Abstract
Invasive pulmonary aspergillosis (IPA) is a life-threatening disease of immunocompromised patients that requires aggressive therapy. Detection of the disease and monitoring of the therapeutic response during IPA are complex, and current molecular diagnostics are not suitably robust. Here, we explored proteomic profiles of bronchoalveolar lavage fluid (BALF) specimens from a persistently neutropenic rabbit model of IPA. Three experimental arms, uninfected control animals, infected untreated animals, and animals infected and treated with ravuconazole/amphotericin B, were studied. Total proteins were evaluated by two-dimensional (2D) gel electrophoresis, followed by matrix-assisted laser desorption ionization–time of flight/time of flight (MALDI-TOF/TOF) mass spectrometry (MS) and quantified by enzyme-linked immunosorbent assay (ELISA). Host-derived proteins haptoglobin (Hp), C-reactive protein (CRP), and annexin A1 (Anx A1) were prominently found in BALF during the IPA infection and showed significant changes in response to antifungal therapy (P < 0.0001). In serum, differences in Hp (P = 0.0001) between infected and treated rabbits were observed. Preliminary in vitro studies revealed that Aspergillus fumigatus-secreted proteases may contribute to the cleavage of Anx A1 during IPA. In summary, host protein biomarkers Hp, CRP, and Anx A1 may have value in monitoring therapeutic response to antifungal agents in IPA patients with confirmed disease.
INTRODUCTION
Invasive pulmonary aspergillosis (IPA) caused by Aspergillus fumigatus is a devastating disease for immunocompromised patients. Successful management of patients depends on early diagnosis, effective therapy, and monitoring of therapeutic response. Detecting IPA and monitoring the response to therapy still remain very difficult, especially in the early stages of the disease. Currently, according to the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG), diagnosis of IPA relies on a positive computed tomography (CT) scan, culture and/or microscopic evidence of disease, and detection of A. fumigatus antigens in a susceptible host (1).
The use of surrogate markers of infection is an important adjunct to diagnosis of IPA. The galactomannan immunoassay is commonly used in the diagnosis of this infection in patients. Yet this test has limitations for monitoring therapeutic response, and it has a potential for false-positive results (2, 3, 4). In recent years, improved methodologies have been developed for detection of Aspergillus nucleic acid in blood and bronchoalveolar lavage fluid (BALF). Developing additional biomarkers could complement existing diagnostic methods by helping to improve detection of IPA and monitoring the response of at-risk patients to therapy.
During the past several years, proteomic techniques have been used to study A. fumigatus during invasive infection (5, 6). However, less is known about the host proteomic profile in response to infection with Aspergillus. Proteomic analysis of primary specimens such as BALF and serum can provide an understanding of complicated host-pathogen interactions during IPA.
In this pilot study, we profiled the proteome of BALF and serum samples from an experimental rabbit model of IPA. Prominent host biomarkers were identified following Aspergillus infection and were useful in assessing the therapeutic response to antifungal agents.
MATERIALS AND METHODS
Animal model.
A well-described (7) persistently neutropenic rabbit model of IPA was used for the experiments. The three experimental arms consisted of uninfected control animals (n = 13), animals infected endotracheally with A. fumigatus and untreated (n = 17), and infected rabbits treated intravenously with one of the following antifungal agents (n = 16): liposomal amphotericin B (LAMB) at 5 mg/kg of body weight, deoxycholate amphotericin B (DAMB) at 1 mg/kg, or ravuconazole (RVZ) at 5 mg/kg. Neutropenia was deliberately chosen for comparison of host biomarkers' responses during the antifungal therapy to simulate a prominent condition for human infection. All procedures in the study were approved by Weill Cornell Medical College's Institutional Animal Care and Use Committee.
Bronchoalveolar lavage.
Bronchoalveolar lavage was performed postmortem on each lung preparation by the instillation and subsequent withdrawal of 10 ml of sterile normal saline twice into the clamped trachea with a sterile 12-ml syringe. The lavage was then centrifuged for 10 min at 400 × g. The upper and lower (2-ml) portions of the supernatant were transferred into centrifuge tubes and stored at −80°C.
Serum collection.
Serum specimens (1 ml) were collected from infected untreated and treated rabbits on 0, 1, 4, 6, and 13 postinoculation days (PID) and stored at −80°C. Serum specimens for the uninfected control rabbit arm were collected from healthy rabbits and stored at −80°C.
