Abstract
Bacterial vaginosis (BV) is a common vaginal disorder characterized by the decrease of lactobacilli and overgrowth of Gardnerella vaginalis and resident anaerobic vaginal bacteria. In the present work, the effects of rifaximin vaginal tablets on vaginal microbiota and metabolome of women affected by BV were investigated by combining quantitative PCR and a metabolomic approach based on 1H nuclear magnetic resonance. To highlight the general trends of the bacterial communities and metabolomic profiles in response to the antibiotic/placebo therapy, a multivariate statistical strategy was set up based on the trajectories traced by vaginal samples in a principal component analysis space. Our data demonstrated the efficacy of rifaximin in restoring a health-like condition in terms of both bacterial communities and metabolomic features. In particular, rifaximin treatment was significantly associated with an increase in the lactobacillus/BV-related bacteria ratio, as well as with an increase in lactic acid concentration and a decrease of a pool of metabolites typically produced by BV-related bacteria (acetic acid, succinate, short-chain fatty acids, and biogenic amines). Among the tested dosages of rifaximin (100 and 25 mg for 5 days and 100 mg for 2 days), 25 mg for 5 days was found to be the most effective.
INTRODUCTION
Bacterial vaginosis (BV) is a complex polymicrobial vaginal disorder associated with an increase in the taxonomic richness and diversity of the vaginal microbiota. BV is microbiologically characterized by replacement of the lactobacillus-predominant vaginal microbiota by potential pathogenic anaerobic bacteria (1, 2). The diagnosis of BV is based on Amsel's criteria (1) and Nugent score determinations (3). The current recommended treatment of BV includes metronidazole (oral or vaginal) or clindamycin (vaginal) (4); however, the short-term (30 days) cure rate is often poor, and recurrences are common (5, 6).
Rifaximin is a new candidate for the local treatment of BV thanks to its antibacterial activity, which covers Gardnerella vaginalis and other pathogens responsible for urogenital infections (7, 8). Rifaximin is a semisynthetic rifamycin derivative characterized by low systemic absorption and good antibacterial activity. In common with the structural analogue rifampin and other members of the rifamycin class, it acts on the β subunit of the bacterial RNA polymerase to inhibit RNA synthesis (9, 10). Recently, the efficacy of rifaximin vaginal tablets in the treatment of BV was demonstrated by evaluating the rate of clinical remission (11) and the restoration of normal vaginal communities (12) and proteome profiles (13).
BV, as well as antibiotics used for the treatment of this disturbance, cause perturbations of the vaginal ecosystem, which are reflected in the overall profile of the metabolites produced by the host-bacterium metaorganism. The study of the comprehensive modifications occurring in the metabolic profiles of living systems actually represents the main field of metabolomics. Metabolomics is, by definition, the study of the effects of an external perturbation on a biological system by looking for changes in its metabolome, that is, the complete set of small-molecule metabolites within it (14). Some of these molecules represent nodes of a high number of metabolic cycles, so that little variations in the balance between the microorganisms of an environment can lead to amplified and more easily studied metabolome modifications (15, 16).
High-resolution 1H nuclear magnetic resonance (HR-NMR) spectroscopy, when applied to metabolomic studies, has provided significant information on a wide range of pathologies, like cancer (17), meningitis (18), and coronary heart disease (19), as well as a variety of gastrointestinal diseases (20). This is because an NMR profile contains qualitative and quantitative information on the hundreds of different small molecules present in a sample at >1 μM concentration (21). Moreover, NMR-based metabolomics makes no assumption on the identity of the metabolites that are relevant for the selected pathology, because information on the significant metabolic pattern alterations is directly obtained through statistical analysis of the NMR profiles (22, 23).
The present work describes a combined approach based on quantitative PCR (qPCR) of bacterial 16S rRNA genes and metabolomics to characterize the vaginal environment of women affected by BV as well as to search for modifications induced by the administration of different dosages of rifaximin vaginal tablets. To the best of our knowledge, this is the first work describing the vaginal environment through a metabolomic approach based on NMR, as well as the first describing, by means of this technique, the evolution of a vaginal syndrome following the administration of a drug.
MATERIALS AND METHODS
Study design and sample preparation.
Vaginal samples from 92 premenopausal women affected by BV were analyzed in the present study. Demographic characteristics and sexual habits of these patients are reported in Table 1. The patients had previously been enrolled in a multicenter, double-blind, randomized, placebo-controlled study (EudraCT; 2009-011826-32) performed to compare the efficacy of rifaximin vaginal tablets to that of the placebo for the treatment of BV (11, 12). Diagnosis of BV was made using both Amsel's criteria and Nugent score at the screening visit (V1). Patients with a Nugent score of >3 and positive for ≥3 of Amsel's criteria were considered positive for BV. At the randomization visit (V2), the patients were distributed into 4 treatment groups: group A received a 100-mg rifaximin vaginal tablet once daily for 5 days (n = 23), group B received a 25-mg rifaximin vaginal tablet once daily for 5 days (n = 23), group C received a 100-mg rifaximin vaginal tablet once daily for the first 2 days and a placebo vaginal tablet for the remaining 3 days (n = 23), and group D received a placebo vaginal tablet once daily for 5 days (n = 23). The medication was dispensed by the investigator in accordance with a computer-generated random allocation sequence (SAS release 9.2; SAS Institute, Cary, NC, USA) prepared by a statistician who was not blind to the treatment regimen. Seven days after the end of the therapy, the first follow-up visit (V3) was performed. Patients showing remission according to Amsel's criteria (≤2) and Gram stain Nugent score (≤3) attended the second follow-up visit (V4) 28 days after the end of the treatment. Participating women were required to abstain from intercourse during the 5-day treatment period and for 3 days before the follow-up visits, to avoid the use of intravaginal products, including douches, sprays, tampons, spermicides, gels, foams, and diaphragms, and to adopt an adequate contraceptive method during the study. Women were excluded from the study if they were pregnant or breast-feeding, anticipated menses at screening or follow-up visits, or had received systemic or vaginal antimicrobial therapy in the 2 weeks before the study.
TABLE 1.
