Abstract
Traditionally, the pharmacokinetics of antimicrobials in bone have been investigated using bone biopsy specimens, but this approach suffers from considerable methodological limitations. Consequently, new methods are needed. The objectives of this study were to assess the feasibility of microdialysis (MD) for measuring cefuroxime in bone and to obtain pharmacokinetic profiles for the same drug in porcine cortical and cancellous bone. The measurements were conducted in bone wax sealed and unsealed drill holes in cortical bone and in drill holes in cancellous bone and in subcutaneous tissue. As a reference, the free and total plasma concentrations were also measured. The animals received a bolus of 1,500 mg cefuroxime over 30 min. No significant differences were found between the key pharmacokinetic parameters for sealed and unsealed drill holes in cortical bone. The mean ± standard error of the mean area under the concentration-time curve (AUC) values from 0 to 5 h were 6,013 ± 1,339, 3,222 ± 1086, 2,232 ± 635, and 952 ± 290 min · μg/ml for free plasma, subcutaneous tissue, cancellous bone, and cortical bone, respectively (P < 0.01, analysis of variance). The AUC for cortical bone was also significantly different from that for cancellous bone (P = 0.04). This heterogeneous tissue distribution was also reflected in other key pharmacokinetic parameters. This study validates MD as a suitable method for measuring cefuroxime in bone. Cefuroxime penetration was impaired for all tissues, and bone may not be considered one distinct compartment.
INTRODUCTION
Osteomyelitis and periprosthetic bone and joint infections (PJIs) are among the most severe orthopedic conditions. Treatment includes surgical debridement, removal of implants, and long-lasting antimicrobial therapy, and it calls for a multidisciplinary approach (1). Nevertheless, treatment failure is common. One of the reasons for this may be incomplete or heterogeneous tissue distribution of antimicrobials, which has been demonstrated in a number of studies for different combinations of drug and tissue (2–8).
Determining the penetration of antimicrobials into bone remains a difficult task. Traditionally, bone biopsy has been the predominant method used. Obtaining pharmacokinetic data by means of tissue biopsy specimens may, however, be misleading and ultimately harmful to patients (9, 10). When analyzing tissue biopsy specimens, no selective measurement of the free extracellular concentration or distinction between the intra- and extracellular compartments can be made. Furthermore, temporal resolution is poor or nonexistent, and the concentrations are given by weight and not by volume. Consequently, pharmacokinetic parameters obtained by tissue biopsy specimens are difficult to relate to relevant pharmacodynamic endpoints.
In several studies, microdialysis (MD) has been used to determine the concentrations of antimicrobials in the tissue of interest (11–16). By being minimally invasive, the MD technique permits clinical investigations, and at the same time, it provides the possibility of continuous sampling of the unbound fraction of a drug in the interstitial space. From this perspective, MD offers an attractive alternative to using bone biopsy specimens in order to assess antimicrobial penetration into the bone. However, due to the compact nature of bone, MD probes cannot readily be implanted. This issue has been overcome by inserting the probes into drill holes in the bone (17–23). This approach obviously raises the question of whether MD measurement of antimicrobials in drill holes truly reflects bone drug concentration or rather a mixed concentration stemming partly from the presence in the bone, and partly from the presence of the substance in the surrounding soft tissue. Additionally, it is a challenge to create drill holes in cortical bone and verify that they are strictly intracortical and that the MD catheters remain in the drill holes during the entire study period.
In the present study, we investigated the suitability of MD for cefuroxime measurements in a laboratory setting. Second, the feasibility of applying MD to measure cefuroxime in cortical bone was investigated in anesthetized pigs. With an identical methodological setup, parallel in vivo measurements of cefuroxime were also performed in cancellous bone and subcutaneous tissue. Studies evaluating the distribution of cefuroxime in cortical and cancellous bone by use of MD have not been published.
MATERIALS AND METHODS
This study was conducted at the Institute of Clinical Medicine and the Department of Clinical Biochemistry, Aarhus University Hospital, Denmark. The study was approved by the Danish Animal Experiments Inspectorate and carried out under existing laws. All chemical analyses were performed at the Department of Biochemistry, Aarhus University Hospital.
Microdialysis.
An in-depth description of MD can be found elsewhere (24, 25). Briefly, the basic principles of MD rely on the facts that diffusion across the semipermeable membrane of the microdialysis probe is quantitatively equal in both directions, and that relative recovery of solutes is independent of the concentration gradient between the tissue and perfusate. In this study, the MD equipment from M Dialysis AB (Stockholm, Sweden) was used in all the experiments. Specifically, the catheters used were CMA 63 (membrane length, 10 mm; molecular cutoff, 20 kDa), and CMA 107 precision pumps produced a flow rate of 2 μl/min.
When calculating the relative recovery by loss (RRloss) and relative recovery by gain (RRgain), the following equations were applied:
| (1) |
| (2) |
where Cin is the concentration in the perfusate, Cout is the concentration of the dialysate, and Cm the concentration in the medium surrounding the catheter.
As microdialysates are gathered continuously, the measured concentrations were attributed to the midpoint of the sampling intervals for the subsequent data analysis. In the in vivo studies, absolute tissue drug concentrations were obtained by correcting for relative recovery using the following equation:
| (3) |
Individual in vivo probe calibration was performed for all animal experiments.
Handling of samples.
The dialysates were immediately frozen and stored at −80°C until analysis. Venous blood samples were stored at 5°C for a maximum of 20 h before being centrifuged at 3,000 × g for 10 min. The plasma aliquots were then frozen and stored at −80°C until analysis.