2D gel electrophoresis and protein identification.
Total proteins in BALF samples were evaluated by two-dimensional (2D) gel electrophoresis as previously described (8). Protein spots of interest were excised from the gel, trypsin digested, and analyzed by mass spectroscopy (MS) on a 4800 matrix-assisted laser desorption ionization –time of flight/time of flight (MALDI-TOF/TOF) mass analyzer. Protein identification was performed by searching the combined tandem mass spectra against the National Center for Biotechnology Information (NCBI) mammals sequence database, using a local MASCOT search engine (V. 1.9) on a GPS (V. 3.5, ABI) server.
ELISA.
Enzyme-linked immunosorbent assay (ELISA) was performed using a human haptoglobin (Hp) ELISA kit (GenWay), a rabbit C-reactive protein (CRP) ELISA kit (Immunology Consultant Laboratory, Inc.), and a rabbit annexin A1 (Anx A1) ELISA kit (Uscn Life Science Inc.) following the manufacturers' protocols.
A. fumigatus growth conditions.
A. fumigatus wild-type strain R21 was grown for 4 days at 37°C on potato dextrose agar (PDA; Becton Dickenson, Sparks, MD). At the end of the incubation period, conidia were harvested with 0.01% Tween 20 solution. For degradation assays, 100 ml of Aspergillus minimal medium (AMM) were inoculated with 1 × 108 conidia and incubated at 37°C with shaking (225 rpm) for 72 h.
Annexin A1 degradation assay.
Five milliliters of 72-h-old A. fumigatus culture were filtered through a 0.45-μm filter (Fisher Scientific) to separate conidia and hyphae from the culture supernatant. Two micrograms of purified recombinant human annexin A1 (RD Systems) or 10 μl of K-562 (human erythromyeloblastoid leukemia cell line; Santa Cruz Biotechnology, Inc.) whole-cell lysate was added to 20 μl of A. fumigatus culture filtrate. The solutions were incubated at 37°C for 2 h, and Western blotting of the samples was performed.
Western blotting.
Samples were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Invitrogen) using iBlot Gel Transfer Device (Program 3) (Invitrogen). After the transfer, cleavage products were detected by Western blotting using the iBlot gel transfer device (Program 9) and the iBlot Western detection kit (Invitrogen). Annexin A1 monoclonal antibodies (EMD Millipore) were used for the detection in a dilution of 1:1,000.
Statistical analysis.
Comparisons between two groups were made by Fisher's exact tests for categorical variables and the Mann-Whitney test or the unpaired t test for continuous variables. Multivariate analysis of variance (ANOVA) was performed to compare continuous variables repeatedly measured at multiple time points among different groups. Significance was defined at P values of ≤0.05 (two tailed).
RESULTS
Identification of BAL fluid host proteins responding to infection and therapy by 2D gel electrophoresis.
BALF samples from uninfected controls, infected untreated, and infected treated rabbits were analyzed by 2D gel electrophoresis, in which the samples were first separated on a pH 5 to 8 linear gradient followed by 12.5% SDS-PAGE in the second dimension. Figure 1 shows a typical 2D gel after staining with Sypro ruby. The images of 2D gels were analyzed using PDQuest 2.3 to determine those prominent proteins responsive to both IPA infection and subsequent therapy. Proteins corresponding to spots 1, 2, and 3 were absent in BALF from healthy animals, appeared with strong signals in samples from infected rabbits, and disappeared in samples collected after the treatment. In contrast, the protein level at spot 4 decreased following IPA infection and was partially restored after therapy.
FIG 1.
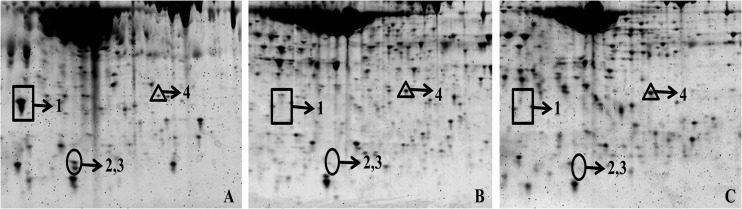
2D gels of bronchoalveolar lavage fluid (BALF) samples. An aliquot of 50 μg of protein was subjected to 2-dimensional gel electrophoresis using strips of pI 5 to 8 followed by 12.5% SDS-PAGE gel in the second dimension. The marked spots were excised, trypsin digested, and analyzed by MS. Spot 1, haptoglobin (Hp); spots 2 and 3, C-reactive protein (CRP); spot 4, annexin A1 (Anx A1). (A) BALF from infected untreated animal demonstrates abundant presence of both Hp and CRP, whereas Anx A1 is almost undetectable. (B) BALF from treated rabbit is characterized by the absence of Hp and CRP with the presence of Anx A1 (similar to the uninfected control). (C) BALF from uninfected control (healthy rabbit) shows the absence of Hp and CRP, while Anx A1 is present. Gels were stained with Sypro ruby for visualization.