Demographic characteristics and sexual habits of the patients analyzed
| Characteristica | Result by dosing regimenb |
|||
|---|---|---|---|---|
| A (100 mg/day for 5 days) (n = 23) | B (25 mg/day for 5 days) (n = 23) | C (100 mg/day for 2 days) (n = 23) | D (placebo for 5 days) (n = 23) | |
| Age (yrs) | 35.0 ± 8.8 | 36.1 ± 9.2 | 37.0 ± 7.9 | 35.3 ± 10.8 |
| % Caucasian | 100 | 100 | 100 | 100 |
| Weight (kg) | 63.4 ± 13.0 | 64.5 ± 14.8 | 62.9 ± 13.6 | 63.9 ± 10.3 |
| Height (cm) | 165.8 ± 6.3 | 166.7 ± 5.2 | 165.0 ± 7.3 | 167.0 ± 6.4 |
| BMI (kg/m2) | 23.1 ± 4.7 | 23.2 ± 5.0 | 23.1 ± 4.7 | 22.9 ± 3.2 |
| History of STDs (%) | 14.3 | 3.8 | 3.8 | 7.7 |
| Previous vaginal intercourse (%) | 100 | 100 | 100 | 100 |
| Contraception method (%) | ||||
| Male partner with vasectomy | 0.0 | 7.7 | 7.7 | 11.5 |
| Abstinence | 25.0 | 23.1 | 11.5 | 15.4 |
| Intrauterine device | 14.3 | 26.9 | 38.5 | 23.1 |
| Steroidal contraceptive | 42.9 | 42.3 | 38.5 | 46.2 |
| Other | 0.0 | 0.0 | 0.0 | 3.8 |
| Surgically sterile | 17.8 | 0.0 | 3.8 | 0.0 |
| No. current sexual partners (%) | ||||
| 0 | 7.1 | 15.4 | 3.8 | 11.6 |
| 1 | 89.3 | 80.8 | 88.5 | 84.6 |
| >1 | 3.6 | 3.8 | 7.7 | 3.8 |
BMI, body mass index; STDs, sexually transmitted diseases.
Values are given as means ± standard deviations or percentages.
A control group consisted of 10 healthy subjects who had no signs of vaginal tract infection and had never had BV. These patients were enrolled in the Heilig Hart Hospital of Tienen (Belgium) and signed informed consent in accordance with the approval from the local ethics committee.
Rifaximin and placebo vaginal tablets were purchased from Alfa Wassermann S.p.A., Bologna, Italy.
Standardized vaginal rinsings with 2 ml of saline were collected at V1, V3, and V4 by flushing and reaspirating the fluid through a 22-gauge needle in the left, central, and right upper vaginal vaults (24) and stored at −80°C until use.
One ml of each vaginal rinsing was centrifuged at 9,500 × g for 15 min to separate the pellet, which was processed for bacterial DNA isolation, from the supernatant used for metabolomic analysis.
DNA isolation was carried out as previously described (25). The DNA amount was quantified using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE).
qPCR.
Quantitative PCR (qPCR) was performed on DNA samples extracted from vaginal fluids using a LightCycler instrument (Roche, Mannheim, Germany) and SYBR green I as the reporter fluorophore. Genus- or species-specific primer sets targeted to the 16S rRNA gene or 16S-23S rRNA spacer region were used to amplify bacteria belonging to Lactobacillus (Bact-0011f/Lab-0677r [26]), Gardnerella vaginalis (F-GV1/R-GV3 [27]), Atopobium (c-Atopo-f/c-Atopo-r [28]), Prevotella (g-Prevo-f/g-Prevo-r [29]), Veillonella (VeilloF/VeilloR [30]), Mycoplasma hominis (MycF/MycR [31]), and Mobiluncus (Mob-s/Mob-as [32]). The reaction mixture contained each primer at 0.5 μM, 4 μl of LightCycler-FastStart DNA master SYBR green I (Roche), and 2 μl of template (final volume of 20 μl). The thermal cycling conditions were optimized as an initial denaturation step at 95°C for 10 min followed by 30 (Lactobacillus, Atopobium, G. vaginalis, Veillonella, and Mobiluncus), 35 (Prevotella), or 40 (M. hominis) cycles of denaturation at 95°C for 15 s; primer annealing at 63°C (Lactobacillus and Mobiluncus), 62°C (Veillonella and M. hominis), or 60°C (Atopobium, Prevotella, and G. vaginalis) for 20 s; extension at 72°C for 45 s (Lactobacillus, Atopobium, Prevotella, G. vaginalis, and Veillonella) or 30 s (M. hominis and Mobiluncus); and a fluorescence acquisition step at 82°C (M. hominis), 85°C (Lactobacillus, Atopobium, G. vaginalis, and Veillonella), 87°C (Prevotella), or 88°C (Mobiluncus) for 5 s. Amplifications were performed in triplicate for each primer set. Data were expressed as ng of DNA of the targeted genus or species per μg of total DNA extracted from the vaginal sample.
1H NMR measurements.
One ml of thawed sample was centrifuged at 14,000 × g for 5 min and then added to 160 μl of a D2O solution of 6.25 mM 3-(trimethylsilyl)-propionic-2,2,3,3–d4 acid sodium salt (TSP). The pH was adjusted to 7.00 with the addition of 0.5 M solution of HCl or NaOH. 1H-NMR spectra were recorded at 298 K with an Avance spectrometer (Bruker, Milan, Italy) operating at a frequency of 600.13 MHz and equipped with an autosampler with 60 holders. The HOD residual signal was suppressed by applying the first increment of the nuclear Overhauser effect spectroscopy (NOESY) pulse sequence and a spoil gradient (33, 34). This was done by employing the NOESYGPPR1D sequence, part of the standard pulse sequence library. Each spectrum was acquired using 32,000 data points over a 7,211.54 Hz spectral width and adding 256 transients. A recycle delay of 5 s and a 90° pulse of 11.4 μs were set up. Acquisition time (2.27 s) and recycle delay were adjusted to be 5 times longer than the longitudinal relaxation time of the protons under investigation, which was considered to be not longer than 1.4 s.
The peaks were assigned by comparing their chemical shift and multiplicity to data in the literature (35) and the Amix software data bank (ver. 3.9.7; Bruker, Italy).
Data analysis.
Statistical analyses were performed using the R computational language (36). Each NMR spectrum was processed by means of scripts developed in-house as follows. The spectrum baseline was adjusted by employing the peak identification algorithm described by Coombes et al. (37) and implemented by Liland et al. (38) under the name “baseline.peakDetection”. Spectra were normalized by means of the probabilistic quotient normalization method (39). Similarities among the analyzed samples, as well as trends in their microbial composition or metabolome profile, were investigated by means of principal component analysis (PCA). For this purpose, the data were means centered and subjected to singular value decomposition (40). PCA calculates linear combinations of the calculated variables, points of NMR spectra, or bacterial concentrations to build new axes, called principal components (PC), oriented along the maximum variance directions. The results of PC analysis were presented in the text as biplots that are superimpositions of scoreplots and loadingplots. The scoreplot is the representation of the samples in the PC space, while the loadingplot is a graphical representation of the variables which contribute most to the spreading of the samples. The scoreplot is one of the best options to highlight the trends characterizing the samples, while the loadingplot highlights the molecules determining the trends so identified. Differences in the amounts of bacterial genera and species, in metabolite concentrations, and in PCA data were analyzed using Wilcoxon's signed-rank test. Unless differently specified, a P value below 0.01 was considered significant in all statistical tests.
RESULTS
Clinical results.
