Abstract
IMP-type metallo-β-lactamase enzymes have been reported in different geographical areas and in various Gram-negative bacteria. However, the risk factors and epidemiology pertaining to IMP-type metallo-β-lactamase-producing Enterobacter cloacae (IMP-producing E. cloacae) have not been systematically evaluated. We conducted a retrospective, matched case-control study of patients from whom IMP-producing E. cloacae isolates were obtained, in addition to performing thorough molecular analyses of the clinically obtained IMP-producing E. cloacae isolates. Unique cases with IMP-producing E. cloacae isolation were included. Patients with IMP-producing E. cloacae were matched to uninfected controls at a ratio of 1 to 3. Fifteen IMP-producing E. cloacae cases were identified, with five of the isolates being obtained from blood, and they were matched to 45 uninfected controls. All (100%) patients from whom IMP-producing E. cloacae isolates were obtained had indwelling devices at the time of isolation, compared with one (2.2%) uninfected control. Independent predictors for isolation of IMP-producing E. cloacae were identified as cephalosporin exposure and invasive procedures within 3 months. Although in-hospital mortality rates were similar between cases and controls (14.3% versus 13.3%), the in-hospital mortality of patients with IMP-producing E. cloacae-caused bacteremia was significantly higher (40%) than the rate in controls. IMP-producing E. cloacae isolates were frequently positive for other resistance determinants. The MICs of meropenem and imipenem were not elevated; 10 (67%) and 12 (80%) of the 15 IMP-producing E. cloacae isolates had a MIC of ≤1 μg/ml. A phylogenetic tree showed a close relationship among the IMP-producing E. cloacae samples. Indwelling devices, exposure to cephalosporin, and a history of invasive procedures were associated with isolation of IMP-producing E. cloacae. Screening for carbapenemase production is important in order to apply appropriate clinical management and infection control measures.
INTRODUCTION
The emergence of extended spectrum β-lactamase (ESBL)-producing Enterobacteriaceae has been observed globally in the health care-associated setting and in the community (1). The importance of carbapenem as a treatment option for ESBL-producing organisms, as well as chromosomal cephalosporinase-producing organisms, has been increasing (1). The recent spread of carbapenemase-producing Gram-negative bacteria is a significant public health problem. Class A enzymes, represented by Klebsiella pneumoniae carbapenemase (KPC)-type enzymes, and acquired class B metallo-β-lactamases (MBLs) are disseminated among bacteria internationally (2). The MBLs include various clinically and epidemiologically important types, such as VIM, NDM, and IMP types (3). IMP-type enzymes were first detected in Japan in the late 1980s (4). Since this time, IMP-type enzymes have been reported from different geographical areas, including Japan, in various Gram-negative bacteria (mostly in Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter spp., and Serratia marcescens) (3–6). IMP-type enzymes have broad substrate specificity that includes cephalosporins and carbapenems (7, 8).
Since 2011, metallo-β-lactamase-producing Enterobacter cloacae isolates have been obtained from multiple patients at the National Center for Global Health and Medicine in Tokyo, Japan (9). E. cloacae is a common nosocomial pathogen. E. cloacae is ubiquitous in the hospital environment and can survive on skin and dry surfaces (10). E. cloacae is known to possess inducible ampC chromosomal β-lactamase and may also carry plasmid-mediated ESBLs (11). Carbapenemase (e.g., IMP, VIM, KPC, and NDM)-producing E. cloacae isolates have been reported (2, 3, 9, 12). However, to the best of our knowledge, the risk factors, epidemiology, and clinical effects pertaining to IMP-type MBL-producing E. cloacae have not been systematically evaluated, in contrast to other carbapenemase-producing pathogens, such as KPC producers (13, 14).
Therefore, we conducted a case-control study of patients from whom IMP-type metallo-β-lactamase-producing E. cloacae (IMP-producing E. cloacae) isolates were obtained, in addition to thorough molecular analyses of the clinically obtained IMP-producing E. cloacae isolates.
MATERIALS AND METHODS
Study setting and design.
A retrospective matched case-control investigation of risk factors and outcomes was conducted at the National Center for Global Health and Medicine (NCGM). NCGM has more than 800 inpatient beds and serves as a tertiary referral hospital for metropolitan Tokyo. Institutional review boards at the NCGM approved the study before its initiation.
Patients and variables.
Patients from whom clinical isolates of IMP-producing E. cloacae were obtained from 1 October 2011 to 31 December 2012 were matched in a 1-to-3 ratio to uninfected controls who did not have E. cloacae isolated during the study period (15). The matching parameters for uninfected controls included (i) the hospital unit where the patient was being treated when the IMP-producing E. cloacae isolate was recovered, (ii) the calendar year and month, and (iii) the time at risk, i.e., time from admission to culture for patients with IMP-producing E. cloacae. For uninfected controls, the total duration of the hospital stay was considered to be the time at risk, and it had to be at least as long as the time at risk of the matched IMP-producing E. cloacae case. Once an eligible pool of controls was identified for each case, controls were randomly selected using the randomization function in Excel (Microsoft). For patients who had more than one strain of IMP-producing E. cloacae isolated during the study period, only the first episode was analyzed for the purpose of epidemiological analyses (i.e., the epidemiological part of the study included only unique patient episodes). Surveillance stool cultures were not routinely conducted at the NCGM during the study period.
The parameters retrieved from patient records included the following: (i) demographics; (ii) background conditions and comorbid conditions (including Charlson scores [16]); (iii) recent health care-associated exposure, such as a stay in a health care facility, an invasive procedure, and the presence of an indwelling device; (iv) the severity of underlying disease, including the McCabe score (17); (v) recent (within 3 months) exposure to antimicrobials prior to isolation of IMP-producing E. cloacae (or prior to admission for controls); and (vi) outcome, including in-hospital and 90-day mortality, length of hospital stay, deterioration in functional status (defined as deterioration from admission to discharge in at least one activity of daily living according to the Katz criteria [18]), and discharge to a long-term facility after being admitted from home. Infectious clinical syndromes in patients from whom IMP-producing E. cloacae was isolated were determined according to the Centers for Disease Control and Prevention definitions (19) and, when present, according to consultation notes from the infectious diseases consult service. IMP-producing E. cloacae isolates were considered to be colonizers if patients did not have any sign of infection based on the above-described criteria and in cases of asymptomatic bacteriuria.
Antimicrobial susceptibility, detection of IMP-type metallo-β-lactamases, and bacterial strains.
Bacteria were identified to the species level, and susceptibilities to predefined antimicrobials were determined by using an automated broth microdilution system (MicroScan WalkAway; Siemens AG, Germany) and in accordance with Clinical and Laboratory Standards Institute (CLSI) criteria (document M100–S19) (20). Clinical isolates of E. cloacae that were resistant to one or multiple agents in the extended-spectrum cephalosporin class and/or that demonstrated elevated MICs (>1 μg/ml) to imipenem and/or meropenem were screened for ESBL, MBL, and AmpC production using the Cica-Beta-Test I with HMRZ-86 (Kanto Chemical, Tokyo, Japan) (21). Subsequently, the isolates deemed positive for MBL production by the Cica-Beta-Test I were tested for IMP-type-metallo-β-lactamase production by using an immunochromatographic assay kit (Mizuho Medy Co., Saga, Japan) (22). The broth microdilution method was also performed manually according to the guidelines of the CLSI (document M100–S22) to determine the susceptibility of isolates included in the study (23). A total of 17 isolates (from 15 patients) were included in the molecular analyses. In addition, 2 E. cloacae isolates from NCGM in 2007 and 2 isolates of IMP-producing E. cloacae from other facilities in Japan were included in the phylogenetic analyses.