Protein spots of interest 1, 2, 3, and 4 were excised from the gel, trypsin digested, and analyzed by MALDI-TOF/TOF MS. The MS analysis revealed that spot 1 was haptoglobin (Hp), spots 2 and 3 were found to be C-reactive protein, and spot 4 was identified as annexin A1 (Anx A1). Spots 2 and 3 represented the same protein (CRP), possibly due to the proteolytic cleavage of CRP subunits or various posttranscriptional modifications.
The three proteins listed above appeared to respond to IPA infection, as well as to treatment with antifungal drugs. Therefore, they were chosen as potential host-based biomarkers of IPA useful in monitoring therapeutic response.
Quantification of host biomarkers in BALF.
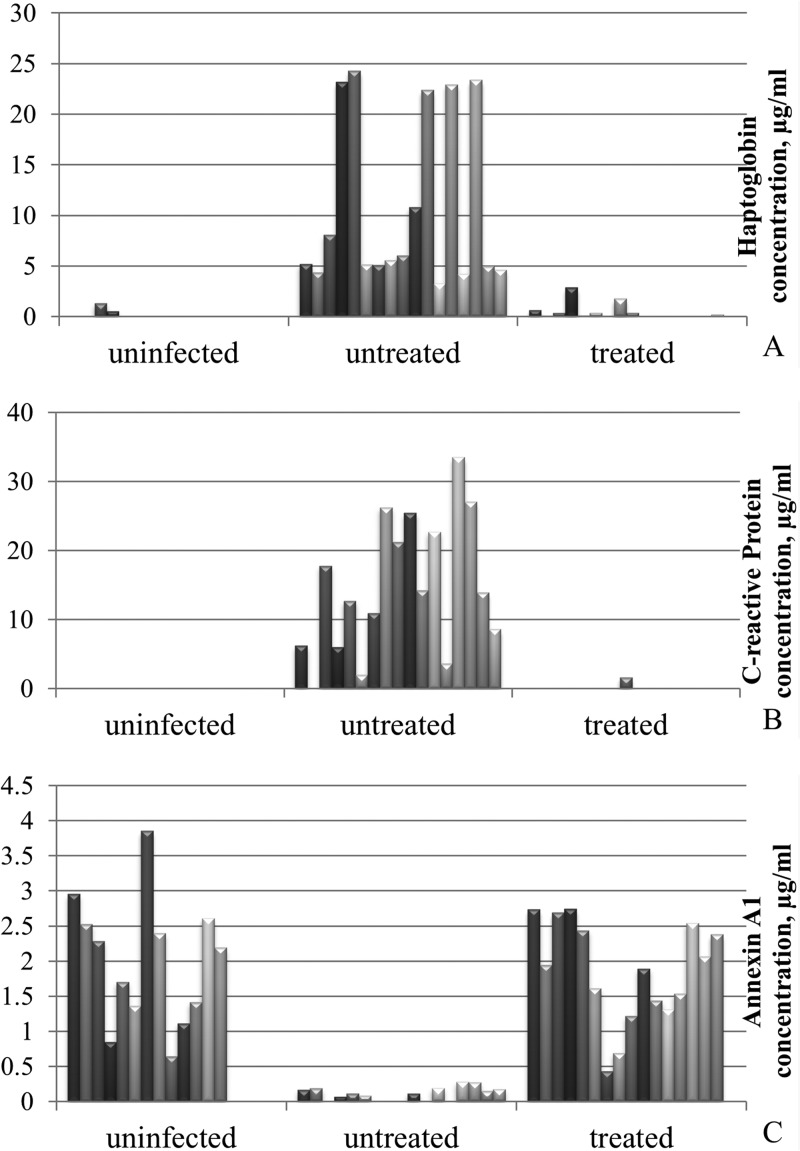
To quantify the amount of Hp, CRP, and Anx A1 in rabbit BALF samples, ELISA was performed for each protein biomarker (Fig. 2). Hp abundance in BALF from infected untreated rabbits was highly pronounced (10.8 ± 2.1 μg/ml), while it was minimal in BALF from uninfected and infected-treated animals (0.14 ± 0.10 μg/ml and 0.44 ± 0.19 μg/ml, respectively). The level of Hp in BALF from rabbits with IPA was significantly greater than either that of uninfected or that of treated animals (P < 0.0001) (Fig. 2A). There was no significant difference in Hp between the uninfected control and treated groups (P > 0.05). CRP was detected in 16 (94%) of 17 samples of infected untreated rabbits (14.84 ± 2.41 μg/ml) and undetectable in 100% of BALF from uninfected control animals (Fig. 2B). In treated animals, CRP was found in 1 (6%) of 16 samples (0.1 ± 0.06 μg/ml). The amount of CRP in BALF from infected untreated rabbits was significantly higher than in the samples from uninfected and infected-treated animals (P < 0.0001). The Anx A1 level was significantly lower in BALF from infected untreated