Abstract
Ertapenem, a carbapenem, relies on time-dependent killing. Therapeutic drug monitoring (TDM) should be considered, when ertapenem is used in specific populations, to achieve optimal bactericidal activity and optimize drug-dosing regimens. No validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method has been reported using deuterated ertapenem as the internal standard. A new simple and robust LC-MS/MS method using a quadrupole mass spectrometer was developed for analysis of ertapenem in human plasma, using deuterated ertapenem as the internal standard. The calibration curve was linear over a range of 0.1 (lower limit of quantification [LLOQ]) to 125 mg/liter. The calculated accuracy ranged from −2.4% to 10.3%. Within-run coefficients of variation (CV) ranged from 2.7% to 11.8%, and between-run CV ranged from 0% to 8.4%. Freeze-thaw stability had a bias of −3.3% and 0.1%. Storage of QC samples for 96 h at 4°C had a bias of −4.3 to 5.6%, storage at room temperature for 24 h had a bias of −10.7% to −14.8%, and storage in the autosampler had a bias between −2.9% and −10.0%. A simple LC-MS/MS method to quantify ertapenem in human plasma using deuterated ertapenem as the internal standard has been validated. This method can be used in pharmacokinetic studies and in clinical studies by performing TDM.
INTRODUCTION
Carbapenems belong to the beta-lactam antibiotics and are widely used against a broad spectrum of aerobic and anaerobic Gram-positive and Gram-negative bacteria (1, 2). Ertapenem, approved by the FDA in 2001, is one of these carbapenems. Since ertapenem has an approximate half-life of 4 h, it can be administered once daily. Therefore, ertapenem can be favored above other carbapenems (3). The pharmacodynamic (PD) parameter of ertapenem correlates with time-dependent killing, which means that the plasma concentration of ertapenem has to exceed the MIC for a percentage of time of its dosing interval (4).
Pharmacokinetic data obtained in healthy volunteers are difficult to extrapolate to specific patient populations. Due to this high variability in pharmacokinetic parameters, exposure of ertapenem might be suboptimal in these specific populations (5–10). Since ertapenem is a time-dependent antibiotic, therapeutic drug monitoring (TDM) should therefore be considered, when this drug is used in specific populations, to achieve optimal bactericidal activity and optimize drug-dosing regimens. More PK studies have to be performed to determine the efficacy and safety of ertapenem in specific populations (11, 12).
Ertapenem has been suggested as having potential use against Mycobacterium tuberculosis (1). However, according to Caminero et al., carbapenems are used as fifth-line drugs in the treatment of tuberculosis (TB) and can be used only in severe cases, since carbapenems are administered intravenously, costs are high, and clinical experience is restricted (13). Clinical studies assessing efficacy or safety profiles for carbapenems are scarce but showed favorable preliminary results (14–16). Before clinical efficacy in TB can be determined, a dose-finding study should be performed to evaluate pharmacokinetic parameters in this specific patient population. Therefore, an analytical method to measure ertapenem concentrations is mandatory.
There are a few published methods for liquid chromatography-tandem mass spectrometry (LC-MS/MS) quantification and validation of ertapenem in human plasma (17, 18). Since LC-MS/MS is easy to use frequently in a daily routine, more pharmacokinetic studies are being performed to quantify drugs. It is therefore important to have established standards in order to compare results of PK studies between laboratories. Present validated LC-MS/MS methods for ertapenem use extensive sample preparation, e.g., liquid-liquid extraction (LLE), solid-phase extraction (SPE), and nitrogen gas drying (17, 18). These methods are time-consuming and less cost-effective. The choice of an internal standard for LC-MS/MS is important, as it corrects for extraction, injection, and ionization variability. In particular, the latter (ion suppression and ion enhancement) is a source of variability. Only a deuterated internal standard is suitable to compensate for this and ensure a robust, high-throughput bioanalytical method (19). Since no LC-MS/MS method has been validated using a deuterated internal standard, the purpose of this study was to develop a new simple and robust LC-MS/MS method using a quadrupole mass spectrometer without extensive sample processing, using deuterated ertapenem as an internal standard to quantify concentrations of ertapenem in human plasma for pharmacokinetic studies.
