in Mueller-Hinton broth and Mueller-Hinton agar (both from Guangdong Huankai Microbial Sci. & Tech. Co., Ltd., Guangzhou, China).
In vitro susceptibility studies.
The MIC values were determined by a broth microdilution assay according to National Committee for Clinical Laboratory Standards (NCCLS) reference methods (17). These determinations were performed in duplicate on three separate occasions. The final results were expressed as the geometric means of these results.
Animals.
Six-week-old specific-pathogen-free female imprinting control region (ICR) mice (Laboratory Animal Center of Zhejiang University, China) weighing between 24 and 27 g were used for all studies. All animal studies were approved by the animal research committees of South China Agriculture University.
The animals were maintained in accordance with the American Association for Accreditation of Laboratory Animal Care criteria (18).
Neutropenic mouse thigh model.
The mice were rendered neutropenic (polymorphonuclear leukocyte count, <100/mm3) by injecting cyclophosphamide (Puboxin Biotechnology Co., Ltd., Beijing, China) intraperitoneally at 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) prior to experimental infection (19). The mice were infected by an intramuscular injection of 0.1 ml of inoculum in each thigh (four thighs per group per time point). Broth cultures of bacteria were grown to logarithmic phase overnight to an absorbance at 580 nm of 0.3 (UV-2550 spectrophotometer; Shimadzu, Kyoto, Japan). After a 1:10 dilution in fresh Mueller-Hinton broth, the bacterial counts of the inocula ranged from 106 to 107 CFU/ml.
Cefquinome was administered subcutaneously at various time points beginning 2 h after infection. At specified time points, the animals were sacrificed by CO2 asphyxiation. After the mice were sacrificed, the thighs were immediately removed and homogenized in 10 ml of 0.9% sterile iced saline. Serial 10-fold dilutions of the homogenized material were plated on Mueller-Hinton agar plates for CFU determination. Each symbol represents the data from two mice (four thighs).
PK parameters.
The single-dose PK parameters of cefquinome were determined in individual infected neutropenic mice following the administration of 10, 40, 160, and 320 mg/kg administered in 0.2-ml volumes by subcutaneous injection. Blood samples were obtained by retro-orbital puncture from each of three mice at the following time points: 0.08, 0.17, 0.25, 0.5, 0.75, 1, 2, 3, and 4 h after dosing. The samples were then centrifuged for 5 min at 10,000 × g, and the serum was removed. Cefquinome concentrations in the serum were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The lower limit of detection was 0.005 μg/ml. The intraday variation was <6%. Protein binding in the sera of the neutropenic infected mice was analyzed using ultrafiltration methods (12). The degree of binding was measured using cefquinome concentrations of 10 and 100 μg/ml.
Pharmacokinetic constants, including elimination half-life, area under the concentration-time curve (AUC), and maximum concentration of drug in serum (Cmax), were calculated by using a one-compartment model with first-order absorption and first-order elimination. For treatment doses for which no kinetics were determined, the PK parameters were extrapolated from the values obtained in the PK studies described above.
In vivo postantibiotic effect.
Infection in neutropenic mice was produced as described above. Two hours after infection with a standard strain of S. aureus (ATCC 29213), single subcutaneous doses of 100 mg/kg cefquinome were administered to ∼60 to 70% of the mice in the experiment. The remaining mice served as untreated controls. Control growth was determined at six sampling times (two mice per time point) over 12 h (at 0, 2, 4, 6, 8, and 12 h). The treated groups (two mice per time point) were sampled eight times over 24 h (at 1, 2, 3, 4, 6, 8, 12, and 24 h). The postantibiotic effect (PAE) was calculated using the following equation: PAE = T − C, where C is the time for the growth of 1-log10 CFU/thigh in control animals and T is the time for the growth of 1-log10 CFU/thigh in treated animals after the free-drug levels in serum had fallen below the MIC.
PD parameter determination.
The neutropenic mice were infected with the standard S. aureus strain ATCC 29213 2 h prior to the start of therapy. Twenty-five dosing regimens were chosen to determine the impact of dose level and dosing interval on cefquinome efficacy. These 25 regimens comprised five total dose levels (2.5, 10, 40, 160, and 320 mg/kg/24 h). The five total dose levels were fractionated by using five dosing intervals (every 2, 4, 8, 12, and 24 h). Groups of two mice were treated with each dosing regimen. The drug was administered subcutaneously in 0.2-ml volumes. The mice were sacrificed after 24 h of therapy, and the thighs were immediately removed and processed for CFU determination, as described above. The untreated control mice were sacrificed just before treatment and after 24 h.
