Abstract
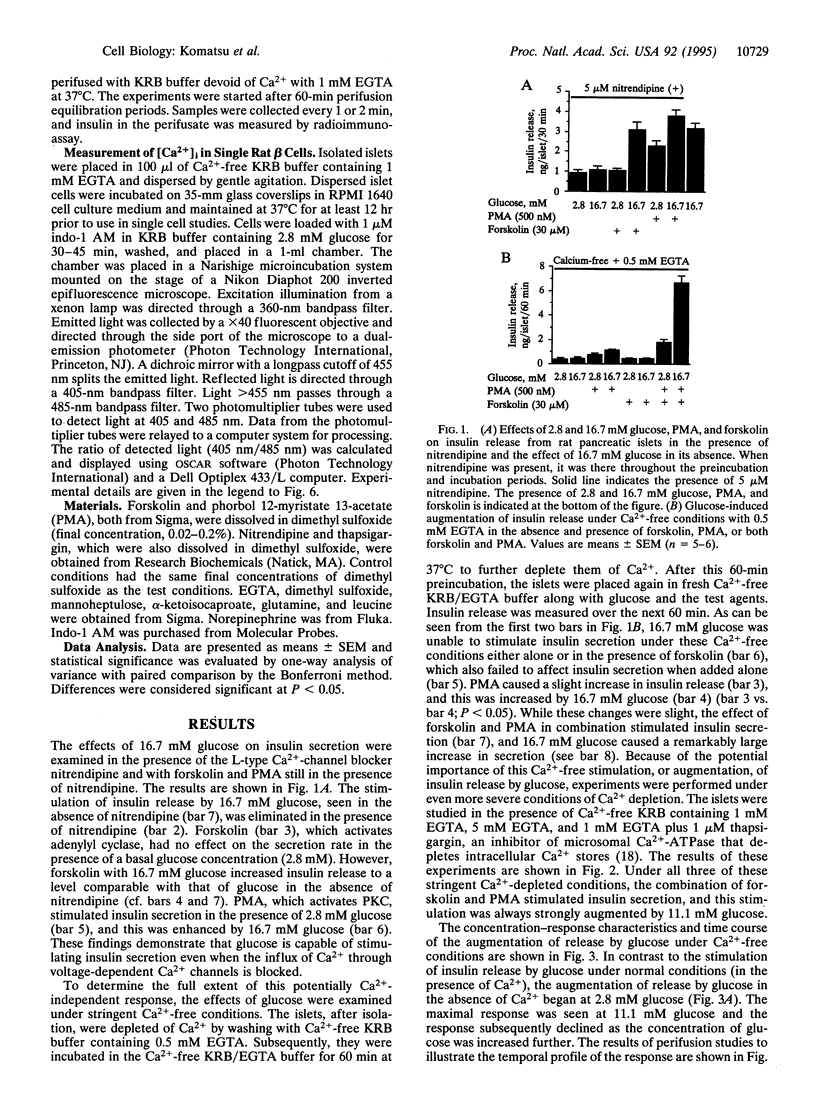
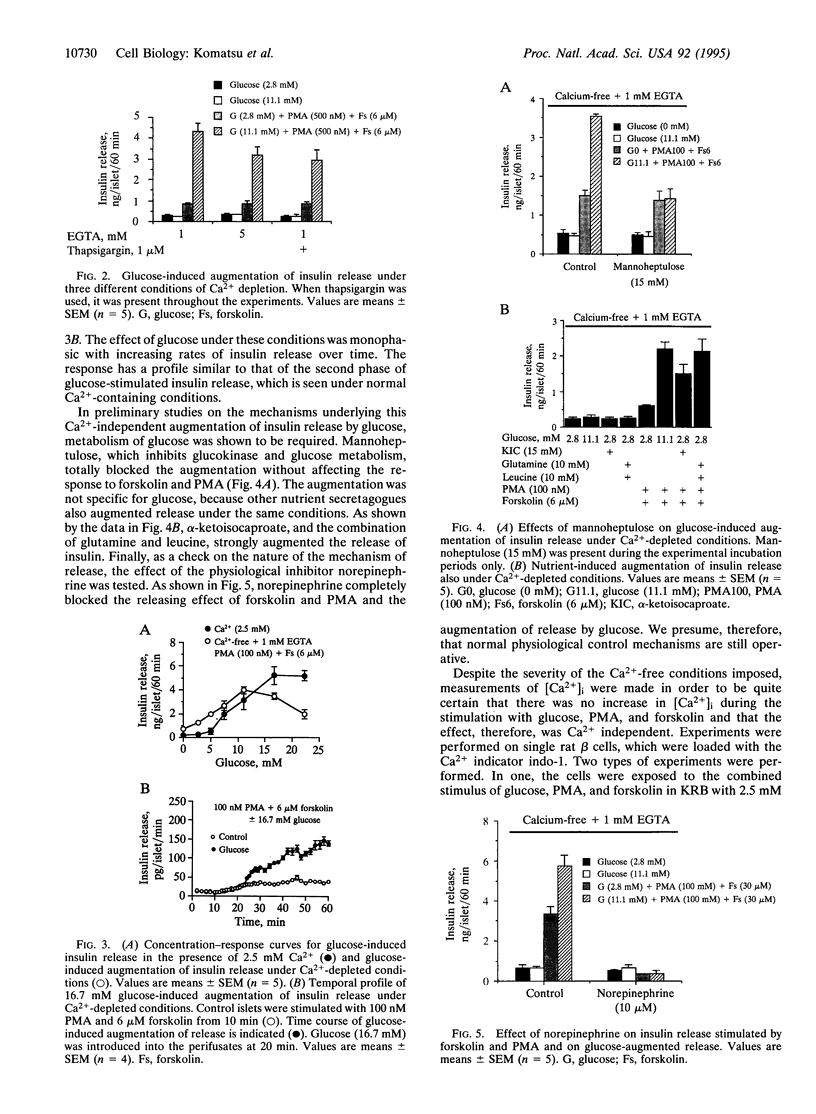
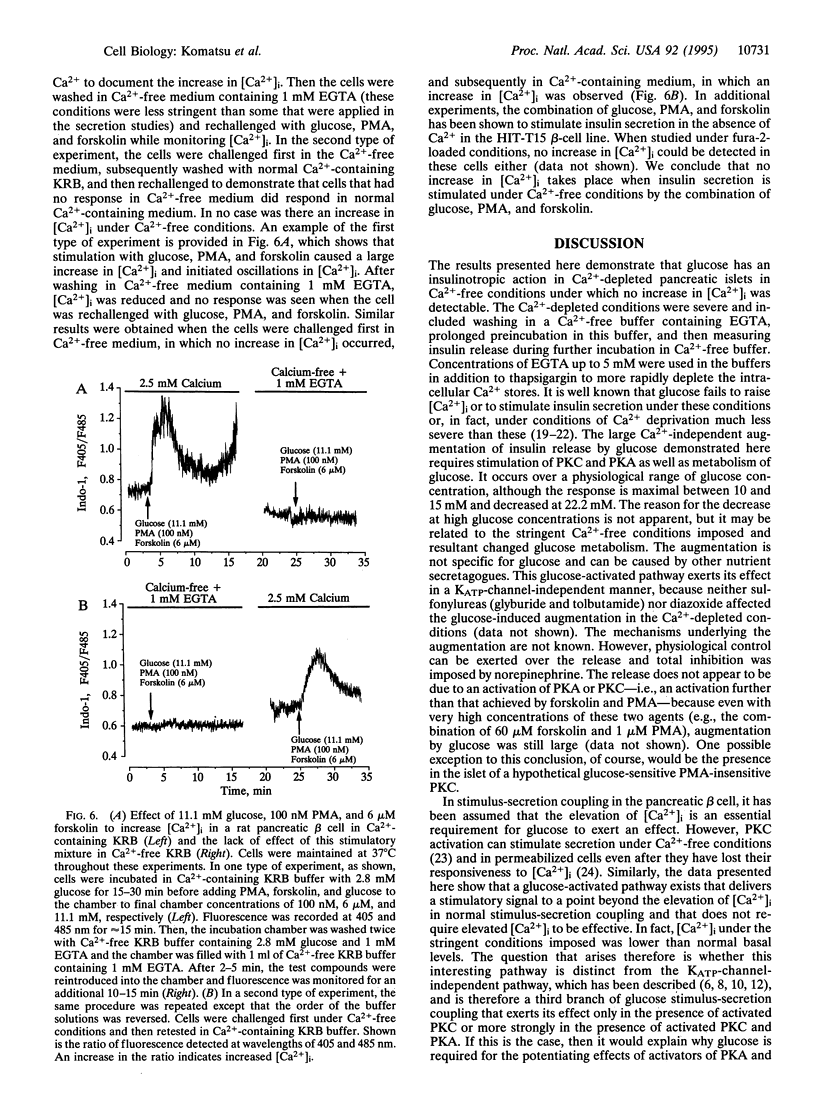
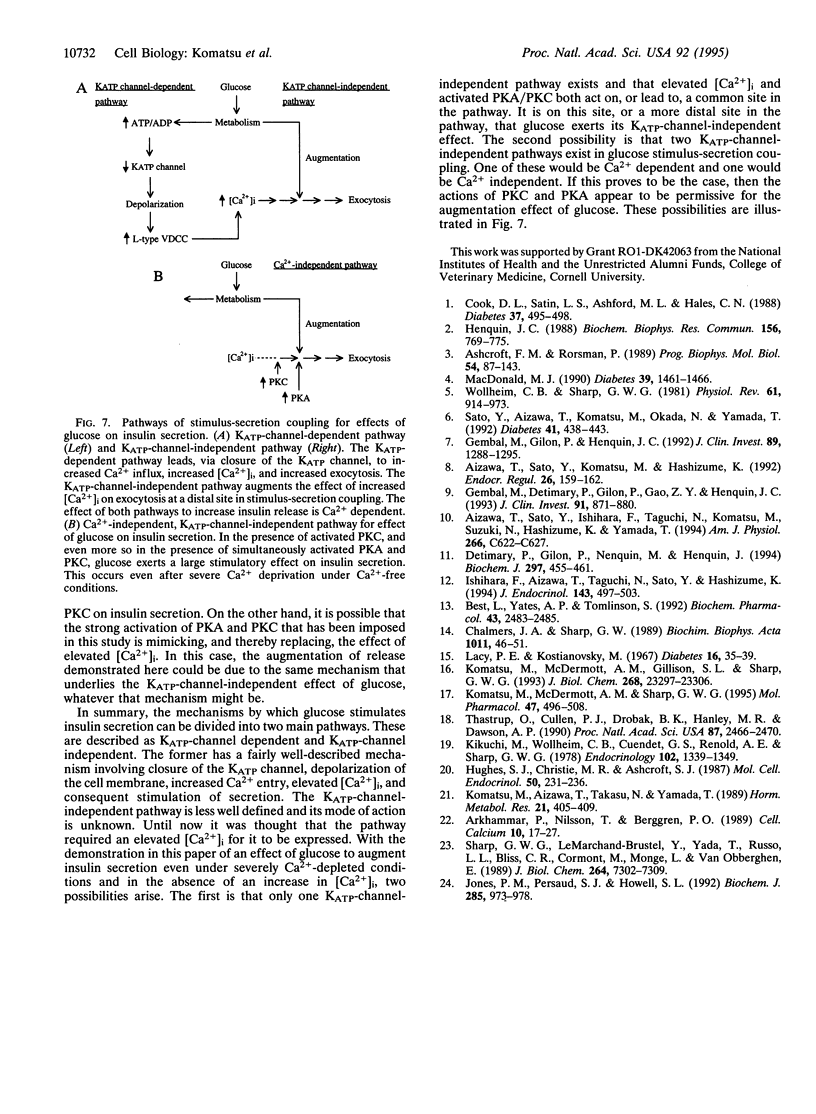
Insulin secretion has been studied in isolated rat pancreatic islets under stringent Ca(2+)-depleted, Ca(2+)-free conditions. Under these conditions, the effect of 16.7 mM glucose to stimulate insulin release was abolished. Forskolin, which activates adenylyl cyclase, also failed to stimulate release in the presence of either low or high glucose concentrations. A phorbol ester (phorbol 12-myristate 13-acetate; PMA) increased the release rate slightly and this was further increased by 16.7 mM glucose. Remarkably, in the presence of both forskolin and PMA, 16.7 mM glucose strongly augmented insulin release. The augmentation was concentration dependent and monophasic and had a temporal profile similar to the "second phase" of glucose-stimulated insulin release, which is seen under normal conditions when Ca2+ is present. Metabolism is required for the effect because mannoheptulose abolished the glucose response. Other nutrient secretagogues, alpha-ketoisocaproate, and the combination of leucine and glutamine augmented release under the same conditions. Norepinephrine, a physiological inhibitor of insulin secretion, totally blocked the stimulation of release by forskolin and PMA and the augmentation of release by glucose. Thus, under the stringent Ca(2+)-free conditions