MATERIALS AND METHODS
Analysis.
Ertapenem (Fig. 1) and the internal standard used, ertapenem-D4, were purchased from Alsachim (Illkirch, Graffenstaden, France). Acetonitrile for LC-MS/MS was purchased from BioSolve (Valkenswaard, The Netherlands). The chemicals used, including methanol Lichrosolv and trifluoroacetic anhydride, were of high-pressure liquid chromatography (HPLC) or analytical grade and were purchased from VWR (Amsterdam, The Netherlands). Purified water was obtained from a Milli-Q water purifying system (Millipore Corporation, Billerica, MA, USA).
FIG 1.
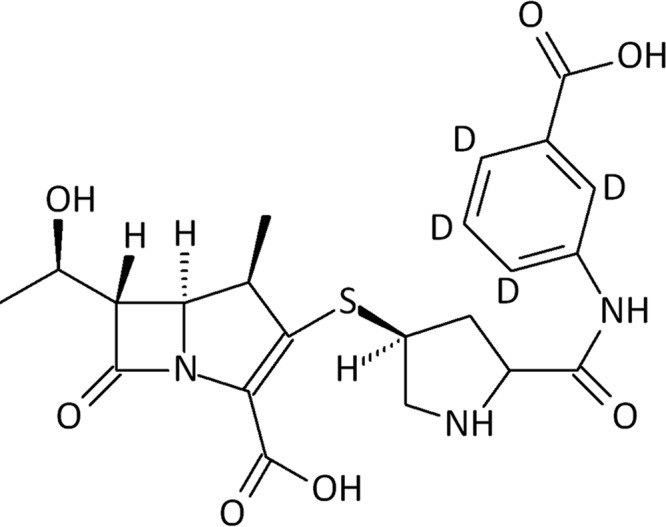
Chemical structure of ertapenem.
The precipitation reagent consisted of a mixture of methanol and acetonitrile (4:21, vol/vol). Pooled human plasma samples with EDTA as anticoagulant and pooled human serum samples were made available according to the standard operating procedures of University Medical Center Groningen.
The calibration standards, blanks, and QC samples were fully thawed at room temperature. To 100 μl of each sample, a volume of 500 μl of precipitation reagents and 10 μl of ertapenem-D4 (250 mg/liter) were added in a vial. The samples were vortexed for 1 min. To promote protein precipitation, the vials were stored at −20°C for 30 min. The vials were centrifuged for 5 min at 11,000 rpm. Five microliters of the upper layer was injected into the LC-MS/MS. QC samples and calibration standards were stored at −20°C.
The analysis was performed on a triple-quadrupole LC-MS/MS (Thermo Scientific, San Jose, CA, USA) with an MS Pump Plus (Finnigan Surveyor) and autosampler (Finnigan Surveyor). The mass spectrometer was a triple-stage quadrupole Quantum Access Max mass spectrometer. The autosampler temperature was set at 10°C. Liquid chromatographic separation was performed on a HyPURITY C18 analytical column (50 by 2.1 mm, 3-μm particle size; Thermo Scientific, Interscience, Breda, The Netherlands), and the temperature was set at 20°C. The mobile phase had a flow rate of 300 μl/min and consisted of purified water, acetonitrile, and an aqueous buffer (containing ammonium acetate [10 g/liter], acetic acid [35 mg/liter], and trifluoroacetic anhydride [2 mg/liter water]. The method had a run time of 4 min, and the elution gradient is shown in Table 1.
TABLE 1.
Elution gradient
| Time (min) | % of eluent ina: |
||
|---|---|---|---|
| A | B | C | |
| 0.00 | 5 | 95 | 0 |
| 0.50 | 5 | 35 | 60 |
| 1.30 | 5 | 35 | 60 |
| 1.40 | 5 | 0 | 95 |
| 2.80 | 5 | 0 | 95 |
| 3.00 | 5 | 95 | 0 |
| 4.00 | 5 | 95 | 0 |
A, aqueous buffer; B, purified water, C, acetonitrile.
