Abstract
Avibactam is a novel non-β-lactam β-lactamase inhibitor that is currently undergoing phase 3 clinical trials in combination with ceftazidime. Ceftazidime is hydrolyzed by a broad range of β-lactamases, but avibactam is able to inhibit the majority of these enzymes. The studies described here attempt to provide insight into the amount of avibactam required to suppress bacterial growth in an environment where the concentrations of both agents are varying as they would when administered to humans. Following the simulation of a single intravenous dose of the drug, ceftazidime alone had no effect on any test organism, but a ceftazidime-avibactam combination resulted in rapid killing of all of the strains, with growth suppressed for the 8 h of the study. For seven of eight strains, this was achieved with a 1-g–250-mg profile, but a 2-g–500-mg profile was necessary to completely suppress a high-level-AmpC-producing isolate. When ceftazidime was infused continuously for 24 h with a single bolus dose of avibactam, rapid killing of all of the strains was again observed, with growth suppressed for 10 to >24 h. Regrowth appeared to commence once the avibactam concentration dropped below a critical concentration of approximately 0.3 μg/ml. In a third series of studies, ceftazidime was administered every 8 h for 24 h with avibactam administered at fixed concentrations for short periods during each ceftazidime dose profile. Simulating a 1-g dose of ceftazidime, an avibactam pulse of >0.25 and <0.5 μg/ml was required to suppress growth for 24 h.
INTRODUCTION
Avibactam, formerly NXL104 or AVE1330A, is the first of a new class of non-β-lactam β-lactamase inhibitors, referred to as diazabicyclooctanes (1). It displays a broad spectrum of inhibitory activity against both class A and class C β-lactamases, including Klebsiella pneumoniae carbapenemase (KPC) enzymes (2), the AmpC β-lactamase of Pseudomonas aeruginosa (3), and extended-spectrum β-lactamases such as TEM, SHV, and CTX-M variants (4, 5). In studies with isolated enzymes, avibactam inactivates β-lactamases at low 50% inhibitory concentrations and with low turnover numbers (6, 7). It also inhibits some class D β-lactamases, OXA-48, for example (8). Avibactam has little intrinsic antibacterial activity but efficiently protects β-lactams from β-lactamase-catalyzed hydrolysis in a range of members of the family Enterobacteriaceae and in P. aeruginosa (3–5, 9). That a combination of ceftazidime and avibactam protects against bacterial infections by β-lactamase-producing bacteria has been demonstrated in animal models (10, 11), and two successful phase 2 human studies have been reported (12, 13).
Like the pharmacodynamic (PD) indices of other cephalosporins (14) and β-lactams generally (15), that of ceftazidime is the time during which its free (non-protein-bound) concentration exceeds the MIC for the infecting pathogen (fT>MIC) (14–19). However, little is known about β-lactam–β-lactamase-inhibitor pharmacokinetic (PK)-PD relationships (20–22), which is a prerequisite for the optimum design of dosage regimens.
The objective of this study was to determine the effect of PK-like dynamic changes in avibactam concentrations on the PD of ceftazidime plus avibactam against examples of ceftazidime-resistant, β-lactamase-positive Enterobacteriaceae by using an in vitro hollow-fiber infection model.
(Some of this work has been reported previously in abstract form [23–25].)
MATERIALS AND METHODS
Compounds.
Avibactam was synthesized in the laboratories of Novexel SA (Paris, France). Ceftazidime was purchased from Sandoz (France).
Bacterial isolates.
The eight Enterobacteriaceae strains used in this study were all clinical isolates. The β-lactamases produced by these strains and their susceptibilities to the agents under test are reported in Table 1.
TABLE 1.