PD parameter magnitude determination.
Studies similar to those described above, using 4-fold increasing total cefquinome doses administered every 4 h, were utilized to treat animals infected with six additional strains of S. aureus (CR30, BKED, SAP00, SAP01, AP02, and AP04). The total daily dose of cefquinome varied from 2.5 to 320 mg/kg. Groups of two mice were again used for each dosing regimen. At the end of study, the mice were euthanized and the thighs were immediately processed for CFU determination.
Data analysis.
The results of these studies were analyzed by using the sigmoid maximum effect (Emax) PD model derived from the Hill equation, E = (Emax × CN)/(EC50N + CN), where E is the effect, or in this case the log change in CFU per thigh, comparing treated mice and untreated controls after the 24-h period of the study; Emax is the maximum effect; C is the PK-PD parameter being examined (e.g., percent time that serum concentrations were above the MIC [%T>MIC], 24-h AUC/MIC ratio, or the Cmax/MIC ratio); EC50 is the C value required to achieve 50% of the Emax; and N is a sigmoidicity factor that controls the steepness of the curve. Nonlinear regression analysis was used to determine which PK-PD index best correlated with the CFU/thigh at 24 h. The coefficient of determination, or R2, was used to estimate the variance that might be due to regression with each of the PK-PD indices.
The best model for each data set was established by using the Akaike information criterion (20).
RESULTS
In vitro susceptibility testing.
The MICs of cefquinome are shown in Table 1. The MICs for the posttreatment isolates recovered from the mouse thighs did not change after exposure to cefquinome therapy.
TABLE 1.
In vitro antibacterial activities of cefquinome against S. aureus
| S. aureus strain | MIC (μg/ml) |
|---|---|
| MSSA | |
| ATCC 29213 | 0.25 |
| SAP00 | 0.5 |
| SAP01 | 0.5 |
| AP02 | 0.5 |
| AP04 | 0.5 |
| MRSA | |
| BKED | 0.5 |
| CR30 | 0.5 |
Pharmacokinetics.
The time course of the mean serum cefquinome concentrations in infected neutropenic mice following single subcutaneous doses of 10, 40, 160, and 320 mg/kg are shown in Fig. 1 and Table 2. The decline in the concentrations was best described using a one-compartment model with first-order absorption. The kinetics at these doses were linear for both Cmax and AUC. The half-life varied from 0.35 to 0.37 h. Protein binding values at 10 and 100 μg/ml were <8%, with a mean of 7.4%.
FIG 1.
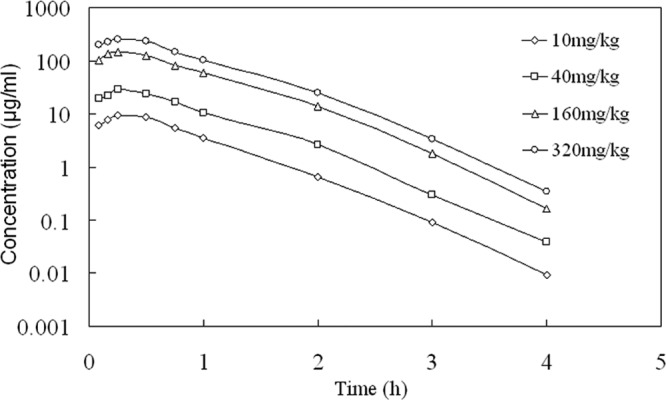
Serum cefquinome concentrations over time in infected neutropenic mice after a single subcutaneous dose.
TABLE 2.
Serum PK parameters of cefquinome following a single subcutaneous injection in infected neutropenic mice
| Cefquinome dose (mg/kg of body wt) | Cmaxa (mg/liter) | Tmaxb (h) | AUCc (mg h · liter−1) | t1/2d (h) |
|---|---|---|---|---|
| 10 | 9.57 | 0.31 | 8.84 | 0.35 |
| 40 | 28.39 | 0.30 | 26.75 | 0.37 |
| 160 | 152.21 | 0.30 | 141.67 | 0.36 |
| 320 | 276.67 | 0.30 | 257.10 | 0.37 |
Cmax, maximum concentration of drug in serum.