UHPLC analysis of cefuroxime.
The assessment of cefuroxime was performed with ultrahigh-performance liquid chromatography (UHPLC). For measuring the total plasma drug concentrations, 100 μl plasma was added to 300 μl acetonitrile (Sigma-Aldrich, Denmark) containing 30 μg/ml ceftriaxone (Sigma-Aldrich) as an internal standard and filtered through a protein precipitation plate (Captiva ND plate; Agilent Technologies, USA). Subsequently, 400 μl of 10 mM phosphate buffer (PB) (pH 3) (NaH2PO4, H2O adjusted with HCl; Merck, Germany) was added to the filtrate. Standards (6.25, 25, and 100 μg/ml) were prepared by adding cefuroxime sodium (Fresenius Kabi AB, Sweden) to human donor plasma.
For measuring the free fraction of cefuroxime, 300 μl plasma was placed into an ultrafilter 96-well plate with a 30-kDa molecular mass cutoff (AcroPrep 30K Omega; Pall Corporation, USA) and centrifuged for 30 min at 1,000 × g. Fifteen microliters of plasma ultrafiltrate, dialysate, or in vitro sample was added to 20 μl PB containing 10 μg/ml ceftriaxone. For these measurements, standards of cefuroxime (0.6, 1.3, 2.5, 5.0, and 10 μg/ml) in 0.9% NaCl-water were used. The UHPLC system (Agilent 1290 Infinity; Agilent Technologies, USA) was equipped with a 1.7 μm 100 by 2.1 mm C18 column (Kinetex; Phenomenex, USA), and chromatography was performed with a gradient of acetonitrile (5 to 10% over 4 min) in PB as the eluent. For analysis, 5 μl prepared sample was injected, and the analytes were detected at 275 nm. Quantification was based on the areas of the cefuroxime and ceftriaxone peaks and was performed with the ChemStation software (Agilent Technologies). The limit of quantification was 0.25 μg/ml for the measurement of the total cefuroxime concentration in plasma and 0.06 μg/ml for the measurement of the free concentrations in plasma, dialysate, and samples from the in vitro study. Intrarun (interrun) imprecisions (percent coefficients of variation [%CV]) were 5.3% (8.2%) at 12.5 μg/ml and 4.1% (4.3%) at 50 μg/ml for quantification of the total plasma drug concentrations, and 4.3% (4.7%) at 2.5 μg/ml and 1.6% (6.2%) at 38 μg/ml for the quantification of the free concentrations. The accuracy of the assay was judged by repeated measurements of 5 different cefuroxime formulations obtained from the pharmacy at Aarhus University Hospital and was found to be between −3.3% and 5.8%.
In vitro experiments.
In vitro relative recovery by gain (RRgain) and by loss (RRloss) were determined using isotonic saline solutions containing cefuroxime concentrations of 1, 10, and 50 μg/ml. Using 20-min intervals, 3 samples of 40 μl were harvested for each concentration, starting at 1 μg/ml. An equilibration period of 15 min was allowed whenever the concentrations were changed. The same catheter was used for both RRgain and RRloss at all concentrations, and the entire experiment was conducted on the same day. The temperature was maintained at 30 ± 1°C.
The effect of temperature was also assessed. This was done in a series of RRgain experiments, where the temperature was increased in a stepwise manner at fixed concentrations of 1, 10, and 50 μg/ml, respectively. Three samples of 40 μl were harvested at each temperature step: 22°C ± 1°C, 30°C ± 1°C, and 40°C ± 1°C.
In vivo studies. (i) Animal, anesthetic, and surgical procedures.
Fifteen pigs were included in the study (60-kg Danish Landrace breed). The animals were kept under general anesthesia during the entire study using a combination of fentanyl (0.25 to 0.5 mg/h, continuous infusion), propofol (150 mg/h, continuous infusion), and sevoflurane (minimal alveolar concentration, 1.1% ± 0.1%). pH, which is known to affect RR (24), was evaluated with arterial gas analysis and kept within the reference range (7.36 to 7.42) throughout the study by controlling ventilation. Normal kidney function, assessed by plasma creatinine, was confirmed for all pigs before inclusion in the study. Body temperature was kept within the range of 36.5°C to 39.5°C. Immediately after the induction of anesthesia, the surgical procedures were performed. MD catheters were placed either in drill holes in cortical bone of the anterior margin of the tibia or in cancellous bone within the femoral condyles. The tibia was accessed by an anteromedial approach, while the femur was accessed by a lateral approach. The depths of the drill holes were 15 mm and 20 mm for the cortical and cancellous drill holes, respectively. Regardless of the anatomical location, the holes were made using a 2-mm drill. Cessation of drilling occurred every few seconds to prevent overheating the tissue. The catheters were fixed to the skin with sutures. The correct locations of the catheters were verified by autopsy. For all pigs, in places where a catheter was implanted into cortical bone, postmortem computed tomography (CT) scans of the tibia were performed in order to document the correct intracortical location of the drill hole.
(ii) Assessment of stability of recovery over time.
In order to assess whether RRloss remains constant over a relevant period of time, retrodialysis was performed for 7 h in three pigs. The first pig had two catheters implanted in cortical and cancellous bone, the second had three catheters implanted in cortical bone, cancellous bone, and subcutaneous tissue, and the third pig had catheters implanted in cancellous bone and subcutaneous tissue. The cefuroxime concentrations in the perfusates were 5 μg/ml for cortical bone catheters and 10 μg/ml for cancellous bone and subcutaneous tissue catheters. The samples were collected at 60-min intervals.
