Abstract
Nutritional supplementation to tuberculosis (TB) patients has been associated with increased weight and reduced mortality, but its effect on the pharmacokinetics of first-line anti-TB drugs is unknown. A cohort of 100 TB patients (58 men; median age, 35 [interquartile range {IQR}, 29 to 40] years, and median body mass index [BMI], 18.8 [17.3 to 19.9] kg/m2) were randomized to receive nutritional supplementation during the intensive phase of TB treatment. Rifampin plasma concentrations were determined after 1 week and 2 months of treatment. The effects of nutritional supplementation, HIV, time on treatment, body weight, and SLCO1B1 rs4149032 genotype were examined using a population pharmacokinetic model. The model adjusted for body size via allometric scaling, accounted for clearance autoinduction, and detected an increase in bioavailability (+14%) for the patients in the continuation phase. HIV coinfection in patients not receiving the supplementation was found to decrease bioavailability by 21.8%, with a median maximum concentration of drug in serum (Cmax) and area under the concentration-time curve from 0 to 24 h (AUC0–24) of 5.6 μg/ml and 28.6 μg · h/ml, respectively. HIV-coinfected patients on nutritional supplementation achieved higher Cmax and AUC0–24 values of 6.4 μg/ml and 31.6 μg · h/ml, respectively, and only 13.3% bioavailability reduction. No effect of the SLCO1B1 rs4149032 genotype was observed. In conclusion, nutritional supplementation during the first 2 months of TB treatment reduces the decrease in rifampin exposure observed in HIV-coinfected patients but does not affect exposure in HIV-uninfected patients. If confirmed in other studies, the use of defined nutritional supplementation in HIV-coinfected TB patients should be considered in TB control programs. (This study has the controlled trial registration number ISRCTN 16552219.)
INTRODUCTION
Tuberculosis (TB) is commonly associated with HIV and undernutrition in resource-limited settings (1, 2). Worldwide, of the 8.6 million people who developed TB in 2012, 13% of them were HIV positive. Sub-Saharan Africa, including Tanzania, bears the highest incidence of HIV (1, 3, 4). Although recent estimates show that TB incidence is decreasing globally (4), the emergence of drug-resistant strains of Mycobacterium tuberculosis is threatening the global TB control effort. Methods to further improve current TB treatment outcomes are warranted (5). The World Health Organization (WHO) recommends the first-line anti-TB regimen to consist of 6 months of daily rifampin and isoniazid, supplemented with pyrazinamide plus ethambutol during the first 2 months (4). Drugs should be administered as fixed-dose combination (FDC) tablets under the “directly observed treatment short course” (DOTS) protocol, applying strategies to avoid unfavorable treatment outcomes (4, 6). However, a recent meta-analysis of clinical studies reported pharmacokinetic (PK) variability of just a single drug in the regimen to be associated with treatment failure and acquired drug resistance (ADR) (7). The pharmacokinetics of rifampin, the most effective anti-TB drug, is highly variable and has in some studies been shown to be affected by HIV and/or poor nutritional status (8, 9). TB patients in general are at risk of nutritional deficiencies, especially in countries with high burdens of TB, where the majority of patients are moderately to severely wasted at the time of diagnosis (10, 11). Daily micronutrient supplementation given together with TB treatment considerably increases weight gain and reduces mortality during treatment (12, 13) and is therefore considered an option for managing malnourished TB patients (14). We investigated the role of nutritional supplementation in rifampin pharmacokinetics among pulmonary TB patients during the intensive phase of treatment.
MATERIALS AND METHODS
Study design, participants, and setting.
This study was an open-label, randomized, controlled clinical trial conducted from September 2010 to August 2011 in Mwanza, Tanzania, and was granted permission from the National Ethics Committee in Tanzania. We assessed the effect of a nutritional intervention (high-energy and vitamin/mineral-fortified biscuits) on newly diagnosed sputum-smear-positive pulmonary TB patients with or without HIV coinfection on rifampin exposure. Patients were tested for TB and HIV at four TB clinics located in Mwanza City serving both urban and suburban populations. Only sputum-smear-positive patients, regardless of HIV status, were enrolled and requested to provide an extra sputum sample in a sterile bottle for confirmatory smear microscopy and culture at the Zonal TB reference laboratory at Bugando Medical Centre, Mwanza City. Patients were eligible if aged 15 years or above, and written informed consent was obtained. We excluded all HIV patients receiving antiretroviral treatment (ART), pregnant women, terminally sick patients not likely to survive >48 h, and nonresidents of Mwanza City. TB treatment was given as FDC tablets each containing isoniazid (75 mg), rifampin (150 mg), pyrazinamide (400 mg), and ethambutol (275 mg) during intensive phase, and for continuation phase, the combination contained isoniazid (75 mg) and rifampin (150 mg) only. Patients with body weights of ≤50 kg received 3 tablets, and those of >50 kg received 4 tablets daily. TB treatment was initiated and performed within the framework of the National Tuberculosis and Leprosy Control Program (NTLP) (6). Simple randomization, stratified by HIV status, was computed using the website www.randomization.com to allocate patients to receive high-energy and vitamin/mineral-fortified biscuits as a nutritional supplement or no supplements. The trial aimed at recruiting at least 100 individuals, with 50% having HIV coinfection in each arm. With 50 patients in each group, we would be able to detect an 0.65-standard-deviation (SD) difference in any exposure with a 90% power.
