Abstract
Sutezolid (PNU-100480 [U-480]) is an oxazolidinone antimicrobial being developed for the treatment of tuberculosis. An active sulfoxide metabolite (PNU-101603 [U-603]), which reaches concentrations in plasma several times those of the parent, has been reported to drive the killing of extracellular Mycobacterium tuberculosis by sutezolid in hollow-fiber culture. However, the relative contributions of the parent and metabolite against intracellular M. tuberculosis in vivo are not fully understood. The relationships between the plasma concentrations of U-480 and U-603 and intracellular whole-blood bactericidal activity (WBA) in ex vivo cultures were examined using a direct competitive population pharmacokinetic (PK)/pharmacodynamic 4-parameter sigmoid model. The data set included 690 PK determinations and 345 WBA determinations from 50 tuberculosis patients enrolled in a phase 2a sutezolid trial. The model parameters were solved iteratively. The median U-603/U-480 concentration ratio was 7.1 (range, 1 to 28). The apparent 50% inhibitory concentration of U-603 for intracellular M. tuberculosis was 17-fold greater than that of U-480 (90% confidence interval [CI], 9.9- to 53-fold). Model parameters were used to simulate in vivo activity after oral dosing with sutezolid at 600 mg twice a day (BID) and 1,200 mg once a day (QD). Divided dosing resulted in greater cumulative activity (−0.269 log10 per day; 90% CI, −0.237 to −0.293 log10 per day) than single daily dosing (−0.186 log10 per day; 90% CI, −0.160 to −0.208 log10 per day). U-480 accounted for 84% and 78% of the activity for BID and QD dosing, respectively, despite the higher concentrations of U-603. Killing of intracellular M. tuberculosis by orally administered sutezolid is mainly due to the activity of the parent compound. Taken together with the findings of other studies in the hollow-fiber model, these findings suggest that sutezolid and its metabolite act on different mycobacterial subpopulations.
INTRODUCTION
Mycobacterium tuberculosis resistance to standard first-line drugs (drug-resistant tuberculosis [DR-TB]) is a serious and growing global health threat, causing at least 444,000 new tuberculosis (TB) cases and 150,000 deaths annually (1). Oxazolidinone antimicrobials are increasingly viewed as candidates for inclusion in new regimens for DR-TB, as they have a distinct mechanism of action (binding to the 23S ribosome) without cross-resistance to current drugs. Linezolid is the only currently licensed oxazolidinone. Sutezolid (PNU-100480 [U-480]) is a thiomorpholinyl analog of linezolid with superior efficacy against M. tuberculosis in the hollow-fiber, mouse, and whole-blood models (2–4). Time-dependent killing of M. tuberculosis by sutezolid has been reported in whole blood and hollow fibers (2, 3).
Orally administered sutezolid is rapidly oxidized in vivo to an active sulfoxide metabolite (PNU-101603 [U-603]), which then undergoes renal excretion. The concentrations of U-603 achieved in human plasma are severalfold greater than those of the parent. Some studies have reported similar MICs for both U-480 and U-603 (0.25 μg/ml) for reference strains and clinical isolates, regardless of the method of testing (5, 6); however, others have reported lower median MIC values (≤0.062 μg/ml) for the parent when clinical isolates are tested in liquid culture (6).
The relative contributions of the parent and metabolite to killing of mycobacteria appear to differ according to cellular location. Studies in the hollow-fiber model using concentrations of parent and metabolite that are achieved in human plasma have indicated that killing of extracellular mycobacteria by sutezolid is mainly due to the metabolite (2). In contrast, studies in which sutezolid or its metabolite were added separately to cultures of M. tuberculosis-infected mammalian cells have found the parent to be at least 10-fold more potent than the metabolite (7). However, these studies have not examined the activity of sutezolid as it would occur in the cells of patients with tuberculosis, which may differ in immune function and drug metabolism.
The present study used mathematical modeling to examine the relative contributions of U-480 and U-603 to the killing of intracellular M. tuberculosis in ex vivo cultures measuring whole-blood bactericidal activity (WBA) that were conducted in clinical trial B1171003, the first study of sutezolid in patients with pulmonary tuberculosis.