imposed, the stimulation of insulin release by forskolin and PMA, as well as the augmentation of release by glucose, is under normal physiological control. As no increase in intracellular [Ca2+] was observed, the results demonstrate that glucose can increase the rate of exocytosis and insulin release by pancreatic islets in a Ca(2+)-independent manner. This interesting pathway of stimulus-secretion coupling for glucose appears to exert its effect at a site beyond the usual elevation of intracellular [Ca2+] and is not due to an activation by glucose of protein kinase A or C.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa T., Sato Y., Ishihara F., Taguchi N., Komatsu M., Suzuki N., Hashizume K., Yamada T. ATP-sensitive K+ channel-independent glucose action in rat pancreatic beta-cell. Am J Physiol. 1994 Mar;266(3 Pt 1):C622–C627. doi: 10.1152/ajpcell.1994.266.3.C622. [DOI] [PubMed] [Google Scholar]
- Aizawa T., Sato Y., Komatsu M., Hashizume K. ATP-sensitive K+ channel-independent, insulinotropic action of glucose in the B-cells. Endocr Regul. 1992 Dec;26(4):159–162. [PubMed] [Google Scholar]
- Arkhammar P., Nilsson T., Berggren P. O. Glucose-induced changes in cytoplasmic free Ca2+ concentration and the significance for the regulation of insulin release. Measurements with fura-2 in suspensions and single aggregates of mouse pancreatic beta-cells. Cell Calcium. 1989 Jan;10(1):17–27. doi: 10.1016/0143-4160(89)90040-7. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Best L., Yates A. P., Tomlinson S. Stimulation of insulin secretion by glucose in the absence of diminished potassium (86Rb+) permeability. Biochem Pharmacol. 1992 Jun 9;43(11):2483–2485. doi: 10.1016/0006-2952(92)90330-l. [DOI] [PubMed] [Google Scholar]
- Chalmers J. A., Sharp G. W. The importance of Ca2+ for glucose-induced priming in pancreatic islets. Biochim Biophys Acta. 1989 Mar 28;1011(1):46–51. doi: 10.1016/0167-4889(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Satin L. S., Ashford M. L., Hales C. N. ATP-sensitive K+ channels in pancreatic beta-cells. Spare-channel hypothesis. Diabetes. 1988 May;37(5):495–498. doi: 10.2337/diab.37.5.495. [DOI] [PubMed] [Google Scholar]
- Detimary P., Gilon P., Nenquin M., Henquin J. C. Two sites of glucose control of insulin release with distinct dependence on the energy state in pancreatic B-cells. Biochem J. 1994 Feb 1;297(Pt 3):455–461. doi: 10.1042/bj2970455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gembal M., Detimary P., Gilon P., Gao Z. Y., Henquin J. C. Mechanisms by which glucose can control insulin release independently from its action on adenosine triphosphate-sensitive K+ channels in mouse B cells. J Clin Invest. 1993 Mar;91(3):871–880. doi: 10.1172/JCI116308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gembal M., Gilon P., Henquin J. C. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest. 1992 Apr;89(4):1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. ATP-sensitive K+ channels may control glucose-induced electrical activity in pancreatic B-cells. Biochem Biophys Res Commun. 1988 Oct 31;156(2):769–775. doi: 10.1016/s0006-291x(88)80910-0. [DOI] [PubMed] [Google Scholar]
- Hughes S. J., Christie M. R., Ashcroft S. J. Potentiators of insulin secretion modulate Ca2+ sensitivity in rat pancreatic islets. Mol Cell Endocrinol. 1987 Apr;50(3):231–236. doi: 10.1016/0303-7207(87)90021-9. [DOI] [PubMed] [Google Scholar]
- Ishihara F., Aizawa T., Taguchi N., Sato Y., Hashizume K. Differential metabolic requirement for initiation and augmentation of insulin release by glucose: a study with rat pancreatic islets. J Endocrinol. 1994 Dec;143(3):497–503. doi: 10.1677/joe.0.1430497. [DOI] [PubMed] [Google Scholar]
- Jones P. M., Persaud S. J., Howell S. L. Ca2(+)-induced insulin secretion from electrically permeabilized islets. Loss of the Ca2(+)-induced secretory response is accompanied by loss of Ca2(+)-induced protein phosphorylation. Biochem J. 1992 Aug 1;285(Pt 3):973–978. doi: 10.1042/bj2850973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M., Wollheim C. B., Cuendet G. S., Renold A. E., Sharp G. W. Studies on the dual effects of glucose on 45Ca++ efflux from isolated rat islets. Endocrinology. 1978 May;102(5):1339–1349. doi: 10.1210/endo-102-5-1339. [DOI] [PubMed] [Google Scholar]