In vitro susceptibilities of strains used in hollow-fiber studies
| Strain | β-Lactamase(s) | Regimensa | MIC (μg/ml) |
|||
|---|---|---|---|---|---|---|
| Ceftazidime | Avibactam | Ceftazidime + 4 μg/ml avibactam | Ceftazidime-avibactam at 4:1 | |||
| E. cloacae 293HT96 | Stably derepressed AmpC | 1, 2, 3 | >128 | >32 | 4 | 8:2 |
| K. pneumoniae Tunisie K4 | CTX-M-15, TEM-1b, OXA-1b | 1, 2 | >128 | >32 | 1 | 4:1 |
| K. pneumoniae 283KB5 | SHV-11, AmpC | 1, 2 | >128 | >32 | 0.5 | NTc |
| K. pneumoniae 283CF5 | SHV-5 | 1, 2 | 64 | >32 | 2 | NT |
| K. pneumoniae 181 | SHV-5, TEM-10 | 1, 2 | >128 | >32 | 2 | 8:2 |
| K. pneumoniae 236 | SHV-5, TEM-10 | 1, 2 | >128 | >32 | 2 | 1:0.25 |
| K. pneumoniae 5761 | KPC-3 | 1, 3 | >128 | >32 | 4 | 8:2 |
| Citrobacter freundii 261GR3 | AmpC, TEM-1b | 1, 2 | >128 | >32 | ≤0.125 | 2:0.5 |
Dosing regimens for which this organism was used (see the text for further details).
β-Lactamase does not hydrolyze ceftazidime to any great extent.
NT, not tested.
Susceptibility testing.
MICs were determined by using Clinical and Laboratory Standards Institute methods for antibiotic susceptibility testing (26) with cation-adjusted Mueller-Hinton broth (MHB) and incubation at 35°C for 16 to 18 h. For ceftazidime-avibactam combinations, results were obtained by two methods. (i) In the CLSI-approved method, using doubling dilutions of ceftazidime in the presence of a fixed 4 μg/ml avibactam, the MIC is recorded as the concentration of ceftazidime at the endpoint. (ii) In the second method, doubling dilutions of a fixed 4:1 ceftazidime-avibactam ratio are used and the MIC is recorded as the concentrations of both ceftazidime and avibactam at the endpoint. The MIC endpoint was defined as the lowest concentration that inhibited all of the visual growth. Susceptibility testing was performed in triplicate, and modal MICs are reported.
Bioassay.
Avibactam was assayed by using 150 ml of Antibiotic Agar Medium No. 1 (Merck) inoculated with 1% (vol/vol) of an overnight culture of S. aureus 011SJ3 in MHB with amoxicillin diluted in dimethyl sulfoxide at a final concentration of 40 mg/liter poured into square plastic plates (25 by 25 cm; Nunc). After cooling and solidification, 4-mm wells were made in the agar to take 0.025-ml samples. Plates were prepared on the day of the assay, and short-term storage was at 4°C. Each sample was plated in duplicate on two separate plates, and after 18 h of incubation at 37°C, diameters of inhibition zones were measured and concentrations were calculated by using a standard curve of log concentration against zone diameter. The assay was linear over an avibactam concentration range of 0.25 to 64 μg/ml.
For the ceftazidime assay, 150 ml of Antibiotic Agar Medium No. 2 (Merck) was inoculated with 2% (vol/vol) of an overnight culture of Escherichia coli 250UC5 in MHB and the plates were prepared and assays were run as for avibactam. The assay was linear over a ceftazidime concentration range of 1 to 128 μg/ml.
Hollow-fiber infection model.
The hollow-fiber system was set up as described by Tam et al. (27), by using cellulosic cartridges (FiberCell Systems, Inc., Frederick, MD) and Masterflex peristaltic pumps from the Cole-Parmer Instrument Co. (Vernon Hills, IL). An automated syringe pump delivers the antibiotics into a 150-ml central reservoir in the required amounts on the desired schedule of administration. Approximately 30 ml of the culture under test was loaded into the peripheral compartment to give a final inoculum of approximately 105 CFU/ml. Fresh medium (with or without dissolved test compounds) was kept in circulation by means of a peristaltic pump. Samples were taken from the peripheral compartment at different time points, and the viable bacterial count was determined by serial 2-fold dilutions on Mueller-Hinton agar plates with a limit of detection of 20 CFU/ml. Samples were also taken from the central compartment and stored at −20°C for later analysis by bioassay.
Dose regimen 1.
For dose regimen 1, a ceftazidime-avibactam combination was administered to simulate a single human dose equivalent to a 30-min infusion. The avibactam human-like concentration-time profile was obtained by imposing biexponential elimination by using the half-lives observed in healthy volunteers following a 30-min infusion of a 500-mg dose (t1/2α = 0.16 h, t1/2β = 2.0 h) (28). The same biexponential elimination rate was used to model ceftazidime. The doses used were 2 g ceftazidime (Cmax = 105 μg/ml) plus 500 mg avibactam (Cmax = 24 μg/ml) and 1 g ceftazidime (Cmax = 52 μg/ml) plus 250 mg avibactam (Cmax = 12 μg/ml).