rabbits (0.11 ± 0.02 μg/ml) than in the samples from control (1.99 ± 0.25 μg/ml) and treated animals (1.83 ± 0.19 μg/ml), (P < 0.0001) (Fig. 2C). There was no significant difference found between ravuconazole and amphotericin B treatment in terms of the three biomarkers' profiles. Both drugs led to reduced levels of Hp and CRP and a restored level of Anx A1.
FIG 2.
Histogram of haptoglobin, C-reactive protein, and annexin A1 ELISA results (means of three tests) for BALF from uninfected control (n = 13), infected untreated (n = 17), and infected treated (n = 16) rabbits. (A) Hp concentration is high in BALF from untreated rabbits and minimal in BALF from uninfected and treated animals. (B) CRP level is abundant in BALF from untreated rabbits, while in BALF from treated and uninfected animals it is almost undetectable. (C) Anx A1 is present in BALF from uninfected animals, decreased following IPA infection, and restored after therapy.
Abundance of putative host biomarkers in serum.
The abundance of all three biomarkers in serum was measured by ELISA and compared in cohorts of uninfected control, infected untreated, and infected treated animals (Fig. 3). While serum was collected from all treated rabbits at specified time points (PID 0, 1, 4, 6, and 13), only a few samples or none were collected from the untreated infected animals on or after PID 6 due to euthanasia using humane endpoints.
FIG 3.
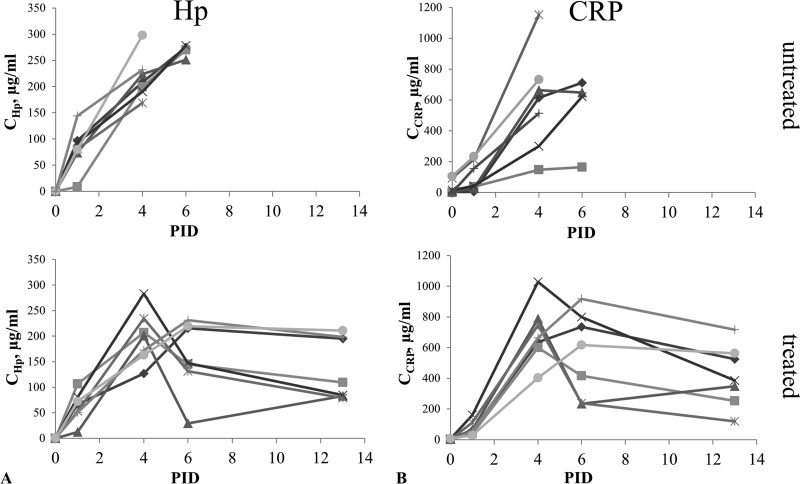
Kinetics of haptoglobin (Hp) and C-reactive protein (CRP) concentrations (means of two tests) in sera of infected untreated (n = 7) and treated (n = 7) rabbits. (A) Hp concentration in sera from untreated and treated rabbits measured on postinoculation days (PID) 0, 1, 4, 6, and 13. (B) CRP concentration in sera from untreated and treated rabbits measured on PID 0, 1, 4, 6, and 13.