Nutritional intervention.
The nutritional supplement was in the form of high-energy and vitamin/mineral-enriched biscuits produced by Compact A/S (Bergen, Norway), identical to those used in the experimental arm of our previous study (15). All assigned participants received daily 5 high-energy biscuit bars that contained approximately 1,000 kcal and additional vitamins and minerals, including zinc and selenium (for further details on content, see reference 15). The supplements were given during the first 2 months of TB treatment, and the intake was monitored by the patient treatment supporter using a specifically designed logbook verified by the study team.
Data collection and laboratory procedures.
Study nurses used a standardized questionnaire to collect information on social demographic characteristics, smoking habits, and alcohol consumption. Anthropometric measurements were obtained at recruitment (day 0) and at the end of the intensive phase. Weight was determined using a digital weighing scale, while height was determined using a height board. Mid-upper arm circumference (MUAC) and triceps skin fold thickness (TSFT) were determined using a tape measure and a Harpenden caliper, respectively. Body mass index (BMI), arm muscle area (AMA), and arm fat area (AFA) were calculated using the following formulas: weight (kg)/(height [m])2, [MUAC − (TSFT × π)]2/(4 × π), and [MUAC2/(4 × π)] − AMA, respectively (16).
Venous blood samples were obtained for HIV testing, determination of CD4 count, and genotyping. HIV status was determined in parallel using two tests: SD Bioline HIV-1/2 3.0 (Standard Diagnostics Inc., Kyonggi-do, South Korea) and Determine HIV-1/HIV-2 (Inverness Medical Innovations Inc., Delaware, USA). HIV coinfection was diagnosed if both tests gave a positive result, and an HIV-negative diagnosis was made if both tests gave a negative result. Indeterminate results were resolved using enzyme-linked immunosorbent assay (ELISA) (Organon Uniform II; Organon Teknika Ltd., Boxtel, Netherlands). Blood for CD4 quantification was drawn into 5-ml EDTA tubes, and CD4 count was then determined as cells/μl using a Coulter Epics XL-MCL flow cytometer (Beckman Coulter, Brea, CA).
Measurement of plasma levels of rifampin.
A pharmacokinetic (PK) profile was obtained on two occasions: the first around 7 ± 2 days after initiation of anti-TB therapy (except for 4 patients who were sampled between days 10 and 14) and the second around 56 days (the majority of patients were sampled on day 60, while on continuation phase). Participants did not eat or take any supplement overnight before the day of the pharmacokinetic evaluation and until 4 h after the intake of anti-TB medication, at which time they were offered a standardized meal. Venous blood for rifampin concentration measurements was drawn in lithium heparin tubes at 2, 4, and 6 h postdose. Plasma was immediately separated by centrifugation (3,000 rpm for 10 min), and aliquots were frozen within 30 min at −80°C until they were transported on dry ice to the Division of Clinical Pharmacology, University of Cape Town (UCT), Cape Town, South Africa. Rifampin plasma levels were determined using a validated tandem mass spectrometry–high-performance liquid chromatography (LC-MS/MS) method developed at the same site. An AB Sciex API mass spectrometer was operated at unit resolution in the multiple-reaction monitoring (MRM) mode, monitoring the transition of the protonated molecular ions at m/z 823.4 to the product ions at m/z 791.4 for rifampin and the protonated molecular ions at m/z 826.5 to the product ions at m/z 794.4 for the internal standard. The assay was validated over the concentration range of 0.117 to 30 μg/ml. During interday sample analysis, the accuracies (% Nom) for rifampin were 99.2%, 98.1%, and 99.4% at the low, medium, and high quality control (QC) levels, respectively. The level of precision had a percent coefficient of variation (%CV) of less than 3% at low, medium, and high QC levels. Rifampin concentrations of <0.117 μg/ml were reported as below the limit of quantification (BLQ).
Human gene analyses.
Genomic DNA was isolated from whole blood using the QIAamp DNA minikit obtained from Qiagen GmbH, Hilden, Germany. Genotyping was carried out at the Statens Serum Institut, Copenhagen, Denmark. Single nucleotide polymorphisms (SNPs) of the transporter gene (SLCO1B1) were detected by TaqMan real-time PCR. An annealing temperature of 60°C was used in all PCRs. The PCR products were sequenced using BigDye Terminator v1.1 cycle resequencing (ABI) and analyzed on an ABI 3730 DNA analyzer. Primer sequences are available on request. The resulting sequences were compared to NCBI accession no. NG_011745 (SLCO1B1) using Sequencher 5.0 software (Gene Codes, Ann Arbor, MI, USA). The resulting SLCO1B1 sequences were annotated for SNP rs4149032 (NG_011741: g.38664C>T).
Pharmacokinetics and statistical analysis.