The MS was configured in positive electrospray ionization mode and selected reaction monitoring (SRM) with a spray voltage of 3,500 V, a capillary temperature of 350°C, and a sheath gas pressure and auxiliary pressure of 35 and 5 arbitrary units, respectively.
Mass transitions were 476.1 m/z to 432.1 m/z for ertapenem and 480.1 m/z to 436.1 m/z for ertapenem-D4, using a scan width of 0.5 m/z. Collision energy was determined at 10 eV for both transitions. Peak height integration for all components was calculated by Xcalibur software version 2.0.7 (Thermo Fisher, San Jose, CA, USA).
Analytical method validation.
Validation of the method included selectivity and sensitivity, linearity, accuracy and precision, recovery and dilution integrity conform the guidance for Industry of the Food and Drug Administration. Since the FDA did not postulate a maximum coefficient of variation (CV) requirement for stability, a maximum CV of 15% was employed according to European Medicines Agency (EMA) guidelines (20, 21).
In some cases, for example, in pharmacokinetic studies, human serum is collected for the quantification of ertapenem. To determine whether there is a difference between the analysis of ertapenem in human plasma and in human serum, a matrix comparison was performed.
The calibration curve of ertapenem consisted of 8 samples with concentrations of 0.1, 0.5, 2.0, 7.5, 20, 50, 90, and 125 mg/liter. Quality control (QC) samples with 4 different concentrations of ertapenem were used, where the lower limit of quantitation (LLOQ) is 0.1 mg/liter, LOW is 2.5 mg/liter, MED is 40 mg/liter, and HIGH is 120 mg/liter. For selectivity, 6 pooled human plasma samples were examined for interference, and their responses were compared with those of the LLOQ samples. Over 3 days, each day a single calibration curve in plasma and in serum was analyzed, and accuracy was measured by evaluation of five determinations per QC sample on three consecutive days. Precision was divided into within-run and between-run values using the same method as accuracy. The coefficient of variation for the LLOQ could not exceed 20%, and that for other QC levels could not exceed 15%.
The recovery was determined on three levels (LOW, MED, and HIGH) and was done in five replicates. As protein precipitation is used as the only means of sample preparation in this method, relative recovery was measured by comparing the ratios of integrated peak heights of ertapenem and the internal standard of the processed QC samples with the average peak heights of the recovery samples. Recovery samples (LOW, MED, and HIGH) were postextraction blank samples spiked at the same concentrations as the QC samples.
The stability of ertapenem was tested at different test conditions, including storage stability and freeze-thaw stability. Storage stability of ertapenem was examined by storing QC samples at room temperature (20°C to 25°C) in a refrigerator at 4°C and after sample preparation in the autosampler at 10°C. Stability was also tested using three freeze-thaw cycles at −20°C. All stability tests were done using three different QC levels (LOW, MED, and HIGH) in five determinations per concentration. Stability is defined as a change in concentration of ≤15%. Since FDA guidelines have no requirements for the coefficient of variation of each QC sample (LOW, MED, and HIGH), EMA guideline requirements were taken into account; these state that the CV of each QC sample should not exceed 15% (20, 21).
To determine the dilution integrity, on three consecutive days, a sample with a concentration of 500 mg ertapenem/liter plasma was diluted 10 times and then prepared in five replicates.
Statistics.
Results were analyzed using one-way analysis of variance (ANOVA) and validated Excel sheets (Microsoft, Redmond, WA).