Remission (R) or nonremission (N) was evaluated at the first (V3) and second (V4) follow-up visits by the combined assessment of Amsel's criteria (clinical cure) and the Gram stain Nugent scoring system (Fig. 1). The highest remission rate at V3 was found for group B (12/23), which also showed the best remission maintenance at V4 (7/12). Notably, the lowest rate of remission at V3 was found for the placebo group (4/19), which recorded a good maintenance of spontaneous remission at V4 (2/4). Similarly, in the work of Donders et al. (11), treatment group B showed the best therapeutic cure and maintenance versus placebo. Demographic parameters and sexual behavior (Table 1) did not influence clinical remission, as previously reported (11).
FIG 1.
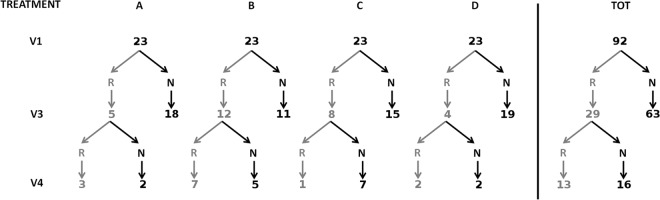
Remission (R) or nonremission (N) evaluated at the follow-up visits V3 and V4 for the treatment groups A (100 mg/day for 5 days), B (25 mg/day for 5 days), C (100 mg/day for 2 days), and D (placebo for 5 days) and for the totality of the women (TOT).
Short-term impact of rifaximin/placebo on vaginal microbiota.
Lactobacillus, G. vaginalis, Atopobium, Prevotella, Veillonella, M. hominis, and Mobiluncus were quantified by qPCR (see Table S1 in the supplemental material) in order to investigate the effects of rifaximin on the principal bacterial genera and species which are unbalanced in the presence of BV (41–44). Table 2 lists the bacterial groups that significantly changed between V1 and V3 in women going into remission at V3. A significant decrease was observed for G. vaginalis, Atopobium, and Prevotella, confirming that these bacteria are specific markers for BV condition (42). To obtain an overview of the variations in bacterial populations associated with remission, a PCA was performed on the qPCR data related to BV-affected women at V1 and V3 and to healthy controls (Fig. 2A). The first two PCs accounted for 44.5% of the whole variance of the investigated samples. Each group of women was spread considerably on the built space, showing that a great source of variability was represented by the individual differences existing among the vaginal microbial communities of different subjects. The general impact exerted by rifaximin/placebo treatment on the vaginal microbiota composition was highlighted by plotting the medians from the groups of women. The medians from the samples collected at V1 and V3 from BV-affected women going into remission (V1→R-V3) were significantly different, showing that a part of the sample variability was due to the evolution of BV syndrome. For these women, remission caused an increase of the lactobacillus/BV-related bacteria ratio and, consequently, a trajectory approaching that of healthy controls in the scoreplot. In this respect, Atopobium, Prevotella, Veillonella, and M. hominis underwent the greatest changes, as revealed by the loadingplot superimposed on the scoreplot. Interestingly, the lactobacillus/BV-related bacteria ratio also significantly increased in the women where BV persisted at V3 (V1→N-V3). In detail, the distance covered by the medians of such samples was 74% of the one covered by women going into remission. Notably, on average, women going into remission showed at the baseline a more favorable microbiological environment than the others.
TABLE 2.
Concentration of bacterial groups which significantly varied (P < 0.05) between V1 and V3 in women going into remission at V3 (n = 29)
| Bacterial group | V1a | V3a | V3–V1b |
|---|---|---|---|
| G. vaginalis | 4.48E + 01 | 0.00E + 00 | −4.48E + 01 |
| Atopobium | 5.97E + 01 | 7.88E + 00 | −2.51E + 01 |
| Prevotella | 8.88E + 00 | 7.61E − 02 | −6.73E + 00 |
Values are expressed as medians of ng of target DNA/μg vaginal genomic DNA.
Values are expressed as medians of the paired differences.
FIG 2.
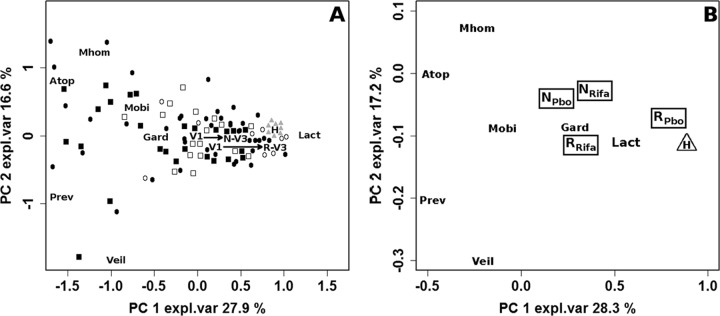
(A) Biplot of a PCA performed on the autoscaled qPCR data related to BV-affected women at visits V1 and V3 and healthy control women. The squares and circles represent BV-affected women at V1 and V3, respectively, with the open and filled symbols indicating women in remission (R) or not in remission (N), respectively, after rifaximin or placebo treatment. The gray triangles represent healthy women (H). Median values of the sample groups are indicated as V1, R-V3, N-V3, and H. (B) Biplot of a PCA performed on qPCR data related to BV-affected women at visit V1 and to healthy women. R and N indicate the median values of women grouped according to the response to rifaximin (RRifa [n = 25] and NRifa [n = 44], respectively) or placebo (RPbo [n = 4] and NPbo [n = 19], respectively) at V3, while H indicates the median values of healthy women (H; n = 10). Both trajectories (V1→R-V3 and V1→N-V3) indicate significant differences. Abbreviations: Lact, Lactobacillus; Gard, G. vaginalis; Atop, Atopobium; Prev, Prevotella; Veil, Veillonella; Mhom, M. hominis; Mobi, Mobiluncus; expl.var, explained variance.
Correlation between baseline microbiota and remission.
Samples collected from BV-affected women at V1 were evaluated by PCA in a relationship between remission condition at V3 (N or R) and the type of treatment applied (rifaximin or placebo) and were compared to healthy controls (H) (Fig. 2B). Although no significant difference was found among the microbiota associated with the 5 analyzed groups, women treated with placebo showed an interesting trend, which suggested a cause/effect relationship between high lactobacillus/BV-related bacteria ratios at the baseline and the possibility of going into spontaneous remission. This trend also suggests that women who went into spontaneous remission did not have true cases of BV.
Long-term impact of placebo on vaginal microbiota.
Figure 3 illustrates the trajectories covered by women who underwent the second follow-up visit, V4, in the space built by means of PCA on the qPCR data. In the case of placebo treatment (Fig. 3A), women who still had BV at V3 and women who were in remission at both V3 and V4 were characterized by the lowest and highest ratios between lactobacilli and BV-related bacteria registered at V1, respectively. This ratio was intermediate in women who went into remission at V3 but presenting relapse at V4. After the first visit, neither group of women showed any significant modification of the microbiota or correlations with BV evolution.
FIG 3.