A nonlinear mixed-effect model was employed to interpret the data and implemented in NONMEM 7.2 (17) using first-order conditional estimation with ETA-EPS interaction. A previously published model for rifampin pharmacokinetics (18) was used as a starting point for the structural model—one-compartment kinetics with first-order elimination and transit compartment absorption (19)—and the statistical model was optimized, including between-subject and between-occasion random effects supported by the current data and assumed to follow a log-normal distribution. Allometric scaling with either total body weight (WT) or fat-free mass (FFM) was applied to clearance (CL) and volume of distribution (V), as advocated in reference 20, and the effect of other covariates was tested and included in the model based on significant decreases in the value of the objective function value (OFV) and physiological plausibility. Covariates tested while investigating effects on pharmacokinetic parameters were HIV coinfection, nutritional supplementation, age, sex, CD4 count, BMI, daily weight-adjusted dose, time on TB treatment, and SLCO1B1 rs4149032 genotype. The effect of “time on TB treatment” on rifampin clearance autoinduction was captured using an exponential model based on a previous report (21) using the equation:
| (1) |
where CLday0 and CLSS are the values of CL on the first day of treatment and the end of the induction, respectively; t1/2 is the induction half-life; and t is the time on treatment in days.
A combined additive and proportional error model was used for the residual unexplained variability in the data, and BLQ data were handled similarly to the M6 method proposed in reference 22, imputing a lower limit of quantification (LLOQ/2) with the difference that only a large additive error was used for the BLQ samples, in order to minimize the impact of the imputation on the fit and on the estimates of the error structure of the measurements above the LLOQ. The OFV, goodness-of-fit plots, and visual predictive checks guided model development. The robustness of the final parameter estimates was assessed with a nonparametric bootstrap.
RESULTS
Of 469 screened patients, 100 met the inclusion criteria and were enrolled and randomized to nutritional supplementation or not and followed for 2 months (Fig. 1). Baseline characteristics of the 100 patients are shown in Table 1. The median (interquartile range [IQR]) age was 35 (29.5; 40.0) years, the median BMI was 18.8 (17.3; 19.8) kg/m2, and 58% of patients were male. There were no differences in baseline characteristics across the randomized groups. During follow-up, 3 patients died and 3 defaulted on the standard arm while only 1 died and 1 defaulted on the intervention arm. The organic anion-transporting polypeptide encoded by SLCO1B1 rs149032 responsible for hepatic drug disposition, which has been associated with lower rifampin exposure, was identified in 98 (98%) of the patients, of whom 6.0% were wild type (CC), 46% were heterozygous (CT), and 48.0% were homozygous (TT).
FIG 1.
Flow chart for pulmonary TB-positive patients randomized to receive a nutritional supplement or no supplement and followed for the effects of nutritional supplementation on rifampin exposure at the end of the second month of the intensive phase of treatment.
TABLE 1.
Comparison of baseline characteristics among 100 sputum-positive TB patients starting treatment randomized to nutritional supplementsa
| Characteristic | HIV uninfected |
HIV coinfected |
||
|---|---|---|---|---|
| Not supplemented (n = 25) | Supplemented (n = 25) | Not supplemented (n = 24) | Supplemented (n = 26) | |
| Median age (IQR), yr | 30 (27; 36) | 33 (30; 40) | 36 (32; 40) | 36 (32; 45) |
| Male sex, no. (%) | 16 (64.0) | 14 (56.0) | 14 (58.3) | 14 (53.8) |
| Current smoker, no. (%) | 6 (25.0) | 10 (40.0) | 7 (29.2) | 6 (23.1) |
| Drink alcohol (any vs none), no. (%) | 13 (52.0) | 8 (32.0) | 14 (60.9) | 16 (61.5) |
| TB treatment adherence of <95%, no. (%) | 3 (12.0) | 1 (4.0) | 3 (14.3) | 1 (3.9) |
| Median wt at day 0 (IQR), kg | 52 (44.6; 58.8) | 50.7 (48.2; 57.7) | 51.9 (49.2; 56.1) | 52.3 (49.9; 56.6) |
| Median wt at 2 mo (IQR), kg | 55.4 (45.3; 62.9) | 53 (51.0; 60.1) | 54.8 (51.0; 59.8) | 55.1 (49.4; 59.8) |
| Median BMI (IQR), kg/m2 | 18.6 (17.3; 19.6) | 18.0 (16.9; 19.7) | 18.8 (17.4; 20.1) | 19.4 (18.3; 20.5) |
| Median AFA (IQR), cm2 | 4.7 (3.9; 6.1) | 4.8 (5.5; 7.8) | 4.9 (3.8; 7.7) | 5.9 (4.6; 8.5) |
| Median AMA (IQR), cm2 | 41.2 (32.6; 46.6) | 38.2 (33.2; 47.2) | 39.2 (35.2; 41.5) | 43.3 (35.1; 47.7) |
| Median CD4 countb (IQR), cells/μl | 637 (433; 812) | 611 (457; 726) | 168 (64; 338) | 243.5 (140.5; 337.5) |
| SLCO1B1 rs4149032 genotype, no. (%)b | ||||
| CC | 1 (4.2) | 2 (8.0) | 1 (4.2) | 2 (8.0) |
| CT | 10 (41.7) | 13 (60.0) | 13 (54.2) | 11 (44.0) |
| TT | 13 (54.2) | 10 (40.0) | 10 (41.7) | 12 (48.0) |
Abbreviations: BMI, body mass index; AFA, arm fat area; AMA, arm muscle area; IQR, interquartile range.