Detection of antibiotic resistance genes.
The blaIMP and aac(6′)-Iae genes were amplified using PCR primers as described previously (24). All of the PCR products were sequenced using an ABI Prism 3130 sequencer (Applied Biosystems, Foster City, CA). The class 1 integron was amplified using the PCR primer set 5′CS and 3′CS (24). All of the PCR products were sequenced to identify the contents of the genes (25).
Multilocus sequence typing.
Multilocus sequence typing was performed as described elsewhere (26). To analyze the clonality of the strains/isolates, phylogenetic analysis using the concatenated sequence comprising the loci was performed (26).
Statistical analysis.
All analyses were performed using IBM SPSS Statistics 20 (2011) and SAS software, version 9.3 (SAS Institute). Matched bivariate analyses were conducted using a conditional logistic regression model. Matched multivariable models were constructed using Cox proportional hazards regression, accounting for clustering in matched pairs. All variables with a P value of <0.1 in the bivariate matched analyses were considered for inclusion in the multivariate matched analyses. A stepwise selection procedure was used to select variables for inclusion in the final model. The final selected model was tested for confounding. If a covariate affected the β-coefficient of a variable in the model by >10%, then the confounding variable was maintained in the multivariable model. The percentages reported are the “valid percentage,” i.e., the percentage excluding data missing from the denominator, unless otherwise stated. A two-sided P value of <0.05 was considered statistically significant.
RESULTS
A total of 15 patients with IMP-producing E. cloacae were identified among 260 unique patients from whom E. cloacae was isolated during the study period. In these patients, IMP-producing E. cloacae isolates were identified from blood (n = 5), wounds (n = 4; 3 were intraabdominal), urine (n = 3), sputum (n = 2), and stool (n = 1). A patient who had IMP-producing E. cloacae isolated from stool was suspected as having infectious colitis. Therefore, a stool culture was performed, which grew IMP-producing E. cloacae. The characteristics of the 15 patients who had IMP-producing E. cloacae isolated are summarized in Table 1. The mean age of patients was 70.9 ± 19.4 years. Eight (53%) patients were admitted for diseases associated with the gastrointestinal tract (including the biliary tract), and 3 (20%) were admitted for neurological problems, including cerebral vascular accidents. With regard to infectious clinical syndromes associated with IMP-producing E. cloacae, 3 (20%) had catheter-related bloodstream infections (2 peripheral line associated and 1 central line associated), 3 (20%) had cholangitis, 2 (13%) had catheter-associated urinary tract infection, and 2 (13%) had catheter-associated asymptomatic bacteriuria. Overall, 10 cases were considered to have infection, and 5 (including 2 catheter-associated asymptomatic bacteriuria) cases had IMP-producing E. cloacae colonization. The median length of hospital stay prior to IMP-producing E. cloacae isolation was 47 days (interquartile range [IQR], 13 to 101 days).
TABLE 1.
Characteristics of patients from whom IMP-type metallo-β-lactamase-producing Enterobacter cloacae was isolated
| Patient | Age (yr) | Sexa | Isolation site | Infectious clinical syndrome associated with IMP-producing E. cloacaeb | Reason for admissionc | Underlying conditionsc | Treatment for IMP-producing E. cloacaed | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 91 | M | Blood | CRBSI, peripheral line, polymicrobial (P. vulgaris) | CI | Hepatitis C virus-related cirrhosis, dementia, aspiration pneumonia | Peripheral line removal, Emp Tx with MEM, Tx with LVX for 7 days | Died 7 days after IMP-producing E. cloacae isolation, due to septic shock and pneumonia |
| 2 | 77 | M | Blood | CRBSI, central line | Esophageal cancer surgery | DM, ASO, HTN, CHF, CRF | Central line removal, Emp Tx with MEM, Tx with MEM + GEN for 5 days | Died 5 days after IMP-producing E. cloacae isolation, due to renal failure and respiratory failure |
| 3 | 49 | F | Urine | CA-UTI, polymicrobial (E. faecalis) | Subdural hematoma | SAH (status post-ventriculoperitoneal shunt placement) | Emp Tx with LVX, Tx with LVX for 7 days | Improved and discharged to subacute facility |
| 4 | 88 | M | Blood | SSI | Colon cancer surgery | AAA (status post-stent placement) | Emp Tx with MEM, Tx with LVX for 14 days | Improved and discharged to subacute facility |
| 5 | 83 | F | Urine | CA-ASB, polymicrobial (E. faecium) | CAPD peritonitis | DM, CRF, HTN, CI | No Tx for CA-ASB, but CAZ used for CAPD peritonitis | Improved and discharged home |
| 6 | 64 | M | Bile | Cholangitis, polymicrobial (E. coli, K. pneumonia, E. agglomerans) | Duodenal stenosis | Pancreatic cancer, DM | No Tx for IMP-producing E. cloacae, but MEM used for other organisms from bile | Improved and discharged to subacute facility |
| 7 | 69 | M | Bile | Cholangitis, polymicrobial (E. faecium, E. casseliflavus) | Cholangitis, pulmonary tuberculosis | DM | Emp Tx with PIP-TZB, VAN, Tx with LVX for 14 days, VAN and PIP-TZB also given | Improved and transferred back to another hospital |
| 8 | 74 | F | Urine | CA-ASB, polymicrobial (P. rettgeri) | DVT, CRBSI with K. pneumonia, C. glabrata, CoNS, Rhodotorula spp., also CDI | MCTD | No Tx against IMP-producing E. cloacae, but MEM, TEC, L-AMB used for CRBSI due to other organisms | Improved and discharged home |
| 9 | 87 | M | Stool | Colonization | CDI | Ileus | No Tx against IMP-producing E. cloacae | Transferred to another hospital |
| 10 | 79 | M | Bile | Cholangitis, polymicrobial (P. aeruginosa, E. faecium) | Cholangitis, choledocholithiasis, peritonitis | CRF, HTN, IHD (status post-stent placement) | Emp Tx with VAN, MEM, Tx with MEM for 14 days, VAN also given | Improved and discharged home |
| 11 | 24 | F | Sputum | Pneumonia, polymicrobial (K. pneumonia, α-hemolytic Streptococcus spp.) | SLE flare | SLE, schizophrenia | Emp Tx with VAN, MEM, Tx with MEM for 14 days | Improved and discharged to subacute facility |
| 12 | 47 | F | Wound, left leg | Colonization | Alveolar hemorrhage | SLE, CRF, DM, CDI, HTN | No antibiotic Tx | Still hospitalized |
| 13 | 86 | M | Blood | CRBSI, peripheral line | Duodenal stenosis | Duodenum papilla cancer (status post-stent placement) | Catheter removal, Emp Tx with VAN, MEM, Tx with LVX for 9 days | Improved and discharged home |
| 14 | 42 | F | Sputum | Colonization, polymicrobial (γ-hemolytic Streptococcus spp., CoNS) | Spinal injury | HIV | No antibiotic Tx | Improved and discharged to subacute facility |
| 15 | 84 | F | Blood | CA-UTI | Rectal ulcer | CHF | Emp Tx with CRO, Tx with LVX for 9 days | Improved and discharged to subacute facility |
F, female; M, male.