RESULTS
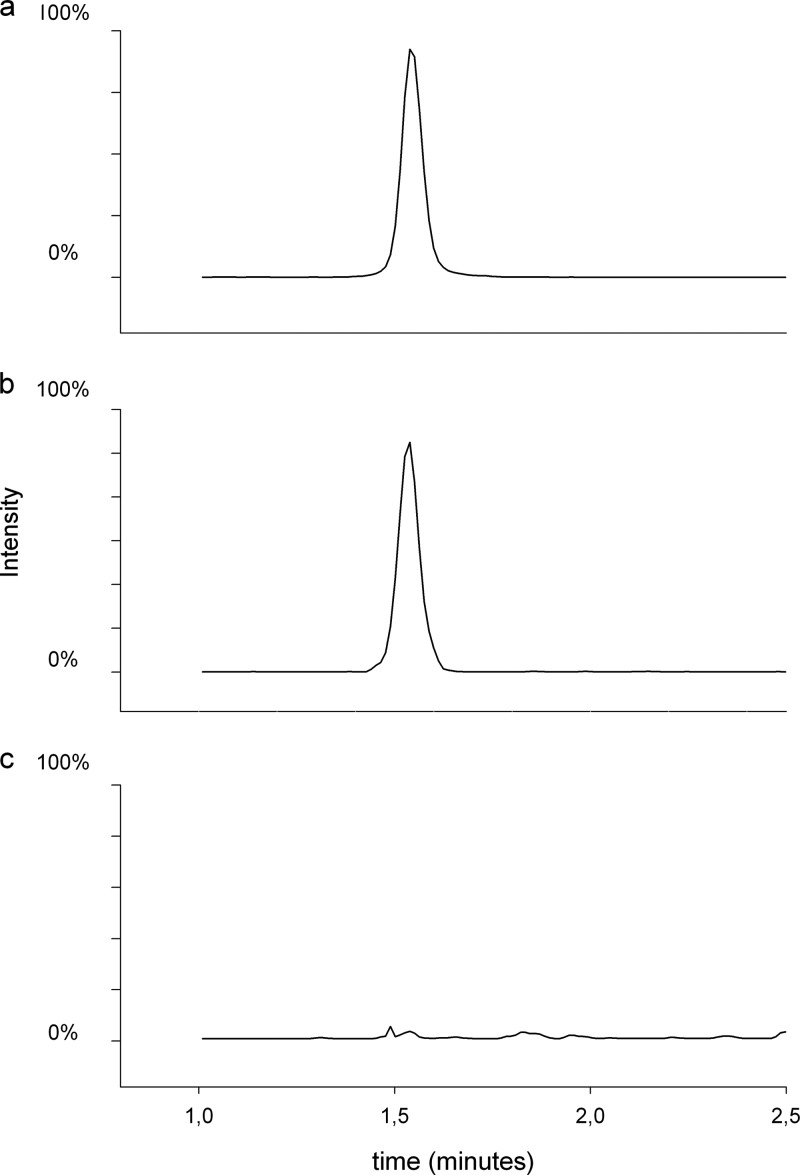
Mean retention times of ertapenem and ertapenem-D4 were 1.5 min. Examination of the selectivity of this analytical method showed that there were no interfering peaks observed at the retention time of ertapenem or ertapenem-D4 in any of the six lots of pooled human plasma (Fig. 2).
FIG 2.
Chromatogram. (a) Ertapenem D4 at the LLOQ; (b) ertapenem at the LLOQ; (c) blank plasma.
The calibration curve in plasma was linear over a range of 0.1 (LLOQ) to 125 mg/liter, and the correlation coefficient (R2) was 0.9988. The calibration curve parameters are as follows: slope, 0.487 ± 0.00519 (average ± standard deviation); intercept, 0.0149 ± 0.0257; average regression coefficient, 0.99762; correlation coefficient, 0.99881.
For comparison of the analysis in plasma and in serum, the peak height ratios of ertapenem and the internal standard in plasma were compared to those in serum. Analyzing both data sets using Passing-Bablok regression showed no statistically significant difference between the two matrices: y = 1.01(0.95–1.02 [range])x + 0.00 (−0.01 to 0.04) at the 95% confidence level.
Accuracy and precision, divided into within-run and between-run measurements, were calculated using spiked samples for 5 determinations per concentration on 3 consecutive days. The calculated accuracy ranged from −2.4% to 10.3%. Within-run precision ranged from 2.7% to 11.8%, and between-run precision ranged from 0% to 8.4%. The results of accuracy and precision for all QC levels are shown in Table 2.