The number of observations does not sum to 100 due to missing values.
In this study, the overall mean weight gain at the end of the intensive phase was 2.52 kg (P < 0.01). Among HIV-coinfected patients, mean weight gain was 2.4 kg for the group that received the supplementation and 1.8 kg for the unsupplemented group (P = 0.74). All but one patient sputum culture converted at the end of the intensive phase.
Population pharmacokinetic model.
A total of 574 rifampin concentrations were available for analysis, and only one was BLQ. The population pharmacokinetic final parameter estimates are shown in Table 2, and a prediction-corrected visual predictive check (pcVPC) (23) stratified by HIV status and nutritional supplementation is provided in Fig. 2. The structural component of the model was, as expected, a one-compartment, first-order absorption with transit compartment absorption. The model supported between-subject variability in clearance and between-occasion variability in bioavailability, absorption rate constant (ka), and mean absorption transit time (MTT). A negative correlation term between bioavailability and MTT was also found to be significant and was included in the model.
TABLE 2.
Rifampin pharmacokinetics parameter estimates among 100 newly diagnosed sputum-smear-positive TB patients
| Parameter description or variability | Typical value |
|
|---|---|---|
| Estimate | 90% CI | |
| Description (abbreviation) | ||
| Clearance on day 7a (CLday7) (liters/h) | 13.9 | 12.7; 15.0 |
| Clearance at steady statea (CLSS) (liters/h) | 16.5 | 15.0; 18.0 |
| Induction half-life (t1/2) (days) | 6 FIXED | NAb |
| Vol of distribution (V) (liters) | 55.8 | 51.4; 60.1 |
| Absorption rate constant (ka) (1/h) | 1.77 | 1.41; 2.34 |
| Absorption mean transit time (MTT) (h) | 1.50 | 1.35; 1.66 |
| No. of absorption transit compartment (NN) | 27.6 | 19.5; 46.7 |
| Bioavailability (BIO) | 1 FIXED | NA |
| % change in bioavailability for HIV+, no supplementation (+%) | −21.8 | −31.5; −10.3 |
| % change in bioavailability for HIV+, with supplementation (+%) | −13.3 | −22.1; −2.1 |
| % change in patients during the continuation phase of treatment (+%) | 13.7 | 3.5; 23.8 |
| % change in MTT (+%) and −ka (−%) in patients on supplementation | 26.9 | 14.0; 41.6 |
| Proportional error (%) | 13.7 | 11.6; 15.7 |
| Additive error (μg/ml) | 0.0417 | 0.0147; 0.0994 |
| Variability | ||
| Between-subject variability of clearance (%CV) | 24.0 | 18.3; 29.1 |
| Between-occasion variability of absorption rate constant (%CV) | 67.6 | 51.8; 85.5 |
| Between-occasion variability of mean transit time (%CV) | 34.0 | 25.1; 41.6 |
| Between-occasion variability of bioavailability (%CV) | 31.1 | 25.7; 35.5 |
| MTT-BIO correlation (%) | −25.2 | −44.7; 1.6 |
Steady state was estimated to last about 30 days or more.
NA, not applicable.
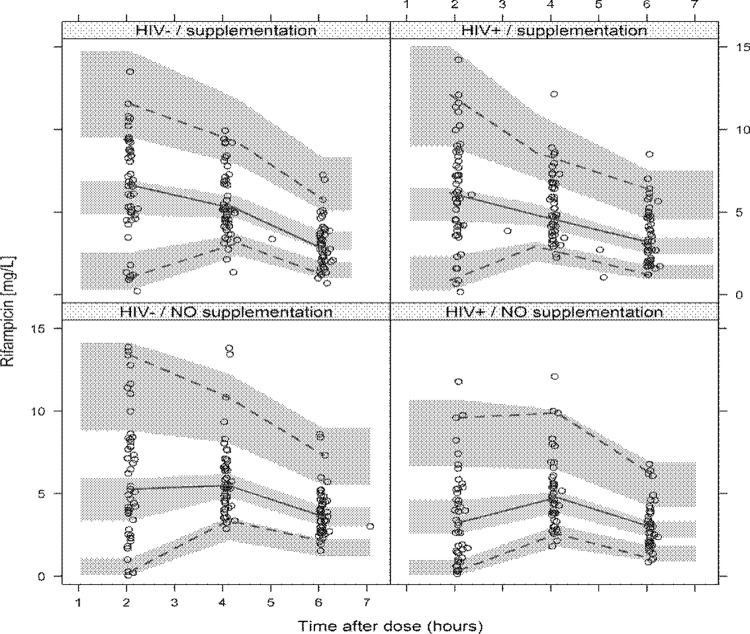
FIG 2.
Prediction-corrected visual predictive check (pcVPC) for rifampin concentration versus time, stratified by HIV status and use of nutritional supplementation. The circles represent the original data, and the dashed and solid lines are the 5th, 50th, and 95th percentiles of the original data, while the shaded areas are the corresponding 95% confidence intervals, as predicted by the model.