Anx A1 was undetectable in sera from all three groups of animals. Hp and CRP were mainly found in sera from both infected untreated and treated rabbits at all time points except day 0 (baseline). As shown in Fig. 3, Hp levels in serum appeared to increase with the progression of infection and decrease in response to treatment. CRP levels in serum showed an upward trend in 6 of 7 untreated rabbits and decreased with antifungal therapy.
The time courses of each protein biomarker were compared between infected untreated and treated groups. Examination of the estimated marginal means indicated that serum Hp concentrations were not significantly different between infected untreated and treated rabbits from PID 0 to 4. On PID 6, however, Hp concentration in the sera of treated animals was significantly lower than that in untreated animals (F = 17.831, P = 0.0001). Serum CRP showed no significant difference between infected untreated and treated rabbits from PID 0 to 6. However, CRP concentration in serum showed a downward trend in response to antifungal treatment from PID 6 to 13.
Effect of Aspergillus fumigatus-secreted proteases on Anx A1.
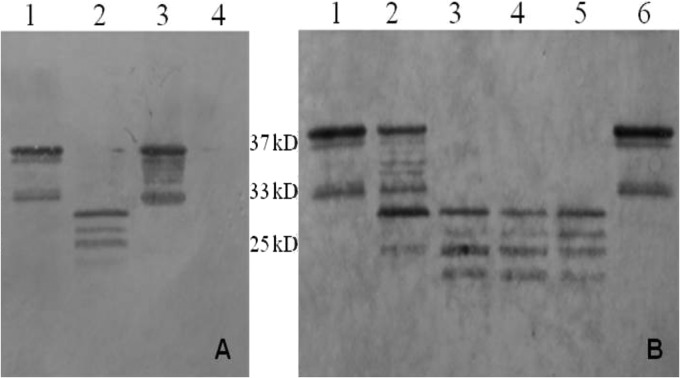
To test whether A. fumigatus plays a role in Anx A1 degradation, supernatant of a 5-ml 72-h-old A. fumigatus liquid culture was filtered and subsequently incubated with purified Anx A1 (Fig. 4A, lane 3) as well as with K562 cell line lysate (Fig. 4A, lane 1) for 2 h at 37°C. Cleavage of Anx A1 was visualized by Western blotting. The analysis showed that after 2 h of incubation Anx A1 was completely degraded (Fig. 4A, lane 4). In the case of K562 cell lysate incubation, low-molecular-weight products were visible (Fig. 4A, lane 2).
FIG 4.
Effect of A. fumigatus culture filtrate on annexin A1 (Anx A1) analyzed by Western blotting. (A) Cleavage of purified Anx A1 (lane 4) and Anx A1 from K562 cell lysate (lane 2) after 2 h of incubation (37°C) with 72-h-old A. fumigatus culture filtrate. In controls, Anx A1 (lane 3) and K562 cell lysate (lane 1) were incubated with phosphate-buffered saline (PBS) instead of culture filtrate. (B) K562 cell lysate was incubated with 2.5, 5, 10, 15, and 20 μl (lanes 1 to 5, respectively) of 72-h-old A. fumigatus culture filtrate for 2 h at 37°C. In control (lane 6), K562 cell lysate was incubated with 20 μl of PBS.
Different volumes of A. fumigatus culture filtrate were incubated with K562 cell line lysate to investigate if different cleavage products could be obtained depending on the volume of culture filtrate added (Fig. 4B). When a volume of culture filtrate greater than 10 μl was added to K562 cell lysate, it resulted in cleavage of Anx A1 into four major low-molecular-weight products (Fig. 4B, lanes 3 to 5). Degradation of Anx A1 also was observed after incubation with 5 μl of culture filtrate, but it was less prominent (Fig. 4B, lane 2). After incubation with 2.5 μl of culture filtrate, the protein remained intact (Fig. 4B, lane 1).
DISCUSSION
The development of host biomarkers of IPA may provide a better understanding of the disease, improve diagnostics, and help monitor treatment response. In this pilot study, we identified several biomarkers that responded to infection and subsequent therapy.