MATERIALS AND METHODS
Subjects.
As previously reported (8), subjects consisted of men and women aged 18 to 65 years with chest radiographs with findings consistent with pulmonary tuberculosis, positive sputum acid-fast smears, culture or molecular confirmation of drug-susceptible M. tuberculosis, a random blood glucose level of <150 mg/dl, reasonably normal renal and hepatic function, and a willingness to provide written informed consent according to International Conference on Harmonization guidelines. Subjects were either HIV-1 uninfected or HIV-1 infected with CD4 T cell counts of >350/mm3 and not currently receiving antiretroviral therapy. This study received ethical approval from the University of Cape Town Faculty of Health Sciences Human Research Ethics Committee, Cape Town, South Africa, and from Pharma-Ethics, Lyttelton Manor, South Africa.
Treatments.
Subjects were randomly assigned to receive sutezolid at 600 mg twice a day (BID; n = 25) or 1,200 mg once a day (QD; n = 25) or to receive a positive control of fixed-dose combination tablets consisting of isoniazid, rifampin, pyrazinamide, and ethambutol (HRZE; Rifafour e275; n = 9). The data for HRZE-treated subjects were not included in the present analysis.
Evaluations.
Blood was collected for WBA at the baseline (WBA0) and for pharmacokinetic (PK) and WBA determinations on days 13 and 14 (at 0, 1, 2, 3, 6, 8, and 12 h postdosing). Plasma was separated immediately after collection and stored at −20°C for PK determinations. Total plasma concentrations of PNU-100480 and PNU-101603 were determined using a validated high-pressure liquid chromatography-tandem mass spectrometry method by Advion BioServices (Ithaca, NY), as previously described (9).
WBA was determined as previously described (9). Briefly, an M. tuberculosis H37Rv stock was prepared in mycobacterial growth indicator tubes (MGITs; Becton, Dickinson, Sparks, MD) containing oleic acid, albumin, dextrose, and catalase (OADC; Becton, Dickinson) and polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin (PANTA; Becton, Dickinson). For each batch of stock, a titration experiment described the relationship between the inoculum volume and MGIT time to positivity (TTP). Whole-blood cultures consisted of heparinized blood, an equal volume of RPMI 1640 tissue culture medium (Highveld Biological, Lyndhurst, South Africa), and bacilli of the volume of the M. tuberculosis H37Rv stock identified by the titration curve to have an MGIT TTP of 5.5 days. Direct inoculation of this volume into an MGIT culture served as a growth control. Whole-blood cultures were incubated with slow constant mixing for 72 h, after which cells were sedimented, the liquid phase was removed, and blood cells were disrupted by hypotonic lysis. Bacilli were recovered and inoculated into MGIT cultures. The log change in viability was calculated as log(final) − log(initial), where final and initial were the volumes corresponding to the TTPs of the completed whole-blood culture and its inoculum, respectively, on the basis of the titration curve of the stock. Results were expressed as the log change per day (Δlog/d) of whole-blood culture. The cumulative WBA over 24 h was calculated as the area under the concentration-time curve from time zero to 24 h of values measured at discrete time points, using the trapezoidal method. Results were expressed as Δlog/d · d, or simply as the log change.
Mathematical modeling.
A population PK/pharmacodynamic (PD) model was developed to simultaneously describe the relationship between the concentrations of U-480 and U-603 observed in plasma and the bactericidal activity observed in whole-blood cultures. The relationship between drug concentration and bactericidal activity was examined using a 4-parameter sigmoid curve, on the basis of previous observations that killing was concentration dependent at low concentrations but did not increase further as a maximal response (Imax) was approached (3). The activities of U-480 and U-603 were assumed to be competitive, on the basis of previous in vitro observations that the addition of U-603 to optimal concentrations of U-480 did not further increase intracellular drug activity (10). The equations used to describe bactericidal activity were as follows: WBA = WBA0 − {Imax[(P + M)/(P + M + 1)]}, P = (C4800.5)/IC50480)γ480, and M = (C6030.5)/IC50603)γ603, where WBA is the model-predicted effect; WBA0 is the baseline effect; Imax is the maximum effect; IC50480 is the 50% inhibitory concentration of U-480, or the concentration of U-480 for 50% of the maximum effect; IC50603 is the 50% inhibitory concentration of U-603, or the concentration of U-603 for 50% of the maximum effect; γ480 is the shape factor (Hill coefficient) of the concentration-effect relationship for U-480, γ603 is the shape factor (Hill coefficient) of the concentration-effect relationship for U-603, C480 is the observed plasma concentration of U-480, and C603 is the observed plasma concentration of U-603.