The best size predictor for allometric scaling of both CL and V was FFM, and CL was found to increase between the two occasions, due to autoinduction. This was described using an exponential increase model, with the half-life of the induction process fixed to 6 days, as previously reported (21). The current data did not allow proper characterization of this parameter, since PK profiles were mostly available around either day 7 or day 56, but a sensitivity analysis confirmed the small impact of this choice on the value of the other model parameters. Moreover, the model was parameterized to provide the typical values of CL on day 7 (CLday7, 13.9 liters/h) and when the steady state of the autoinduction was reached (CLSS, 16.6 liters/h). The model also identified increased bioavailability (+14%) for the patients who had already started the continuation phase on the second occasion sampled after day 56 of 2 months of treatment (Table 2).
After correcting for the above-mentioned effects and using the HIV-uninfected unsupplemented group as a reference, the model shows that bioavailability in HIV-coinfected patients without supplementation was 21.8% (90% confidence interval [CI], −31.5%; −10.3%) lower. In HIV-coinfected TB patients receiving supplementation, the reduction in bioavailability was only 13.3% (90% CI, −22.1%; −2.1%). Among HIV-uninfected patients, nutritional supplementation was not found to have any significant effect on bioavailability or CL. Nutritional supplementation, regardless of HIV status, was found to increase the rate of absorption (MTT, −27%, and ka, +27%). None of the other covariates tested in the model (age, sex, CD4 count, BMI, total dose, and SLCO1B1 rs4149032 genotype) were found to have a significant effect the on pharmacokinetic parameters.
Based on these parameter values, the model was used to predict the typical effect of nutritional supplementation and HIV coinfection on rifampin exposure (peak concentration [Cmax] and 0- to 24-h area under the concentration-time curve [AUC0–24]). The model was applied to a standard subject, a patient with 43 kg of FFM, given 600 mg of rifampin daily and sampled on day 56 of treatment while still in the intensive phase of treatment. The simulation was repeated 5,000 times for each of the four possible scenarios: HIV coinfected, non-HIV infected, supplemented, and unsupplemented. For HIV coinfection on supplementation, the median Cmax and AUC0–24 (90% range) were 6.4 (3.5; 11.2) μg/ml and 31.6 (16.4; 60.3) μg · h/ml, respectively, compared to a Cmax of 5.6 (3.0; 9.9) μg/ml and an AUC0–24 of 28.6 (15.0; 54.8) μg · h/ml without supplementation. For no HIV coinfection, supplementation resulted in slightly higher median Cmax (7.4 μg/ml versus 7.1 μg/ml), but this difference is minimal, and no difference was predicted in AUC0–24 (Table 3).
TABLE 3.
Predicted pharmacokinetic exposures for a typical patient with or without HIV coinfection, randomized to nutritional supplementationa
| Group | Rifampin estimate parameter |
|
|---|---|---|
| Median Cmaxb (90% range) | Median AUC0–24c (90% range) | |
| HIV uninfected | ||
| Unsupplemented | 7.1 (3.9; 12.6) | 36.6 (19.1; 70.3) |
| Supplemented | 7.4 (4.1; 12.9) | 36.5 (19.1; 69.8) |
| HIV coinfected | ||
| Unsupplemented | 5.6 (3.0; 9.9) | 28.6 (15.0; 54.8) |
| Supplemented | 6.4 (3.5; 11.2) | 31.6 (16.4; 60.3) |
Model-predicted rifampin pharmacokinetics: values were obtained by simulating (5,000 times for each group) the pharmacokinetic profile of a subject with 43 kg of fat-free mass, receiving 600 mg of rifampin during the intensive phase of treatment, and with the autoinduction of rifampin CL already at steady state.
Cmax, peak plasma concentrations (μg/ml).
AUC0–24, steady-state area under the plasma drug concentration-time curve from time zero to 24 h (μg · h/ml).
DISCUSSION
We undertook a randomized, controlled trial in order to evaluate the effect of nutritional supplementation on rifampin pharmacokinetics in pulmonary TB patients with or without HIV coinfection. We found that nutritional supplementation during the first 2 months of TB treatment had a positive effect on rifampin exposure in HIV-coinfected patients, significantly mitigating the negative effect of HIV coinfection, which caused a 22% lower bioavailability, a median Cmax of only 5.6 μg/ml, and an AUC0–24 of 28.6 μg · h/ml. In fact, intake of nutritional supplementation diminished the decrease in bioavailability among HIV-coinfected patients to 13.3% and thus attained higher rifampin exposure (Cmax, 6.4 μg/ml, and AUC0–24, 31.6 μg · h/ml). Moreover, the use of supplementation per se, irrespective of HIV status, was found to enhance absorption speed by 27%, thus slightly increasing Cmax. These results offer an explanation for the beneficial effect of nutritional supplementation reported previously in other studies where enhanced clearance of bacteria, radiographic improvement, and greater weight gain have been reported (24–26). In this study, all patients had sputum culture converted with the exception of one patient at the end of the intensive phase; thus, we could not determine the effect of supplementation on sputum culture conversion at 2 months, as the study was not powered to detect it. In addition, we did not determine culture between PK samplings, and thus, we do not know whether supplementation had any role in the overall time to sputum conversion.