(iii) Assessment of the effect of bone wax sealing of drill holes.
This part of the study was designed to assess whether MD measurements of cefuroxime in drill holes in cortical bone solely reflect bone drug concentrations. In six pigs, four holes were drilled in the cortical part of the tibia, two at each side. Each hole was symmetrical with a hole on the contralateral side, leaving a total of two symmetrical pairs. When all holes were fitted with a catheter, one hole in each pair was randomly allocated for sealing with bone wax, while the corresponding contralateral hole was left open. Prior to implantation, the catheters were perfused with Ringer's acetate containing cefuroxime at a concentration of 10 μg/ml. When surgery was completed, a 30-min tissue equilibration period followed. The probes were then calibrated using the retrodialysis method (26) by collecting a sample over a 30-min interval. Following calibration, the perfusate was changed to blank Ringer's acetate, and a 75-min washout period was conducted. A dialysate was collected during the last 20 min of this period in order to assess the efficacy of the washout. Fifteen hundred milligrams of cefuroxime was then administered intravenously over a 30-min period. The dialysates were collected at 30-min intervals for the first 2 h and at 60-min intervals at 3 to 5 h.
(iv) Measurement of cefuroxime in cancellous bone and subcutaneous tissue.
The methodological setup of this part of the study is analogous to the one outlined above. However, the MD catheters were placed in cancellous bone of the femur and in subcutaneous tissue of the abdomen. The dialysates were collected every 20 min for the first 3 h and every 30 min for the next 5 h, giving a total sampling time of 8 h. Additionally, venous blood samples were collected in the middle of every dialysate sampling interval. The blood samples were drawn from a central venous catheter. Six pigs were included in this part of the study.
Pharmacokinetic analysis and statistics.
The pharmacokinetic parameters were determined separately for each subject by noncompartmental analysis (NCA) using Stata (version 12.0; StataCorp, USA). The exception is the time to 50% of Cmax (T50% of Cmax), which was determined using WinNonlin software (version 5.3; Pharsight Corporation, Mountain View, CA). The washout concentrations were low and as such were neglected in the analysis. The area under the concentration-time curve (AUC) values for the sampling periods were calculated using the trapezoidal rule. As cefuroxime measurements were conducted for only 5 h in cortical bone, the AUC from 0 to 5 h (AUC0–5) was also calculated for the other compartments to allow for a relevant statistical comparison. The terminal half-life (t1/2) was calculated as ln2/λeq, where λeq is the terminal elimination rate constant estimated by linear regression of the log concentration on time. The appropriate number of points used for the calculation was determined separately by inspection of the concentration-time profiles.
In order to assess whether RRloss remains constant, the relation of RRloss to mean RRloss for the entire 7 h (RRloss/mRRloss) was calculated for every catheter at each time point. In the following analysis, the data were pooled for all catheters and for each distinct location, respectively.
All values are given as the mean ± standard error of the mean (SEM) unless stated otherwise. An unpaired t test was used for comparing relative recovery at room temperature and 40°C. A paired t test was used for comparing the pharmacokinetic parameters between sealed and unsealed drill holes within the same animal. A general comparison of the pharmacokinetic parameters was conducted using one-way analysis of variance (ANOVA) with a random animal effect. Finally, post hoc pairwise comparisons were made for selected pairs of pharmacokinetic parameters. When comparisons were made across the two animal experiments, the variability of Cmax and AUC increased slightly. Normality improved and was confirmed when transforming to the log scale, and as such, comparisons for these parameters were made on a log scale. A P value of <0.05 was considered significant. As a measure of tissue penetration, the ratio of the free tissue AUC (fAUCtissue) to free plasma AUC (fAUCplasma) was also calculated for subcutaneous tissue and cancellous bone. Statistical analyses were also performed using Stata (version 12.0; StataCorp, USA).
RESULTS
Effects of concentration, temperature, and time.
The mean RRgain and RRloss for cefuroxime were 42.1% and 42.9% at 1 μg/ml, 46.0% and 48.8% at 10 μg/ml, and 51.3% and 48.0% at 50 μg/ml, respectively (Fig. 1a). Figure 1b shows RRgain using different concentrations of cefuroxime at different temperatures. When pooling data for the three different concentrations, the average difference in recovery between 20°C and 40°C was 7.5% (95% confidence interval [CI], −2.4 to 17.4) (P = 0.12).
FIG 1.
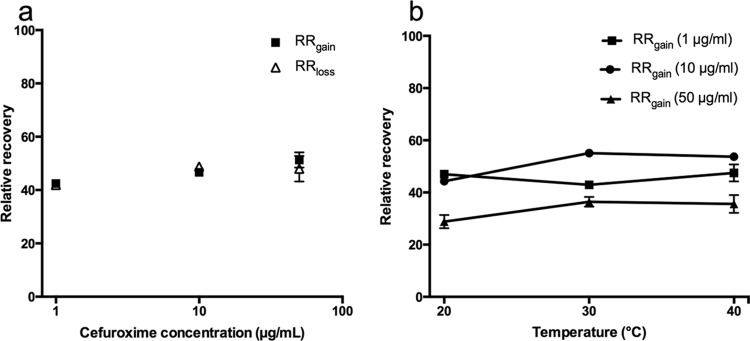
(a) Mean RRgain and RRloss values at different concentrations of cefuroxime. (b) Effects of temperature on recovery at different concentrations of cefuroxime. Bars represent the SEM.
