Abstract
We compared the dynamics and mechanisms of resistance development to ceftazidime, meropenem, ciprofloxacin, and ceftolozane-tazobactam in wild-type (PAO1) and mutator (PAOMS, ΔmutS) P. aeruginosa. The strains were incubated for 24 h with 0.5 to 64× MICs of each antibiotic in triplicate experiments. The tubes from the highest antibiotic concentration showing growth were reinoculated in fresh medium containing concentrations up to 64× MIC for 7 consecutive days. The susceptibility profiles and resistance mechanisms were assessed in two isolated colonies from each step, antibiotic, and strain. Ceftolozane-tazobactam-resistant mutants were further characterized by whole-genome analysis through RNA sequencing (RNA-seq). The development of high-level resistance was fastest for ceftazidime, followed by meropenem and ciprofloxacin. None of the mutants selected with these antibiotics showed cross-resistance to ceftolozane-tazobactam. On the other hand, ceftolozane-tazobactam resistance development was much slower, and high-level resistance was observed for the mutator strain only. PAO1 derivatives that were moderately resistant (MICs, 4 to 8 μg/ml) to ceftolozane-tazobactam showed only 2 to 4 mutations, which determined global pleiotropic effects associated with a severe fitness cost. High-level-resistant (MICs, 32 to 128 μg/ml) PAOMS derivatives showed 45 to 53 mutations. Major changes in the global gene expression profiles were detected in all mutants, but only PAOMS mutants showed ampC overexpression, which was caused by dacB or ampR mutations. Moreover, all PAOMS mutants contained 1 to 4 mutations in the conserved residues of AmpC (F147L, Q157R, G183D, E247K, or V356I). Complementation studies revealed that these mutations greatly increased ceftolozane-tazobactam and ceftazidime MICs but reduced those of piperacillin-tazobactam and imipenem, compared to those in wild-type ampC. Therefore, the development of high-level resistance to ceftolozane-tazobactam appears to occur efficiently only in a P. aeruginosa mutator background, in which multiple mutations lead to overexpression and structural modifications of AmpC.
INTRODUCTION
The growing prevalence of nosocomial infections produced by multiresistant Pseudomonas aeruginosa strains severely compromises the selection of appropriate treatments and is therefore associated with significant morbidity and mortality (1–3). While the incidences of concerning transferable resistance determinants, such as those encoding class B carbapenemases (or metallo-β-lactamases), are increasing, especially in certain areas (4, 5), the current global threat of antimicrobial resistance in P. aeruginosa mainly still results from the extraordinary capacity of this microorganism to develop resistance to almost any available antibiotic by the selection of mutations in chromosomal genes (6, 7). Among the particularly noteworthy mutation-mediated resistance mechanisms are those leading to the repression or inactivation of the carbapenem porin OprD, the hyperproduction of the chromosomal cephalosporinase AmpC, or the upregulation of one of the several efflux pumps encoded in the P. aeruginosa genome (8, 9). Furthermore, the accumulation of these various chromosomal mutations can lead to the emergence of multiresistant strains that eventually may be responsible for notable outbreaks in the hospital setting (7, 10). Therefore, strategies to overcome P. aeruginosa mutation-driven resistance mechanisms are urgently needed.
Ceftolozane (formerly CXA-101) is a new cephalosporin under clinical development in combination with tazobactam (ceftolozane-tazobactam, formerly CXA-201) that shows promising characteristics for the treatment of P. aeruginosa infections. Although tazobactam does not have a major impact on the activity of ceftolozane against P. aeruginosa, it significantly enhances the coverage of Enterobacteriaceae isolates producing extended-spectrum β-lactamases (11). Indeed, several recent studies revealed a potent in vitro activity of ceftolozane against P. aeruginosa, including in many cystic fibrosis and multiresistant strains not producing horizontally acquired β-lactamases (12–16). Additionally, in vitro studies have shown that ceftolozane appears to be stable against the most common resistance mechanisms driven by mutation in this species, particularly the overexpression of the chromosomal cephalosporinase AmpC or efflux pumps, conserving activity against pan-β-lactam-resistant clinical strains (17, 18). Previous studies have also revealed that the spontaneous mutation rate for the development of 4× MIC of ceftolozane-resistant mutants was below the detection limit (<10−10) even for DNA mismatch-repair-deficient mutator strains (19). Based on these previous findings, the objective of this work was to compare the dynamics and mechanisms of in vitro development of resistance to ceftolozane-tazobactam with the currently available antipseudomonal agents, using wild-type and mutator strains, under long-term exposure to growing drug concentrations.
MATERIALS AND METHODS
Strains.
The wild-type reference strain P. aeruginosa PAO1 and its mismatch-repair-deficient (ΔmutS) mutator derivative (PAOMS) were used (19).
Dynamics of resistance development.
To determine the dynamics of resistance development to ceftazidime, meropenem, ciprofloxacin, and ceftolozane-tazobactam, 10-ml Mueller-Hinton tubes containing 0.5×, 1×, 2×, 4×, 8×, 16×, 32×, and 64× MIC values of each antibiotic were inoculated with approximately 106 CFU/ml of exponentially growing PAO1 or PAOMS strains and incubated for 24 h at 37°C and 180 rpm. All experiments were performed in triplicate. The tubes from the highest antibiotic concentration showing growth were reinoculated (at a 1:1,000 dilution) in fresh medium containing concentrations up to 64× MIC for 7 consecutive days. Two colonies per strain, antibiotic, resistance step, and replicate experiment were purified in antibiotic-free LB agar plates for further characterization.
Susceptibility testing.
The MICs of ceftolozane, ceftolozane-tazobactam, ceftazidime, cefepime, piperacillin, piperacillin-tazobactam, aztreonam, imipenem, meropenem, and ciprofloxacin were determined by broth microdilution according to CLSI guidelines (20).
Characterization of resistance mechanisms.
The expression of the genes encoding the chromosomal β-lactamase AmpC (ampC) and four P. aeruginosa efflux pumps, MexAB-OprM (mexB), MexCD-OprJ (mexD), MexXY-OprM (mexY), and MexEF-OprN (mexF), were determined from late-log-phase Luria-Bertani (LB) broth cultures at 37°C and 180 rpm by real-time reverse transcription-PCR (RT-PCR), as previously described (8). The quinolone resistance determining regions (QRDR) of gyrA, gyrB, parC, and parE were sequenced in ciprofloxacin-resistant mutants (7). Outer membrane protein (OMP) profiles were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie blue (8). The obtained OprD profiles were compared with those of PAO1 and its OprD-deficient mutant. Penicillin-binding proteins (PBPs) were labeled with Bocillin FL fluorescent penicillin, separated through SDS-PAGE, and visualized using a Bio-Rad FX Pro molecular imager (21).
RNA sequencing.