Tmax, time to maximum concentration of drug in serum.
AUC, area under the concentration-time curve.
t1/2, half-life.
In vivo PAE.
When single doses of 100 mg/kg were given, the mice exhibited 106.21 CFU of S. aureus per thigh. The time course of the antimicrobial activity of cefquinome against S. aureus ATCC 29213 is shown in Fig. 2. The growth of the organism in the thighs of the untreated control mice increased 1.87 ± 0.12 log10 CFU/thigh (mean ± standard deviation) over 12 h. The growth of 1-log10 CFU/thigh in untreated animals occurred within 5.3 h in S. aureus-infected animals. Based on the above pharmacokinetics extrapolation, the dose of cefquinome produced free-drug serum concentrations that exceeded the MIC of S. aureus ATCC 29213 for 3.6 h. The dose reduced the number of bacteria by 0.9 ± 0.03 log10 CFU/thigh. However, regrowth did not start immediately after the drug levels fell below the MIC of S. aureus ATCC 29213. The duration of the in vivo PAE was 2.9 h for cefquinome against S. aureus.
FIG 2.
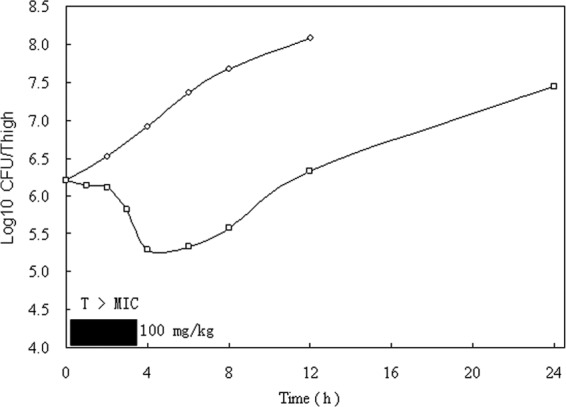
In vivo PAE of cefquinome at doses of 100 mg/kg against S. aureus in neutropenic mice. Each symbol represents the mean values from data from two mice (four thighs). The width of the solid bar represents the duration of time that free drug levels remained above the MIC of the infecting pathogen.
Correlation of PK and PD parameters with efficacy.
At the start of therapy, mice had 7.19 log10 CFU/thigh of S. aureus. The relationships between the antibacterial effects and each of the PD parameters for cefquinome with the number of CFU of S. aureus ATCC 29213 remaining in the thigh after 24 h of treatment are shown in Fig. 3. The %T>fMIC was the parameter that most strongly correlated with efficacy (R2 = 86%), whereas correlation with the other parameters was not nearly as strong (R2 = 57% for the fCmax/MIC ratio and only 17% for the 24-h fAUC/MIC ratio for the drug). In this study with S. aureus, even the highest dose levels did not achieve a net bacteriostatic effect with intervals of every 24 h. The above studies indicate that treatment efficacy was dependent upon the dosing intervals studied and suggest that efficacy was driven by the %T>fMIC index.
FIG 3.
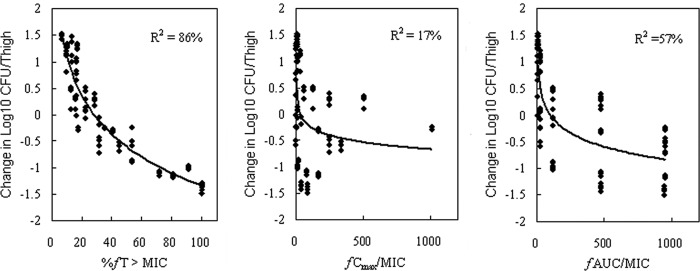
Relationships between %T>fMIC, fCmax/MIC, and fAUC/MIC and the change in the log10 number of CFU/thigh of S. aureus ATCC 29213. The lines represent the best model fits of the data. R2 is the correlation coefficient.
Magnitudes of PK and PD parameters determining efficacy against multiple strains.
The dose-response relationships with 4-h dosing of cefquinome for multiple strains of S. aureus were evaluated using a sigmoid Emax model. The calculated 4-h doses for the percentage of time that free-drug serum concentrations were above the MIC (%T>fMIC) with cefquinome required for the static, 0.5-log kill, and 1-log kill doses are listed in Table 3; the %T>fMICs ranged from 30.28 to 36.84% for stasis, 34.38 to 46.70% for a 0.5-log kill, and 43.50 to 54.01% for a 1-log kill.