Dose regimen 2.
For dose regimen 2, the concentration of ceftazidime was set at a constant 16 or 8 μg/ml to be in excess of the MIC of ceftazidime-avibactam but below that of ceftazidime alone for all of the strains. In combination with the constant ceftazidime concentration, two different regimens of avibactam were used so as to achieve similar avibactam area under the concentration-time curve to infinity (AUC) values, as follows: (i) a 24-h continuous constant-rate infusion and (ii) a single simulated human-like profile. The 500-mg avibactam profile from dose regimen 1 was used here. For each strain, the AUC was calculated by using WinNonlin version 4.1.
In both test cartridges, at time zero, a mixture of ceftazidime and avibactam was injected into the central compartment at the doses described below. For the cartridge where both components were infused continuously, a flow of fresh MHB containing an appropriate concentration of both ceftazidime (8 or 16 μg/ml) and avibactam (1, 2, or 4 μg/ml) was infused for 24 h. For the cartridge with the single-dose avibactam profile, fresh MHB containing an appropriate concentration of ceftazidime was infused to generate the simulated avibactam human terminal half-life (t1/2) of 2 h. In order to maintain an isovolumetric environment, MHB was pumped from the central reservoir at a fixed rate. Test strains were injected and confined to the hollow-fiber cartridge, and test compounds and fresh medium were exchanged between the central reservoir and the hollow-fiber cartridge by diffusion through a semipermeable membrane. Drug infusions were terminated at 24 h, but viable counts were monitored from 0 to 24 h in some experiments and from 0 to 48 h in others. The dose-organism combinations used in these studies (see Table 3) were single runs.
TABLE 3.
Growth suppression windowsa obtained for 24-h ceftazidime infusion with avibactam infused for 24 h or administered once at time zero
| Strain | Constant ceftazidime-avibactam infusionb |
Ceftazidime constant infusion, avibactam single dose at t = 0 hc |
||||
|---|---|---|---|---|---|---|
| Ceftazidime infused (μg/ml) | Avibactam infused (μg/ml) | Growth suppression window (h) | Ceftazidime infused (μg/ml) | Avibactam Cmax (μg/ml) | Growth suppression window (h) | |
| E. cloacae 293HT96 | 8 | 4 | 22 | 8 | 50 | 24 |
| 16 | 4 | 24 | 16 | 60 | 14 | |
| 8 | 4 | 24 | 8 | 20 | 24 | |
| 8 | 2 | 24 | 8 | 30 | 16 | |
| 8 | 2 | 24 | 8 | 30 | 16 | |
| 8 | 2 | 24 | 8 | 30 | 24 | |
| K. pneumoniae 181 | 16 | 4 | 24 | 8 | 60 | 10 |
| K. pneumoniae 236 | 16 | 4 | 24 | 8 | 60 | 24 |
| K. pneumoniae 283CF5 | 16 | 2 | 24 | 16 | 60 | 24 |
| 16 | 4 | 24 | 8 | 60 | 24 | |
| 8 | 2 | 24 | 8 | 30 | 24 | |
| 8 | 1 | 24 | 8 | 12 | 24 | |
| K. pneumoniae 283KB5 | 8 | 4 | 24 | 8 | 20 | 24 |
| 8 | 2 | 24 | 8 | 30 | 24 | |
| 8 | 1 | 24 | 8 | 12 | 24 | |
| Citrobacter freundii 261GR3 | 8 | 4 | 24 | 8 | 20 | 24 |
The growth suppression window is the period of time for which the viable count remained at least 103-fold lower than the starting inoculum.
Both ceftazidime and avibactam were infused for 24 h at the concentrations listed.
Ceftazidime was infused for 24 h at the concentrations listed, and avibactam was administered as a single dose with a t½ of 2 h.
In the paired experiments, where a continuous infusion of ceftazidime (8 or 16 μg/ml) was administered with the avibactam dose simulating a human biexponential profile, the avibactam Cmax values were somewhat higher than those in human volunteers reported by Merdjan et al. (28), as avibactam was administered such that the 24-h AUC equaled the AUC in the paired study where avibactam was infused for 24 h. Thus, for example, where the 24-h infusion experiment employed avibactam at a constant 4 μg/ml, the profile in the parallel single-dose experiment used an avibactam Cmax of 60 μg/ml; for a 1-μg/ml constant infusion, the single-dose experiment used an avibactam Cmax of 12 μg/ml.