CA-ASB, catheter-associated asymptomatic bacteriuria; CA-UTI, catheter-associated urinary tract infection; CoNS, coagulase-negative staphylococci; CRBSI, catheter-related bloodstream infection; IMP-producing E. cloacae, IMP-type metallo-β-lactamase-producing Enterobacter cloacae; polymicrobial, bacteria other than IMP-producing E. cloacae were isolated from the same culture specimen; SSI, surgical site infection.
AAA, abdominal aortic aneurysm; ASO, arteriosclerosis obliterans; CAPD, continuous ambulatory peritoneal dialysis; CDI, C. difficile infection; CHF, congestive heart failure; CI, cerebral infarction; CRF, chronic renal failure; DM, diabetes mellitus; DVT, deep vein thrombosis; HTN, hypertension; IHD, ischemic heart disease; MCTD, mixed connective tissue disease; SAH, subarachnoid hemorrhage; SLE, systemic lupus erythematosus.
Treatment (Tx) is defined as effective antimicrobial therapy provided from 24 h before to 28 days after IMP-producing E. cloacae isolation. Effective antimicrobial therapy was defined based on in vitro activity as reported by the NCGM clinical microbiology laboratory, in accordance with the Clinical and Laboratory Standards Institute (CLSI) criteria. Meropenem was considered effective definite therapy when the MIC of meropenem was <1μg/ml. Empirical treatment (Emp Tx) is defined as antimicrobial therapy provided from 24 h before to 48 h after the IMP-producing E. cloacae culture. CAZ, ceftazidime; CRO, ceftriaxone; GEN, gentamicin; L-AMB, liposomal amphotericin B; LVX, levofloxacin; MEM, meropenem; PIP-TZB, piperacillin-tazobactam; TEC, teicoplanin; VAN, vancomycin.
Two of the 15 patients (13%; 40% of 5 patients with bacteremia and 20% of 10 patients with infection [not colonization]) died during their hospital stay despite receiving effective therapy. Five patients (33%) from whom IMP-producing E. cloacae isolates were obtained only had asymptomatic colonization, and therefore, no antibiotics targeting IMP-producing E. cloacae were given. Two patients (patients 3 and 13) did not receive appropriate antibiotics for IMP-producing E. cloacae based on in vitro susceptibility. However, both of these patients improved clinically, probably because of the infected site (the urinary tract, where high antibiotic concentrations can be expected) and removal of devices (urinary catheter and peripheral line). The rest of the patients received effective therapy. Nine (60%) patients had bacteria other than IMP-producing E. cloacae isolated from the same culture specimen (i.e., polymicrobial isolation).
Table 2 shows the susceptibility profiles and resistance genes of the IMP-producing E. cloacae isolates. All 15 clinical isolates were susceptible to aminoglycosides (amikacin and gentamicin) (23) and colistin (27). The MICs of fluoroquinolone (ciprofloxacin) varied from ≤0.25 to 32 μg/ml; 7 (47%) of the 15 isolates were resistant per CLSI criteria (M100–S22) (23). The MICs of meropenem and imipenem were not elevated; 10 (67%) and 12 (80%) of the isolates, respectively, were categorized as susceptible (≤1 μg/ml) according to recent CLSI criteria (M100–S22) (23). Ten clinical isolates were positive for the blaIMP-1 gene, and 5 for the blaIMP-11 gene. IMP-producing E. cloacae isolates were positive for multiple resistance genes (Table 2).
TABLE 2.
Susceptibility profiles and resistance genes among IMP-type metallo-β-lactamase-producing Enterobacter cloacae isolates
| Patient | Isolate | IMP ICGAa result | Resistance gene |
MICb (μg/ml) and susceptibility interpretation |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| blaIMP | aac(6′) | aac/aad | gyrA | qnrS | blaTEM | PIP-TZB | CTX | CAZ | FEP | IPM | MEM | CIP | AMK | GEM | ATM | CST | ||||||||||||||
| 1 | EC4 | + | 1 | IIc | 128/4 | R | 512 | R | 512 | R | 32 | R | 1 | S | 2 | I | 0.5 | S | 1 | S | 0.5 | S | 64 | R | 0.5 | S | ||||
| 2 | EC5 | + | 11 | aacA1 | S83Ic | + | 32/4 | I | 32 | R | 128 | R | 8 | S | 1 | S | 1 | S | 32 | R | 16 | S | ≤0.125 | S | ≤4 | S | 1 | S | ||
| 3 | EC7 | + | 11 | IIc | aacA1 | S83I | + | + | 64/4 | I | 64 | R | 128 | R | ≤4 | S | 0.5 | S | 1 | S | 8 | R | 8 | S | ≤0.125 | S | ≤4 | S | 0.5 | S |
| 4 | EC10 | + | 1 | IIc | 64/4 | I | 512 | R | 512 | R | 32 | R | 1 | S | 1 | S | ≤0.25 | S | 1 | S | 0.5 | S | 64 | R | 0.5 | S | ||||
| 5 | EC13 | + | 1 | IIc | 128/4 | R | 512 | R | >512 | R | 32 | R | 1 | S | 2 | I | 4 | R | 0.5 | S | 0.5 | S | 512 | R | 0.5 | S | ||||
| 6 | EC14 | + | 11 | Ib | aacA4 | + | 64/4 | I | 256 | R | >512 | R | 256 | R | 32 | R | 32 | R | ≤0.25 | S | 16 | S | 0.25 | S | 512 | R | 0.25 | S | ||
| 7 | EC15 | + | 1 | IIc | 8/4 | S | 128 | R | 512 | R | 16 | I | 2 | I | 4 | R | ≤0.25 | S | 1 | S | 0.5 | S | ≤4 | S | 0.5 | S | ||||
| 8 | EC16 | + | 1 | IIc | 128/4 | R | 512 | R | 512 | R | 32 | R | 1 | S | 1 | S | ≤0.25 | S | 0.5 | S | 1 | S | 64 | R | 0.5 | S | ||||
| 9 | EC17 | + | 1 | IIc | S83Yc | ≤4/4 | S | 512 | R | 512 | R | 8 | S | 4 | R | 4 | R | 1 | S | 1 | S | 0.5 | S | ≤4 | S | 0.25 | S | |||
| 10 | EC18 | + | 11 | aacA1 | S83Y | + | ≤4/4 | S | 64 | R | 128 | R | ≤4 | S | 1 | S | 1 | S | 1 | S | 16 | S | 0.25 | S | ≤4 | S | 0.5 | S | ||
| 11 | EC19 | + | 11 | aacA1 | S83Y | + | + | 32/4 | I | 32 | R | 64 | R | ≤4 | S | 0.5 | S | 0.5 | S | 8 | R | 8 | S | ≤0.125 | S | ≤4 | S | 0.5 | S | |
| 12 | EC20 | + | 1 | aacA1 | S83Y | + | 32/4 | I | 128 | R | 256 | R | 8 | S | 0.5 | S | 1 | S | 8 | R | 8 | S | 0.5 | S | ≤4 | S | 0.5 | S | ||
| 13 | EC21 | + | 1 | IIc | 64/4 | I | 512 | R | 512 | R | 16 | I | 1 | S | 1 | S | 4 | R | 4 | S | 0.5 | S | 64 | R | 0.5 | S | ||||
| 14 | EC22 | + | 1 | IIc | 128/4 | R | 512 | R | 512 | R | 32 | R | 1 | S | 1 | S | ≤0.25 | S | 0.5 | S | 0.5 | S | 128 | R | 0.5 | S | ||||
| 15 | EC24 | + | 1 | IIc | 64/4 | I | 512 | R | 512 | R | 16 | I | 1 | S | 1 | S | ≤0.25 | S | 1 | S | 0.5 | S | 64 | R | 0.5 | S | ||||
ICGA, immunochromatographic assay.