Over 7 h, the pooled relationship of RRloss/mRRloss ranged from 0.96 to 1.04, while the ranges for subcutaneous tissue, cortical, and cancellous bone were 0.93 to 1.10, 0.93 to 1.12, and 0.92 to 1.12, respectively (see Fig. 2). No distinct patterns were recognized for the pooled relationship or for any of the separate anatomical locations.
FIG 2.
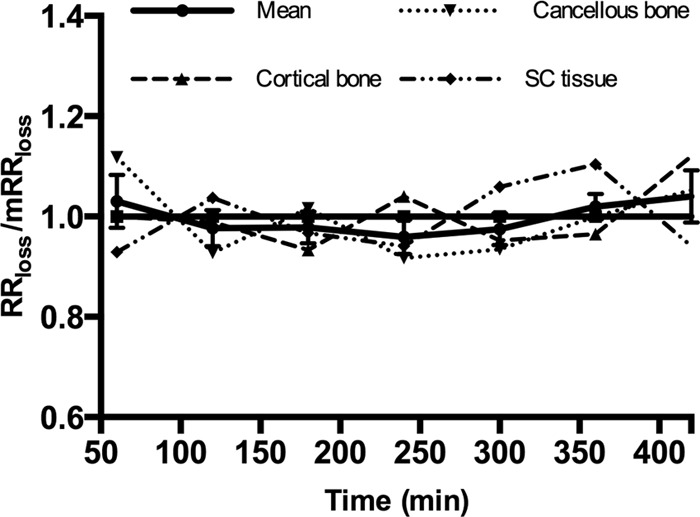
The relationship of RRloss/mRRloss for each anatomical location (dashed lines) and the means for all locations (solid line). The horizontal solid line represents a relationship of 1. Bars represent the SEM.
Assessment of the effect of bone wax sealing of drill holes.
Of the 6 pigs included in this part of the study, only 5 were eligible for analysis. In the excluded pig, one pair of drill holes was excluded because the postmortem CT scan revealed penetration to the surroundings in the distal part of the hole. For one of the catheters in the other pair of drill holes, two concentration analyses failed, and not enough material was left for a third reanalysis. In another pig, the calibration resulted in an RR of 4%. This was considered unreliably low, and therefore, all the samples from this catheter were reanalyzed. The mean RR values were 18.7% ± 2.5% and 17.7% ± 1.7% for the sealed and unsealed holes, respectively. The mean concentrations in the washout samples were 0.26 ± 0.08 μg/ml and 0.31 ± 0.05 μg/ml for the same holes, respectively. The concentration-time profiles for the sealed and unsealed drill holes are depicted in Fig. 3. No significant differences were detected between the key pharmacokinetic parameters (Table 1).
FIG 3.
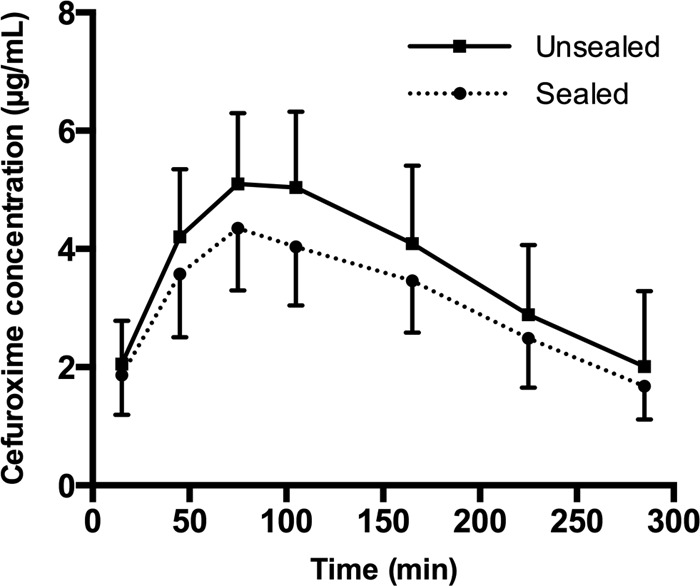
Mean concentration-time profiles for sealed and unsealed drill holes. Bars represent the SEM.
TABLE 1.
Key pharmacokinetic parameters for sealed and unsealed drill holes in cortical bone
| Pharmacokinetic parametera | Sealed drill holes (mean ± SEM) | Unsealed drill holes (mean ± SEM) | P |
|---|---|---|---|
| AUC0-last (min · μg/ml) | 868 ± 233 | 1,037 ± 318 | 0.38 |
| Cmax (μg/ml) | 4.8 ± 1.0 | 5.7 ± 1.4 | 0.42 |
| Tmax (min) | 84 ± 12.7 | 105 ± 11.8 | 0.33 |
| T50% of Cmax (min) | 31.8 ± 5.8 | 28.7 ± 4.7 | 0.61 |
| t1/2 (min) | 136 ± 42.0 | 142.2 ± 59.3 | 0.93 |
Cmax, peak drug concentration in serum; Tmax, time to Cmax; T50% of Cmax, time to 50% of Cmax; t1/2, half-life at β phase; AUC0-last, area under the concentration-time curve from 0 to the last measured value.
Pharmacokinetics of cefuroxime in subcutaneous tissue and cancellous bone.