RNA sequencing (RNA-seq) was performed in duplicate on the parental strains PAO1 and PAOMS and on two low-level (MICs, 4 to 8 μg/ml) PAO1 and 3 high-level (MICs, 32 to 128 μg/ml) PAOMS ceftolozane-tazobactam-resistant mutants. Total RNA was isolated from three replicate cultures (optical density at 600 nm [OD600], 1; using LB broth and at 37°C and 180 rpm) and after rRNA depletion by the use of a commercial capture and depletion system (MICROBExpress kit; Ambion), strand-specific bar-coded cDNA libraries were generated, and all samples were sequenced using a lane of an Illumina HiSeq 2500. The raw sequence output consisted of 263.7 million reads, with a length of 100 nucleotides. Computational analysis was slightly modified from that used by Dötsch et al. (22). Briefly, the reads were mapped using Stampy (23), differential gene expression was calculated using the DESeq package (24), and mutations were identified using SAMtools (25). The Pseudomonas genome database was used for gene function analysis (26, 27).
Characterization of ampC mutations.
The obtained ampC mutant derivatives were cloned in parallel with the wild-type ampC gene from PAO1. For this purpose, PCR products obtained with upstream (AmpC-F-EcoRI, 5′-TCGAATTCACGACAAAGGACGCCAATCC-3′) and downstream (AmpC-R-HinDIII, TCAAGCTTTCAGCGCTTCAGCGGCACC) primers were digested with EcoRI or HinDIII, ligated to pUCP24 (28), and transformed into Escherichia coli XL1-Blue made competent by CaCl2. Transformants were selected in 5 μg/ml gentamicin MacConkey agar plates. The cloned genes obtained from three independent experiments were fully sequenced to ascertain the absence of mutations introduced during PCR amplification. The resulting plasmids were transformed into an ampC knockout mutant of PAO1 (PAΔC) (29) and characterized through the determination of the MICs for ceftolozane, ceftolozane-tazobactam, ceftazidime, cefepime, piperacillin, piperacillin-tazobactam, aztreonam, and imipenem using broth microdilution, according to CLSI guidelines.
In vitro competition experiments.
In vitro competition experiments between each of the resistant mutants and a gentamicin-tagged (att intergenic neutral chromosomal locus) wild-type PAO1 were performed (29, 30). Exponentially growing cells were mixed in a 1:1 ratio and diluted in 0.9% saline solution. Approximately 103 cells from each of the mixtures were inoculated into eight 10-ml LB broth flasks and grown at 37°C and 180 rpm for 16 to 18 h, corresponding to approximately 20 generations. Serial 10-fold dilutions were plated in duplicate onto LB agar alone and with 15 μg/ml of gentamicin. The competition index (CI) was defined as the mutant-to-wild-type ratio.
RESULTS AND DISCUSSION
Analysis of the dynamics of resistance development to ceftolozane-tazobactam and comparators in wild-type and mutator strains.
The basal MICs for both PAO1 and PAOMS strains of ceftazidime, meropenem, ciprofloxacin, and ceftolozane-tazobactam were 1, 0.5, 0.125, and 0.5 μg/ml, respectively.
As shown in Fig. 1A, the development of high-level resistance in the PAO1 strain was fastest for ceftazidime, reaching 64× MIC by day 4, followed by meropenem and ciprofloxacin, which reached 64× MIC at day 6. In contrast, resistance development was much slower for ceftolozane-tazobactam, with modal concentrations reaching only 8× MIC after the completion of the 7-day experiments (Fig. 1A). Moreover, a 64× MIC was not reached in any of three cultures even after extended 14-day exposure experiments (not shown).
FIG 1.
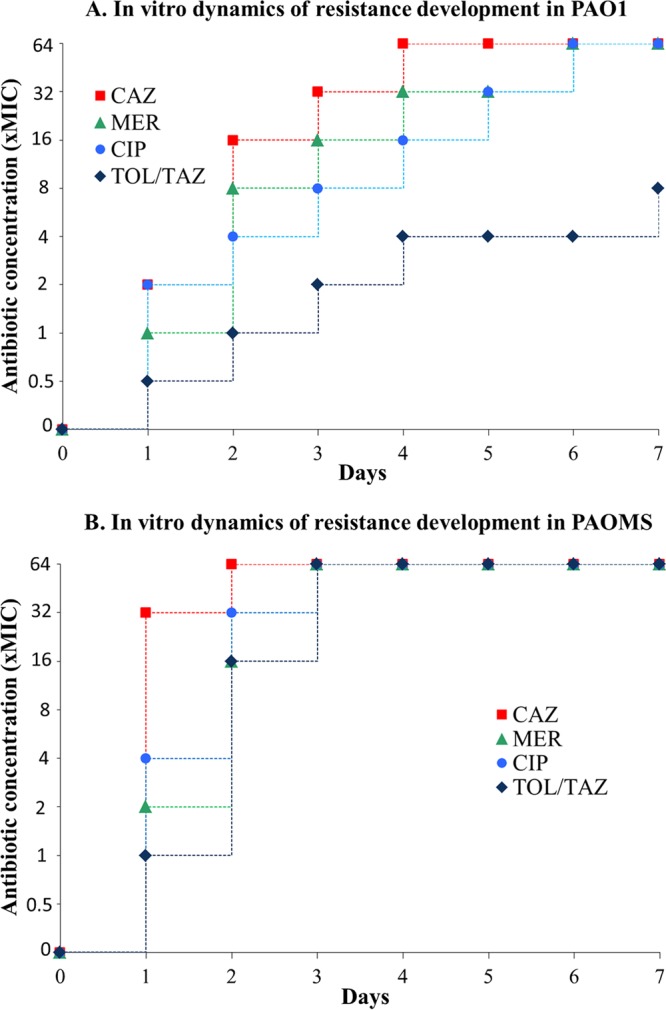
Dynamics of resistance development to ceftolozane-tazobactam and comparators. The modal values for three experiments are shown.
As shown in Fig. 1B, the development of resistance was dramatically enhanced for all compounds in the mutator strain; 64× MICs were reached by day 2 for ceftazidime and by day 3 for meropenem, ciprofloxacin, and ceftolozane-tazobactam. However, first-step ceftolozane-tazobactam resistance development was very limited even for the mutator strain, with concentrations reaching only 1× MIC after day 1. Thus, these results are consistent with previous findings suggesting that resistance development to ceftolozane cannot be achieved by single-step mutations (19).
Analysis of the susceptibility profiles and resistance mechanisms of the mutants selected during stepwise antibiotic exposure.
All mutants selected upon ceftazidime exposure showed high-level resistance to ceftazidime, cefepime, piperacillin-tazobactam, and aztreonam (Table 1), caused by the overexpression of the chromosomal cephalosporinase AmpC (Table 1). Cross-resistances to carbapenems and/or fluoroquinolones were not detected in any of the ceftazidime-selected mutants, and none of them showed efflux pump overexpression. Remarkably, ceftolozane-tazobactam MICs remained at ≤4 μg/ml in all ceftazidime-selected mutants, confirming the much higher stability of ceftolozane-tazobactam against AmpC hydrolysis (17, 18).
TABLE 1.