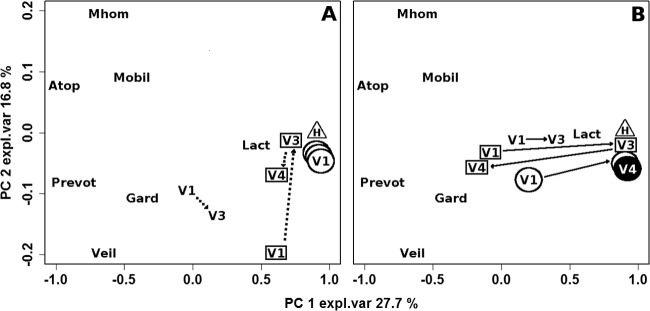
Biplot of a PCA calculated on the autoscaled qPCR data related to healthy women (triangle) and BV-affected women in remission at both V3 and V4 (circles), in remission at V3 but not at V4 (squares), and not in remission at V3 (no symbol). To limit the sample superimposition, data are represented separately for women subjected to placebo (A) and rifaximin (B) treatment. Median values of the samples collected from healthy women (H) and BV-affected women at V1, V3, and V4 are shown. The number of women (n) for each sample group is reported in Fig. 1. Continuous lines indicate significant differences between V1–V3 and V3–V4 trajectories, while dashed lines indicate nonsignificant differences. Full V4 symbols indicate significant differences between V1 and V4. expl.var, explained variance.
Long-term impact of rifaximin on vaginal microbiota.
The trajectories covered by women treated with rifaximin (Fig. 3B) were clearly different from those of women treated with placebo. V3 and V4 samples of women in remission at both follow-up visits were localized in the same position, characterized by a favorable balance between lactobacilli and BV-related bacteria. This position was very close to that of healthy controls and significantly distant from V1. In contrast to those of women in remission at both follow-up visits, the trajectories of V4 samples of women who were not in remission at the second follow-up visit moved away significantly from the V3 trajectory in the direction of V1. For these women, V1 and V4 samples were characterized by a less favorable balance in the microbiota than V3 samples. In women who still had BV after rifaximin treatment, V3 samples were closer to V1 samples and were characterized by the highest concentrations of BV-related bacteria. Nevertheless, the V3–V1 distance was statistically significant even for this group of patients.
Impact of rifaximin on metabolome.
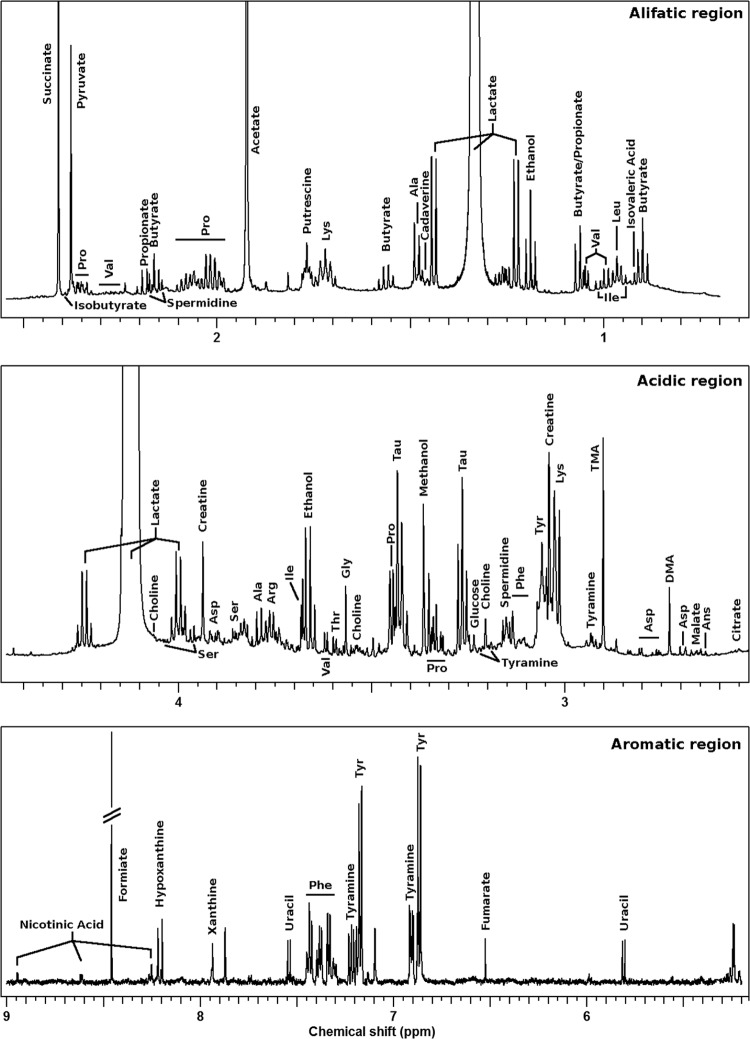
An example of an HR-NMR spectrum, describing the metabolomic profile of vaginal fluid collected from a woman affected by BV, is shown in Fig. 4. The spectrum was dominated in intensity and variance by the peaks ascribable to lactate, whose area ranged between 7% and 72% of the entire spectrum. Molecules associated with the growth of BV-related bacteria could be identified, such as short-chain fatty acids (acetate, propionate, butyrate, isobutyrate, and isovaleric acid) (45, 46), organic acids (succinate, formiate, and fumarate) (47), and biogenic amines (putrescine, spermidine, and tyramine) (6, 48). Peaks from amino acids and molecules from purine catabolism (hypoxanthine and xanthine) were also identified.
FIG 4.
1H-NMR spectrum of a vaginal fluid sample collected from a BV-affected woman at the baseline.
Table 3 lists the molecules whose concentrations significantly changed between V1 and V3 in women who were in remission at V3. Most of these molecules are known to be modulated by the balance between lactobacilli and BV-related bacteria, such as lactate, which increased with remission, and biogenic amines, short-chain fatty acids, acetate, and succinate, which decreased in parallel (6, 45–48).
TABLE 3.
Concentration of metabolites which significantly varied (P < 0.01) between V1 and V3 in women going into remission at V3 (n = 29)
| Compound | Chemical shift (ppm) | Concn. of metabolite at treatment visit: |
||
|---|---|---|---|---|
| V1a | V3a | V3–V1b (%) | ||
| Formiate | 8.46 | 0.74 | 0.17 | −82.55 |
| Tyramine | 2.93 | 1.59 | 0.37 | −73.33 |
| Succinate/isobutyrate | 2.41 | 10.38 | 1.56 | −87.17 |
| Propanoate/spermidine | 2.19 | 0.95 | 0.25 | −77.90 |
| Proline | 2.05 | 10.31 | 8.79 | −19.90 |
| Acetate | 1.95 | 80.73 | 3.75 | −94.13 |
| Putrescine | 1.78 | 5.34 | 1.04 | −79.08 |
| Butyrate | 1.55 | 2.92 | 0.71 | −43.39 |
| Alanine | 1.47 | 9.95 | 3.40 | −62.92 |
| Lactate | 1.24 | 144.29 | 225.40 | 17.57 |
Values are expressed as medians of μmol/liter.