The plasma concentrations of U-480 and U-603 were adjusted by a factor of 0.5 to account for the dilution of blood with tissue culture medium in the whole-blood cultures.
The model was developed using the $PRED subroutine and FOCE-I in the NONMEM (version 7.1.2) program (11). Intersubject variability was tested for all model-estimated parameters alone or in combination. Residual variability was described with an additive error model. Model adequacy was assessed by goodness-of-fit plots and visual predictive checks (n = 1,000), using the R package (version 2.12.2) (12). The precision of the parameter estimates was obtained by nonparametric bootstrap analysis (n = 1,000), using the PsN (version 3.2.12) program (13). The WBA time course was simulated for U-480, U-603, and U-480–U-603 on the basis of final model parameters and median plasma concentration-time profiles.
RESULTS
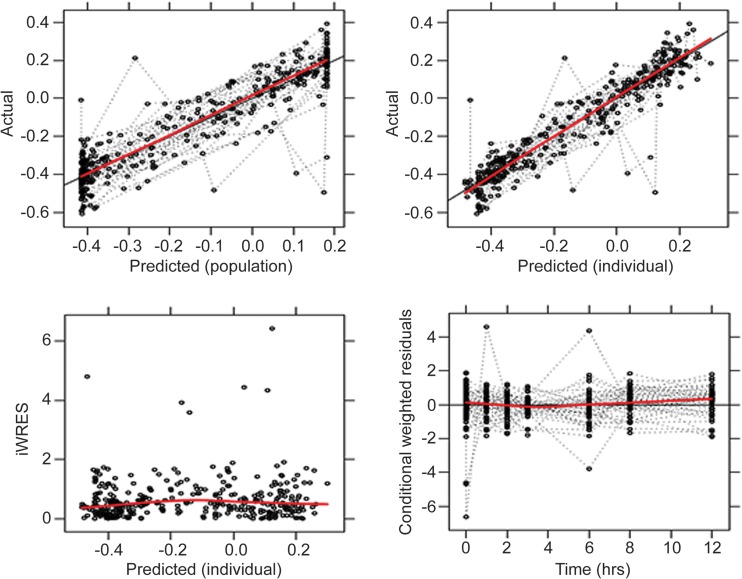
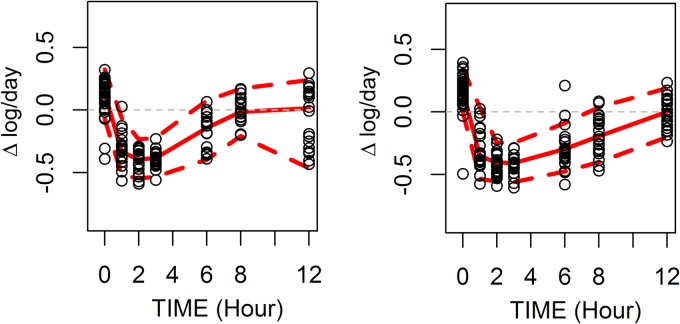
A total of 690 PK determinations and 345 WBA determinations from 50 subjects were analyzed. The median ratio of the U-603/U-480 plasma concentrations was 7.1 (range, 1 to 28). High ratios mainly occurred at late time points in the dosing interval. The wide range of values facilitated modeling of the relative contributions of parent and metabolite to overall activity. The model was solved iteratively to determine the parameter values that most closely predicted actual WBA results. Final parameter estimates are indicated in Table 1. The concentration of U-603 required for a half-maximal effect (IC50603) was 17-fold greater than that for U-480 (90% confidence interval [CI], 9.9 to 53). The value of γ (the shape factor of the concentration-effect relationship) was determined for both U-480 and U-603. Its value differed significantly from 1 only for U-480. Intersubject variability was tested for IC50480, IC50603, and WBA0; however, only intersubject variability in WBA0 remained significant in the final model. The relatively narrow confidence intervals (Table 1) supported the adequacy of the fit of the model. Diagnostic plots (Fig. 1) did not indicate any systematic errors in the model. The eta shrinkage was 20.87%. A visual predictive check (Fig. 2) revealed an adequate correspondence of the observed and predicted values throughout the dosing interval.