HIV is known to target the small intestine, especially the gut-associated lymphoid tissue, and HIV-mediated enteropathy is associated with malabsorption (27). Decrease in the functional absorptive area of the intestine has been associated with reduced TB drug availability (28–31). The recovery mechanism whereby nutritional supplementation may restore the pathophysiology and immunological changes is not explained and warrants further studies (26). Semba has suggested that nutritional supplementation may play a role in maintenance of intestinal mucosal epithelia responsible for absorption (32). Lack of significant differences in the effect of nutritional supplementation on rifampin exposure among HIV-uninfected patients suggests that the effect of the intervention in our study is mainly repair of an HIV-induced defect. The model detected a larger bioavailability (+14%) during the continuation phase of treatment. Most patients on the second PK sampling occasion were on continuation phase, but the model provided a better fit when “continuation phase,” rather than “second PK occasion,” was used as a predictor in the fit.
This greater bioavailability could be explained by a formulation effect, in that the continuation phase consists of only two drugs (isoniazid and rifampin). Another possible explanation is the recovery of the intestinal mucosa responsible for absorption after 2 months of treatment.
Poor nutritional status is commonly seen among TB patients and has been associated with unfavorable treatment outcome, as reported in a study from Malawi (33). A complex set of interactions between the host and the virulent infecting organisms alters body metabolism and may result in patients wasting due to loss of appetite, nutrient malabsorption, and increase of energy expenditure (26, 34). The majority of our participants were moderately wasted, and among unsupplemented patients, the HIV-coinfected patients had lower rifampin Cmax and AUC0–24 than did HIV-uninfected patients. Low rifampin exposure among undernourished TB HIV-coinfected patients has been reported previously; a study by Polasa et al. assessing rifampin kinetics in undernourished individuals shows that AUC and Cmax were reduced in undernourished individuals compared to individuals with normal BMI (9). McIlleron et al. also reported reduced rifampin exposure among undernourished HIV-coinfected TB patients in studies from South Africa (8, 35). A recent study from South Africa by Pasipanodya et al. has also shown that serum drug concentrations predicted clinical outcome in TB patients and that low rifampin and isoniazid Cmax and AUC preceded therapy failure and occurrence of multidrug resistance or extremely drug-resistant TB (36). The previously reported benefits associated with nutritional supplementation (24–26) could be explained by the achievement of higher drug concentrations, and the results presented here seem to corroborate this hypothesis. We have no indication of increased adverse events in the patients receiving the nutritional intervention (data not presented). Therefore, this simple intervention could be suggested in the management of HIV-coinfected, underweight TB patients at risk of poor treatment outcome.
In this study, we found no effect of SLCO1B1 genotype on rifampin exposure. SLCO1B1 encodes a liver-specific transmembrane protein belonging to the solute carrier organic anion transporter protein family 1B1. The protein is involved in hepatic uptake of a number of drugs from the portal blood, including rifampin, and polymorphisms in this gene have been suggested to explain differences in drug levels. We found a high frequency of SLCO1B1 rs4149032 polymorphisms in our study population, comparable to that reported from other parts of Africa (37). Surprisingly, SLCO1B1 genotypes were not found to affect drug exposure in our study population. These results are in contrast to those reported by Weiner et al. and Chigutsa et al., who have reported that SLCO1B1 rs4149032 was associated with substantially reduced rifampin exposure (37, 38). However, our results are in line with those from a Chinese study (39). This suggests that studies are warranted to further elucidate the effect of SLCO1B1 genotype on the pharmacokinetics of rifampin in different populations.
Study strengths and limitations.
The study was well conducted and based on a randomized controlled trial in a country in which TB is highly endemic, the planned sample size was reached, and the overall follow-up was good. The trial was conducted while it was not mandatory that HIV-coinfected patients initiate ART immediately after the HIV diagnosis, which reduced the chance of ART and anti-TB drug interaction. A limitation of this trial is the small number of time points for pharmacokinetic assessment, thus limiting characterization of the absorption phase of the pharmacokinetic curve. But, the use of nonlinear mixed-effect modeling managed nonetheless to detect significant differences among the different cohorts. The study was not powered to assess the outcome of TB treatment.
Conclusion.
In summary, we demonstrated that among HIV-coinfected TB patients, the use of nutritional supplementation results in higher rifampin exposure, mitigating the detrimental effect of HIV coinfection on rifampin drug levels. The SLCO1B1 genotype did not alter rifampin exposure in this population. The use of defined nutritional supplementation for HIV-coinfected TB patients should be considered in TB control programs if these data are confirmed in other studies.
ACKNOWLEDGMENTS
We thank all the health staff and study participants involved in the study. Special thanks go to Christian Ritz, a senior statistician at the Department of Nutrition, Exercise and Sports, Faculty of Science, University of Copenhagen, Denmark, for his statistical analysis advice and to Oswald Kaswamila, Lucy Magawa, and David Madili at the National Institute for Medical Research-Mwanza Center for excellent laboratory and clinical work assistance.
This work was supported by the Danish Ministry of Foreign Affairs (DANIDA, DFC file no. 09-026RH) through Denmark's International Development Cooperation.