TABLE 2.
Concentrations of calibration standards and QC samples
| QC sample | Concn (mg/liter) | Accuracy (% bias) | Precision (% CV) |
|
|---|---|---|---|---|
| Within run | Between run | |||
| LLOQ | 0.1 | −2.4 | 11.8 | 8.4 |
| LOW | 2.5 | 9.3 | 5.6 | 0 |
| MED | 40 | 7.3 | 3.1 | 1.5 |
| HIGH | 120 | 10.3 | 2.7 | 1.6 |
QC samples designated LOW (2.5 mg/liter), MED (40 mg/liter), and HIGH (120 mg/liter) were used to determine recovery; relative recovery was 101.7%, 97.9%, and 95.1% for these three samples, respectively. Dilution integrity was determined in 5-fold on 3 consecutive days. Accuracy was 1% and within-run and between-run precisions were 3.2% and 2.3%, respectively.
Stability of ertapenem using different test conditions is shown in Table 3. Measured concentrations of QC samples (LOW, MED, and HIGH) for the freeze-thaw had a bias between −3.3% and 0.1% and therefore complied with the guidelines. Stability was determined by measuring QC samples stored for 96 h at 4°C and differed by −4.3 to 5.6% from the nominal concentrations. After storage at room temperature for 24 h, the concentration of ertapenem had a bias of −10.7% to −14.8%, compared to the initial concentrations. After sample preparation, the concentration of ertapenem stored in the autosampler had a bias between −2.9% and −10.0% of the nominal concentrations.
TABLE 3.
Stability testing results for ertapenem
| Test conditiona | % bias for sample |
||
|---|---|---|---|
| LOW | MED | HIGH | |
| Benchtop, RT, 24 h | −11.9 | −10.7 | −14.8 |
| Refrigerator, 4°C, 96 h | 5.6 | −0.1 | −4.3 |
| Freeze-thaw, −20°C, 3 cycles | −0.2 | 0.1 | −3.3 |
| Autosampler, 10°C, 24 h | −10.0 | −2.9 | −4.8 |
RT, room temperature.
DISCUSSION
This is the first design and validation of a new, simple, and rapid analysis method using a triple-quadrupole LC-MS/MS for the quantification of ertapenem in human plasma and deuterated ertapenem as the internal standard.
This LC-MS/MS method was validated for accuracy and precision according to FDA guidelines, having biases of <20% for LLOQ and <15% for other QC levels (20). The calibration curve was linear within a range of 0.1 (LLOQ) to 125 μg/ml, compared to other studies, which were validated up to 50 μg/ml and had LLOQs of 0.5 and 1.0 μg/ml, respectively (17, 18) This method used deuterated ertapenem as the internal standard, resulting in better interday variation, intraday variation, and accuracy than the methods of Pickering and Brown (18) and Koal et al. (17), which used beta-lactam analogues as internal standards.
Matrix comparison showed no difference between the analysis of ertapenem in human plasma and in human serum. However, because of the poor stability of ertapenem at room temperature, it is recommended to draw whole blood (with EDTA as the anticoagulant), as it can be placed on ice for a short time during transport from the nursing ward to the analyzing laboratory.
As mentioned in the introduction, a major advantage of this LC-MS/MS method is that a simple protein precipitation is used instead of LLE, SPE, or nitrogen gas drying, resulting in a less time-consuming and a less expensive method, compared to other LC-MS/MS methods (17, 18). The run time is very short, since the retention time of ertapenem is 1.5 min. This facilitates a high sample throughput. This is a great advantage for laboratories that have only one LC-MS/MS to support their clinical TDM service.
Ertapenem in plasma stored at room temperature degrades within a short period with 10 to 15%. Therefore, it is crucial to store samples in the freezer until analysis and to process samples containing ertapenem within the validated time frame of stability to ensure accurate and precise results. Reinjection of processed samples stored at 10°C in the autosampler is tolerated within 24 h.