MIC interpretive criteria (I, intermediate; R, resistant; S, susceptible) are according to CLSI document M100–S22 (23), except for colistin. Colistin MIC interpretive criteria are according to EUCAST (27). AMK, amikacin; ATM, aztreonam; CAZ, ceftazidime; FEP, cefepime; CST, colistin; CIP, ciprofloxacin; CTX, cefotaxime; GEM, gentamicin; IPM, imipenem; MEM, meropenem; PIP-TZB, piperacillin-tazobactam.
S-to-I or S-to-Y change at position 83 encoded by gyrA.
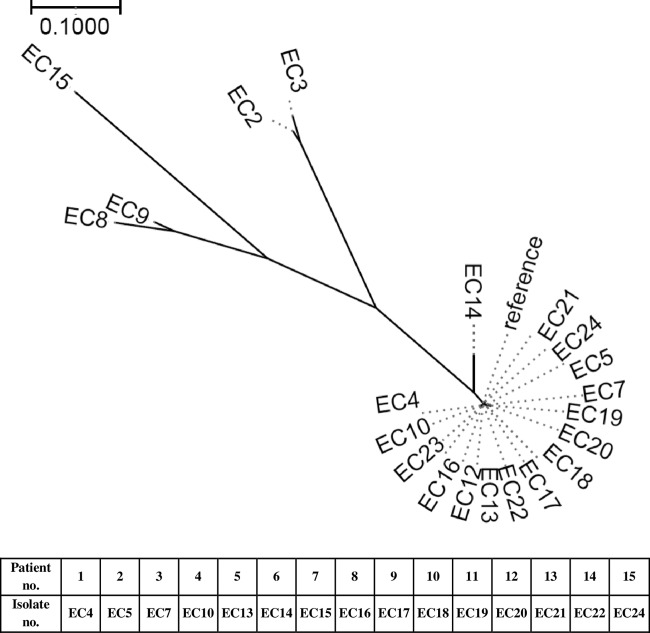
A phylogenetic tree (Fig. 1) showed a close relationship among IMP-producing E. cloacae samples isolated from the NCGM during the study period, except for one isolate (EC15) obtained from a patient who had been transferred from another hospital. The time line of the hospital location of patients with IMP-producing E. cloacae is shown in Table 3. This time line suggested possible transmission of IMP-producing E. cloacae in particular wards (C, D, H, and K).
FIG 1.
Phylogenetic tree of IMP-metallo-β-lactamase-producing Enterobacter cloacae isolates. The group of isolates included EC4, EC5, EC7, EC10, EC12, EC13, EC14, EC16, EC17, EC18, EC19, EC20, EC21, EC22, EC23, EC24, and the reference. Outliers included EC2, EC3, EC8, EC9, and EC15. EC2 and EC3 were E. cloacae isolates from NCGM in 2007. EC8 and EC9 were IMP-producing E. cloacae isolates from other facilities in Japan. EC15 was obtained from a patient who had been transferred from another hospital. EC10 (blood) and EC12 (wound) were IMP-producing E. cloacae isolates from the same patient (patient 4). EC15 (blood) and EC23 (urine) were IMP-producing E. cloacae isolates from the same patient (patient 15). Reference, de novo assembled contigs of all E. cloacae isolates used in this study were used as the reference.
TABLE 3.
Time line of the hospital locations of patients from whom IMP-type metallo-β-lactamase-producing Enterobacter cloacae was isolated
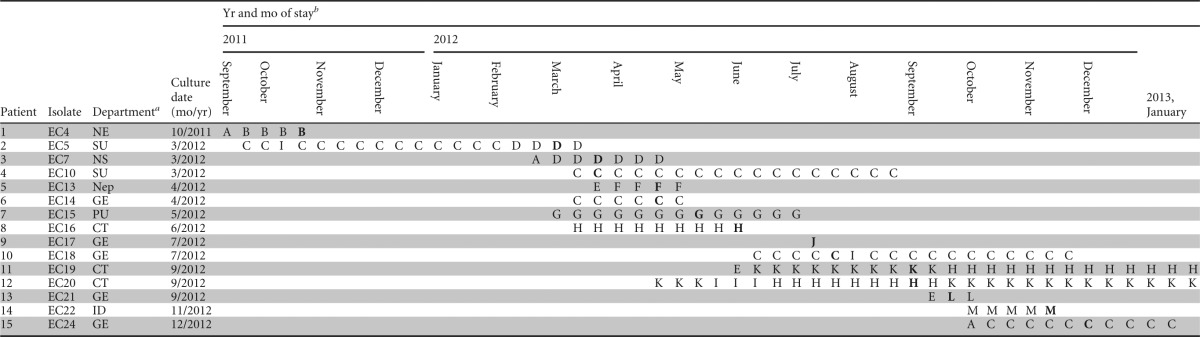
CT, rheumatology; GE, gastroenterology; ID, infectious diseases; NE, neurology; Nep, nephrology; NS, neurosurgery; PU, pulmonology; SU, surgery.
Each capital letter represents a hospital unit where the patient stayed. A boldface letter represents the unit where the patient was staying when the IMP-producing E. cloacae was isolated.
To further determine the risk factors for isolation of IMP-producing E. cloacae, 15 IMP-producing E. cloacae cases were matched to 45 uninfected controls. The overall mean age of the study cohort (n = 60) was 66 ± 18.7 years, and 28 (46.7%) of the patients were men. Bivariate analysis comparing IMP-producing E. cloacae cases and uninfected controls is shown in Table 4. Patients with isolation of IMP-producing E. cloacae were more likely to have health care-associated exposure, such as recent hospitalization, invasive procedures, and surgery within 3 months. Patients with isolation of IMP-producing E. cloacae had indwelling devices more frequently than uninfected controls. All patients with isolation of IMP-producing E. cloacae had at least 1 indwelling device, including a central line (n = 4, 27%), urinary catheter (n = 9, 60%), tracheostomy tube (n = 2, 13%), dialysis catheter (n = 2, 13%), biliary drainage tube/stent (n = 4, 27%), or nasogastric tube or percutaneous endoscopic gastrostomy (n = 4, 27%) at the time of IMP-producing E. cloacae isolation. Antibiotic exposure was more common in the IMP-producing E. cloacae group than in controls. All of the patients with isolation of IMP-producing E. cloacae had antimicrobial exposure within 3 months; the most frequent exposures were to cephalosporins (n = 9, 60%), followed by glycopeptides (n = 6, 40%), penicillins (n = 6, 40%), and carbapenems (n = 6, 40%).