Values are expressed as medians of the paired differences.
A complete overview of all samples considered in the present investigation could be obtained by means of PCA, as shown in Fig. 5A. The first PC, representing 99.1% of the total variance among the samples, was dominated by lactate, while the second, representing 0.5% of the total variance, brought information mainly on acetate and succinate, molecules produced by BV-related bacteria. PCA shows 3 types of trends with significant differences registered on PC2: (i) when the remission condition persisted at V4, the medians from V3 and V4 samples were superimposed and were very close to those of healthy samples and different from those of V1 samples; (ii) the average metabolome of women going into remission at V3 but presenting relapse at V4 showed a high variation at V3 with a return to the baseline at V4; and (iii) the metabolome of women who were not in remission at V3 underwent minor modifications compared to women going into remission, but these variations were still significant along PC2 (P < 0.05).
FIG 5.
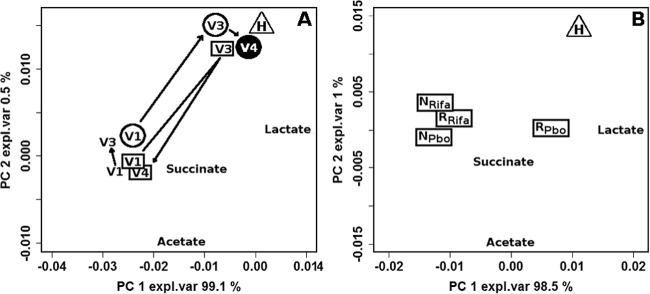
(A) Biplot of a PCA calculated on the entire set of NMR spectra. Median values of the samples collected from healthy women (triangle) and BV-affected women in remission at both V3 and V4 (circles), in remission at V3 but not at V4 (squares), and not in remission at V3 (no symbols) are shown. (B) Biplot of a PCA calculated on NMR data related to BV-affected women at visit V1 and to healthy women. R and N indicate the median values of women grouped according to the response to rifaximin (RRifa, n = 25; NRifa, n = 44) or placebo (RPbo, n = 4; NPbo, n = 19) at V3, while H indicates the median values of healthy women (H; n = 10). Continuous lines in panel A indicate significant differences between V1–V3 and V3–V4 trajectories, while dashed lines indicate nonsignificant differences. Full V4 symbols indicate significant differences between V1 and V4. expl.var, explained variance.
Correlation between baseline metabolome and remission.
Figure 5B illustrates the correlation between remission at V3 and vaginal fluid metabolome at V1. Treatment with placebo showed a positive correlation between lactate concentration and the possibility to go into remission, while the opposite was observed when rifaximin was employed. These data, although not statistically significant, suggest that a high lactate concentration is beneficial for spontaneous remission, while the success of rifaximin treatment is independent of lactate presence. In analogy with the considerations on the microbiota, the high lactate concentration at baseline of women going into spontaneous remission suggests that Amsel and Nugent criteria could be not exhaustive for a correct diagnosis of BV.
Correlation between placebo treatment and BV-related metabolites.
To study in detail the relationship between BV and short-chain fatty acids, organic acids, and biogenic amine concentration, a PC model was built on the concentrations of these molecules, as shown in Fig. 6. A picture coherent with clinical and microbiological data seemed to emerge. In the case of placebo treatment (Fig. 6A), the highest concentrations of BV-related molecules were found in V1 samples of women still affected by BV at V3, while the lowest concentrations of these metabolites were detected in V1 samples of women who maintained remission for the long term. Women in remission at V3 but still affected by BV at V4 showed an intermediate situation. This means that a connection between the position of the samples along PC1 at V1 and the possibility to go into remission could be noticed. However, the treatment did not cause significant modifications to the metabolome, so the samples collected at V3 and V4 appeared along PC1 very close to the samples collected at V1.
FIG 6.
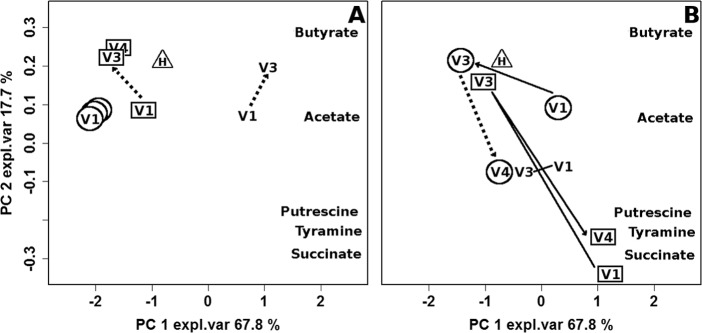
Biplot of a PCA calculated on the autoscaled concentrations of the molecules associated with the presence of BV-related bacteria measured in healthy women (triangle) and BV-affected women in remission at both V3 and V4 (circles), in remission at V3 but not at V4 (squares), and not in remission at V3 (no symbol). To limit the sample superimposition, data are represented separately for women subjected to placebo (A) or rifaximin (B) treatment. Median values of the samples collected from healthy women (H) and BV-affected women at V1, V3, and V4 are shown. The number of women (n) for each sample group is reported in Fig. 1. Continuous lines indicate significant differences between V1–V3 and V3–V4 trajectories, while dashed lines indicate nonsignificant differences. Full V4 symbols indicate significant differences between V1 and V4. expl.var, explained variance.
Correlation between rifaximin treatment and BV-related metabolites.
In women treated with rifaximin (Fig. 6B), clinical remission and microbial restoration were associated with a significant reduction of the concentration of the molecules produced by BV-related bacteria (acetate, succinate, butyrate, putrescine, and tyramine). V4 samples collected from women who maintained remission were very close to V3 samples, while a relapse resulted in a return of V4 samples very close to V1 samples. A slight but still significant reduction in concentration of molecules produced by BV-related bacteria also could be observed in women failing to go into remission at V3.
Effects of different dosages of rifaximin on the vaginal microbiota.
Figure 7A shows the scoreplot of a PCA calculated on the differences existing for each woman between the vaginal microbiota composition at V1 and V3. The two PCs accounted for 46.4% of the entire variability. The proximity of results for placebo (RD) and those for women who had still BV at V3 (N) indicates that spontaneous remission is not associated with modifications induced in the microbial communities by placebo, as changes are minor and similar to those determined by an ineffective treatment. This finding further confirms the association between spontaneous remission and favorable microbiota at the baseline. Differences in the microbiota were more marked for rifaximin treatment groups A, B, and C, with the highest variations registered for group B.
FIG 7.