Susceptibility profiles and resistance mechanisms of mutants selected during stepwise exposure to ceftolozane-tazobactam, ceftazidime, meropenem, and ciprofloxacin in each of three experiments
| Strain informationa | MIC (μg/ml) by antibiotic (CLSI breakpoint)b |
Resistance mechanism(s)c | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TOL-TAZ | CAZ (S ≤ 8) | FEP (S ≤ 8) | PIP-TAZ (S ≤ 16) | AZT (S ≤ 8) | IMP (S ≤ 4) | MER (S ≤ 4) | CIP (S ≤ 1) | ||
| PAO1 | 0.5 | 1 | 1 | 4 | 4 | 1 | 0.5 | 0.12 | None |
| 1, P1.1, TOL-TAZr (0.5×) | 1 | 4 | 2 | 8 | 16d | 1 | 1 | 0.12 | None |
| 1, P1.2, TOL-TAZr (0.5×) | 1 | 4 | 2 | 8 | 8 | 1 | 1 | 0.12 | None |
| 1, P1.3, TOL-TAZr (0.5×) | 1 | 4 | 2 | 8 | 8 | 1 | 1 | 0.12 | None |
| 2, P1.1, TOL-TAZr (1×) | 1 | 4 | 2 | 8 | 16 | 1 | 1 | 0.12 | None |
| 2, P1.2, TOL-TAZr (1×) | 1 | 4 | 2 | 8 | 16 | 1 | 1 | 0.12 | None |
| 2, P1.3, TOL-TAZr (1×) | 1 | 4 | 2 | 8 | 16 | 1 | 1 | 0.12 | None |
| 3, P1.1, TOL-TAZr (2×) | 2 | 4 | 4 | 16 | 16 | 0.5 | 2 | 0.25 | None |
| 3, P1.2, TOL-TAZr (2×) | 2 | 8 | 4 | 8 | 16 | 1 | 1 | 0.25 | None |
| 3, P1.3, TOL-TAZr (2×) | 2 | 4 | 4 | 16 | 16 | 1 | 1 | 0.25 | None |
| 4, P1.1, TOL-TAZr (4×) | 4 | 4 | 4 | 16 | 16 | 2 | 2 | 0.25 | None |
| 4, P1.2, TOL-TAZr (4×) | 4 | 8 | 4 | 16 | 8 | 2 | 2 | 0.25 | None |
| 4, P1.3, TOL-TAZr (4×) | 4 | 8 | 4 | 16 | 16 | 2 | 2 | 0.25 | None |
| 7, P1.1, TOL-TAZr (8×) | 4 | 4 | 8 | 32 | 16 | 1 | 2 | 0.25 | None |
| 7, P1.2, TOL-TAZr (8×) | 8 | 8 | 4 | 16 | 16 | 2 | 4 | 0.5 | None |
| 7, P1.3, TOL-TAZr (8×) | 8 | 8 | 4 | 16 | 16 | 4 | 4 | 0.12 | None |
| 1, PmS.1, TOL-TAZr (1×) | 2 | 32 | 16 | 128 | 128 | 1 | 0.5 | 0.25 | ampC (619) |
| 1, PmS.2, TOL-TAZr (1×) | 2 | 32 | 8 | 128 | 128 | 4 | 1 | 0.5 | ampC (602) |
| 1, PmS.3, TOL-TAZr (1×) | 4 | 64 | 16 | 256 | 256 | 4 | 1 | 0.25 | ampC (2,863) |
| 2, PmS.1, TOL-TAZr (16×) | 16 | 32 | 2 | 8 | 32 | 0.25 | 1 | 0.25 | ampC (264) |
| 2, PmS.2, TOL-TAZr (16×) | 16 | 32 | 4 | 8 | 32 | 0.25 | 1 | 0.25 | ampC (444) |
| 2, PmS.3, TOL-TAZr (16×) | 32 | 64 | 8 | 8 | 32 | 0.25 | 1 | 0.5 | ampC (734) |
| 3, PmS.1, TOL-TAZr (64×) | 128 | 256 | 8 | 8 | 128 | 0.12 | 1 | 0.25 | ampC (169) |
| 3, PmS.2, TOL-TAZr (64×) | 64 | 256 | 8 | 16 | 64 | 0.12 | 0.5 | 0.5 | ampC (804) |
| 3, PmS.3, TOL-TAZr (64×) | 32 | 64 | 4 | 16 | 32 | 0.06 | 0.5 | 0.03 | ampC (4,459) |
| 7, PmS.1, TOL-TAZr (64×) | 128 | >256 | 16 | 16 | 128 | 0.06 | 0.5 | 0.06 | ampC (128) |
| 7, PmS.2, TOL-TAZr (64×) | 32 | 256 | 8 | 16 | 128 | 0.12 | 0.5 | 0.03 | ampC (268) |
| 7, PmS.3, TOL-TAZr (64×) | 64 | 128 | 8 | 32 | 64 | 0.12 | 1 | 0.06 | ampC (458) |
| 7, P1.1, CAZr (64×) | 2 | 128 | 64 | >256 | 128 | 2 | 2 | 0.12 | ampC (350) |
| 7, P1.2, CAZr (64×) | 4 | 128 | 64 | >256 | 128 | 2 | 2 | 0.12 | ampC (32) |
| 7, P1.3, CAZr (64×) | 4 | 128 | 64 | >256 | 128 | 4 | 2 | 0.12 | ampC (67) |
| 7, PmS.1, CAZr (64×) | 4 | 128 | 32 | 128 | 128 | 1 | 0.25 | 0.12 | ampC (244) |
| 7, PmS.2, CAZr (64×) | 4 | 128 | 16 | 128 | 128 | 1 | 0.5 | 0.12 | ampC (672) |
| 7, PmS.3, CAZr (64×) | 4 | 256 | 64 | >256 | 256 | 1 | 1 | 0.12 | ampC (254) |
| 7, P1.1, MERr (64×) | 2 | 16 | 4 | 256 | 128 | 16 | 64 | 1 | OprD− + mexB (4.5) + mexF (48) |
| 7, P1.2, MERr (64×) | 1 | 16 | 4 | 128 | 64 | 16 | 128 | 1 | OprD− + mexB (4.4) |
| 7, P1.3, MERr (64×) | 2 | 16 | 4 | 128 | 128 | 16 | 64 | 1 | OprD− + mexB (5.8) |
| 7, PmS.1, MERr (64×) | 0.25 | 2 | 8 | 32 | 64 | 16 | 64 | 1 | OprD− + ampC (17) + mexB (5.3) + mexY (11) + mexF (44) |
| 7, PmS.2, MERr (64×) | 1 | 8 | 16 | 128 | 256 | 16 | 64 | 0.5 | OprD− + mexB (16) |
| 7, PMS.3, MERr (64×) | 1 | 8 | 16 | 64 | 64 | 8 | 64 | 0.5 | OprD− + mexB (13) |
| 7, P1.1, CIPr (64×) | 0.25 | 0.5 | 4 | 2 | 2 | <0.12 | 0.25 | >32 | mexD (515) + GyrA T83I + ParC S87L |
| 7, P1.2, CIPr (32×) | 0.25 | 1 | 4 | 4 | 1 | <0.12 | 0.25 | 8 | mexD (739) + GyrA E153K + ParC S87L |
| 7, P1.3, CIPr (64×) | 0.5 | 2 | 2 | 16 | 32 | 1 | 2 | >32 | mexB (8.4) + GyrA T83I + ParC D117E |
| 7, PmS.1, CIPr (16×) | 0.25 | 0.5 | 4 | 4 | 2 | 0.5 | 0.5 | >32 | mexD (534) + GyrA T83I + ParC E91K |
| 7, PmS.2, CIPr (64×) | 0.25 | 0.5 | 4 | 4 | 2 | 0.25 | 0.5 | >32 | mexD (441) + GyrA T83I + ParC S87L |
| 7, PmS.3, CIPr (64×) | 0.25 | 0.5 | 2 | 4 | 2 | 0.5 | 0.5 | >32 | mexD (430) + GyrA T83I + ParC S87L |
Format for strains: number of days of exposure, P1 (PAO1) or PmS (PAOMS), antibiotic resistance (concentration of antibiotic in tubes from which the mutants were selected). For ceftazidime, meropenem, and ciprofloxacin, only mutants obtained in the final step of the experiment (day 7) are included. For ceftolozane-tazobactam, mutants from the intermediate selection steps (according to data from Fig. 1) are also included with the day 7 mutants.