- Komatsu M., Aizawa T., Takasu N., Yamada T. Glucose raises cytosolic free calcium in the rat pancreatic islets. Horm Metab Res. 1989 Aug;21(8):405–409. doi: 10.1055/s-2007-1009249. [DOI] [PubMed] [Google Scholar]
- Komatsu M., McDermott A. M., Gillison S. L., Sharp G. W. Mastoparan stimulates exocytosis at a Ca(2+)-independent late site in stimulus-secretion coupling. Studies with the RINm5F beta-cell line. J Biol Chem. 1993 Nov 5;268(31):23297–23306. [PubMed] [Google Scholar]
- Komatsu M., McDermott A. M., Sharp G. W. Sodium fluoride stimulates exocytosis at a late site of calcium interaction in stimulus-secretion coupling: studies with the RINm5F beta cell line. Mol Pharmacol. 1995 Mar;47(3):496–508. [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J. Elusive proximal signals of beta-cells for insulin secretion. Diabetes. 1990 Dec;39(12):1461–1466. doi: 10.2337/diab.39.12.1461. [DOI] [PubMed] [Google Scholar]
- Sato Y., Aizawa T., Komatsu M., Okada N., Yamada T. Dual functional role of membrane depolarization/Ca2+ influx in rat pancreatic B-cell. Diabetes. 1992 Apr;41(4):438–443. doi: 10.2337/diab.41.4.438. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Le Marchand-Brustel Y., Yada T., Russo L. L., Bliss C. R., Cormont M., Monge L., Van Obberghen E. Galanin can inhibit insulin release by a mechanism other than membrane hyperpolarization or inhibition of adenylate cyclase. J Biol Chem. 1989 May 5;264(13):7302–7309. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]