TABLE 3.
Relationship between cefquinome %T>fMIC required to achieve various degrees of antibacterial effects in vivo
| Organism | Emax | %T>fMIC of cefquinome required to achieve: |
||
|---|---|---|---|---|
| Static effect | 0.5-log drop | 1-log drop | ||
| MSSA | ||||
| ATCC 29213 | 2.80 | 31.61 | 38.48 | 54.01 |
| SAP00 | 2.37 | 32.11 | 36.72 | 53.07 |
| SAP01 | 2.87 | 30.98 | 35.29 | 45.18 |
| AP02 | 2.07 | 36.84 | 46.70 | NAa |
| AP04 | 2.61 | 31.38 | 34.38 | 43.50 |
| MRSA | ||||
| BKED | 2.63 | 30.42 | 34.92 | 49.71 |
| CR30 | 2.18 | 30.28 | 37.60 | NA |
| Mean ± SD | 2.50 ± 0.31 | 31.95 ± 2.25 | 37.72 ± 4.23 | 49.09 ± 4.66 |
NA, not applicable.
DISCUSSION
Gram-positive pathogens, including MRSA, are a major cause of serious nosocomial infections worldwide (21). Today, S. aureus is recognized as the most commonly implicated organism (22, 23). Cefquinome possesses a broad antibacterial spectrum (against, e.g., S. aureus, including MRSA) and favorable pharmacokinetics; thus, it merits further investigation into its chemotherapeutic properties (7). The use of antimicrobial PK-PD analyses to identify the pharmacodynamic activity of antimicrobial agents through the integration of the PK properties, in vitro potency (MIC), and outcome is one approach that has proven helpful for the design of effective dosing regimens in humans and animals, as well as in the development of appropriate in vitro susceptibility breakpoints (15, 24, 25).
The present analyses characterized the in vivo pharmacokinetic-pharmacodynamic profile of cefquinome against S. aureus. As was determined for other β-lactams (10, 13, 15, 26–28), data from the current multiple-dosing regimen studies confirmed that %T>MIC was the major PK-PD index that best correlated with the in vivo antibacterial effects of cefquinome, given this pattern of antimicrobial activity. Furthermore, PAE analyses have exhibited minimal persistent effects with drugs from the β-lactam class against most bacteria. The one exception is with staphylococci, for which the duration of the PAE with cephalosporins induces a PAE against S. aureus but not against streptococci or Gram-negative bacilli (19, 29). In our animal study, exposure of the organisms to cefquinome led to a PAE of 2.9 h for S. aureus ATCC 29213. These data are very similar to those for the PAEs of other cephalosporins against staphylococci. Craig and Andes (26) found that the in vivo PAE of cephalosporins is 2 to 6 h in a neutropenic murine S. aureus thigh infection model. Vogelman et al. (19) found that the in vivo PAE of cefamandole (100 mg/kg) against S. aureus was 4.6 h.
In addition, our dose fractionation studies suggest that for any of the five doses studied, the lowest colony counts were encountered when the total dose was given as divided doses rather than as a single dose, and prior studies with several cephalosporins have also shown that treatment outcome is dependent on the dosing interval (prolonging the time that serum levels exceed the MIC of the infecting bacteria) and not the total amount of drug. The 24-h AUC/MIC ratios also showed a poor correlation with efficacy, despite the presence of a significant in vivo postantibiotic effect of cefquinome against S. aureus.
Numerous in vitro and in vivo models and clinical trials have suggested that the magnitude of the %T>MIC predictive of cephalosporin efficacy ranges from 25 to 70%, depending upon the defined therapeutic endpoint (15, 30, 31). An analysis of the results of the experiments with S. aureus revealed that a static effect is achieved with a %T>fMIC of 30.28 to 36.84%, which is similar to those for the activities of other cephalosporins against S. aureus. The cephalosporin %T>MIC target to produce a net bacteriostatic effect has been reported most commonly in the range of 30 to 40%, as long as free-drug levels are considered. Moreover, an analysis of the results of the experiments shows that a 0.5-log drop and a 1-log drop were not achieved until the %T>fMIC had reached 34.38 to 46.70% and 43.50 to 54.01%, respectively. These two values are higher than those for the activities of other cephalosporins against S. aureus. Previous reports found that cefotaxime, ceftriaxone, ceftazidime, PPI-0903 (ceftaroline), RWJ-54428, and cefpirome achieved a 1-log drop against S. aureus, with %T>MICs of 18% to 44% (11, 12, 31), and in the same model, the %T>MIC required for ceftobiprole to achieve a 2-log drop against various S. aureus strains, including some resistant strains, was only 24 to 39% of the dosing interval (32). Furthermore, the magnitudes of the PK and PD parameters predicting a bacteriostatic effect and killing of 0.5-log10 CFU/thigh over 24 h were relatively similar among the various strains, despite the presence of methicillin resistance in some strains.