TABLE 1.
Final parameter estimates and 90% CIs
| Parameter | Estimate | 90% CI |
|---|---|---|
| Fixed effects | ||
| WBA0 (Δlog/d) | 0.182 | 0.154–0.209 |
| Imax (Δlog/d) | 0.597 | 0.559–0.636 |
| IC50480 (ng/ml) | 70.4 | 56.0–84.9 |
| IC50603 (ng/ml) | 1,200 | 839–2,960 |
| γ480 | 2.25 | 1.7–3.2 |
| γ603 | (0.94)a | |
| Random effects | ||
| WBA0 (%) | 27.4 | 19.4–35.2 |
| Sigmab | 0.096 | 0.075–0.114 |
The initial estimate for γ603 (0.94) could not be distinguished from 1 and was omitted from the final model.
Sigma, residual error.
FIG 1.
Diagnostic plots to evaluate goodness of fit. iWRES, individual weighted residuals. Actual and predicted values indicate the log change in mycobacterial viability per day (Δlog/d) of whole-blood culture.
FIG 2.
Visual predictive check of model. (Left) Dosing at 600 mg BID; (right) dosing at 1,200 mg QD. Symbols indicate individual observations; solid lines, model predictions; dashed lines, 5th to 95th prediction intervals. The wide confidence interval at 12 h in the 600-mg-BID arm was due to a delay of up to 1 h postdosing in obtaining the 12-h specimen from 7 subjects.
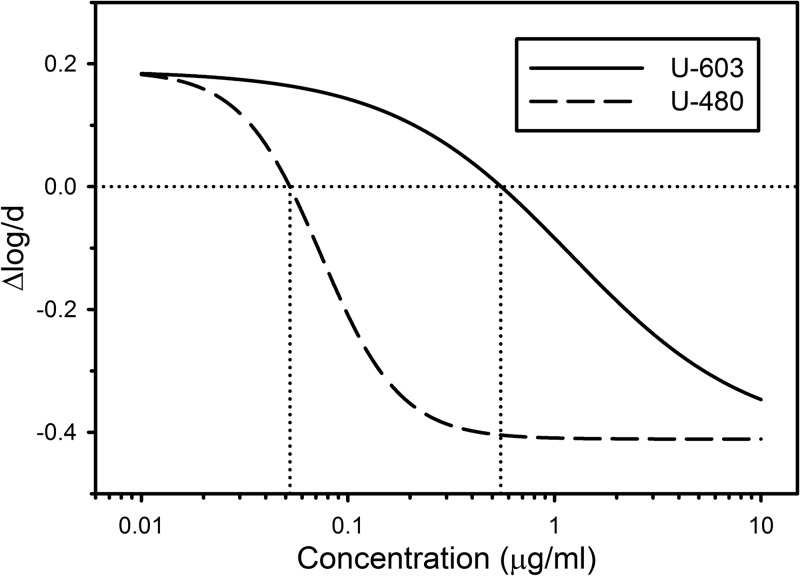
The concentration-activity relationships predicted by the model for sutezolid (U-480) and its metabolite (U-603) on the basis of the fixed parameter estimates in Table 1 are illustrated in Fig. 3. Compared to the curve for U-603, the curve for U-480 was shifted to the left and had a steeper slope. The mean apparent intracellular MIC (the extracellular concentration required for intracellular bacteriostasis) for U-480 was 0.05 μg/ml, whereas that for U-603 was 0.55 μg/ml, which was 11-fold greater.