Of the 6 pigs included in this part of the study, only 5 were included in the analysis. For the excluded pig, the perfusate accidentally was not changed to pure Ringer's acetate following calibration. Thus, it was not possible to calculate absolute tissue drug concentrations. For another pig, the RR of the subcutaneous catheter was not reliably determined. Consequently, the subcutaneous measurements in this pig were also left out of the analysis. The mean RR values were 29.1% ± 11.0% and 29.4% ± 14.1% for the cancellous and subcutaneous catheters, respectively. The mean concentrations in the washout samples were 0.11 ± 0.05 μg/ml and 0.10 ± 0.05 μg/ml for the same holes, respectively. The concentration-time profiles of the two locations and for plasma are depicted in Fig. 4. The pharmacokinetic parameters are shown in Table 2. Significant differences among the means were found for AUC, Cmax, and T50% of Cmax values. Significant differences were also found for pairwise comparisons of free plasma versus subcutaneous tissue and free plasma versus cancellous bone for the same pharmacokinetic parameters.
FIG 4.
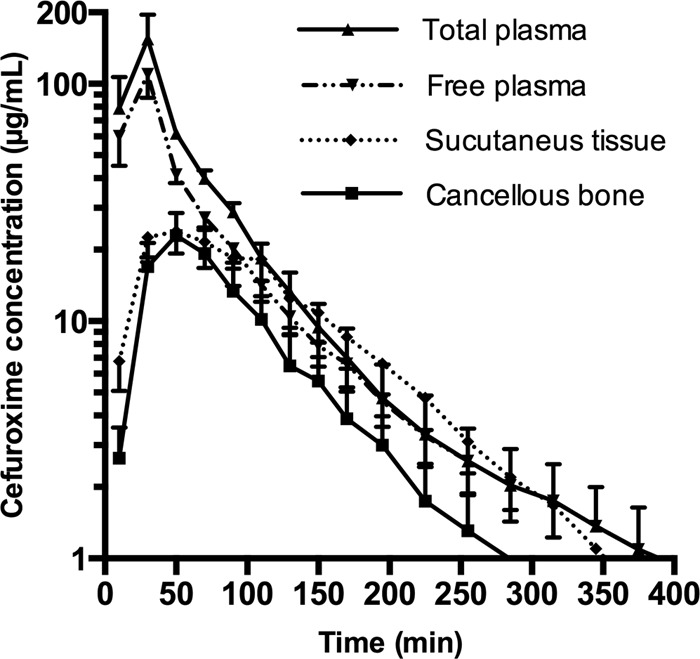
Mean concentration-time profiles for plasma, subcutaneous tissue, and cancellous bone. Bars represent the SEM.
TABLE 2.
Key pharmacokinetic parameters for plasma, subcutaneous tissue, and cancellous boneb
| Pharmacokinetic parametera | Total plasma | Free plasma | Subcutaneous tissue | Cancellous bone | Pc |
|---|---|---|---|---|---|
| AUC0-last (min · μg/ml) | 8,450 ± 1,911 | 6,227 ± 1,449 | 3,386 ± 1,120d | 2,292 ± 660e | 0.01 |
| Cmax (μg/ml) | 154.4 ± 45.7 | 109.9 ± 31.4 | 24.0 ± 6.5e | 22.9 ± 6.3e | <0.01 |
| Tmax (min) | 30.0 ± 0 | 30.0 ± 0 | 50.0 ± 8.2 | 50.0 ± 0 | |
| t1/2 (min) | 60.8 ± 9.6 | 66.6 ± 11.5 | 85.4 ± 34.9 | 56.4 ± 6.6 | 0.40 |
| T50% of Cmax (min) | 11.0 ± 1.7 | 10.7 ± 2.2 | 16.8 ± 1.4e | 22.5 ± 1.5e | <0.01 |
| fAUCISF tissue/fAUCplasma | 0.58 ± 0.19 | 0.45 ± 0.13 | 0.57f |
Cmax, peak drug concentration in serum; Tmax, time to Cmax; t1/2, half-life at β phase; AUC0-last, area under the concentration-time curve from 0 to the last measured value; fAUCISF tissue/fAUCplasma, free area under the concentration-time curve ratio of interstitial fluid (ISF) tissue to free plasma.
All values (other than P values) are expressed as the mean ± SEM.
One-way ANOVA for free plasma, subcutaneous tissue, and cancellous bone.
P < 0.05 for comparison with the corresponding free plasma value.
P < 0.01 for comparison with the corresponding free plasma value.
By t test.
Comparison of AUC0–5, Cmax, and time to 50% of Cmax for free plasma, subcutaneous tissue, and cancellous and cortical bone.
The AUC0–5 values were 6,013 ± 1,339, 3,222 ± 1,086, 2,232 ± 635, and 952 ± 290 min μg/ml for free plasma, subcutaneous tissue, cancellous bone, and cortical bone, respectively (P < 0.01, ANOVA). The value for cortical bone is the average of the sealed and unsealed drill holes. A subsequent comparison of cancellous versus cortical bone showed a P value of 0.04. Statistically significant differences among the means were also found for the Cmax (P < 0.01) and T50% of Cmax (P < 0.01) values for plasma and the three different tissues (data not shown). For these parameters, a significant difference was found for the Cmax (P < 0.01) for the subsequent pairwise comparison of cancellous and cortical bone but not for the T50% of Cmax.
DISCUSSION
In this study, we have demonstrated that in vitro RRgain equaled RRloss over a relevant range of concentrations and that RR was independent of the concentration. When temperature was increased from 20°C to 40°C, an insignificant increase in the recovery of 7.6% was found. It was also shown that in vivo RRloss remained constant over a relevant period of time. Accordingly, MD seems to be a valuable tool for assessing tissue distribution of cefuroxime in studies lasting several hours and with possible physiological changes in temperature. This is in agreement with the findings in previous studies (7, 27, 28).