Hp levels were found to be high in BALF from rabbits with IPA infection and significantly decreased after the treatment (Fig. 2A). Hp is an acute-phase protein, which takes part in various processes of immune responses, including activation of the innate and adaptive immune response and tissue repair and regeneration. Hp release induces leukocyte activation, modulation of cytokine patterns, prostaglandin synthesis, and tissue repair (9). Hp has a bacteriostatic role by binding hemoglobin and preventing the utilization of iron by pathogenic bacteria that require iron for their growth (10). Iron uptake is crucial for A. fumigatus growth in the lung environment (11). Thus, high levels of Hp during IPA suggest the host sequestration of iron to prevent its use by A. fumigatus along with an immunomodulatory response to the infection.
A. fumigatus secretes large quantities of hemolysin (12). During IPA, angioinvasion results in hemorrhagic infarction and release of hemoglobin. The importance of the host response of releasing Hp to bind hemoglobin during invasive aspergillosis was recently reported by Goetting et al., who found that plasma iron was significantly increased in infected animals (13). Fusarium infection also has been associated with elevated Hp concentrations in the tears of patients with fusarial keratitis (14). Given the increasingly recognized role of Hp in invasive mycoses, understanding its role as a host-based diagnostic biomarker of therapeutic response is important. Combining an organism-based biomarker, such as galactomannan, for measuring therapeutic response (15) with a pathophysiologically based host biomarker, such as Hp, may further strengthen our ability to assess therapeutic efficacy and predict outcome.
The analysis of rabbit BALF specimens during IPA also revealed a substantial increase of another acute-phase protein, CRP (Fig. 2B). It is noteworthy that CRP levels in BALF from treated rabbits were almost undetectable, which makes it a promising marker to monitor the response to antifungal therapy. At the molecular level, production of CRP is induced by proinflammatory cytokines interleukin-1 (IL-1), IL-6, and IL-17 in the liver in response to microbial infection, tissue injury, and autoimmune disorders (16). Human endothelial cells and murine macrophages exposed to CRP in vitro express chemokine monocyte chemoattractant protein 1 (MCP-1) (17). CRP acts as an opsonin, supporting the ingestion of apoptotic cells by human macrophages, and plays a role in the clearance of apoptotic cells, especially during acute-phase reactions (18, 19). Our data are consistent with other studies that also detected a high level of CRP in BALF during the fungal infections (20, 21).
Finally, our study indicates that Anx A1 levels in BALF from infected rabbits were significantly reduced compared to samples from uninfected and treated animals (Fig. 2C). Anx A1 is a member of a phospholipid and calcium binding family of proteins. Large amounts of this protein were found in BALF from healthy volunteers, while the degradation of Anx A1 was seen in cystic fibrosis patients (22, 23). Both Anx A1 and its biologically active N-terminal peptide are considered to be modulators of systemic anti-inflammatory processes (24, 25). It has been shown that the amounts of Anx A1 increase in response to corticosteroid treatment (26), suggesting an essential role during the inflammatory processes in the lungs. The major site of IPA infection is the lungs, which contains abundant Anx A1 (27). Thus, inactivation of Anx A1 might be critical for A. fumigatus growth, considering its regulatory role in the anti-inflammatory pathway. We demonstrated that culture filtrate form A. fumigatus possessed proteolytic activity toward Anx A1 in vitro (Fig. 4). Our study suggests that secreted proteases of A. fumigatus may play a role in Anx A1 cleavage. However, additional experiments are needed to establish the specificity of the observed cleavage.
The fact that Anx A1 was undetectable in sera from all three groups of animals (uninfected, infected untreated, and infected treated) suggests that it is a biomarker of local inflammation rather than circulating infection. Nevertheless, the state of Anx A1 might still reflect treatment response during IPA using BALF. In contrast, Hp in serum displayed time-dependent patterns associated with IPA development, as well as with response to antifungal therapy, while in healthy normal rabbits it was undetectable (Fig. 3A). The level of circulating Hp increased with the progression of infection and subsequently decreased in treated rabbits with significant difference on PID 6 of the study (P = 0.0001). Therefore, further exploring Hp kinetics may provide additional information on disease progression as well as therapeutic response to antifungals. CRP was found in sera collected from both infected untreated and treated rabbits, but not from uninfected healthy rabbits. There was no significant difference in CRP concentration in serum between untreated and treated groups from PID 0 to 6; however, the level of protein decreased in response to antifungal treatment during the remaining study period (PID 6 to 13) (Fig. 3B).