Experiments in which human-like PK profiles of avibactam were simulated were used to estimate the threshold concentration (CT) of avibactam during the exponentially declining phase below which inhibition of β-lactamase was lost, as inferred from the observation of bacterial regrowth in the presence of a continuous concentration of ceftazidime.
Dose regimen 3.
For dose regimen 3, ceftazidime was administered to simulate a human-like profile (30-min infusion) following a dose of either 1 g (Cmax = 52 μg/ml) or 2 g (Cmax = 105 μg/ml) administered every 8 h (q8h) for 1 day, with avibactam (i) infused continuously at a fixed concentration over the 24-h period, (ii) infused at a fixed concentration for 6 h at the start of each ceftazidime dose administration, (iii) infused at a fixed concentration for 4.5 h at the start of each ceftazidime dose administration, or (iv) infused at a fixed concentration for 3 h at the start of each ceftazidime dose administration. At the end of each infusion period, the avibactam reservoir was replaced with a fresh MHB reservoir, clearing the avibactam with a t1/2 of 2 h.
Ceftazidime 1-g growth suppression windows are averages of two or three results; ceftazidime 2-g growth suppression windows are single datum points. In these experiments, where ceftazidime concentrations simulated a human profile and avibactam was infused at a constant concentration, a slightly different CT was measured, designated CTQ8, to differentiate it from the above CT. CTQ8 was the minimum constant-infusion concentration of avibactam that suppressed bacterial growth for the 24-h duration of the study.
Terminology.
In all of the studies of the ceftazidime-avibactam combination, rapid bactericidal killing was observed (>3-log reduction of the viable count) and growth remained at least 103-fold lower than the starting inoculum for a period of time (Fig. 1 shows a representative example). Bacterial regrowth occurred when the avibactam concentration declined. The bacterial regrowth was interpreted in terms of two concepts, (i) a critical concentration, CT, of avibactam and (ii) a time period of growth suppression termed the “growth suppression window.” These two parameters were defined and measured as follows.
FIG 1.
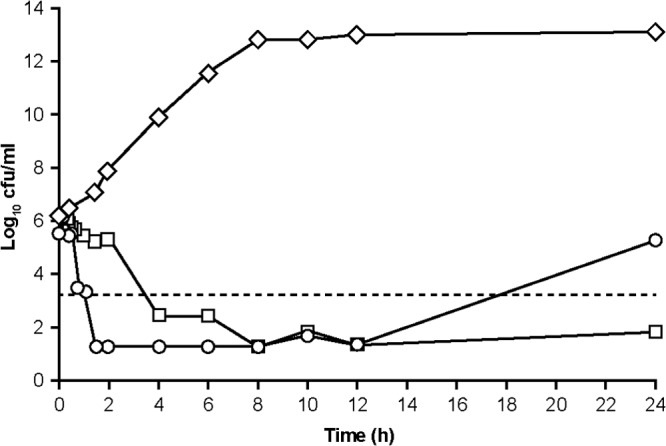
Responses of E. cloacae 293HT96 to continuous infusion of ceftazidime combined with two different concentration-time profiles of avibactam in the hollow-fiber model. Shown are results obtained with an untreated growth control (open diamonds), continuous infusion of ceftazidime (8.2 μg/ml) and avibactam (1.6 μg/ml) (open squares), and continuous infusion of ceftazidime (8.2 μg/ml) with a single-dose profile of avibactam (Cmax, 31 μg/ml at 0.5 h) (open circles) and 99.9% bacterial killing (broken line).
Critical concentration (CT) of avibactam.
This approach was to define a “critical concentration” (CT) of avibactam achieved during its exponential decline phase in the presence of a constant ceftazidime concentration. The CT was defined as the minimum concentration of avibactam able to suppress the growth of a β-lactamase-producing bacterium as judged by the concentration of avibactam in the hollow-fiber system at the time point when regrowth occurred. CT values were experimentally estimated by extrapolation from the exponential-decline curves, because they occurred at times later than the decrease in avibactam concentrations to below the limit of quantitation.