Bone is a compact tissue. Thus, it is necessary to create drill holes in order to implant MD catheters. This inevitably raises the question as to whether MD measurements of cefuroxime in drill holes solely reflect bone drug concentrations or rather a mixed tissue drug concentration stemming from the actual presence of cefuroxime in bone and a contribution of cefuroxime diffusing into the drill hole from the surrounding soft tissues. Our results show identical pharmacokinetic parameters for sealed and unsealed drill holes, with parallel and almost overlapping time-concentration profiles. If a substantial diffusion of cefuroxime should have occurred from the surroundings to the unsealed drill holes, the pharmacokinetic parameters for these holes should have resembled findings in subcutaneous tissue and/or plasma, which was not the case. In addition to the common pharmacokinetic parameters, we included T50% of Cmax in the analysis for the following reasons. Considering the fact that the concentration-time profiles are rather flat around Cmax combined with a temporal resolution of 30 min, Tmax is an insensitive measure for detecting differences in the kinetics. T50% of Cmax, on the other hand, is situated on the steepest part of the curve, making evaluation and comparison of tissue penetration more accurate.
Over the last 2 decades, an increasing number of studies have demonstrated incomplete tissue penetration for different combinations of drug and tissue under both physiological and pathological conditions (2–8). This emphasizes the need not only to characterize the pharmacokinetics of an antimicrobial drug in a specific tissue but also under specific conditions. In this study, tissue distribution was analyzed using a number of pharmacokinetic parameters. For all extravascular tissues, a heterogeneous tissue distribution was demonstrated. Significant differences in AUC values were found for all tissues compared to free plasma. The lowest AUC was found in cortical bone, reaching only about 1/6 of the corresponding free plasma value. The same ratio for cancellous bone was just >1/3. Both the AUC and Cmax values were significantly higher in cancellous than in cortical bone, suggesting that bone may not be considered one distinct compartment. It is noteworthy that cefuroxime penetration was impaired for all investigated tissues, as expressed in several pharmacokinetic parameters. Altogether, the findings in this study support the fact that complete tissue penetration cannot be taken for granted. In turn, it can be speculated that poor bone penetration may partly account for the prolonged treatment needed for osteomyelitis and PJIs and for the high failure rate when treating these infections.
Determining the concentration of antimicrobials in bone remains a difficult task. The vast majority of studies assessing this challenge have done so using bone biopsy specimens. This method, however, has considerable limitations, not only regarding the method but also because of the lack of standardized procedures in terms of sample preparation, drug analysis, data handling, and reporting (10, 29). Regarding the method as such, it allows only for measurement of the total tissue concentration and not the free and unbound extracellular fraction, which is known to be pharmaceutically active (30, 31). Due to the inherent invasiveness of bone biopsy specimens, samples can be harvested only during surgery, providing poor temporal resolution. Moreover, concentrations are given by weight and not by volume, which makes it difficult to relate the findings to established pharmacodynamic endpoints (10, 29). For β-lactams, it is generally recommended that the concentration of the drug exceeds the MIC for suspected microorganisms for ≥50% of a dosing interval, leaving the time exceeding the MIC (TMIC) as the most important pharmacokinetic parameter for this group of antimicrobials (32). Due to the restricted temporal resolution provided with bone biopsy specimens, TMIC cannot be assessed with this method. The present study suggests that microdialysis can solve these major limitations that are encountered with bone biopsy specimens.
When performing MD experiments, it is important to realize that there will often be a trade-off between experimental needs and the ideal setup. Examples of factors contributing to the limitations are the analytical lower limit of quantification, injection volume, membrane length, and flow rate. Also, these are the adjustable experimental factors that will ultimately decide the RR and the temporal resolution. Our setup resulted in an in vivo RR of approximately 18% for cortical bone MD measurements. It is generally recommended that recovery should be >20%, as lower levels of recovery are relatively more exposed to the standard deviations associated with the preanalytical handling as well as chemical analysis (33). The resulting variations will increase exponentially with decreasing recovery. This disadvantage should be remembered when interpreting results obtained with MD. Nevertheless, in our case, where the depth of the drill holes limits membrane length and the relatively short half-life of cefuroxime calls for high temporal resolution, an in vivo RR of 18% seems acceptable.
From a clinical perspective, the findings of the present study are of considerable importance. A drug like cefuroxime reaches a high peak concentration in plasma after a bolus injection, but it is rapidly cleared from plasma because of excretion and redistribution. For the drug to exceed the MIC in its target site for a sufficient period of time, quick tissue equilibration seems mandatory for obtaining relevant antimicrobial action. For cortical bone in particular, penetration seems to be incomplete and delayed, as shown in this study, and it can be questioned if bolus injections of drugs with short half-lives are suitable when treating or preventing infections in bone.
In conclusion, the findings in the present study demonstrate that MD is a valuable and reliable method for evaluating the tissue distribution of cefuroxime. Calibration can be performed by means of retrodialysis, and studies can be prolonged for several hours. The problem of assessing cefuroxime concentrations in bone can be overcome by placing the MD catheters in drill holes, and sealing of these seems unnecessary. As such, we find that MD might be a valuable tool for clinical studies on bone pharmacokinetics. The uneven tissue distribution that was demonstrated in this study is important and may account for treatment failures in the clinical setting.