Our findings suggest that host proteins Hp, CRP, and Anx A1, which play important roles in host defense mechanisms during the pathogenesis of IPA, may have value for monitoring therapeutic effects on patients who were diagnosed with IPA according to the EORTC/MSG diagnosis criteria. Once a definite diagnosis of IPA is made, these biomarkers could be useful in guiding antifungal treatment. However, the response of these biomarkers to both infection and antifungal therapy requires further study.
In conclusion, a proteomic approach was used to identify potential host protein biomarkers of IPA that are responsive to therapy. Detection of the prominent host proteins Hp, CRP, and Anx A1 may have value in monitoring the antifungal therapeutic response in patients with IPA.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI103636 to D.S.P., NINDS grant P30NS046593 to H.L. and by internal funds provided by the Public Health Research Institute.
D. S. Perlin receives research support from Merck, Astellas, and Pfizer and has a patent application on assays for drug-resistant fungal infections. We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 31 March 2014
REFERENCES
- 1.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813–1821. 10.1086/588660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aubry A, Porcher R, Bottero J, Touratier S, Leblanc T, Brethon B, Rousselot P, Raffoux E, Menotti J, Derouin F, Ribaud P, Sulahian A. 2006. Occurrence and kinetics of false-positive Aspergillus galactomannan test results following treatment with beta-lactam antibiotics in patients with hematological disorders. J. Clin. Microbiol. 44:389–394. 10.1128/JCM.44.2.389-394.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petraitiene R, Petraitis V, Witt JR, III, Durkin MM, Bacher JD, Wheat LJ, Walsh TJ. 2011. Galactomannan antigenemia after infusion of gluconate-containing Plasma-Lyte. J. Clin. Microbiol. 49:4330–4332. 10.1128/JCM.05031-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surmont I, Stockman W. 2007. Gluconate-containing intravenous solutions: another cause of false-positive galactomannan assay reactivity. J. Clin. Microbiol. 45:1373. 10.1128/JCM.02373-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh MJ, Fedorova ND, Cagas SE, Hastings S, Fleischmann RD, Peterson SN, Perlin DS, Nierman WC, Pieper R, Momany M. 2012. Development stage-specific proteomic profiling uncovers small, lineage specific proteins most abundant in the Aspergillus fumigatus conidial proteome. Proteome Sci. 10:30. 10.1186/1477-5956-10-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubitschek-Barreira PH, Curty N, Neves GW, Gil C, Lopes-Bezerra LM. 2013. Differential proteomic analysis of Aspergillus fumigatus morphotypes reveals putative drug targets. J. Proteomics 78:522–534. 10.1016/j.jprot.2012.10.022 [DOI] [PubMed] [Google Scholar]
- 7.Francis P, Lee JW, Hoffman A, Peter J, Francesconi A, Bacher J, Shelhamer J, Pizzo P, Walsh TJ. 1994. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 169:356–368. 10.1093/infdis/169.2.356 [DOI] [PubMed] [Google Scholar]
- 8.Cagas SE, Jain MR, Li H, Perlin DS. 2011. Profiling the Aspergillus fumigatus proteome in response to caspofungin. Antimicrob. Agents Chemother. 55:146–154. 10.1128/AAC.00884-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Kinzie E, Berger FG, Lim SK, Baumann H. 2001. Haptoglobin, an inflammation-inducible plasma protein. Redox Rep. 6:379–385. 10.1179/135100001101536580 [DOI] [PubMed] [Google Scholar]
- 10.Eaton JW, Brandt P, Mahoney JR, Lee JT., Jr 1982. Haptoglobin: a natural bacteriostat. Science 215:691–693. 10.1126/science.7036344 [DOI] [PubMed] [Google Scholar]
- 11.Abad A, Fernández-Molina JV, Bikandi J, Ramírez A, Margareto J, Sendino J, Hernando FL, Pontón J, Garaizar J, Rementeria A. 2010. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. Micol. 27:155–182. 10.1016/j.riam.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 12.Wartenberg D, Lapp K, Jacobsen ID, Dahse HM, Kniemeyer O, Heinekamp T, Brakhage AA. 2011. Secretome analysis of Aspergillus fumigatus reveals Asp-hemolysin as a major secreted protein. Int. J. Med. Microbiol. 301:602–611. 10.1016/j.ijmm.2011.04.016 [DOI] [PubMed] [Google Scholar]