TOL-TAZ, ceftolozane-tazobactam; CAZ, ceftazidime, FEP, cefepime; PIP-TAZ, piperacillin-tazobactam; AZT, aztreonam; IMP, imipenem; MER, meropenem; CIP, ciprofloxacin; S, susceptible.
Resistance mechanisms studied. ampC, mexB, mexD, mexF, and mexY expression. Previously described breakpoints (8) were applied for defining overexpression: ampC, mexD, mexY, and mexF, >10-fold compared to wild-type PAO1; mexB, >3-fold compared to wild-type PAO1. Expression levels are indicated in parentheses. The lack of OprD (OprD−), as evidenced by the analysis of outer membrane proteins (OMPs) through SDS-PAGE and mutations in the QRDR regions of GyrA, GyrB, ParC, and ParE are also indicated.
Bold type indicates strains that are not susceptible.
Mutants selected upon meropenem exposure developed resistance to imipenem and meropenem through the loss of the expression of the carbapenem porin OprD (Table 1), but they also showed significantly enhanced MICs for ceftazidime, cefepime, piperacillin-tazobactam, aztreonam, and ciprofloxacin due to the overexpression of the efflux pump MexAB-OprM in all of them, occasionally accompanied by the overexpression of other efflux pumps (MexXY-OprM or MexEF-OprN) or AmpC (Table 1). Thus, meropenem exposure selected multidrug-resistant (MDR) profiles. Nevertheless, in contrast to all other antibiotics tested, the MICs of ceftolozane-tazobactam were not increased in meropenem-selected mutants.
Similarly, all ciprofloxacin-resistant mutants showed two QRDR mutations (gyrA and parC) determining high-level fluoroquinolone resistance. Remarkably, while the mutations detected most frequently included the classical GyrA T83I and ParC S87L, mutations not previously described in GyrA (E153K) and ParC (D117E) were also detected, each in one different mutant (Table 1). The specific effects of these new QRDR mutations in quinolone resistance are under investigation in our laboratory. Additionally, all ciprofloxacin-resistant mutants overexpressed efflux pumps (MexAB-OprM or MexCD-OprJ) conferring reduced susceptibility to unrelated antipseudomonal agents (Table 1).
In contrast, PAO1 ceftolozane-tazobactam mutants reached only moderate resistance (MICs, 4 to 8 μg/ml) after the 7-day exposure experiments. High-level ceftolozane-tazobactam-resistant mutants were selected only in PAOMS experiments and showed cross-resistance to ceftazidime, piperacillin-tazobactam, and aztreonam due to AmpC overexpression. On the other hand, none of the ceftolozane-tazobactam-selected mutants overexpressed efflux pumps. Moreover, high-level ceftolozane-tazobactam-resistant mutants showed increased susceptibility to imipenem and ciprofloxacin (Table 1).
Characterization of ceftolozane-tazobactam resistance mechanisms through whole-genome analysis.
PAO1 ceftolozane-tazobactam mutants, reaching only moderate resistance (MICs, 4 to 8 μg/ml) after the 7-day exposure experiments, showed only two to four mutations in the RNA-seq experiments (Table 2). Both mutants showed a deletion in pilB, whereas PAO1.1 showed a mutation in PA3206 (a probable two-component sensor) and PAO1.2 in the intracellular protease ClpX. PAO1.2 additionally showed a silent and an intergenic mutation. Despite the small number of mutations, a global transcriptome analysis revealed a remarkable number of genes with modified expression both in PAO1.1 (309 genes) and PAO1.2 (395 genes), perhaps related to the broad regulatory functions of PA3206 and ClpX (Fig. 2; see also Table S1 in the supplemental material). However, neither the mutations detected nor the genes showing modified expression were directly linked to classical antibiotic resistance mechanisms. Moreover, both mutants showed greatly reduced expression of genes belonging to the MDR efflux pumps MexXY-OprM (mexX and mexY) and MexCD-OprJ (mexC) (see Table S1 in the supplemental material). The specific effects on β-lactam resistance of the detected mutations are under investigation in our laboratory; the function of PA3206 is currently unknown, but previous works established a role for intracellular proteases in multiple relevant processes, including antibiotic resistance, motility, biofilm formation, and alginate production (31, 32). Moreover, in vitro competition experiments revealed a major fitness cost in both mutants, with a CI of 0.0002 for PAO1.1 and a CI of 0.044 for PAO1.2 (Fig. 3A). Indeed, the CIs of moderately resistant ceftolozane-tazobactam PAO1 mutants were much lower than those of high-level ceftazidime-resistant mutants and comparable only to those of high-level meropenem-resistant mutants (Fig. 4A). Therefore, our data suggest that moderate ceftolozane-tazobactam resistance in PAO1 results from nonspecific mutations with global pleiotropic effects associated with an important fitness cost.
TABLE 2.