FIG 3.
Predicted individual concentration-activity relationships for sutezolid (U-480) and its main metabolite (U-603) against intracellular M. tuberculosis on the basis of the fixed-effect estimates of Table 1. The vertical axis indicates the change in mycobacterial viability per day of whole-blood culture, with negative values indicating killing. Dotted lines indicate the concentrations required for intracellular bacteriostasis.
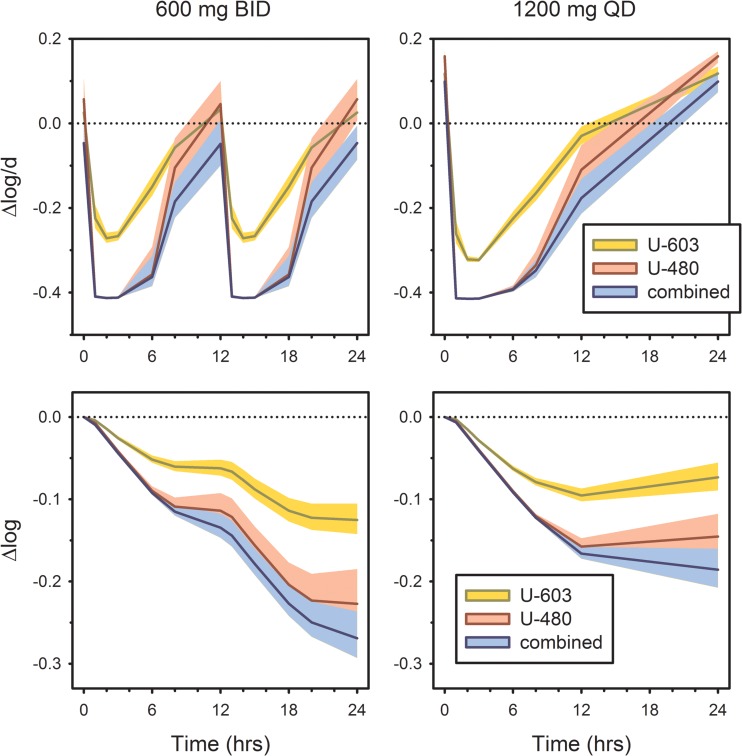
The model was then used to simulate mycobactericidal activity in vivo after oral dosing with sutezolid at 600 mg BID and 1,200 mg QD, on the basis of median plasma drug concentrations from the recently completed phase 2a trial (8). Unlike the results reported in that trial, however, these reflect drug concentrations and effects as they occur in vivo without artifacts resulting from diluting blood with tissue culture medium in vitro. Results are shown in Fig. 4. The upper panels indicate activity at discrete time points. Cumulative activity, calculated as the integral over time of values observed at discrete time points, is shown in the lower panels. These take the form of a time-kill curve, converting a static kill model into a dynamic one. A potential shortcoming of this approach is that postantibiotic effects (PAEs) are not represented; however, studies with linezolid suggest a short PAE (4 h) when oxazolidinones are tested against M. tuberculosis (14).
FIG 4.
Predicted in vivo mycobactericidal activity of U-603 and U-480, individually and in combination, after simulated oral dosing with sutezolid at 600 mg BID and 1,200 mg QD. (Top) Activity at discrete time points; (bottom) cumulative activity, calculated as the integral over time of values at discrete time points. Shading indicates the 90% CI on the basis of PK variability.
Examination of the cumulative intracellular activity of sutezolid using this approach revealed that divided dosing of sutezolid produced a greater cumulative effect (−0.269 log10 per day; 90% CI, −0.237 to −0.293 log10 per day) than single daily dosing (−0.186 log10 per day; 90% CI, −0.160 to −0.208 log10 per day). U-480 accounted for 78% and 84% of the cumulative daily activity when sutezolid was administered at 1,200 mg QD and 600 mg BID, respectively, with the contribution of U-603 becoming significant only at later time points in the dosing interval.