Since ertapenem is a time-dependent antibiotic, it is necessary that the plasma concentration exceed the MIC for at least 40% of its dosing interval. To attain this target in patients suspected of having altered pharmacokinetics due to renal function or high variability in plasma proteins, ertapenem concentration measuring may be of help, especially if more resistant pathogens are targeted with higher MICs. This method meets the criteria for TDM but is also suitable for clinical pharmacokinetic studies or clinical trials to further investigate the use of ertapenem in other infectious diseases or other specific patient populations.
Conclusion.
A simple LC-MS/MS method to quantify ertapenem in human plasma using deuterated ertapenem as the internal standard has been developed and validated. This method can be used in pharmacokinetic studies and in clinical studies by performing TDM.
Footnotes
Published ahead of print 14 April 2014
REFERENCES
- 1.Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE, III, Blanchard JS. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218. 10.1126/science.1167498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta R, Lavollay M, Mainardi JL, Arthur M, Bishai WR, Lamichhane G. 2010. The Mycobacterium tuberculosis protein LdtMt2 is a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nat. Med. 16:466–469. 10.1038/nm.2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majumdar AK, Musson DG, Birk KL, Kitchen CJ, Holland S, McCrea J, Mistry G, Hesney M, Xi L, Li SX, Haesen R, Blum RA, Lins RL, Greenberg H, Waldman S, Deutsch P, Rogers JD. 2002. Pharmacokinetics of ertapenem in healthy young volunteers. Antimicrob. Agents Chemother. 46:3506–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolau DP. 2008. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin. Infect. Dis. 47(Suppl 1):S32–S40. 10.1086/590064 [DOI] [PubMed] [Google Scholar]
- 5.Burkhardt O, Kumar V, Schmidt S, Kielstein JT, Welte T, Derendorf H. 2010. Underdosing of ertapenem in critically ill patients with pneumonia confirmed by Monte Carlo simulations. Int. J. Antimicrob. Agents. 35:96–97. 10.1016/j.ijantimicag.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 6.Brink AJ, Richards GA, Schillack V, Kiem S, Schentag J. 2009. Pharmacokinetics of once-daily dosing of ertapenem in critically ill patients with severe sepsis. Int. J. Antimicrob. Agents. 33:432–436. 10.1016/j.ijantimicag.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 7.Dailly E, Arnould JF, Fraissinet F, Naux E, Letard de la Bouraliere MA, Bouquie R, Deslandes G, Jolliet P, Le Floch R. 2013. Pharmacokinetics of ertapenem in burns patients. Int. J. Antimicrob. Agents. 42:48–52. 10.1016/j.ijantimicag.2013.02.021 [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Nafziger AN, Drusano GL, Ma L, Bertino JS., Jr 2006 Comparative pharmacokinetics and pharmacodynamic target attainment of ertapenem in normal-weight, obese, and extremely obese adults. Antimicrob. Agents Chemother. 50:1222–1227. 10.1128/AAC.50.4.1222-1227.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mistry GC, Majumdar AK, Swan S, Sica D, Fisher A, Xu Y, Hesney M, Xi L, Wagner JA, Deutsch PJ. 2006. Pharmacokinetics of ertapenem in patients with varying degrees of renal insufficiency and in patients on hemodialysis. J. Clin. Pharmacol. 46:1128–1138. 10.1177/0091270006291839 [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Rahman SM, Kearns GL, Topelberg S, Jacobs RF, Mistry GC, Majumdar A, Xu Y, Wagner JA, Kitchen CJ, Groff M, Herman G, Blumer JL. 2010. Pharmacokinetics and tolerability of single-dose intravenous ertapenem in infants, children, and adolescents. Pediatr. Infect. Dis. J. 29:1072–1076. 