TABLE 4.
Bivariate analysis of risk factors and outcomes for isolation of IMP-type metallo-β-lactamase-producing Enterobacter cloacae compared with uninfected controls
| Parametera | No. (%b) or value as indicated for: |
Result for IMP-producing E. cloacae cases vs uninfected controlsc |
||
|---|---|---|---|---|
| IMP-producing E. cloacae cases (n = 15) | Uninfected controls (n = 45) | OR (95% CI) | P value | |
| Demographics | ||||
| Mean age ± SD (yr) | 70.9 ± 19.4 | 64.4 ± 18.5 | NA | 0.249 |
| Male | 8 (53.3) | 20 (44.4) | 1.64 (0.41–6.56) | 0.483 |
| Non-home residence | 3 (20) | 3 (6.7) | 6.46 (0.62–67.72) | 0.119 |
| Acute and chronic conditions on admission | ||||
| Dependent functional status | 9 (60) | 22 (48.8) | 1.66 (0.48–5.78) | 0.427 |
| Impaired consciousness | 6 (40) | 14 (31.1) | 1.64 (0.41–6.56) | 0.483 |
| Current use of H2 blocker or PPI | 13 (86.6) | 19 (42.2) | 12.63 (1.57–101.35) | 0.017 |
| Rapidly fatal McCabe score | 1 (6.7) | 6 (13.3) | 0.39 (0.03–4.39) | 0.446 |
| Cerebrovascular accident | 4 (26.6) | 10 (22.2) | 1.66 (0.23–12.09) | 0.619 |
| Congestive heart failure | 3 (20) | 2 (4.4) | 4.5 (0.75–26.93) | 0.099 |
| Dementia | 3 (20) | 4 (8.9) | 3 (0.47–19.04) | 0.244 |
| Connective tissue disease | 3 (20) | 10 (22.2) | 0.81 (0.13–5.13) | 0.819 |
| Diabetes mellitus | 4 (26.6) | 6 (13.3) | 2.56 (0.54–12.06) | 0.234 |
| Any liver disease | 1 (6.7) | 4 (8.9) | 0.72 (0.07–7.35) | 0.782 |
| Any renal disease | 8 (53.3) | 2 (4.4) | 13.52 (3.07–59.58) | <0.001 |
| Active malignant disease | 4 (26.6) | 17 (37.7) | 0.53 (0.13–2.16) | 0.373 |
| Median Charlson combined condition score (IQR) | 5 (4–10) | 5 (2–9) | NA | 0.341 |
| Immunosuppressive stated | 6 (40) | 14 (31.1) | 1.69 (0.41–7.02) | 0.468 |
| Exposure to health care settings and environments before IMP-producing E. cloacae isolation | ||||
| Hospitalization in the past 3 mo | 10 (66.6) | 13 (28.8) | 5.35 (1.38–20.71) | 0.015 |
| Median no. of days from last hospitalization (IQR) | 10 (0–56) | 15 (3–29) | NA | 0.784 |
| GI tract endoscopy in the past 3 mo | 7 (46.6) | 5 (11.1) | 14.65 (1.74–123.24) | 0.014 |
| Invasive procedure in the past 3 moe | 6 (40) | 6 (13.3) | 4.62 (1.11–19.31) | 0.036 |
| Surgery in the past 3 mo | 5 (33.3) | 0 | 21 (2.99–147.69) | <0.001 |
| GI tract endoscopy, invasive procedure, or surgery in the past 3 mo | 11 (73.3) | 7 (15.5) | 23.9 (3.02–189.32) | 0.003 |
| Any permanent devicef | 15 (100) | 1 (2.2) | 18.55 (6.09–56.51) | <0.001 |
| ICU stay in the past 3 mo | 4 (26.7) | 4 (8.9) | 9 (0.94–86.52) | 0.06 |
| Antimicrobial exposure in the past 3 mo | ||||
| Any antibiotic | 15 (100) | 13 (28.9) | 6.02 (2.13–17.02) | <0.001 |
| Median no. of days from last hospitalization (IQR) | 0 (0–10) | 35 (0–65) | NA | 0.023 |
| Penicillinsg | 6 (40) | 4 (8.9) | 5.44 (1.34–22.01) | 0.018 |
| Oxyimino-cephalosporinsh | 4 (26.7) | 1 (2.2) | 12 (1.34–107.36) | 0.026 |
| Other cephalosporins | 6 (40) | 4 (8.9) | 12.45 (1.45–106.64) | 0.021 |
| Cephalosporins | 9 (60) | 4 (8.9) | 21.27 (2.65–170.43) | 0.004 |
| β-Lactam/β-lactamase inhibitorsi | 5 (33.3) | 5 (11.1) | 5.4 (0.99–29.46) | 0.051 |
| Imipenem or meropenem | 6 (40) | 1 (2.2) | 18 (2.17–149.51) | 0.007 |
| β-Lactam antibiotics | 12 (80) | 6 (13.3) | 27.58 (3.53–215.3) | 0.002 |
| Fluoroquinolones | 5 (33.3) | 7 (15.6) | 2.79 (0.71–11) | 0.144 |
| Aminoglycosides | 1 (6.7) | 1 (2.2) | 3 (1.89–47.96) | 0.44 |
| Glycopeptides | 6 (40) | 1 (2.2) | 15.54 (2.74–88.03) | <0.001 |
| Outcomes | ||||
| In-hospital mortality | 2 (14.3) | 6 (13.3) | 1.26 (0.2–8.03) | 0.809 |
| Mortality within 3 mo | 2 (14.3) | 11 (24.4) | 0.59 (0.11–3.32) | 0.551 |
| Functional status deterioration | 3 (25) | 1 (2.6) | 7.24 (0.73–72.04) | 0.091 |
| Discharge to LTCF after being admitted from home | 5 (55.6) | 12 (32.4) | 2.18 (0.47–10.05) | 0.318 |
| Additional hospitalizations within 6 mo following IMP-producing E. cloacae isolationj | 3 (60) | 6 (24) | 9 (0.37–220.93) | 0.16 |
| Total length of hospital stay [median no. of days (IQR)] | 93 (52–175) | 57 (36–96) | NA | 0.052 |
| Total length of hospital stay excluding stays ending in death [median no. of days (IQR)] | 83 (55–175) | 56 (28–92) | NA | 0.098 |
GI, gastrointestinal; ICU, intensive care unit; IQR, interquartile range; LTCF, long-term care facilities; PPI, proton pump inhibitors.
The percentage is of patients for whom data were available, i.e., excluding the missing cases.
Boldface indicates statistically significant difference between groups (P < 0.05). CI, confidence interval; NA, data not available; OR, odds ratio.
Includes one or more of the following: (i) neutropenia (<500 neutrophils) at time of culture, (ii) glucocorticoid/steroid use in the past month, (iii) chemotherapy in the past 3 months, (iv) radiotherapy in the past 3 months, (v) posttransplantation, (vi) anti-tumor necrosis factor alpha therapy in the past 3 months, or (vii) HIV.
Includes percutaneous interventions, endoscopies, and biopsies.
The presence of any indwelling device (e.g., tracheotomies, central lines, urinary catheters, orthopedic external fixators, percutaneous endoscopic gastrostomy, biliary stent/tube, ventriculoperitoneal shunt, nasogastric tube, continuous ambulatory peritoneal dialysis catheter, or hemodialysis catheter) (i) at the time of IMP-producing E. cloacae isolation or on admission for uninfected controls or (ii) at the time of IMP-producing E. cloacae isolation or on admission for uninfected controls.