DISCUSSION
This study used a direct PK/PD model to simultaneously determine the relationship between intracellular mycobactericidal activity and plasma concentrations of sutezolid and its major metabolite in patients with pulmonary tuberculosis. The parent (U-480) was found to be 17-fold more potent than its metabolite for killing of intracellular M. tuberculosis. Similar results have been reported when the parent and metabolite were added separately to cultures of M. tuberculosis-infected mammalian cells (7). The basis of this observation is not known but may reflect differences in intracellular drug accumulation. Approximately 80% of the intracellular activity could be attributed to the parent, despite substantially greater exposures to the metabolite in vivo. This finding stands in contrast to extracellular bactericidal activity, which, in the hollow-fiber model, appeared to be mainly due to the metabolite (U-603), due to its higher achieved concentrations. Together, these two observations indicate that drugs that affect sutezolid metabolism may influence outcomes when combined with sutezolid in future TB trials.
Distinct mycobacterial subpopulations exist in patients with tuberculosis, with each subpopulation having distinct biological and clinical significance. Extracellular mycobacteria comprise the majority of the mycobacterial burden in patients at the time of TB diagnosis, particularly in patients with cavitary pulmonary disease. These bacilli are essential for TB transmission, through the generation by coughing of M. tuberculosis-infected aerosol droplets. Because these organisms are, for the most part, actively replicating, this subpopulation is likely responsible for the selection and emergence of drug resistance during treatment. Factors that reduce exposure to U-603 may therefore increase the risk of resistance emerging during sutezolid treatment.
The other mycobacterial subpopulation of importance in tuberculosis is that within tissues and cells. M. tuberculosis is readily ingested but not readily killed by host phagocytic cells, resulting in the characteristic pathology of human M. tuberculosis infection, the granuloma. Bacilli in tissues exist both within the necrotic centers of granulomas and within intact macrophages near their periphery. Bacillary replication and metabolism in these circumstances are limited by a mycobacterial dormancy response triggered by a lack of oxygen and nutrients. It is thought that the propensity of tuberculosis to relapse despite apparently successful treatment is due to persistence of this subpopulation.
Mycobacteria added to whole-blood cultures rapidly undergo essentially complete phagocytosis by neutrophils and monocytes (15, 16). The extent of mycobacterial growth or killing in the absence of chemotherapy reflects the balance between microbial pathogenicity and host immune mechanisms (15–21). The effects of administered chemotherapy are reflected in the ex vivo whole-blood model as they occur in cells in vivo. After standard oral doses, the rank order of activity of anti-TB drugs in the model is rifampin > moxifloxacin > isoniazid > pyrazinamide > ethambutol (16). Standard therapy shows 3 times the activity of current regimens for multidrug-resistant TB. In one small study, the cumulative WBA during TB treatment was superior in those patients whose sputum cultures converted by month 2 (22). Culture status at 2 months is, in turn, an independent predictor of relapse risk (23, 24). The whole-blood model may therefore be best described as an emerging biomarker of drug effects associated with durable TB cure. The observations obtained using this model in the present study suggest that reduced exposure to U-480 due to enhanced metabolism may increase the risk of relapse after treatment with sutezolid.
The oxidative metabolism of sutezolid is not fully understood. CYP3A4 and flavin-containing monooxygenases each contribute 20 to 30% toward its metabolism. CYP3A4 inhibitors or inducers may therefore affect the relative concentrations of sutezolid and its metabolite, thus potentially affecting the two main goals of TB treatment, tissue sterilization and resistance prevention. Specific attention will be warranted to examine the PK-PD relationship of sutezolid in future trials in which it is combined with rifampin, ritonavir, or other drugs that may affect its metabolism.
Finally, this model also enhances our understanding of the PK-PD relationship of sutezolid in vivo. Drug concentrations in the ex vivo cultures are reduced by half, due to the dilution of blood with tissue culture medium. Modeling removed this artifact, predicting the drug effects at the concentrations achieved in vivo. The predicted in vivo cumulative activities (−0.269 and −0.186 for BID and QD dosing, respectively) are greater than those previously reported ex vivo (−0.142 and −0.089, respectively) (8). In addition, the difference between divided and single daily dosing is magnified. Longer clinical trials will be required to determine if divided dosing of sutezolid results in accelerated tissue sterilization and a shortened required duration of treatment, as these data would suggest.