Footnotes
Published ahead of print 7 April 2014
REFERENCES
- 1.Raviglione M, Marais B, Floyd K, Lonnroth K, Getahun H, Migliori GB, Harries AD, Nunn P, Lienhardt C, Graham S, Chakaya J, Weyer K, Cole S, Kaufmann SH, Zumla A. 2012. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet 379:1902–1913. 10.1016/S0140-6736(12)60727-2 [DOI] [PubMed] [Google Scholar]
- 2.Van Lettow M, Fawzi WW, Semba RD. 2003. Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutr. Rev. 61:81–90. 10.1301/nr.2003.marr.81-90 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2011. Global HIV/AIDS response epidemic update and health sector progress towards universal access: progress report 2011. World Health Organization, Geneva, Switzerland [Google Scholar]
- 4.World Health Organization. 2013. Global tuberculosis report 2013. World Health Organization, Geneva, Switzerland [Google Scholar]
- 5.World Health Organization. 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB) 2010 global report on surveillance and response. World Health Organization, Geneva, Switzerland [Google Scholar]
- 6.Ministry of Health and Social Welfare. 2006. Manual of the national tuberculosis and leprosy programme in Tanzania, 5th ed. Ministry of Health and Social Welfare, Dar es Salaam, Tanzania [Google Scholar]
- 7.Pasipanodya JG, Srivastava S, Gumbo T. 2012. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin. Infect. Dis. 55:169–177. 10.1093/cid/cis353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. 2006. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob. Agents Chemother. 50:1170–1177. 10.1128/AAC.50.4.1170-1177.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polasa K, Murthy KJ, Krishnaswamy K. 1984. Rifampicin kinetics in undernutrition. Br. J. Clin. Pharmacol. 17:481–484. 10.1111/j.1365-2125.1984.tb02377.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onwubalili JK. 1988. Malnutrition among tuberculosis patients in Harrow, England. Eur. J. Clin. Nutr. 42:363–366 [PubMed] [Google Scholar]
- 11.Range NS, Malenganisho W, Temu MM, Changalucha J, Magnussen P, Krarup H, Andersen AB, Friis H. 2010. Body composition of HIV-positive patients with pulmonary tuberculosis: a cross-sectional study in Mwanza, Tanzania. Ann. Trop. Med. Parasitol. 104:81–90. 10.1179/136485910X12607012373830 [DOI] [PubMed] [Google Scholar]
- 12.Range N, Andersen AB, Magnussen P, Mugomela A, Friis H. 2005. The effect of micronutrient supplementation on treatment outcome in patients with pulmonary tuberculosis: a randomized controlled trial in Mwanza, Tanzania. Trop. Med. Int. Health 10:826–832. 10.1111/j.1365-3156.2005.01463.x [DOI] [PubMed] [Google Scholar]
- 13.Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. 2006. The effect of multi-vitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two-by-two factorial trial in Mwanza, Tanzania. Br. J. Nutr. 95:762–770. 10.1079/BJN20051684 [DOI] [PubMed] [Google Scholar]
- 14.Academy of Science of South Africa. 2007. HIV/AIDS, TB and nutrition: scientific inquiry into the nutritional influences on human immunity with special reference to HIV infection and active TB in South Africa. Academy of Science of South Africa, Pretoria, South Africa [Google Scholar]
- 15.PrayGod G, Range N, Faurholt-Jepsen D, Jeremiah K, Faurholt-Jepsen M, Aabye MG, Jensen L, Jensen AV, Grewal HM, Magnussen P, Changalucha J, Andersen AB, Friis H. 2011. Daily multi-micronutrient supplementation during tuberculosis treatment increases weight and grip strength among HIV-uninfected but not HIV-infected patients in Mwanza, Tanzania. J. Nutr. 141:685–691. 10.3945/jn.110.131672 [DOI] [PubMed] [Google Scholar]
- 16.Frisancho AR. 1990. Anthropometric standards for the assessment of growth and nutritional status. University of Michigan Press, Ann Arbor, MI [Google Scholar]
- 17.Beal S, Sheiner L, Boeckmann A, Bauer R. 2009. NONMEM users guides (1989–2009). ICON Development Solutions, Ellicott City, MD [Google Scholar]
- 18.Wilkins JJ, Savic RM, Karlsson MO, Langdon G, McIlleron H, Pillai G, Smith PJ, Simonsson US. 2008. Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption. Antimicrob. Agents Chemother. 52:2138–2148. 10.1128/AAC.00461-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savic RM, Karlsson MO. 2009. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J. 11:558–569. 10.1208/s12248-009-9133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson BJ, Holford NH. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48:303–332. 10.1146/annurev.pharmtox.48.113006.094708 [DOI] [PubMed] [Google Scholar]
- 21.Denti P, Smythe W, Simonsson US, Rustomjee R, Onyebujoh P, Smith P, McIlleron H. 2010. A population pharmacokinetic model for rifampicin auto-induction, abstr 12. Proc. 3rd Int. Workshop Clin. Pharmacol. TB Drugs, Boston, MA [Google Scholar]