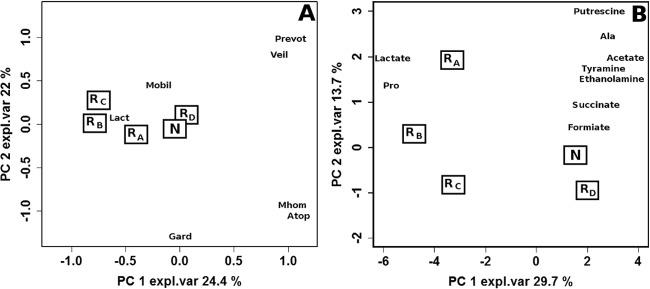
Biplots of PCA models built on the autoscaled differences for V3–V1 between qPCR (A) and metabolic (B) data. The medians of the samples from women in remission or not in remission at V3 are indicated as R and N, respectively, while rifaximin dosing regimens are indicated as subscripts. Abbreviations: Lact, Lactobacillus; Gard, G. vaginalis; Atop, Atopobium; Prev, Prevotella; Veil, Veillonella; Mhom, M. hominis; Mobi, Mobiluncus; expl.var, explained variance.
Effects of different dosages of rifaximin on the vaginal metabolome.
The effects of the different dosages of rifaximin on the metabolome are shown in Fig. 7B. Very similar modifications were found between V1 and V3 in women in spontaneous remission at V3 induced by placebo (RD) and in women who still had BV at V3 (N). Major differences from ineffective treatment were observed for treatment B, followed by treatments C and A. The loadingplot superimposed on the scoreplot shows that treatment B induced the highest increase of lactate and proline, which was associated with the highest decreases of organic acids and biogenic amines.
DISCUSSION
An understanding of the composition and metabolic activities of the vaginal microbial ecosystem in relation to a vaginal disease is essential for comprehensively understanding the etiology of that disease and for rationally designing successful strategies of prevention and therapy. Although BV has been studied in detail regarding the implications of the vaginal microbiota composition (42–44), the vaginal metabolomic framework remains largely unexplored: to date, only one gas chromatography-mass spectrometry-based study has been focused on the vaginal metabolome in BV (49), but no data are available to correlate the metabolomics features with the potential remission induced by antibiotic therapy.
To study BV from a holistic point of view, a combination of two elements are necessary: (i) analytical techniques able to account for the complexity of the vaginal ecosystem, and (ii) multivariate statistical techniques able to bring out the general features of the vaginal samples from the confounding sources of variability that always characterize complex microbial ecosystems.
In the present work, the effects of rifaximin vaginal tablets on vaginal microbiota and metabolome of BV-affected women were investigated by combining qPCR, a well-established technique for the characterization of vaginal bacterial communities (25, 31, 50), and a metabolomic approach based on 1H-NMR, which had never been used to study vaginal ecology. To highlight the general trends of the bacterial communities and metabolomic profiles in response to the antibiotic/placebo therapy, a multivariate statistical strategy was set up based on the trajectories traced by vaginal samples in a PCA space.
In general, our data demonstrated the efficacy of rifaximin in restoring a health-like condition in terms of both bacterial communities and metabolomics features, in agreement with the clinical observations previously reported (11). In particular, a significant increase of the lactobacillus/BV-related bacteria ratio was found in women affected by BV and treated with rifaximin. This increase was observed not only in women in remission but also in women not in remission, even if the trajectory traced in the PC space built on the qPCR data was shorter for the latter.
Metabolomic data showed that the restoration of a Lactobacillus-dominated flora, which occurred following rifaximin treatment, was strongly associated with the increase of lactic acid concentration and the decrease of the pool of molecules associated with the metabolism of BV-related bacteria, such as acetic acid, succinate, short-chain fatty acids, and biogenic amines (6, 45–48). Placebo treatment did not cause any significant variation in the metabolome in cases of spontaneous remission and BV persistence. In addition, the PCA approach developed in the present work allowed us to visualize a BV relapse occurring at the second follow-up visit as a return of the vaginal samples toward the baseline situation in the PC spaces built on both qPCR and metabolomic data.
An interesting finding of the present study is the hypothesis of a spontaneous remission from BV on the basis of the vaginal microbial and metabolomic features present at the baseline. In fact, women going into remission after placebo treatment showed at the baseline the highest ratios of lactobacilli to BV-related bacteria and lactate to BV-related metabolites. These women also could be considered not to be true cases of BV; thus, antibiotic treatment would not be appropriate. On the contrary, the use of rifaximin treatment appeared to be useful to induce remission from BV in women who presented both unfavorable microbiomes and metabolomes. This observation suggests the use of molecular or metabolomics tools to predict the need for a specific antibiotic treatment to induce remission from BV.
The impact of different dosages of rifaximin on the vaginal microbiota and metabolome was also investigated in the present study. The major differences from ineffective treatment were induced by 25 mg of rifaximin for 5 days, in agreement with the findings of Cruciani et al. (12), who described this dosage as the best treatment regimen in terms of restoration of a healthy-like microbiota.
The metabolome of a biological system, being downstream of the genome, transcriptome, and proteome, is particularly sensitive to external agents, even to those determining little effects on the different metabolic fluxes. NMR is ideally tailored for metabolomic investigations due to its high reproducibility, easing the application of pattern recognition techniques and multivariate analyses on minimally optimized spectra (51). A strategy based on the combination of microbiological and metabolomic observations allowed a comprehensive study of the effects of the antibiotic rifaximin on the vaginal environment of women affected by BV. Relationships were found between the doses of the antibiotic agent and restoration of healthy conditions, as well as between initial conditions and evolution of the disease. This allows us to envisage possibilities to finely tailor treatment strategies against BV according to vaginal environmental characteristics.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a research grant provided by Alfa Wassermann S.p.A. (no. 15/2012). G. Donders received advisory fees, lecture fees, and grant support from Alfa Wassermann S.p.A. F. Calanni is an employee of Alfa Wassermann S.p.A.
Footnotes
Published ahead of print 7 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02469-14.