Includes β-lactam/β-lactamase inhibitor combinations.
Includes ceftriaxone, cefepime, and ceftazidime.
Includes ampicillin-sulbactam, piperacillin-tazobactam, ticarcillin-clavulanate, and amoxicillin-clavulanate.
For uninfected controls, within 6 months following admission.
Although in-hospital mortality was similar between cases and controls (14.3% versus 13.3%), the in-hospital mortality of patients with isolation of IMP-producing E. cloacae bacteremia was significantly higher (40%) than that in controls (P = 0.014). Functional deterioration was more common in the IMP-producing E. cloacae group than in uninfected controls (25% versus 2.6%, P = 0.05).
Independent predictors for the isolation of IMP-producing E. cloacae were determined by multivariate analyses (Table 5). Invasive procedures in the past 3 months and exposure to cephalosporins in the past 3 months were independently associated with isolation of IMP-producing E. cloacae, and thus, these were considered risk factors for the isolation of IMP-producing E. cloacae, in addition to the patients' locations in the hospital.
TABLE 5.
Multivariate analysis of risk factors for the isolation of IMP-producing E. cloacae
| Variable | IMP-producing E. cloacae cases vs uninfected controls |
|
|---|---|---|
| OR (95% CI) | P value | |
| Invasive procedure in the previous 3 moa | 21.48 (1.88–246.18) | 0.014 |
| Exposure to cephalosporins in the previous 3 mo | 19.1 (1.5–243.46) | 0.023 |
Includes percutaneous interventions, endoscopies, urological procedures, and biopsies.
DISCUSSION
This study examined molecular and epidemiological characteristics of clinically obtained metallo-β-lactamase-producing E. cloacae isolates. To the best of our knowledge, this is the first study to systematically elucidate independent risk factors for the isolation of IMP-producing E. cloacae. For the risk factor analyses, we carefully included risk factors known to be associated with the isolation of multidrug-resistant Enterobacteriaceae species (28–30). In addition, two individuals (an infectious diseases specialist and an infection preventionist) independently reviewed the patients' medical records and visited each ward as often as possible to rule out any common source of infection other than those included in the risk factor analyses. We chose to collect samples from patients admitted to the hospital who were at risk of acquiring the antimicrobial-resistant organism (control type 2) rather than from patients with cultures positive for the antibiotic-susceptible form of the organism of interest (control type 1). This is because a previous study showed that the selection of control patients from the type 1 group can falsely identify certain antibiotics and overestimate the odds ratio (OR) of the use of antimicrobial agents as risk factors (15).
In our study, IMP-producing E. cloacae isolates were obtained from elderly, debilitated individuals whose length of hospital stay prior to the isolation of IMP-producing E. cloacae was long (median, 47 days; IQR, 13 to 101 days) (Table 1). Catheter-related bloodstream infections and cholangitis were two major infectious clinical syndromes caused by IMP-producing E. cloacae. Notably, all 15 patients with isolation of IMP-producing E. cloacae had exposure to antibiotics in the 3 months prior to IMP-producing E. cloacae isolation. Additionally, all patients with isolation of IMP-producing E. cloacae had at least one indwelling device at the time of IMP-producing E. cloacae isolation. These were apparent risk factors for the isolation of IMP-producing E. cloacae, although they could not be incorporated into the final multivariate models to identify independent risk factors for the isolation of IMP-producing E. cloacae because all (100%) IMP-producing E. cloacae cases had these exposures (exposures to antibiotics and/or indwelling devices). Other independent risk factors for the isolation of IMP-producing E. cloacae were invasive procedures and exposure to cephalosporins in the 3 months prior. Carbapenem and cephalosporin exposure has been reported as a risk factor for the isolation of carbapenem-resistant organisms, such as IMP-type metallo-β-lactamase-producing organisms (31), multidrug-resistant P. aeruginosa producing SPM-type metallo-β-lactamase (32), and carbapenem-resistant K. pneumonia (13). In our study, carbapenem exposure was much more common in the IMP-producing E. cloacae group than in uninfected controls, but this was not identified as an independent risk factor.
The phylogenetic tree (Fig. 1) and time line of hospital locations of patients with IMP-producing E. cloacae (Table 3) suggested possible transmission of IMP-producing E. cloacae in particular wards in our hospital. An infection control team emphasized the importance of infection control measures, especially strict compliance with contact isolation procedures in each ward. The incidence of new isolations of IMP-producing E. cloacae has eventually decreased to 2 cases over 5 months since March 2013.
IMP-producing E. cloacae possesses many other resistance genes (Table 2). Seven (47%) of the isolates were resistant to ciprofloxacin. This is in accordance with previous reports of metallo-β-lactamase-producing organisms with low susceptibilities to different classes of antibiotics (33). Isolates with aacA1/aacA4 resistance genes had elevated MICs (8 to 16 μg/ml) to amikacin but not to gentamicin. The difference is probably related to the resistance mechanism of AAC(6′)-I, associated with the aacA1/aacA4 gene, which is known to acetylate tobramycin and amikacin but not gentamicin.
As previously reported, the MICs of meropenem and imipenem were not elevated in our study. Bloodstream infections caused by IMP-type metallo-β-lactamase-producing E. cloacae isolates with a MIC of 2 μg/ml have been previously reported; however, in these reports, the exact methods of measuring the MIC were not described (9). In our study, even when using the revised CLSI criteria (defining susceptibility as a MIC of ≤1 μg/ml), 67% and 80% of IMP-producing E. cloacae isolates were categorized as susceptible to meropenem and imipenem, respectively (23). This finding underscores the difficulties in identifying metallo-β-carbapenemase-producing organisms solely based on MIC results, as previously reported (34–36). In geographical areas where the IMP-type carbapenemase is endemic, such as Asia (34–36), both ESBLs and metallo-β-lactamase might need to be considered when assessing a patient with infection due to Enterobacteriaceae species with elevated MICs to penicillins and cephalosporins, including oxyimino-cephalosporins (2, 37). This is particularly important for patients who fail to respond to carbapenem treatment despite the low MICs to carbapenems (9).
In our study, all of the blaIMP-positive isolates were positive for IMP in the immunochromatographic assay, with 2 false-positive results during the study period. The immunochromatographic assay is technically easy to use as a screening method in hospital microbiology laboratories (22); further investigations are warranted to evaluate the diagnostic usefulness of detecting IMP-metallo-β-carbapenemase. The IMP-containing integron has been suggested to spread through horizontal transfer (38), so early recognition of IMP-producing organisms is of particular importance.
Two (patients 1 and 2) of the 15 patients (13%; 40% of 5 patients with bacteremia and 20% of patients with infection [not colonization]) died during their hospital stay. The IMP-producing E. cloacae isolates from these 2 patients had relatively low MICs to meropenem (MICs of 2 μg/ml and 1 μg/ml), and both patients received meropenem as empirical therapy; however, they both deteriorated clinically. Their multiple comorbid conditions and old age are likely to have contributed to their unfortunate clinical courses. However, our results raise some concern for relying on carbapenem as a treatment option in infections, especially for elderly individuals and/or those with comorbidities. Previous reports have suggested conflicting results (34, 39–41). Therefore, further studies are needed on this issue.