- 22.Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481–504. 10.1023/A:1012299115260 [DOI] [PubMed] [Google Scholar]
- 23.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 13:143–151. 10.1208/s12248-011-9255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karyadi E, West CE, Schultink W, Nelwan RH, Gross R, Amin Z, Dolmans WM, Schlebusch H, van der Meer JW. 2002. A double-blind, placebo-controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. Am. J. Clin. Nutr. 75:720–727 [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishnan CV, Rajendran K, Jacob PG, Fox W, Radhakrishna S. 1961. The role of diet in the treatment of pulmonary tuberculosis. An evaluation in a controlled chemotherapy study in home and sanatorium patients in South India. Bull. World Health Organ. 25:339–359 [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta KB, Gupta R, Atreja A, Verma M, Vishvkarma S. 2009. Tuberculosis and nutrition. Lung India 26:9–16. 10.4103/0970-2113.45198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delezay O, Yahi N, Tamalet C, Baghdiguian S, Boudier JA, Fantini J. 1997. Direct effect of type 1 human immunodeficiency virus (HIV-1) on intestinal epithelial cell differentiation: relationship to HIV-1 enteropathy. Virology 238:231–242. 10.1006/viro.1997.8829 [DOI] [PubMed] [Google Scholar]
- 28.Facanha MC, Gondim AM, Pinheiro VG, Barroso EC, Peloquin CA, Guerrant RL, Lima AA. 2009. Intestinal barrier function and serum concentrations of rifampin, isoniazid and pyrazinamide in patients with pulmonary tuberculosis. Braz. J. Infect. Dis. 13:210–217. 10.1590/S1413-86702009000300011 [DOI] [PubMed] [Google Scholar]
- 29.Pinheiro VG, Ramos LM, Monteiro HS, Barroso EC, Bushen OY, Facanha MC, Peloquin CA, Guerrant RL, Lima AA. 2006. Intestinal permeability and malabsorption of rifampin and isoniazid in active pulmonary tuberculosis. Braz. J. Infect. Dis. 10:374–379. 10.1590/S1413-86702006000600003 [DOI] [PubMed] [Google Scholar]
- 30.Gurumurthy P, Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Padmapriyadarsini C, Swaminathan S, Venkatesan P, Sekar L, Kumar S, Krishnarajasekhar OR, Paramesh P. 2004. Malabsorption of rifampin and isoniazid in HIV-infected patients with and without tuberculosis. Clin. Infect. Dis. 38:280–283. 10.1086/380795 [DOI] [PubMed] [Google Scholar]
- 31.Gurumurthy P, Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Padmapriyadarsini C, Swaminathan S, Bhagavathy S, Venkatesan P, Sekar L, Mahilmaran A, Ravichandran N, Paramesh P. 2004. Decreased bioavailability of rifampin and other antituberculosis drugs in patients with advanced human immunodeficiency virus disease. Antimicrob. Agents Chemother. 48:4473–4475. 10.1128/AAC.48.11.4473-4475.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semba RD. 1998. The role of vitamin A and related retinoids in immune function. Nutr. Rev. 56:38–48 [DOI] [PubMed] [Google Scholar]
- 33.Zachariah R, Spielmann MP, Harries AD, Salaniponi FM. 2002. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans. R. Soc. Trop. Med. Hyg. 96:291–294. 10.1016/S0035-9203(02)90103-3 [DOI] [PubMed] [Google Scholar]
- 34.Macallan DC, McNurlan MA, Kurpad AV, de Souza G, Shetty PS, Calder AG, Griffin GE. 1998. Whole body protein metabolism in human pulmonary tuberculosis and undernutrition: evidence for anabolic block in tuberculosis. Clin. Sci. (Lond.) 94:321–331 [DOI] [PubMed] [Google Scholar]
- 35.McIlleron H, Rustomjee R, Vahedi M, Mthiyane T, Denti P, Connolly C, Rida W, Pym A, Smith PJ, Onyebujoh PC. 2012. Reduced antituberculosis drug concentrations in HIV-infected patients who are men or have low weight: implications for international dosing guidelines. Antimicrob. Agents Chemother. 56:3232–3238. 10.1128/AAC.05526-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J. Infect. Dis. 208:1464–1473. 10.1093/infdis/jit352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chigutsa E, Visser ME, Swart EC, Denti P, Pushpakom S, Egan D, Holford NH, Smith PJ, Maartens G, Owen A, McIlleron H. 2011. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob. Agents Chemother. 55:4122–4127. 10.1128/AAC.01833-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiner M, Peloquin C, Burman W, Luo CC, Engle M, Prihoda TJ, Mac Kenzie WR, Bliven-Sizemore E, Johnson JL, Vernon A. 2010. Effects of tuberculosis, race, and human gene SLCO1B1 polymorphisms on rifampin concentrations. Antimicrob. Agents Chemother. 54:4192–4200. 10.1128/AAC.00353-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He YJ, Zhang W, Chen Y, Guo D, Tu JH, Xu LY, Tan ZR, Chen BL, Li Z, Zhou G, Yu BN, Kirchheiner J, Zhou HH. 2009. Rifampicin alters atorvastatin plasma concentration on the basis of SLCO1B1 521T>C polymorphism. Clin. Chim. Acta 405:49–52. 10.1016/j.cca.2009.04.003 [DOI] [PubMed] [Google Scholar]