In conclusion, the present study characterized the in vivo drug exposure response of cefquinome against S. aureus in a neutropenic murine model. The data for cefquinome in the mouse models suggest that animal dosage regimens should supply free-drug %T>MIC of cefquinome for 40 to 50% of the interval for S. aureus. However, it is apparent that optimal cefquinome PK–PD targets are not achieved in pigs, sheep, and cattle at the current recommended doses (1 to ∼2 mg/kg).These studies suggest that cefquinome, if used for the treatment of S. aureus infection with an MIC90 of 1.56 μg/ml (7), would benefit from larger (5 to ∼6 mg/kg) and more frequent doses (twice daily) than those commonly used in clinical practice.
ACKNOWLEDGMENT
This work is supported financially by the National Natural Science Foundation of China (grant 31372480) and the National Key Basic Research Program of China (grant 2013CB127200).
Footnotes
Published ahead of print 10 March 2014
REFERENCES
- 1.de Neeling AJ, van den Broek MJ, Spalburg EC, van Santen-Verheuvel MG, Dam-Deisz WD, Boshuizen HC, van de Giessen AW, van Duijkeren E, Huijsdens XW. 2007. High prevalence of methicillin resistant Staphylococcus aureus in pigs. Vet. Microbiol. 122:366–372. 10.1016/j.vetmic.2007.01.027 [DOI] [PubMed] [Google Scholar]
- 2.Khanna T, Friendship R, Dewey C, Weese JS. 2008. Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Vet. Microbiol. 128:298–303. 10.1016/j.vetmic.2007.10.006 [DOI] [PubMed] [Google Scholar]
- 3.Graveland H, Wagenaar JA, Heesterbeek H, Mevius D, van Duijkeren E, Heederik D. 2010. Methicillin resistant Staphylococcus aureus ST398 in veal calf farming: human MRSA carriage related with animal antimicrobial usage and farm hygiene. PLoS One 5:e10990. 10.1371/journal.pone.0010990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weese J, Avery BP, Rousseau J, Reid-Smith R. 2009. Methicillin-resistant Staphylococcus aureus contamination of retail meat: Canada. Abstr. 49A. ASM-ESCMID Conference on Methicillin-Resistant Staphylococci in Animals, London, United Kingdom, 22 to 25 September 2009 [Google Scholar]
- 5.Ogata K, Narimatsu H, Suzuki M, Higuchi W, Yamamoto T, Taniguchi H. 2012. Commercially distributed meat as a potential vehicle for community-acquired methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol. 78:2797–2802. 10.1128/AEM.07470-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2002. Public health dispatch: vancomycin-resistant Staphylococcus aureus—Pennsylvania. MMWR Morb. Mortal. Wkly. Rep. 51:902. [PubMed] [Google Scholar]
- 7.Limbert M, Isert D, Klesel N, Markus A, Seeger K, Seibert G, Schrinner E. 1991. Antibacterial activities in vitro and in vivo and pharmacokinetics of cefquinome (HR 111V), a new broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 35:14–19. 10.1128/AAC.35.1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maes A, Meyns T, Sustronck B, Maes D, De Backer P, Croubels S. 2007. Determination of cefquinome in pig plasma and bronchoalveolar lavage fluid by high-performance liquid chromatography combined with electrospray ionization mass spectrometry. J. Mass Spectrom. 42:657–663. 10.1002/jms.1199 [DOI] [PubMed] [Google Scholar]
- 9.Committee for Veterinary Medicinal Products (CVMP). 1995. Cefquinome: summary report 2. EMEA/MRL/005/95. The European Agency for the Evaluation of Medicinal Products, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500011877.pdf [Google Scholar]
- 10.Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig WA. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J. Infect. Dis. 158:831–847. 10.1093/infdis/158.4.831 [DOI] [PubMed] [Google Scholar]