ACKNOWLEDGMENTS
We thank Bo Martin Bibby (Department of Biostatistics, University of Aarhus) for statistical counseling. We thank consultant of orthopedic surgery Klaus Kjær Petersen (Department of Orthopaedic Surgery, Aarhus University Hospital) for advice regarding the surgical procedures.
This study was supported by a grant from the Department of Clinical Medicine, University of Aarhus.
Footnotes
Published ahead of print 24 March 2014
REFERENCES
- 1.Lew DP, Waldvogel FA. 1997. Osteomyelitis. N. Engl. J. Med. 336:999–1007. 10.1056/NEJM199704033361406 [DOI] [PubMed] [Google Scholar]
- 2.Andreas M, Zeitlinger M, Hoeferl M, Jaeger W, Zimpfer D, Hiesmayr JM, Laufer G, Hutschala D. 2013. Internal mammary artery harvesting influences antibiotic penetration into presternal tissue. Ann. Thorac. Surg. 95:1323–1329, discussion 1329–1330. 10.1016/j.athoracsur.2012.10.088 [DOI] [PubMed] [Google Scholar]
- 3.Brill MJ, Houwink AP, Schmidt S, Van Dongen EP, Hazebroek EJ, van Ramshorst B, Deneer VH, Mouton JW, Knibbe CA. 2013. Reduced subcutaneous tissue distribution of cefazolin in morbidly obese versus non-obese patients determined using clinical microdialysis. J. Antimicrob. Chemother. 10.1093/jac/dkt444 [DOI] [PubMed] [Google Scholar]
- 4.Brunner M, Pernerstorfer T, Mayer BX, Eichler HG, Müller M. 2000. Surgery and intensive care procedures affect the target site distribution of piperacillin. Crit. Care Med. 28:1754–1759. 10.1097/00003246-200006000-00009 [DOI] [PubMed] [Google Scholar]
- 5.Joukhadar C, Frossard M, Mayer BX, Brunner M, Klein N, Siostrzonek P, Eichler HG, Müller M. 2001. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit. Care Med. 29:385–391. 10.1097/00003246-200102000-00030 [DOI] [PubMed] [Google Scholar]
- 6.Tegeder I, Schmidtko A, Bräutigam L, Kirschbaum A, Geisslinger G, Lötsch J. 2002. Tissue distribution of imipenem in critically ill patients. Clin. Pharmacol. Ther. 71:325–333. 10.1067/mcp.2002.122526 [DOI] [PubMed] [Google Scholar]
- 7.Barbour A, Schmidt S, Rout WR, Ben-David K, Burkhardt O, Derendorf H. 2009. Soft tissue penetration of cefuroxime determined by clinical microdialysis in morbidly obese patients undergoing abdominal surgery. Int. J. Antimicrob. Agents 34:231–235. 10.1016/j.ijantimicag.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 8.De La Peña A, Dalla Costa T, Talton JD, Rehak E, Gross J, Thyroff-Friesinger U, Webb AI, Müller M, Derendorf H. 2001. Penetration of cefaclor into the interstitial space fluid of skeletal muscle and lung tissue in rats. Pharm. Res. 18:1310–1314. 10.1023/A:1013042128791 [DOI] [PubMed] [Google Scholar]
- 9.Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. 2008. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 61:235–237. 10.1093/jac/dkm476 [DOI] [PubMed] [Google Scholar]
- 10.Pea F. 2009. Penetration of antibacterials into bone: what do we really need to know for optimal prophylaxis and treatment of bone and joint infections? Clin. Pharmacokinet. 48:125–127. 10.2165/00003088-200948020-00003 [DOI] [PubMed] [Google Scholar]
- 11.Barbour A, Schmidt S, Sabarinath SN, Grant M, Seubert C, Skee D, Murthy B, Derendorf H. 2009. Soft-tissue penetration of ceftobiprole in healthy volunteers determined by in vivo microdialysis. Antimicrob. Agents Chemother. 53:2773–2776. 10.1128/AAC.01409-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim A, Suecof LA, Sutherland CA, Gao L, Kuti JL, Nicolau DP. 2008. In vivo microdialysis study of the penetration of daptomycin into soft tissues in diabetic versus healthy volunteers. Antimicrob. Agents Chemother. 52:3941–3946. 10.1128/AAC.00589-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller M, Haag O, Burgdorff T, Georgopoulos A, Weninger W, Jansen B, Stanek G, Pehamberger H, Agneter E, Eichler HG. 1996. Characterization of peripheral-compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob. Agents Chemother. 40:2703–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buerger C, Plock N, Dehghanyar P, Joukhadar C, Kloft C. 2006. Pharmacokinetics of unbound linezolid in plasma and tissue interstitium of critically ill patients after multiple dosing using microdialysis. Antimicrob. Agents Chemother. 50:2455–2463. 10.1128/AAC.01468-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joukhadar C, Klein N, Mayer BX, Kreischitz N, Delle-Karth G, Palkovits P, Heinz G, Müller M. 2002. Plasma and tissue pharmacokinetics of cefpirome in patients with sepsis. Crit. Care Med. 30:1478–1482. 10.1097/00003246-200207000-00013 [DOI] [PubMed] [Google Scholar]