Mutations detected by RNA-seq of PAO1 and PAOMS derivative mutants after 7 days of exposure to increasing concentrations of ceftolozane-tazobactam
| Gene information |
Mutation(s) by strain typea |
||||||
|---|---|---|---|---|---|---|---|
| Locus | Name | Product name | 7, P1.1, TOL-TAZr | 7, P1.2, TOL-TAZr | 7, PmS.1, TOL-TAZr | 7, PmS.2, TOL-TAZr | 7, PmS.3, TOL-TAZr |
| Locus | Name | Product name | |||||
| PA0123 | Probable transcriptional regulator | R131H | R131H | ||||
| PA0136 | Probable ATP-binding component of ABC transporter | Silent | |||||
| PA0201 | Hypothetical protein | Silent | |||||
| Intergenic | A266314G | ||||||
| PA0347 | glpQ | Glycerophosphoryl diester phosphodiesterase, periplasmic | Y75H | ||||
| PA0352 | yicE | Probable transporter | P371L | ||||
| PA0378 | mgtA | Probable transglycosylase | Y23H | ||||
| PA0413 | chpA | Component of chemotactic signal transduction system | Q947R | ||||
| PA0615 | Hypothetical protein | Silent | |||||
| Intergenic | C788723T | ||||||
| Intergenic | G811242A | ||||||
| PA0747 | Probable aldehyde dehydrogenase | I5T | |||||
| PA0811 | Probable major facilitator superfamily (MFS) transporter | Silent | Silent | ||||
| PA0877 | Probable transcriptional regulator | Silent | |||||
| PA0887 | acsA | Acetyl-coenzyme A synthetase | Silent | Silent | |||
| PA0895 | aruC | N-Succinylglutamate 5-semialdehyde dehydrogenase | Silent | ||||
| PA0902 | Hypothetical protein | nt858Ins(G) | nt858Ins(G); A43V | ||||
| PA0919 | Hypothetical protein | D40N | |||||
| PA0920 | Hypothetical protein | L437P | L437P | ||||
| PA0923 | dinB | DNA polymerase IV, DinB | L316F | ||||
| PA0928 | gacS | Sensor/response regulator hybrid | G489S | L531P | |||
| Intergenic | A1037625G | A1037625G | |||||
| PA0964 | pmpR | pqsR-mediated PQS regulator, PmpR | E225G | ||||
| PA0971 | tolA | TolA protein | S12G | S12G | |||
| PA0997 | pqsB | PqsB | Silent | ||||
| Intergenic | nt1083918Ins(G) | ||||||
| PA1026 | Hypothetical protein | S148P | |||||
| PA1069 | Hypothetical protein | Silent | |||||
| PA1124 | dgt | Deoxyguanosinetriphosphate triphosphohydrolase | H344R | H344R | |||
| Intergenic | G1220630A | ||||||
| PA1223 | Probable transcriptional regulator | Silent | Silent | ||||
| Intergenic | G1397241A | ||||||
| PA1269 | Probable 2-hydroxy acid dehydrogenase | Silent | |||||
| PA1310 | phnW | 2-Aminoethylphosphonate: pyruvate aminotransferase | A176V | A176V | |||
| PA1458 | cheA | Probable two-component sensor | nt2167Δ1 | ||||
| PA1480 | ccmF | Cytochrome c-type biogenesis protein CcmF | F292L | ||||
| Intergenic | C1674355T | C1674355T | |||||
| PA1622 | Probable hydrolase | A114V | |||||
| PA1662 | clpV2 | ClpV2 | A128V | ||||
| PA1690 | pscU | Translocation protein in type III secretion | A11T | ||||
| PA1730 | Conserved hypothetical protein | Silent | Silent | ||||
| PA1797 | Hypothetical protein | Silent | |||||
| PA1802 | clpX | ClpX | G266D | ||||
| PA2023 | galU | UTP-glucose-1-phosphate uridylyltransferase | D64G | ||||
| PA2040 | pauA4 | Glutamylpolyamine synthetase | Silent | ||||
| PA2138 | Probable ATP-dependent DNA ligase | G260S | |||||
| Intergenic | T2545663C | ||||||
| PA2232 | pslB | PslB | V414A | V414A | |||
| PA2250 | lpdV | Lipoamide dehydrogenase-Val | W118X | W118X | |||
| Intergenic | T2581013C | ||||||
| PA2387 | fpvI | FpvI | Silent | ||||
| PA2399 | pvdD | Pyoverdine synthetase D | R1576Q | ||||
| PA2402 | Probable nonribosomal peptide synthetase | A2368T | |||||
| PA2443 | sdaA | l-Serine dehydratase | Silent | ||||
| PA2540 | Conserved hypothetical protein | Silent | |||||
| PA2597 | Hypothetical protein | Silent | Silent | ||||
| PA2615 | ftsK | Cell division protein FtsK | Silent | ||||
| PA2643 | nuoH | NADH dehydrogenase I chain H | V101A | ||||
| Intergenic | T3108446C | T3108446C | |||||
| PA2938 | Probable transporter | Silent | |||||
| PA3047 | dacB | Probable d-alanyl-d-alanine carboxypeptidase | G427D | E84K | |||
| PA3060 | pelE | PelE | W102R | W102R | |||
| Intergenic | A3481349G | A3481349G | |||||
| PA3158 | wbpB | UDP-2-acetamido-2-deoxy-d-glucuronic acid 3-dehydrogenase, WbpB | Silent | Silent | |||
| PA3179 | yciL | Conserved hypothetical protein | Silent | ||||
| PA3187 | gltK | Probable ATP-binding component of ABC transporter | A299T | ||||
| PA3206 | Probable two-component sensor | I279T | |||||
| PA3346 | Two-component response regulator | P300S | |||||
| PA3516 | Probable lyase | T186I | |||||
| PA3614 | Hypothetical protein | G380D | G380D | ||||
| PA3624 | pcm | l-Isoaspartate protein carboxyl methyltransferase type II | T86A | ||||
| PA3666 | dapD | Tetrahydrodipicolinate succinylase | Silent | ||||
| PA3795 | Probable oxidoreductase | Silent | |||||
| PA3803 | gcpE | Probable isoprenoid biosynthetic protein GcpE | V224A | ||||
| PA3900 | Probable transmembrane sensor | R278Q | |||||
| PA3919 | ylaK | Conserved hypothetical protein | L267P | ||||
| Intergenic | nt4388937Δ1 | nt4388937Δ1 | |||||
| PA3935 | tauD | Taurine dioxygenase | N241S | ||||
| PA3974 | ladS | Lost adherence sensor | N230S | ||||
| Intergenic | T4544558C | ||||||
| PA4069 | Hypothetical protein | Silent | |||||
| PA4109 | ampR | Transcriptional regulator AmpR | D135N | ||||
| PA4110 | ampC | β-Lactamase precursor | F147L, Q157R, E247K, V356I | E247K, V356I | G183D | ||
| PA4120 | Probable transcriptional regulator | Silent | Silent | ||||
| PA4147 | acoR | Transcriptional regulator AcoR | nt547Ins(C) | ||||
| PA4186 | Hypothetical protein | G249S | |||||
| PA4208 | opmD | Probable outer membrane protein precursor | E142G | ||||
| PA4290 | Probable chemotaxis transducer | Q185X | Q185X | ||||
| PA4311 | Conserved hypothetical protein | E298K | |||||
| PA4313 | Hypothetical protein | Silent | |||||
| Intergenic | G4903413A | ||||||
| PA4526 | pilB | Type 4 fimbrial biogenesis protein PilB | nt1478Δ3 | nt1478Δ3 | |||
| PA4548 | yfiT | Probable d-amino acid oxidase | Silent | ||||
| PA4556 | pilE | Type 4 fimbrial biogenesis protein PilE | N133S | ||||
| PA4571 | Probable cytochrome c | C15Y | |||||
| Intergenic | C5141232T | ||||||
| PA4622 | Probable major facilitator superfamily (MFS) transporter | E361K | |||||
| PA4659 | Probable transcriptional regulator | R239H | |||||
| PA4673.1 | tRNA-Met | T5242034C | |||||
| Intergenic | C5282588T | ||||||
| PA4745 | nusA | N utilization substance protein A | P87L | ||||
| PA4771 | lldD | l-Lactate dehydrogenase | R50W | R50W | |||
| PA4783 | yedA | Conserved hypothetical protein | Silent | ||||
| PA4819 | Probable glycosyl transferase | I268T | |||||
| Intergenic | G5431174A | ||||||
| PA4840 | yciH | Conserved hypothetical protein | R57C | ||||
| PA4846 | aroQ1 | 3-Dehydroquinate dehydratase | R65H | ||||
| PA4848 | accC | Biotin carboxylase | Silent | Silent | |||
| PA4856 | retS | RetS (regulator of exopolysaccharide and type III secretion) | G197S | ||||
| PA4911 | Probable permease of ABC branched-chain amino acid transporter | T172A | |||||
| Intergenic | G5599844A | G5599844A | |||||
| PA5006 | - | Hypothetical protein | Silent | ||||
| PA5197 | rimK | Ribosomal protein S6 modification protein | nt871Δ1 | ||||
| PA5398 | dgcA | DgcA, dimethylglycine catabolism | T276A | T276A | |||
| PA5474 | Probable metalloprotease | G167D | |||||
| PA5490 | cc4 | Cytochrome c4 precursor | D172G | ||||
| PA5538 | amiA | N-Acetylmuramoyl-l-alanine amidase | P18S | ||||
| Intergenic | G6252699A | ||||||
Format for strains: number of days of exposure, P1 (PAO1) or PmS (PAOMS), antibiotic resistance.