The majority of the isolates are clonally related, and thus, an outbreak might have occurred in the hospital. However, they possessed different resistance genes, as shown in Table 2. Although we suspected that mobile elements may have been transferred among strains that had the same drug resistance genes, the exact mechanisms for the closely related strains to acquire different drug resistance genes are not certain. Even though a close relationship among IMP-positive isolates was found by MLST, these isolates had two different types of blaIMP genes, IMP-1 and IMP-11. Although IMP-type metallo-β lactamase enzymes are thought to be located within a variety of integron structures (38), in this study, we did not determine the exact mechanism of how each E. cloacae isolate acquired the IMP-type metallo-β-lactamase. Further studies are warranted to determine the exact mechanisms by which E. cloacae acquires IMP-type metallo-β-lactamase.
In conclusion, we identified the risk factors for isolation of IMP-producing E. cloacae, as well as molecular and microbiological characteristics of these isolates. Considering the clinical outcomes in our patient cohort, a lower threshold for screening for carbapenemase production is recommended in patients who have previous exposure to antimicrobials, indwelling devices, and recent invasive procedures and from whom E. cloacae that is resistant to one or multiple agents in the extended-spectrum cephalosporin class and/or shows elevated MICs (>1 μg/ml) to imipenem and/or meropenem has been isolated. Choosing an appropriate antimicrobial therapy, as well as applying strong infection control measures, are clinically important measures for patients from whom IMP-producing E. cloacae isolates have been obtained.
ACKNOWLEDGMENTS
The authors declare no potential conflicts of interest.
K.H. and T.M.-A. were supported by Grants for International Health Research (24S-5 and 26A-103, respectively) from the Ministry of Health, Labor, and Welfare of Japan. T.K. was supported by a grant from the Ministry of Health, Labor and Welfare of Japan (H24-Shinko-Ippan-010).
Footnotes
Published ahead of print 7 April 2014
REFERENCES
- 1.Pitout JD, Laupland KB. 2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect. Dis. 8:159–166. 10.1016/S1473-3099(08)70041-0 [DOI] [PubMed] [Google Scholar]
- 2.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin. Microbiol. Rev. 25:682–707. 10.1128/CMR.05035-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornaglia G, Giamarellou H, Rossolini GM. 2011. Metallo-beta-lactamases: a last frontier for beta-lactams? Lancet Infect. Dis. 11:381–393. 10.1016/S1473-3099(11)70056-1 [DOI] [PubMed] [Google Scholar]
- 4.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. 1994. Molecular characterization of an enterobacterial metallo beta-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob. Agents Chemother. 38:71–78. 10.1128/AAC.38.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castanheira M, Mendes RE, Rhomberg PR, Jones RN. 2008. Rapid emergence of blaCTX-M among Enterobacteriaceae in U.S. medical centers: molecular evaluation from the MYSTIC Program (2007). Microb. Drug Resist. 14:211–216. 10.1089/mdr.2008.0827 [DOI] [PubMed] [Google Scholar]
- 6.Fukigai S, Alba J, Kimura S, Iida T, Nishikura N, Ishii Y, Yamaguchi K. 2007. Nosocomial outbreak of genetically related IMP-1 beta-lactamase-producing Klebsiella pneumoniae in a general hospital in Japan. Int. J. Antimicrob. Agents 29:306–310. 10.1016/j.ijantimicag.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 7.Docquier JD, Riccio ML, Mugnaioli C, Luzzaro F, Endimiani A, Toniolo A, Amicosante G, Rossolini GM. 2003. IMP-12, a new plasmid-encoded metallo-beta-lactamase from a Pseudomonas putida clinical isolate. Antimicrob. Agents Chemother. 47:1522–1528. 10.1128/AAC.47.5.1522-1528.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miriagou V, Cornaglia G, Edelstein M, Galani I, Giske CG, Gniadkowski M, Malamou-Lada E, Martinez-Martinez L, Navarro F, Nordmann P, Peixe L, Pournaras S, Rossolini GM, Tsakris A, Vatopoulos A, Canton R. 2010. Acquired carbapenemases in Gram-negative bacterial pathogens: detection and surveillance issues. Clin. Microbiol. Infect. 16:112–122. 10.1111/j.1469-0691.2009.03116.x [DOI] [PubMed] [Google Scholar]
- 9.Hamada Y, Watanabe K, Tatsuya T, Mezaki K, Takeuchi S, Shimizu T, Kirikae T, Ohmagari N. 2013. Three cases of IMP-type metallo-β-lactamase-producing Enterobacter cloacae bloodstream infection in Japan. J. Infect. Chemother. 19:956–958. 10.1007/s10156-012-0520-6 [DOI] [PubMed] [Google Scholar]
- 10.Dalben M, Varkulja G, Basso M, Krebs VL, Gibelli MA, van der Heijden I, Rossi F, Duboc G, Levin AS, Costa SF. 2008. Investigation of an outbreak of Enterobacter cloacae in a neonatal unit and review of the literature. J. Hosp. Infect. 70:7–14. 10.1016/j.jhin.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 11.Paterson DL, Bonomo RA. 2005. Extended-spectrum beta-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657–686. 10.1128/CMR.18.4.657-686.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shet V, Gouliouris T, Brown NM, Turton JF, Zhang J, Woodford N. 2011. IMP metallo-beta-lactamase-producing clinical isolates of Enterobacter cloacae in the UK. J. Antimicrob. Chemother. 66:1408–1409. 10.1093/jac/dkr078 [DOI] [PubMed] [Google Scholar]
- 13.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. 2008. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob. Agents Chemother. 52:1028–1033. 10.1128/AAC.01020-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchaim D, Chopra T, Bhargava A, Bogan C, Dhar S, Hayakawa K, Pogue JM, Bheemreddy S, Blunden C, Shango M, Swan J, Lephart PR, Perez F, Bonomo RA, Kaye KS. 2012. Recent exposure to antimicrobials and carbapenem-resistant Enterobacteriaceae: the role of antimicrobial stewardship. Infect. Control Hosp. Epidemiol. 33:817–830. 10.1086/666642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris AD, Samore MH, Lipsitch M, Kaye KS, Perencevich E, Carmeli Y. 2002. Control-group selection importance in studies of antimicrobial resistance: examples applied to Pseudomonas aeruginosa, Enterococci, and Escherichia coli. Clin. Infect. Dis. 34:1558–1563. 10.1086/340533 [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40:373–383. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 17.Bion JF, Edlin SA, Ramsay G, McCabe S, Ledingham IM. 1985. Validation of a prognostic score in critically ill patients undergoing transport. Br. Med. J. (Clin. Res. ed) 291:432–434. 10.1136/bmj.291.6493.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. 1963. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA 185:914–919. 10.1001/jama.1963.03060120024016 [DOI] [PubMed] [Google Scholar]