ACKNOWLEDGMENTS
This study was supported by Pfizer.
T.Z. and R.S.W. are or were Pfizer employees and shareholders. R.S.W. has served as a consultant to Sequella, which has acquired the rights to sutezolid.
Footnotes
Published ahead of print 31 March 2014
REFERENCES
- 1.World Health Organization. 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Report WHO/HTM/TB/2010.3 World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf [Google Scholar]
- 2.Louie A, Eichas K, Files K, Swift M, Bahniuk N, Brown D, Drusano GL. 2011. Activities of PNU-100480 (PNU 480) alone, PNU 480 plus its major metabolite PNU-101603 (PNU 1603) and PNU 480 plus PNU 1603 in combination with rifampin (RIF) against Mycobacterium tuberculosis: comparison with linezolid, abstr A1-1737 Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 3.Wallis RS, Jakubiec W, Kumar V, Bedarida G, Silvia A, Paige D, Zhu T, Mitton-Fry M, Ladutko L, Campbell S, Miller PF. 2011. Biomarker assisted dose selection for safety and efficacy in early development of PNU-100480 for tuberculosis. Antimicrob. Agents Chemother. 55:567–574. 10.1128/AAC.01179-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams KN, Brickner SJ, Stover CK, Zhu T, Ogden A, Tasneen R, Tyagi S, Grosset JH, Nuermberger EL. 2009. Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am. J. Respir. Crit. Care Med. 180:371–376. 10.1164/rccm.200904-0611OC [DOI] [PubMed] [Google Scholar]
- 5.Williams KN, Stover CK, Zhu T, Tasneen R, Tyagi S, Grosset JH, Nuermberger E. 2009. Promising anti-tuberculosis activity of the oxazolidinone PNU-100480 relative to linezolid in the murine model. Antimicrob. Agents Chemother. 53:1314–1319. 10.1128/AAC.01182-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alffenaar JW, van der Laan T, Simons S, van der Werf TS, van de Kasteele PJ, de Neeling H, van Soolingen D. 2011. Susceptibility of clinical Mycobacterium tuberculosis isolates to a potentially less toxic derivate of linezolid, PNU-100480. Antimicrob. Agents Chemother. 55:1287–1289. 10.1128/AAC.01297-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Converse PJ, Lee J, Williams KN, Dionne K, Parrish N, Wallis RS, Nuermberger EL. 2012. Activity of PNU-100480 and its major metabolite in whole blood and broth culture models of tuberculosis, abstr GM-A-2052 Abstr. 112th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC [Google Scholar]
- 8.Wallis RS, Dawson R, Friedrich SO, Venter A, Paige D, Zhu T, Silvia A, Gobey J, Ellery C, Zhang Y, Eisenach K, Miller P, Diacon AH. 2014. Mycobactericidal activity of sutezolid (PNU-100480) in sputum (EBA) and blood (WBA) of patients with pulmonary tuberculosis. PLoS One 9(4):e94462. 10.1371/journal.pone.0094462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallis RS, Jakubiec W, Kumar V, Silvia AM, Paige D, Dimitrova D, Li X, Ladutko L, Campbell S, Friedland G, Mitton-Fry M, Miller PF. 2010. Pharmacokinetics and whole blood bactericidal activity against Mycobacterium tuberculosis of single ascending doses of PNU-100480 in healthy volunteers. J. Infect. Dis. 202:745–751. 10.1086/655471 [DOI] [PubMed] [Google Scholar]
- 10.Wallis RS, Jakubiec W, Mitton-Fry M, Ladutko L, Campbell S, Paige D, Silvia A, Miller PF. 2012. Rapid evaluation in whole blood culture of regimens for XDR-TB containing PNU-100480 (Sutezolid), TMC207, PA-824, SQ109, and pyrazinamide. PLoS One 7:e30479. 10.1371/journal.pone.0030479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Development Solutions, ICON. 2013. NONMEM. ICON plc, Dublin, Ireland [Google Scholar]