- 11.Andes D, Craig WA. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376–1383. 10.1128/AAC.50.4.1376-1383.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith DC, Rodriguez D, Corcoran E, Dudley MN. 2008. Pharmacodynamics of RWJ-54428 against Staphylococcus aureus, Streptococcus pneumoniae, and Enterococcus faecalis in a neutropenic mouse thigh infection model. Antimicrob. Agents Chemother. 52:244–247. 10.1128/AAC.00776-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leggett JE, Fantin B, Ebert S, Totsuka K, Vogelman B, Calame W, Mattie H, Craig WA. 1989. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159:281–292. 10.1093/infdis/159.2.281 [DOI] [PubMed] [Google Scholar]
- 14.Novelli A, Fallani S, Cassetta MI, Conti S. 2000. Pharmacokinetics and pharmacodynamics of oral cephalosporins as critical factors in choice of antibiotics. Int. J. Antimicrob. Agents. 16:5. 10.1016/S0924-8579(00)00192-8 [DOI] [PubMed] [Google Scholar]
- 15.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10; quiz 11–12 [DOI] [PubMed] [Google Scholar]
- 16.Nicolau DP, Onyeji CO, Zhong M, Tessier PR, Banevicius MA, Nightingale CH. 2000. Pharmacodynamic assessment of cefprozil against Streptococcus pneumoniae: implications for breakpoint determinations. Antimicrob. Agents Chemother. 44:1291–1295. 10.1128/AAC.44.5.1291-1295.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NCCLS. 2004. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 18.Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 19.Vogelman B, Gudmundsson S, Turnidge J, Leggett J, Craig WA. 1988. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J. Infect. Dis. 157:287–298. 10.1093/infdis/157.2.287 [DOI] [PubMed] [Google Scholar]
- 20.Akaike AC. 1978. Posterior probabilities for choosing a regression model. Ann. Inst. Math. Sci. 30:9–14 [Google Scholar]
- 21.Woodford N, Livermore DM. 2009. Infections caused by Gram-positive bacteria: a review of the global challenge. J. Infect. 59(Suppl 1):S4–S16. 10.1016/S0163-4453(09)60003-7 [DOI] [PubMed] [Google Scholar]
- 22.Klein E, Smith DL, Laxminarayan R. 2007. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg. Infect. Dis. 13:1840–1846. 10.3201/eid1312.070629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shorr AF, Tabak YP, Gupta V, Johannes RS, Liu LZ, Kollef MH. 2006. Morbidity and cost burden of methicillin-resistant Staphylococcus aureus in early onset ventilator-associated pneumonia. Crit. Care 10:R97. 10.1186/cc4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig WA. 1993. Relevance of animal models for clinical treatment. Eur. J. Clin. Microbiol. Infect. Dis. 12:S55–S57. 10.1007/BF02389879 [DOI] [PubMed] [Google Scholar]
- 25.Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craig WA, Andes D. 1996. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr. Infect. Dis. J. 15:255–259. 10.1097/00006454-199603000-00015 [DOI] [PubMed] [Google Scholar]
- 27.Gustafsson I, Löwdin E, Odenholt I, Cars O. 2001. Pharmacokinetic and pharmacodynamic parameters for antimicrobial effects of cefotaxime and amoxicillin in an in vitro kinetic model. Antimicrob. Agents Chemother. 45:2436–2440. 10.1128/AAC.45.9.2436-2440.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs MR. 2001. Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin. Microbiol. Infect. 7:589–596. 10.1046/j.1198-743x.2001.00295.x [DOI] [PubMed] [Google Scholar]
- 29.Gudmundsson S, Vogelman B, Craig WA. 1986. The in-vivo postantibiotic effect of imipenem and other new antimicrobials. J. Antimicrob. Chemother. 18(Suppl E):67–73 [DOI] [PubMed] [Google Scholar]
- 30.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North Am. 17:479–501. 10.1016/S0891-5520(03)00065-5 [DOI] [PubMed] [Google Scholar]
- 31.Craig WA. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89–96. 10.1016/0732-8893(95)00053-D [DOI] [PubMed] [Google Scholar]
- 32.Craig WA, Andes DR. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob. Agents Chemother. 52:3492–3496. 10.1128/AAC.01273-07 [DOI] [PMC free article] [PubMed] [Google Scholar]