REFERENCES
- 1.Amsel R, Totten PA, Spiegel CA, Chen KCS, Eschenbach D, Holmes KK. 1983. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 74:14–22. 10.1016/0002-9343(83)91112-9 [DOI] [PubMed] [Google Scholar]
- 2.Fredricks DN, Fiedler TL, Marrazzo JM. 2005. Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 353:1899–1911. 10.1056/NEJMoa043802 [DOI] [PubMed] [Google Scholar]
- 3.Nugent RP, Krohn MA, Hillier SL. 1991. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 29:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Workowski KA, Berman SM. 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm. Rep. 59:1–110 [PubMed] [Google Scholar]
- 5.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, Horvath LB, Kuzevska I, Fairley CK. 2006. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 193:1478–1486. 10.1086/503780 [DOI] [PubMed] [Google Scholar]
- 6.Sobel JD, Karpas Z, Lorber A. 2012. Diagnosing vaginal infections through measurement of biogenic amines by ion mobility spectrometry. Eur. J. Obstet. Gyn. R. B. 163:81–84. 10.1016/j.ejogrb.2012.03.022 [DOI] [PubMed] [Google Scholar]
- 7.Hoover WW, Gerlach EH, Hoban DJ, Eliopoulos GM, Pfaller MA, Jones RN. 1993. Antimicrobial activity and spectrum of rifaximin, a new topical rifamycin derivative. Diagn. Microbiol. Infect. Dis. 16:111–118. 10.1016/0732-8893(93)90004-Q [DOI] [PubMed] [Google Scholar]
- 8.Hillier SL, Cosentino L, Petrina M, Rabe L. 2013. Susceptibility of bacterial vaginosis (BV)-associated bacteria and lactobacilli to rifaximin, metronidazole and clindamycin. Sex. Transm. Infect. 89(Suppl 1):A28–A29. 10.1136/sextrans-2013-051184.0090 [DOI] [Google Scholar]
- 9.McClure WR, Cech CL. 1978. On the mechanism of rifampicin inhibition of RNA synthesis. J. Biol. Chem. 253:8949–8956 [PubMed] [Google Scholar]
- 10.Gillis J, Brogden R. 1995. Rifaximin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential in conditions mediated by gastrointestinal bacteria. Drugs 49:467–484 [DOI] [PubMed] [Google Scholar]
- 11.Donders GGG, Guaschino S, Peters K, Tacchi R, Lauro V. 2013. A multicenter, double-blind, randomized, placebo-controlled study of rifaximin for the treatment of bacterial vaginosis. Int. J. Gynecol. Obstet. 120:131–136. 10.1016/j.ijgo.2012.08.022 [DOI] [PubMed] [Google Scholar]
- 12.Cruciani F, Brigidi P, Calanni F, Lauro V, Tacchi R, Donders G, Peters K, Guaschino S, Vitali B. 2012. Efficacy of rifaximin vaginal tablets in treatment of bacterial vaginosis: a molecular characterization of the vaginal microbiota. Antimicrob. Agents Chemother. 56:4062–4070. 10.1128/AAC.00061-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruciani F, Wasinger V, Turroni S, Fiorella C, Donders G, Brigidi P, Vitali B. 2013. Proteome profiles of vaginal fluids from women affected by bacterial vaginosis and healthy controls: outcomes of rifaximin treatment. J. Antimicrob. Chemother. 68:2648–2659. 10.1093/jac/dkt244 [DOI] [PubMed] [Google Scholar]
- 14.Fiehn O. 2002. Metabolomics–the link between genotypes and phenotypes. Plant Mol. Biol. 48:155–171. 10.1023/A:1013713905833 [DOI] [PubMed] [Google Scholar]
- 15.Raamsdonk LM, Teusink B, Broadhurst D, Zhang N, Hayes A, Walsh MC, Berden JA, Brindle KM, Kell DB, Rowland JJ, Westerhoff HV, van Dam K, Oliver SG. 2001. Functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations. Nat. Biotech. 19:45–50. 10.1038/83496 [DOI] [PubMed] [Google Scholar]
- 16.Urbanczyk-Wochniak E, Luedemann A, Kopka J, Selbig J, Roessner-Tunali U, Willmitzer L, Fernie AR. 2003. Parallel analysis of transcript and metabolic profiles: a new approach in systems biology. EMBO Rep. 4:989. 10.1038/sj.embor.embor944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teichert F, Verschoyle RD, Greaves P, Edwards RE, Teahan O, Jones DJL, Wilson ID, Farmer PB, Steward WP, Gant TW, Gescher AJ, Keun HC. 2008. Metabolic profiling of transgenic adenocarcinoma of mouse prostate (TRAMP) tissue by 1H-NMR analysis: evidence for unusual phospholipid metabolism. Prostate 68:1035–1047. 10.1002/pros.20761 [DOI] [PubMed] [Google Scholar]
- 18.Coen M, O'Sullivan M, Bubb WA, Kuchel PW, Sorrell T. 2005. Proton nuclear magnetic resonance-based metabonomics for rapid diagnosis of meningitis and ventriculitis. Clin. Infect. Dis. 41:1582–1590. 10.1086/497836 [DOI] [PubMed] [Google Scholar]
- 19.Brindle JT, Antti H, Holmes E, Tranter G, Nicholson JK, Bethell HW, Clarke S, Schofield PM, McKilligin E, Mosedale DE, Grainger DJ. 2002. Rapid and noninvasive diagnosis of the presence and severity of coronary heart disease using 1H-NMR-based metabonomics. Nat. Med. 8:1439–1445. 10.1038/nm1202-802 [DOI] [PubMed] [Google Scholar]
- 20.Maccaferri S, Vitali B, Klinder A, Kolida S, Ndagijimana M, Laghi L, Calanni F, Brigidi P, Gibson GR, Costabile A. 2010. Rifaximin modulates the colonic microbiota of patients with Crohn's disease: an in vitro approach using a continuous culture colonic model system. J. Antimicrob. Chemother. 65:2556–2565. 10.1093/jac/dkq345 [DOI] [PubMed] [Google Scholar]
- 21.Bertini I, Calabro A, De Carli V, Luchinat C, Nepi S, Porfirio B, Renzi D, Saccenti E, Tenori L. 2009. The metabonomic signature of celiac disease. J. Proteome Res. 8:170–177. 10.1021/pr800548z [DOI] [PubMed] [Google Scholar]
- 22.Hendriks MMWB, Eeuwijk FAV, Jellema RH, Westerhuis JA, Reijmers TH, Hoefsloot HCJ, Smilde AK. 2011. Data-processing strategies for metabolomics studies. Trends Anal. Chem. 30:1685–1698. 10.1016/j.trac.2011.04.019 [DOI] [Google Scholar]
- 23.Picone G, Mezzetti B, Babini E, Capocasa F, Placucci G, Capozzi F. 2011. Unsupervised principal component analysis of NMR metabolic profiles for the assessment of substantial equivalence of transgenic grapes (Vitis vinifera). J. Agric. Food Chem. 59:9271–9279. 10.1021/jf2020717 [DOI] [PubMed] [Google Scholar]
- 24.Donders GG, Van Calsteren K, Bellen G, Reybrouck R, Van den Bosch T, Riphagen I, Van Lierde S. 2009. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG 116:1315–1324. 10.1111/j.1471-0528.2009.02237.x [DOI] [PubMed] [Google Scholar]
- 25.Vitali B, Biagi E, Brigidi P. 2012. Protocol for the use of PCR-denaturing gradient gel electrophoresis and quantitative PCR to determine vaginal microflora constitution and pathogens in bacterial vaginosis, p 177–193 In MacKenzie CR, Henrich B. (ed), Diagnosis of sexually transmitted diseases, vol 903 Humana Press, Totowa, NJ: [DOI] [PubMed] [Google Scholar]