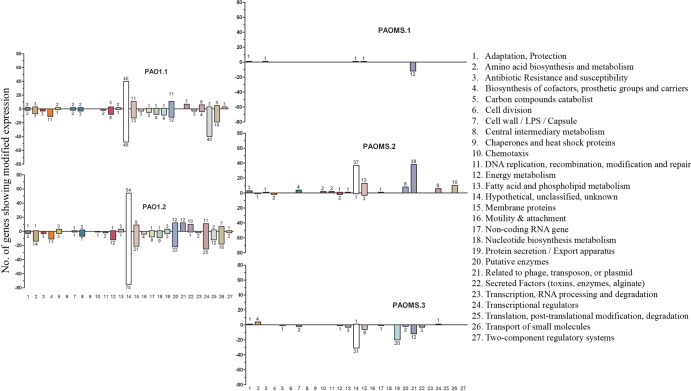
FIG 2.
Numbers of genes showing modified expression in the studied ceftolozane-tazobactam resistant mutants compared with wild-type PAO1 in each of the 27 established functional categories. Negative numbers indicate genes with decreased expression, and positive numbers indicate genes with increased expression.
FIG 3.
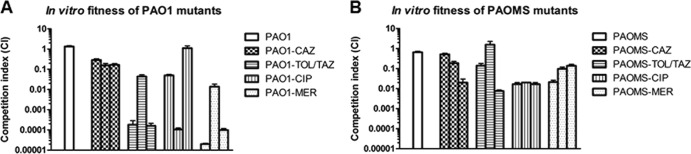
In vitro competition assays to assess the fitness cost associated with the development of resistance to ceftolozane-tazobactam (TOL-TAZ), ceftazidime (CAZ), meropenem (MER), and ciprofloxacin (CIP) in the three day 7 PAO1 and PAOMS mutants described in Table 1. Error bars indicate standard deviation.
FIG 4.
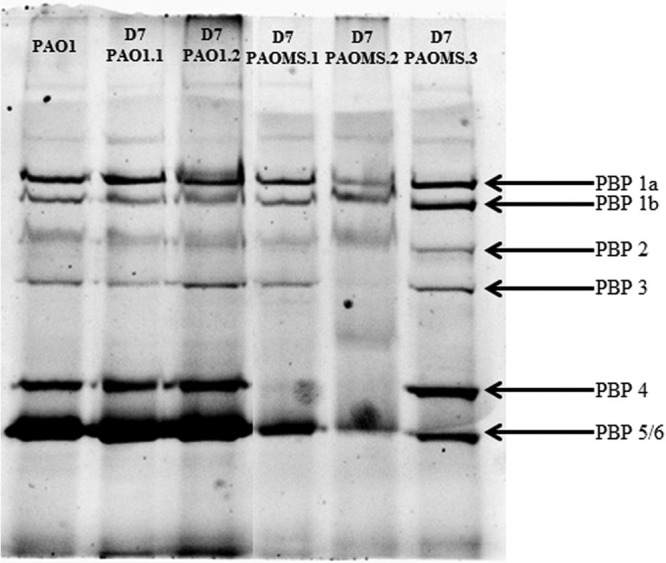
Penicillin-binding protein (PBP) profiles of ceftolozane-tazobactam-resistant mutants obtained from PAO1 and PAOMS strains.
In contrast, high-level (MICs, 32 to 128 μg/ml) ceftolozane-tazobactam-resistant PAOMS mutants showed 45 to 53 mutations (Table 2). However, the number of genes showing modified expression (16 to 136 genes) was lower than for PAO1 mutants but always included ampC overexpression (Fig. 2; see also Table S1 in the supplemental material). Consistently, all PAOMS mutants showed mutations in ampC regulators (dacB [n = 2] or ampR [n = 1]) (Table 2). Interestingly, the detected AmpR mutation (D135N) has been observed among clinical isolates of multiple Gram-negative pathogens, including P. aeruginosa (33). The absence of a functional PBP4 was also evidenced in the two dacB mutants through the analysis of the PBP profiles (Fig. 4). The PBP profiles also revealed an apparent reduction of PBP3 (one of the main targets of cephalosporins) expression in one of the mutants. Moreover, all PAOMS mutants contained one to four mutations in the conserved residues of AmpC (F147L, Q157R, G183D, E247K, or V356I) (Table 2). Complementation studies on PAΔC revealed that these mutations greatly increased ceftolozane-tazobactam and ceftazidime MICs but reduced those of piperacillin-tazobactam and imipenem compared with wild-type ampC (Table 3). Further ongoing structural and biochemical studies with these mutant enzymes will yield relevant information for understanding the plasticity of AmpC enzymes for β-lactam hydrolysis and its impact on resistance.
TABLE 3.
Susceptibility profile of the PAO1 ampC knockout mutant (PAΔC) complemented with wild-type ampC and derivatives from ceftolozane-tazobactam-resistant mutants
| Strain (ampC mutation[s]) | MICs (μg/ml)by antibiotic (CLSI breakpoint)b |
|||||||
|---|---|---|---|---|---|---|---|---|
| TOL | TOL-TAZ | CAZ (S ≤ 8) | PIP (S ≤ 16) | PIP-TAZ (S ≤ 16) | FEP (S ≤ 8) | ATM (S ≤ 16) | IMP (S ≤ 4) | |
| PAO1 | 0.5 | 0.5 | 1 | 2 | 2 | 0.5 | 2 | 1 |
| PAΔC | 0.5 | 0.5 | 1 | 2 | 2 | 0.5 | 2 | 0.25 |
| PAΔC + pUCPACWTa | 1 | 1 | 16 | 128 | 128 | 4 | 32 | 0.5 |
| PAΔC + pUCPACPAOMS.1 (F147L, Q157R, E247K, V356I) | 128 | 128 | 256 | 8 | 8 | 4 | 32 | 0.25 |
| PAΔC + pUCPACPAOMS.2 (E247K, V356I) | 64 | 64 | 128 | 32 | 32 | 8 | 32 | 0.25 |
| PAΔC + pUCPACPAOMS.3 (G183D) | 32 | 32 | 32 | 8 | 8 | 2 | 8 | 0.25 |
WT, wild type.