10.1097/INF.0b013e3181e82608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiskirchen DE, Housman ST, Quintiliani R, Nicolau DP, Kuti JL. 2013. Comparative pharmacokinetics, pharmacodynamics, and tolerability of ertapenem 1 gram/day administered as a rapid 5-minute infusion versus the standard 30-minute infusion in healthy adult volunteers. Pharmacotherapy 33:266–274. 10.1002/phar.1197 [DOI] [PubMed] [Google Scholar]
- 12.Breilh D, Fleureau C, Gordien JB, Joanes-Boyau O, Texier-Maugein J, Rapaport S, Boselli E, Janvier G, Saux MC. 2011. Pharmacokinetics of free ertapenem in critically ill septic patients: intermittent versus continuous infusion. Minerva Anestesiol. 77:1058–1062 [PubMed] [Google Scholar]
- 13.Caminero JA, Sotgiu G, Zumla A, Migliori GB. 2010. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect. Dis. 10:621–629. 10.1016/S1473-3099(10)70139-0 [DOI] [PubMed] [Google Scholar]
- 14.De Lorenzo S, Alffenaar JW, Sotgiu G, Centis R, D'Ambrosio L, Tiberi S, Bolhuis MS, van Altena R, Viggiani P, Piana A, Spanevello A, Migliori GB. 2012. Efficacy and safety of meropenem/clavulanate added to linezolid containing regimens in the treatment of M/XDR-TB. Eur. Respir. J. 41:1386–1392. 10.1183/09031936.00124312 [DOI] [PubMed] [Google Scholar]
- 15.Dauby N, Muylle I, Mouchet F, Sergysels R, Payen MC. 2011. Meropenem/clavulanate and linezolid treatment for extensively drug-resistant tuberculosis. Pediatr. Infect. Dis. J. 30:812–813. 10.1097/INF.0b013e3182154b05 [DOI] [PubMed] [Google Scholar]
- 16.Payen MC, De Wit S, Martin C, Sergysels R, Muylle I, Van Laethem Y, Clumeck N. 2012. Clinical use of the meropenem-clavulanate combination for extensively drug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 16:558–560. 10.5588/ijtld.11.0414 [DOI] [PubMed] [Google Scholar]
- 17.Koal T, Deters M, Resch K, Kaever V. 2006. Quantification of the carbapenem antibiotic ertapenem in human plasma by a validated liquid chromatography-mass spectrometry method. Clin. Chim. Acta 364:239–245. 10.1016/j.cccn.2005.07.004 [DOI] [PubMed] [Google Scholar]
- 18.Pickering M, Brown S. 2013. Quantification and validation of HPLC-UV and LC-MS assays for therapeutic drug monitoring of ertapenem in human plasma. Biomed. Chromatogr. 27:568–574. 10.1002/bmc.2829 [DOI] [PubMed] [Google Scholar]
- 19.Lowes S, Jersey J, Shoup R, Garofolo F, Savoie N, Mortz E, Needham S, Caturla MC, Steffen R, Sheldon C, Hayes R, Samuels T, Di Donato L, Kamerud J, Michael S, Lin ZJ, Hillier J, Moussallie M, de Souza Teixeira L, Rocci M, Buonarati M, Truog J, Hussain S, Lundberg R, Breau A, Zhang T, Jonker J, Berger N, Gagnon-Carignan S, Nehls C, Nicholson R, Hilhorst M, Karnik S, de Boer T, Houghton R, Smith K, Cojocaru L, Allen M, Harter T, Fatmi S, Sayyarpour F, Vija J, Malone M, Heller D. 2011. Recommendations on: internal standard criteria, stability, incurred sample reanalysis and recent 483s by the Global CRO Council for Bioanalysis. Bioanalysis 3:1323–1332. 10.4155/bio.11.135 [DOI] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services Food and Drug Administration. 2001. Guidance for industry, bioanalytical method validation. Center for Drug Evaluation and Research, Rockville, MD [Google Scholar]
- 21.European Medicines Agency, Committee for Medicinal Products for Human Use. 2011. Guideline on bioanalytical validation (EMEA/CHMP/EWP/192217/2009). European Medicines Agency, London, United Kingdom [Google Scholar]