- 19.Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 36:309–332. 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing. Nineteenth informational supplement. Approved standard M100–S19 CLSI, Wayne, PA [Google Scholar]
- 21.Livermore DM, Warner M, Mushtaq S. 2007. Evaluation of the chromogenic Cica-beta-Test for detecting extended-spectrum, AmpC and metallo-beta-lactamases. J. Antimicrob. Chemother. 60:1375–1379. 10.1093/jac/dkm374 [DOI] [PubMed] [Google Scholar]
- 22.Kitao T, Miyoshi-Akiyama T, Tanaka M, Narahara K, Shimojima M, Kirikae T. 2011. Development of an immunochromatographic assay for diagnosing the production of IMP-type metallo-beta-lactamases that mediate carbapenem resistance in Pseudomonas. J. Microbiol. Methods 87:330–337. 10.1016/j.mimet.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 23.The Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing. Twentieth informational supplement. Approved standard M100–S22 CLSI, Wayne, PA [Google Scholar]
- 24.Sekiguchi J, Asagi T, Miyoshi-Akiyama T, Fujino T, Kobayashi I, Morita K, Kikuchi Y, Kuratsuji T, Kirikae T. 2005. Multidrug-resistant Pseudomonas aeruginosa strain that caused an outbreak in a neurosurgery ward and its aac(6′)-Iae gene cassette encoding a novel aminoglycoside acetyltransferase. Antimicrob. Agents Chemother. 49:3734–3742. 10.1128/AAC.49.9.3734-3742.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levesque C, Piche L, Larose C, Roy PH. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185–191. 10.1128/AAC.39.1.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyoshi-Akiyama T, Hayakawa K, Ohmagari N, Shimojima M, Kirikae T. 2013. Multilocus sequence typing (MLST) for characterization of Enterobacter cloacae. PLoS One 8:e66358. 10.1371/journal.pone.0066358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Committee on Antimicrobial Susceptibility Testing. 2013. Breakpoint tables for interpretation of MICs and zone diameters, version 3.1. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_3.1.pdf [Google Scholar]
- 28.Hirakata Y, Yamaguchi T, Nakano M, Izumikawa K, Mine M, Aoki S, Kondoh A, Matsuda J, Hirayama M, Yanagihara K, Miyazaki Y, Tomono K, Yamada Y, Kamihira S, Kohno S. 2003. Clinical and bacteriological characteristics of IMP-type metallo-beta-lactamase-producing Pseudomonas aeruginosa. Clin. Infect. Dis. 37:26–32. 10.1086/375594 [DOI] [PubMed] [Google Scholar]
- 29.Pop-Vicas AE, D'Agata EM. 2005. The rising influx of multidrug-resistant gram-negative bacilli into a tertiary care hospital. Clin. Infect. Dis. 40:1792–1798. 10.1086/430314 [DOI] [PubMed] [Google Scholar]
- 30.Qureshi ZA, Paterson DL, Peleg AY, Adams-Haduch JM, Shutt KA, Pakstis DL, Sordillo E, Polsky B, Sandkovsky G, Bhussar MK, Doi Y. 2012. Clinical characteristics of bacteraemia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae in the era of CTX-M-type and KPC-type beta-lactamases. Clin. Microbiol. Infect. 18:887-893. 10.1111/j.1469-0691.2011.03658.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herbert S, Halvorsen DS, Leong T, Franklin C, Harrington G, Spelman D. 2007. Large outbreak of infection and colonization with gram-negative pathogens carrying the metallo-beta-lactamase gene blaIMP-4 at a 320-bed tertiary hospital in Australia. Infect. Control Hosp. Epidemiol. 28:98–101. 10.1086/508841 [DOI] [PubMed] [Google Scholar]
- 32.Nouer SA, Nucci M, de Oliveira MP, Pellegrino FL, Moreira BM. 2005. Risk factors for acquisition of multidrug-resistant Pseudomonas aeruginosa producing SPM metallo-beta-lactamase. Antimicrob. Agents Chemother. 49:3663–3667. 10.1128/AAC.49.9.3663-3667.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornaglia G, Akova M, Amicosante G, Canton R, Cauda R, Docquier JD, Edelstein M, Frere JM, Fuzi M, Galleni M, Giamarellou H, Gniadkowski M, Koncan R, Libisch B, Luzzaro F, Miriagou V, Navarro F, Nordmann P, Pagani L, Peixe L, Poirel L, Souli M, Tacconelli E, Vatopoulos A, Rossolini GM, ESCMID Study Group for Antimicrobial Resistance Surveillance (ESGARS) 2007. Metallo-beta-lactamases as emerging resistance determinants in Gram-negative pathogens: open issues. Int. J. Antimicrob. Agents 29:380–388. 10.1016/j.ijantimicag.2006.10.008 [DOI] [PubMed] [Google Scholar]
- 34.Carmeli Y, Akova M, Cornaglia G, Daikos GL, Garau J, Harbarth S, Rossolini GM, Souli M, Giamarellou H. 2010. Controlling the spread of carbapenemase-producing Gram-negatives: therapeutic approach and infection control. Clin. Microbiol. Infect. 16:102–111. 10.1111/j.1469-0691.2009.03115.x [DOI] [PubMed] [Google Scholar]
- 35.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798. 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yano H, Ogawa M, Endo S, Kakuta R, Kanamori H, Inomata S, Ishibashi N, Aoyagi T, Hatta M, Gu Y, Yamada M, Tokuda K, Kunishima H, Kitagawa M, Hirakata Y, Kaku M. 2012. High frequency of IMP-6 among clinical isolates of metallo-beta-lactamase-producing Escherichia coli in Japan. Antimicrob. Agents Chemother. 56:4554–4555. 10.1128/AAC.00617-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishio H, Komatsu M, Shibata N, Shimakawa K, Sueyoshi N, Ura T, Satoh K, Toyokawa M, Nakamura T, Wada Y, Orita T, Kofuku T, Yamasaki K, Sakamoto M, Kinoshita S, Aihara M, Arakawa Y. 2004. Metallo-beta-lactamase-producing gram-negative bacilli: laboratory-based surveillance in cooperation with 13 clinical laboratories in the Kinki region of Japan. J. Clin. Microbiol. 42:5256–5263. 10.1128/JCM.42.11.5256-5263.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Queenan AM, Bush K. 2007. Carbapenemases: the versatile beta-lactamases. Clin. Microbiol. Rev. 20:440–458. 10.1128/CMR.00001-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daikos GL, Panagiotakopoulou A, Tzelepi E, Loli A, Tzouvelekis LS, Miriagou V. 2007. Activity of imipenem against VIM-1 metallo-beta-lactamase-producing Klebsiella pneumoniae in the murine thigh infection model. Clin. Microbiol. Infect. 13:202–205. 10.1111/j.1469-0691.2006.01590.x [DOI] [PubMed] [Google Scholar]
- 40.Daikos GL, Petrikkos P, Psichogiou M, Kosmidis C, Vryonis E, Skoutelis A, Georgousi K, Tzouvelekis LS, Tassios PT, Bamia C, Petrikkos G. 2009. Prospective observational study of the impact of VIM-1 metallo-beta-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob. Agents Chemother. 53:1868–1873. 10.1128/AAC.00782-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mantengoli E, Luzzaro F, Pecile P, Cecconi D, Cavallo A, Attala L, Bartoloni A, Rossolini GM. 2011. Escherichia coli ST131 producing extended-spectrum beta-lactamases plus VIM-1 carbapenemase: further narrowing of treatment options. Clin. Infect. Dis. 52:690–691. 10.1093/cid/ciq194 [DOI] [PubMed] [Google Scholar]