TOL, ceftolozane; TOL-TAZ, ceftolozane-tazobactam; CAZ, ceftazidime, PIP, piperacillin; PIP-TAZ, piperacillin-tazobactam; FEP, cefepime; ATM, aztreonam; IMP, imipenem; S, susceptible.
The specific effects on the susceptibility profiles, if any, of each of the other multiple mutations detected in the PAOMS mutants still need to be explored. It is expected that a number of them should just be nonpositively selected random mutations as a consequence of the very high spontaneous mutation rate of PAOMS. This is likely to be the case for the 12 to 14 silent mutations detected in each of the mutants. However, in addition to those related to AmpC, several others of the nonsynonymous mutations might also play a role in the phenotype. Indeed, at least eight (gacS, pqsB, phnW, galU, nuoH, nusA, pvdD, and PA3516) of the mutated genes have been shown to have an impact (increase or decrease) on antimicrobial susceptibility in previous analyses of saturated transposon-mutant libraries (34–37). Among these, mutations in phnW, galU, and nuoH have been shown to increase cephalosporin (ceftazidime) MICs, but remarkably some resulted in increased imipenem (galU and nusA) or ciprofloxacin (gacS and PA3516) susceptibility, consistent with the susceptibility profiles observed in our work (Table 1).
For all antibiotics, the impact on fitness of high-level antibiotic resistance was much lower for PAOMS mutants than for PAO1 mutants, likely reflecting the increased capacity of this strain to acquire cost-compensatory mutations (Fig. 3). Indeed, the fitness costs of high-level ceftolozane-tazobactam-resistant PAOMS mutants were highly variable, ranging from a CI of 0.008 in PAOMS.3 to a CI of 1.57 in PAOMS.2, possibly indicating the absence or presence of cost-compensatory mutations in these mutants (Fig. 3B).
Concluding remarks.
The development of ceftolozane-tazobactam resistance was much slower than that of resistance to other antipseudomonal agents. Moreover, ceftolozane-tazobactam remained active against ceftazidime-, ciprofloxacin-, and meropenem-resistant P. aeruginosa mutants. After 7 days of exposure, the wild-type strain PAO1 developed only moderate resistance (MICs, 4 to 8 μg/ml), which was associated with a high biological cost. High-level resistance occurred only in the mutator strain, in which multiple mutations led to overexpression and structural modifications of AmpC. These mutations increased cephalosporin resistance but reduced resistances to penicillins and carbapenems. Ceftolozane-tazobactam is therefore envisaged as a valuable option for the treatment of P. aeruginosa infections, minimizing the development of self- and cross-resistance and conserving activity against MDR strains selected with other antipseudomonal agents.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Cubist Pharmaceuticals, the Ministerio de Economía y Competitividad of Spain and the Instituto de Salud Carlos III, through the Spanish Network for the Research in Infectious Diseases (RD06/0008 and RD12/0015), the Direcció General d′Universitats, Recerca i Transferència del Coneixement del Govern de les Illes Balears, an EU-funded ERC starter grant (RESISTOME 260276) (given to S.H.), and by the President's Initiative and Networking Funds of the Helmholtz Association of German Research Centers (HGF) (under contract VH-GS-202).
Footnotes
Published ahead of print 17 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02462-13.
REFERENCES
- 1.Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitliks SD. 1998. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J. Intern. Med. 244:379–386. 10.1046/j.1365-2796.1998.00379.x [DOI] [PubMed] [Google Scholar]
- 2.Mesaros N, Nordmann P, Plésiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, Van Laethem Y, Jacobs F, Lebesque P, Malfroot A, Tulkens PM, Van Bambeke F. 2007. Pseudomonas aeruginosa: resistance and therapeutics options in the turn of the new millennium. Clin. Microbiol. Infect. 13:560–578. 10.1111/j.1469-0691.2007.01681.x [DOI] [PubMed] [Google Scholar]
- 3.Peña C, Suarez C, Ocampo-Sosa AA, Murillas J, Almirante B, Pomar V, Aguilar M, Granados A, Calbo E, Rodríguez-Baño J, Rodríguez F, Tubau F, Oliver A, Martínez-Martínez, Spanish Network for Research in Infectious Diseases (REIPI) 2013. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin. Infect. Dis. 57:208–216. 10.1093/cid/cit223 [DOI] [PubMed] [Google Scholar]
- 4.Gales AC, Menezes LC, Silbert S, Sader HS. 2003. Dissemination in distinct Brazilian regions of an epidemic carbapenem-resistant Pseudomonas aeruginosa producing SPM metallo-beta-lactamase. J. Antimicrob. Chemother. 52:699–702. 10.1093/jac/dkg416 [DOI] [PubMed] [Google Scholar]
- 5.Edelstein MV, Skleenova EN, Shevchenko OV, D'souza JW, Tapalski DV, Azizov IS, Sukhorukova MV, Pavlukov RA, Kozlov RS, Toleman MA, Walsh TR. 2013. Spread of extensively resistant VIM-2-positive ST235 Pseudomonas aeruginosa in Belarus, Kazakhstan, and Russia: a longitudinal epidemiological and clinical study. Lancet Infect. Dis. 13:867–876. 10.1016/S1473-3099(13)70168-3 [DOI] [PubMed] [Google Scholar]
- 6.Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 22:582–610. 10.1128/CMR.00040-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabot G, Ocampo-Sosa AA, Domínguez MA, Gago JF, Juan C, Tubau F, Rodríguez C, Moyà B, Peña C, Martínez-Martínez L, Oliver A, Spanish Network for Research in Infectious Diseases (REIPI) 2012. Genetic markers of widespread extensively drug-resistant Pseudomonas aeruginosa high-risk clones. Antimicrob. Agents Chemother. 56:6349–6357. 10.1128/AAC.01388-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabot G, Ocampo-Sosa AA, Tubau F, Macia MD, Rodríguez C, Moya B, Zamorano L, Suárez C, Peña C, Martínez-Martínez L, Oliver A, Spanish Network for Research in Infectious Diseases (REIPI) 2011. Overexpression of AmpC and efflux pumps in Pseudomonas aeruginosa isolates from bloodstream infections: prevalence and impact on resistance in a Spanish multicenter study. Antimicrob. Agents Chemother. 55:1906–1911. 10.1128/AAC.01645-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hocquet D, Berthelot P, Roussel-Delvallez M, Favre R, Jeannot K, Bajolet O, Marty N, Grattard F, Mariani-Kurkdjian P, Bingen E, Husson MO, Couetdic G, Plésiat P. 2007. Pseudomonas aeruginosa may accumulate drug resistance mechanisms without losing its ability to cause bloodstream infections. Antimicrob. Agents Chemother. 51:3531–3536. 10.1128/AAC.00503-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deplano A, Denis O, Poirel L, Hocquet D, Nonhoff C, Byl B, Nordmann P, Vincent JL, Struelens MJ. 2005. Molecular characterization of an epidemic clone of panantibiotic-resistant Pseudomonas aeruginosa. J. Clin. Microbiol. 43:1198–1204. 10.1128/JCM.43.3.1198-1204.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhanel GG, Chung P, Adam H, Zelenitsky S, Denisuik A, Schweizer F, Lagacé-Wiens PR, Rubinstein E, Gin AS, Walkty A, Hoban DJ, Lynch JP, III, Karlowsky JA. 2013. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant Gram-negative bacilli. Drugs 74:31–51. 10.1007/s40265-013-0168-2 [DOI] [PubMed] [Google Scholar]