- 26.Heilig HGHJ, Zoetendal EG, Vaughan EE, Marteau P, Akkermans ADL, de Vos WM. 2002. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl. Environ. Microbiol. 68:114–123. 10.1128/AEM.68.1.114-123.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zariffard MR, Saifuddin M, Sha BE, Spear GT. 2002. Detection of bacterial vaginosis-related organisms by real-time PCR for lactobacilli, Gardnerella vaginalis and Mycoplasma hominis. FEMS Immunol. Med. Microbiol. 34:277–281. 10.1111/j.1574-695X.2002.tb00634.x [DOI] [PubMed] [Google Scholar]
- 28.Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R. 2004. Use of 16S rRNA gene-targeted group-specific primers for Real-Time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microbiol. 70:7220–7228. 10.1128/AEM.70.12.7220-7228.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuki T, Watanabe K, Fujimoto J, Miyamoto Y, Takada T, Matsumoto K, Oyaizu H, Tanaka R. 2002. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl. Environ. Microbiol. 68:5445–5451. 10.1128/AEM.68.11.5445-5451.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A. 2004. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 97:1166–1177. 10.1111/j.1365-2672.2004.02409.x [DOI] [PubMed] [Google Scholar]
- 31.Zozaya-Hinchliffe M, Lillis R, Martin DH, Ferris MJ. 2010. Quantitative PCR assessments of bacterial species in women with and without bacterial vaginosis. J. Clin. Microbiol. 48:1812–1819. 10.1128/JCM.00851-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiveljung A, Forsum U, Monstein H-J. 1996. Classification of the genus Mobiluncus based on comparative partial 16S rRNA gene analysis. Int. J. Syst. Bacteriol. 46:332–336. 10.1099/00207713-46-1-332 [DOI] [PubMed] [Google Scholar]
- 33.Alum M, Shaw P, Sweatman B, Ubhi B, Haselden J, Connor S. 2008. 4,4-Dimethyl-4-silapentane-1-ammonium trifluoroacetate (DSA), a promising universal internal standard for NMR-based metabolic profiling studies of biofluids, including blood plasma and serum. Metabolomics 4:122–127. 10.1007/s11306-008-0103-9 [DOI] [Google Scholar]
- 34.Marzorati M, Bigler P, Vermathen M. 2011. Interactions between selected photosensitizers and model membranes: an NMR classification. Biochim. Biophys. Acta 1808:1661–1672. 10.1016/j.bbamem.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 35.Rohr G, Eggert-Kruse W, Pehlke A, Sahrbacher U, Runnebaum B, Kalbitzer HR. 1992. Biochemical analysis of cervical mucus by nuclear magnetic resonance spectroscopy. Hum. Reprod. 7:915–917 [DOI] [PubMed] [Google Scholar]
- 36.Ihaka R, Gentleman R. 1996. R: a language for data analysis and graphics. J. Comput. Graph. Stat. 5:299–314 [Google Scholar]
- 37.Coombes KR, Fritsche HA, Clarke C, Chen J-N, Baggerly KA, Morris JS, Xiao L-C, Hung M-C, Kuerer HM. 2003. Quality control and peak finding for proteomics data collected from nipple aspirate fluid by surface-enhanced laser desorption and ionization. Clin. Chem. 49:1615–1623. 10.1373/49.10.1615 [DOI] [PubMed] [Google Scholar]
- 38.Liland KH, Almøy T, Mevik B-H. 2010. Optimal choice of baseline correction for multivariate calibration of spectra. Appl. Spectrosc. 64:1007–1016. 10.1366/000370210792434350 [DOI] [PubMed] [Google Scholar]
- 39.Dieterle F, Ross A, Schlotterbeck G, Senn H. 2006. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 78:4281–4290. 10.1021/ac051632c [DOI] [PubMed] [Google Scholar]
- 40.Venables W, Ripley BD. 2000. S programming. Springer, New York, New York [Google Scholar]
- 41.Biagi E, Vitali B, Pugliese C, Candela M, Donders GGG, Brigidi P. 2009. Quantitative variations in the vaginal bacterial population associated with asymptomatic infections: a real-time polymerase chain reaction study. Eur. J. Clin. Microbiol. Infect. Dis. 28:281–285. 10.1007/s10096-008-0617-0 [DOI] [PubMed] [Google Scholar]
- 42.Ling Z, Liu X, Chen W, Luo Y, Yuan L, Xia Y, Nelson K, Huang S, Zhang S, Wang Y, Yuan J, Li L, Xiang C. 2013. The restoration of the vaginal microbiota after treatment for bacterial vaginosis with metronidazole or probiotics. Microb. Ecol. 65:773–780. 10.1007/s00248-012-0154-3 [DOI] [PubMed] [Google Scholar]
- 43.Ling Z, Kong J, Liu F, Zhu H, Chen X, Wang Y, Li L, Nelson K, Xia Y, Xiang C. 2010. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genomics 11:488. 10.1186/1471-2164-11-488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitali B, Pugliese C, Biagi E, Candela M, Turroni S, Bellen G, Donders GGG, Brigidi P. 2007. Dynamics of vaginal bacterial communities in women developing bacterial vaginosis, candidiasis, or no infection, analyzed by PCR-denaturing gradient gel electrophoresis and real-time PCR. Appl. Environ. Microbiol. 73:5731–5741. 10.1128/AEM.01251-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaudry AN, Travers PJ, Yuenger J, Colletta L, Evans P, Zenilman JM, Tummon A. 2004. Analysis of vaginal acetic acid in patients undergoing treatment for bacterial vaginosis. J. Clin. Microbiol. 42:5170–5175. 10.1128/JCM.42.11.5170-5175.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirmonsef P, Gilbert D, Zariffard MR, Hamaker BR, Kaur A, Landay AL, Spear GT. 2011. The effects of commensal bacteria on innate immune responses in the female genital tract. Am. J. Reprod. Immunol. 65:190–195. 10.1111/j.1600-0897.2010.00943.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Mushrif S, Eley A, Jones BM. 2000. Inhibition of chemotaxis by organic acids from anaerobes may prevent a purulent response in bacterial vaginosis. J. Med. Microbiol. 49:1023–1030 [DOI] [PubMed] [Google Scholar]
- 48.Wolrath H, Forsum U, Larsson PG, Borén H. 2001. Analysis of bacterial vaginosis-related amines in vaginal fluid by gas chromatography and mass spectrometry. J. Clin. Microbiol. 39:4026–4031. 10.1128/JCM.39.11.4026-4031.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeoman CJ, Thomas SM, Miller MEB, Ulanov AV, Torralba M, Lucas S, Gillis M, Cregger M, Gomez A, Ho M, Leigh SR, Stumpf R, Creedon DJ, Smith MA, Weisbaum JS, Nelson KE, Wilson BA, White BA. 2013. A multi-omic systems-based approach reveals metabolic markers of bacterial vaginosis and insight into the disease. PLoS One 8:e56111. 10.1371/journal.pone.0056111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cartwright CP, Lembke BD, Ramachandran K, Body BA, Nye MB, Rivers CA, Schwebke JR. 2012. Development and validation of a semiquantitative, multitarget PCR assay for diagnosis of bacterial vaginosis. J. Clin. Microbiol. 50:2321–2329. 10.1128/JCM.00506-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pauli GF. 2001. qNMR–a versatile concept for the validation of natural product reference compounds. Phytochem. Anal. 12:28–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.