- 16.Liu P, Müller M, Grant M, Webb AI, Obermann B, Derendorf H. 2002. Interstitial tissue concentrations of cefpodoxime. J. Antimicrob. Chemother. 50(Suppl):19–22. 10.1093/jac/dkf804 [DOI] [PubMed] [Google Scholar]
- 17.Schintler MV, Traunmüller F, Metzler J, Kreuzwirt G, Spendel S, Mauric O, Popovic M, Scharnagl E, Joukhadar C. 2009. High fosfomycin concentrations in bone and peripheral soft tissue in diabetic patients presenting with bacterial foot infection. J. Antimicrob. Chemother. 64:574–578. 10.1093/jac/dkp230 [DOI] [PubMed] [Google Scholar]
- 18.Stolle L, Arpi M, Holmberg-Jørgensen P, Riegels-Nielsen P, Keller J. 2005. Distribution of gentamicin from a Gentacoll sponge measured by in vivo microdialysis. Scand. J. Infect. Dis. 37:284–287. 10.1080/00365540410021108-1 [DOI] [PubMed] [Google Scholar]
- 19.Stolle LB, Arpi M, Holmberg-Jørgensen P, Riegels-Nielsen P, Keller J. 2004. Application of microdialysis to cancellous bone tissue for measurement of gentamicin levels. J. Antimicrob. Chemother. 54:263–265. 10.1093/jac/dkh291 [DOI] [PubMed] [Google Scholar]
- 20.Stolle LB, Arpi M, Jørgensen PH, Riegels-Nielsen P, Keller J. 2003. In situ gentamicin concentrations in cortical bone: an experimental study using microdialysis in bone. Acta Orthop. Scand. 74:611–616. 10.1080/00016470310018045 [DOI] [PubMed] [Google Scholar]
- 21.Stolle LB, Plock N, Joukhadar C, Arpi M, Emmertsen KJ, Buerger C, Riegels-Nielsen P, Kloft C. 2008. Pharmacokinetics of linezolid in bone tissue investigated by in vivo microdialysis. Scand. J. Infect. Dis. 40:24–29. 10.1080/00365540701509873 [DOI] [PubMed] [Google Scholar]
- 22.Traunmüller F, Schintler MV, Metzler J, Spendel S, Mauric O, Popovic M, Konz KH, Scharnagl E, Joukhadar C. 2010. Soft tissue and bone penetration abilities of daptomycin in diabetic patients with bacterial foot infections. J. Antimicrob. Chemother. 65:1252–1257. 10.1093/jac/dkq109 [DOI] [PubMed] [Google Scholar]
- 23.Traunmüller F, Schintler MV, Spendel S, Popovic M, Mauric O, Scharnagl E, Joukhadar C. 2010. Linezolid concentrations in infected soft tissue and bone following repetitive doses in diabetic patients with bacterial foot infections. Int. J. Antimicrob. Agents 36:84–86. 10.1016/j.ijantimicag.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 24.Joukhadar C, Müller M. 2005. Microdialysis: current applications in clinical pharmacokinetic studies and its potential role in the future. Clin. Pharmacokinet. 44:895–913. 10.2165/00003088-200544090-00002 [DOI] [PubMed] [Google Scholar]
- 25.Müller M. 2002. Science, medicine, and the future: microdialysis. BMJ 324:588–591. 10.1136/bmj.324.7337.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ståhle L, Arner P, Ungerstedt U. 1991. Drug distribution studies with microdialysis. III: extracellular concentration of caffeine in adipose tissue in man. Life Sci. 49:1853–1858 [DOI] [PubMed] [Google Scholar]
- 27.Shukla C, Patel V, Juluru R, Stagni G. 2009. Quantification and prediction of skin pharmacokinetics of amoxicillin and cefuroxime. Biopharm. Drug Dispos. 30:281–293. 10.1002/bdd.658 [DOI] [PubMed] [Google Scholar]
- 28.Tsai TH, Cheng FC, Chen KC, Chen YF, Chen CF. 1999. Simultaneous measurement of cefuroxime in rat blood and brain by microdialysis and microbore liquid chromatography. Application to pharmacokinetics. J. Chromatogr. B Biomed. Sci. Appl. 735:25–31. 10.1016/S0378-4347(99)00410-7 [DOI] [PubMed] [Google Scholar]
- 29.Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sörgel F. 2009. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin. Pharmacokinet. 48:89–124. 10.2165/00003088-200948020-00002 [DOI] [PubMed] [Google Scholar]
- 30.Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug.' Nat. Rev. Microbiol. 2:289–300. 10.1038/nrmicro862 [DOI] [PubMed] [Google Scholar]
- 31.Ryan DM. 1993. Pharmacokinetics of antibiotics in natural and experimental superficial compartments in animals and humans. J. Antimicrob. Chemother. 31(Suppl D):1–16 [DOI] [PubMed] [Google Scholar]
- 32.Craig WA. 2001. Does the dose matter? Clin. Infect. Dis. 33(Suppl 3):S233–S237. 10.1086/321854 [DOI] [PubMed] [Google Scholar]
- 33.Chaurasia CS, Müller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, Bungay PM, DeLange ECM, Derendorf H, Elmquist WF, Hammarlund-Udenaes M, Joukhadar C, Kellogg DL, Jr, Lunte CE, Nordstrom CH, Rollema H, Sawchuk RJ, Cheung BWY, Shah VP, Stahle L, Ungerstedt U, Welty DF, Yeo H. 2005. AAPS-FDA workshop white paper: microdialysis principles, application, and regulatory perspectives report from the Joint AAPS-FDA Workshop, November 4–5, 2005, Nashville, TN. AAPS J. 9:47–59. 10.1208/aapsj0901006 [DOI] [Google Scholar]