- 12.Livermore DM, Mushtaq S, Ge Y, Warner M. 2009. Activity of cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa and Burkholderia cepacia group strains and isolates. Int. J. Antimicrob. Agents 34:402–406. 10.1016/j.ijantimicag.2009.03.021 [DOI] [PubMed] [Google Scholar]
- 13.Juan C, Zamorano L, Pérez JL, Ge Y, Oliver A, Spanish Group for the Study of Pseudomonas, Spanish Network for Research in Infectious Diseases 2010. Activity of a new antipseudomonal cephalosporin, CXA-101 (FR264205), against carbapenem-resistant and multidrug-resistant Pseudomonas aeruginosa clinical strains. Antimicrob. Agents Chemother. 54:846–851. 10.1128/AAC.00834-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamorano L, Juan C, Fernández-Olmos A, Ge Y, Cantón R, Oliver A. 2010. Activity of the new cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa isolates from chronically-infected cystic fibrosis patients. Clin. Microbiol. Infect. 16:1482–1487. 10.1111/j.1469-0691.2010.03130.x [DOI] [PubMed] [Google Scholar]
- 15.Bulik CC, Christensen H, Nicolau DP. 2010. In vitro potency of CXA-101, a novel cephalosporin, against Pseudomonas aeruginosa displaying various resistance phenotypes, including multidrug resistance. Antimicrob. Agents Chemother. 54:557–559. 10.1128/AAC.00912-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrell DJ, Flamm RK, Sader HS, Jones RN. 2013. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. hospitals (2011–2012). Antimicrob. Agents Chemother. 57:6305–6310. 10.1128/AAC.01802-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moya B, Zamorano L, Juan C, Pérez JL, Ge Y, Oliver A. 2010. Activity of a new cephalosporin, CXA-101 (FR264205), against β-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob. Agents Chemother. 54:1213–1217. 10.1128/AAC.01104-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moyá B, Beceiro A, Cabot G, Juan C, Zamorano L, Alberti S, Oliver A. 2012. Pan-β-lactam resistance development in Pseudomonas aeruginosa clinical strains: molecular mechanisms, penicillin-binding protein profiles, and binding affinities Antimicrob. Agents Chemother. 56:4771–4778. 10.1128/AAC.00680-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riera E, Macià MD, Mena A, Mulet X, Pérez JL, Ge Y, Oliver A. 2010. Anti-biofilm and resistance suppression activities of CXA-101 against chronic respiratory infection phenotypes of Pseudomonas aeruginosa strain PAO1. J. Antimicrob. Chemother. 65:1399–1404. 10.1093/jac/dkq143 [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute (CLSI). 2011. Performance standards for antimicrobial susceptibility testing, vol 31; 21st informational supplement. M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 21.Moyá B, Zamorano L, Juan C, Ge Y, Oliver A. 2010. Affinity of the new cephalosporin CXA-101 to penicillin-binding proteins of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54:3933–3937. 10.1128/AAC.00296-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dötsch A, Eckweiler D, Schniederjans M, Zimmermann A, Jensen V, Scharfe M, Geffers R, Häussler S. 2012. The Pseudomonas aeruginosa transcriptome in planktonic cultures and static biofilms using RNA sequencing. PLoS One 7:e31092. 10.1371/journal.pone.0031092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lunter G, Goodson M. 2011. Stampy: a statistical algorithm for sensitive and fast mapping of Illumina sequence reads. Genome Res. 21:936–939. 10.1101/gr.111120.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol. 11:R106. 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winsor GL, Lam DK, Fleming L, Lo R, Whiteside MD, Yu NY, Hancock RE, Brinkman FS. 2011. Pseudomonas Genome Database: improved comparative analysis and population genomics capability for Pseudomonas genomes. Nucleic Acids Res. 39:D596–D600. 10.1093/nar/gkq869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stover KC, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RR, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. 10.1038/35023079 [DOI] [PubMed] [Google Scholar]
- 28.West SE, Schweizer HP, Dall C, Sample AK, Runyen-Janecky LJ. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81–86. 10.1016/0378-1119(94)90237-2 [DOI] [PubMed] [Google Scholar]
- 29.Moya B, Juan C, Albertí S, Pérez JL, Oliver A. 2008. Benefit of having multiple ampD genes for acquiring beta-lactam resistance without losing fitness and virulence in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52:3694–3700. 10.1128/AAC.00172-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulet X, Cabot G, Ocampo-Sosa AA, Domínguez MA, Zamorano L, Juan C, Tubau F, Rodríguez C, Moyà B, Peña C, Martínez-Martínez L, Oliver A, Spanish Network for Research in Infectious Diseases (REIPI) 2013. Biological markers of Pseudomonas aeruginosa epidemic high-risk clones. Antimicrob. Agents Chemother. 57:5527–5535. 10.1128/AAC.01481-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu D, Eisinger VM, Head NE, Pier GB, Yu HD. 2008. ClpXP proteases positively regulate alginate overexpression and mucoid conversion. Microbiology 154:2119–2130. 10.1099/mic.0.2008/017368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernández L, Breidenstein EB, Song D, Hancock RE. 2012. Role of intracellular proteases in the antibiotic resistance, motility, and biofilm formation of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 56:1128–1132. 10.1128/AAC.05336-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagge N, Ciofu O, Hentzer M, Campbell JI, Givskov M, Høiby N. 2002. Constitutive high expression of chromosomal beta-lactamase in Pseudomonas aeruginosa caused by a new insertion sequence (IS1669) located in ampD. Antimicrob. Agents Chemother. 46:3406–3411. 10.1128/AAC.46.11.3406-3411.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fajardo A, Martínez-Martín N, Mercadillo M, Galán JC, Ghysels B, Matthijs S, Cornelis P, Wiehlmann L, Tümmler B, Baquero F, Martínez JL. 2008. The neglected intrinsic resistome of bacterial pathogens. PLoS One 3:e1619. 10.1371/journal.pone.0001619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breidenstein EB, Khaira BK, Wiegand I, Overhage J, Hancock RE. 2008. Complex ciprofloxacin resistome revealed by screening a Pseudomonas aeruginosa mutant library for altered susceptibility. Antimicrob. Agents Chemother. 52:4486–4491. 10.1128/AAC.00222-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dötsch A, Becker T, Pommerenke C, Magnowska Z, Jänsch Häussler LS. 2009. Genomewide identification of genetic determinants of antimicrobial drug resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:2522–2531. 10.1128/AAC.00035-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvarez-Ortega C, Wiegand I, Olivares J, Hancock RE, Martínez JL. 2010. Genetic determinants involved in the susceptibility of Pseudomonas aeruginosa to beta-lactam antibiotics. Antimicrob. Agents Chemother. 54:4159–4167. 10.1128/AAC.00257-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.