- 12.Dalgaard P. 2013. R Center for Statistics, Copenhagen, Denmark. http://www.r-project.org/
- 13.Lindbom L, Pihlgren P, Jonsson EN. 2005. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 79:241–257. 10.1016/j.cmpb.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 14.Hui M, Au-Yeang C, Wong KT, Chan CY, Yew WW, Leung CC. 2008. Post-antibiotic effects of linezolid and other agents against Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 31:395–396. 10.1016/j.ijantimicag.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 15.Janulionis E, Sofer C, Schwander S, Simmons D, Kreiswirth B, Shashkina E, Wallis RS. 2005. Survival and replication of clinical Mycobacterium tuberculosis isolates in the context of human innate immunity. Infect. Immun. 73:2595–2601. 10.1128/IAI.73.5.2595-2601.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallis RS, Palaci M, Vinhas S, Hise AG, Ribeiro FC, Landen K, Cheon SH, Song HY, Phillips M, Dietze R, Ellner JJ. 2001. A whole blood bactericidal assay for tuberculosis. J. Infect. Dis. 183:1300–1303. 10.1086/319679 [DOI] [PubMed] [Google Scholar]
- 17.Cheon SH, Kampmann B, Hise AG, Phillips M, Song HY, Landen K, Li Q, Larkin R, Ellner JJ, Silver RF, Hoft DF, Wallis RS. 2002. Bactericidal activity in whole blood as a potential surrogate marker of immunity after vaccination against tuberculosis. Clin. Diagn. Lab. Immunol. 9:901–907. 10.1128/CDLI.9.4.901-907.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saliu O, Sofer C, Stein DS, Schwander SK, Wallis RS. 2006. Tumor necrosis factor blockers: differential effects on mycobacterial immunity. J. Infect. Dis. 194:486–492. 10.1086/505430 [DOI] [PubMed] [Google Scholar]
- 19.Hoft DF, Worku S, Kampmann B, Whalen CC, Ellner JJ, Hirsch CS, Brown RB, Larkin R, Li Q, Yun H, Silver RF. 2002. Investigation of the relationships between immune-mediated inhibition of mycobacterial growth and other potential surrogate markers of protective Mycobacterium tuberculosis immunity. J. Infect. Dis. 186:1448–1457. 10.1086/344359 [DOI] [PubMed] [Google Scholar]
- 20.Kampmann B, Tena-Coki GN, Nicol M, Levin M, Eley B. 2006. Reconstitution of antimycobacterial immune responses in HIV-infected children receiving HAART. AIDS 20:1011–1018. 10.1097/01.aids.0000222073.45372.ce [DOI] [PubMed] [Google Scholar]
- 21.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, Rainbow SJ, Berry JL, Griffiths CJ. 2007. A single dose of vitamin D enhances immunity to mycobacteria. Am. J. Respir. Crit. Care Med. 176:208–213. 10.1164/rccm.200701-007OC [DOI] [PubMed] [Google Scholar]
- 22.Wallis RS, Vinhas SA, Johnson JL, Ribeiro FC, Palaci M, Peres RL, Sa RT, Dietze R, Chiunda A, Eisenach K, Ellner JJ. 2003. Whole blood bactericidal activity during treatment of pulmonary tuberculosis. J. Infect. Dis. 187:270–278. 10.1086/346053 [DOI] [PubMed] [Google Scholar]
- 23.Wallis RS, Wang C, Doherty TM, Onyebujoh P, Vahedi M, Laang H, Olesen O, Parida S, Zumla A. 2010. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect. Dis. 10:68–69. 10.1016/S1473-3099(10)70003-7 [DOI] [PubMed] [Google Scholar]
- 24.Benator D, Bhattacharya M, Bozeman L, Burman W, Cantazaro A, Chaisson R, Gordin F, Horsburgh CR, Horton J, Khan A, Lahart C, Metchock B, Pachucki C, Stanton L, Vernon A, Villarino ME, Wang YC, Weiner M, Weis S. 2002. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 360:528–534. 10.1016/S0140-6736(02)09742-8 [DOI] [PubMed] [Google Scholar]