- 13.Goetting V, Lee KA, Woods L, Clemons KV, Stevens DA, Tell LA. 2013. Inflammatory marker profiles in an avian experimental model of aspergillosis. Med. Mycol. 51:696–703. 10.3109/13693786.2013.788257 [DOI] [PubMed] [Google Scholar]
- 14.Ananthi S, Venkatesh Prajna N, Lalitha P, Valarnila M, Dharmalingam K. 2013. Pathogen induced changes in the protein profile of human tears from Fusarium keratitis patients. PLoS One 8(1):e53018. 10.1371/journal.pone.0053018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miceli MH, Grazziutti ML, Woods G, Zhao W, Kocoglu MH, Barlogie B, Anaissie E. 2008. Strong correlation between serum Aspergillus galactomannan index and outcome of aspergillosis in patients with hematological cancer: clinical and research implications. Clin. Infect. Dis. 46:1412–1422. 10.1086/528714 [DOI] [PubMed] [Google Scholar]
- 16.Eklund CM. 2009. Proinflammatory cytokines in CRP baseline regulation. Adv. Clin. Chem. 48:111–136. 10.1016/S0065-2423(09)48005-3 [DOI] [PubMed] [Google Scholar]
- 17.Pasceri V, Cheng JS, Willerson JT, Yeh ET, Chang J. 2001. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation 103:2531–2534. 10.1161/01.CIR.103.21.2531 [DOI] [PubMed] [Google Scholar]
- 18.Gershov D, Kim S, Brot N, Elkon KB. 2000. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J. Exp. Med. 192:1353–1364. 10.1084/jem.192.9.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogt B, Führnrohr B, Müller R, Sheriff A. 2007. CRP and the disposal of dying cells: consequences for systemic lupus erythematosus and rheumatoid arthritis. Autoimmunity 40:295–298. 10.1080/08916930701358925 [DOI] [PubMed] [Google Scholar]
- 20.Bulpa PA, Dive AM, Garrino MG, Delos MA, Gonzalez MR, Evrard PA, Glupczynski Y, Installé EJ. 2001. Chronic obstructive pulmonary disease patients with invasive pulmonary aspergillosis: benefits of intensive care? Intensive Care Med. 27:59–67. 10.1007/s001340000768 [DOI] [PubMed] [Google Scholar]
- 21.Gonzales DA, De Torre C, Wang H, Devor CB, Munson PJ, Ying SX, Kern SJ, Petraitiene R, Levens DL, Walsh TJ, Suffredini AF. 2010. Protein expression profiles distinguish between experimental invasive pulmonary aspergillosis and Pseudomonas pneumonia. Proteomics 10:4270–4280. 10.1002/pmic.200900768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsao FH, Meyer KC, Chen X, Rosenthal NS, Hu J. 1998. Degradation of annexin I in bronchoalveolar lavage fluid from patients with cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 18:120–128. 10.1165/ajrcmb.18.1.2808 [DOI] [PubMed] [Google Scholar]
- 23.Bensalem N, Ventura AP, Vallée B, Lipecka J, Tondelier D, Davezac N, Dos Santos A, Perretti M, Fajac A, Sermet-Gaudelus I, Renouil M, Lesure JF, Halgand F, Laprévote O, Edelman A. 2005. Down-regulation of the anti-inflammatory protein annexin A1 in cystic fibrosis knock-out mice and patients. Mol. Cell. Proteomics 4:1591–1601. 10.1074/mcp.M500019-MCP200 [DOI] [PubMed] [Google Scholar]
- 24.D'Acquisto F, Perretti M, Flower RJ. 2008. Annexin-A1: a pivotal regulator of the innate and adaptive immune systems. Br. J. Pharmacol. 155:152–169. 10.1038/bjp.2008.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalli J, Jones CP, Cavalcanti DM, Farsky SH, Perretti M, Rankin SM. 2012. Annexin A1 regulates neutrophil clearance by macrophages in the mouse bone marrow. FASEB J. 26:387–396. 10.1096/fj.11-182089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambrose MP, Hunninghake GW. 1990. Corticosteroids increase lipocortin I in BAL fluid from normal individuals and patients with lung disease. J. Appl. Physiol. 68:1668–1671 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Huang L, Zhao W, Rigas B. 2010. Annexin 1 induced by anti-inflammatory drugs binds to NF-kappaB and inhibits its activation: anticancer effects in vitro and in vivo. Cancer Res. 70:2379–2388. 10.1158/0008-5472.CAN-09-4204 [DOI] [PMC free article] [PubMed] [Google Scholar]