The magnitude of CT for Enterobacteriaceae was estimated from hollow-fiber experiments with three β-lactamase-producing isolates: Enterobacter cloacae 293HT96, K. pneumoniae 283CF5, and K. pneumoniae Tunisie K4 (Tables 1 and 2). In three of the experiments used for these estimations, ceftazidime was maintained at a constant background concentration of about 8 mg/liter while avibactam was instilled with simulated human-PK-like profiles with a Cmax of 9, 31, or 37 mg/liter and exponential-decline half-lives of 2 to 3 h. Viable counts were monitored in the perfused compartment, starting with inocula of 1 × 105 to 3 × 105 CFU/ml at time zero. In all three experiments, bacterial counts declined to below detectable in about 2 h and stayed undetectable for a further 10 h (i.e., t = 12 h). After that, samples were not taken until t = 24 h, by which time growth had restarted (Fig. 1). The magnitude of CT was estimated as being equal to or lower than the concentration of avibactam remaining in the hollow-fiber system at the time point at which growth suppression was last experimentally demonstrated. That time point was t = 12 h in three experiments (as was the case in the experiment shown in Fig. 1) but t = 20 h in one experiment with E. cloacae 293HT96 (Fig. 2). As stated above, in these estimations, the concentration of avibactam at which regrowth occurred was below the limit of quantification, and so the concentration at the given time point was estimated by extrapolation of the monoexponential decline of the terminal phase. Moreover, the estimate was made at the last time point when growth was experimentally confirmed to be suppressed, which was an indeterminate time before growth actually recurred. The concentration of avibactam estimated by this method was thus a maximum, so that CT is reported here as less than or equal to the magnitude estimated at the given time point. Table 2 shows four estimated CT values and summarizes the experimental conditions under which the estimations were made.
TABLE 2.
Estimates of the avibactam CT in hollow-fiber experiments
| Strain | Ceftazidime |
Avibactam |
Time (h) when CT was estimated | Estimated CT (μg/ml) | ||
|---|---|---|---|---|---|---|
| Constant concn (μg/ml) | AUC0-24h (mg · h · liter−1) | Cmax (μg/ml) | AUC0-24h (mg · h · liter−1) | |||
| E. cloacaea 293HT96 | 8.2 | 195 | 31.0 | 54.7 | 12 | ≤0.15b |
| K. pneumoniaea 283CF5 | 8.3 | 198 | 8.9 | 16.4 | 12 | ≤0.22b |
| K. pneumoniaea Tunisie K4 | 9.4–9.8 | 232 | 36.9 | 63.8 | 12 | ≤0.28b |
| E. cloacaec 293HT96 | 20 | 480 | ∼60 | 126 | 18–20 | ∼0.2 |
Concentrations were estimated at the 12-h sampling time (last sample showing growth suppression [Fig. 1]). Regrowth started at some time between the 12- and 24-h sampling times.
The magnitude of CT is expressed as less than or equal to the stated value, as estimates were made at 12 h, whereas regrowth occurred between 12 and 24 h (Fig. 1).
CT was estimated at 18 to 20 h, as the 2-h sampling times allowed greater precision in identifying the time at which regrowth occurred (Fig. 2). However, the declining avibactam concentrations were modeled rather than measured in this experiment (23), and so the estimate of CT is approximate.
FIG 2.

Responses of E. cloacae 293HT96 to continuous infusion of ceftazidime combined with two different concentration-time profiles of avibactam in the hollow-fiber model with monitoring of growth during the critical regrowth period of 18 to 24 h. Shown are results obtained with an untreated growth control (open diamonds), continuous infusion of ceftazidime (16 μg/ml) and avibactam (4 μg/ml) (open squares), and continuous infusion of ceftazidime (16 μg/ml) with a single-dose profile of avibactam (Cmax, ∼55 μg/ml at 0.5 h) (open circles) and 99.9% bacterial killing (broken line).
Growth suppression window.
For the ceftazidime-avibactam treatment regimens used in this study, the bacterial viable count fell rapidly and remained low for some time. The period of time for which the viable count remained at least 103-fold lower than the starting inoculum was defined as the growth suppression window.
RESULTS
The organisms used, the enzymes they produced, and their susceptibilities to ceftazidime and ceftazidime-avibactam are given in Table 1. Strain selection was based on high ceftazidime MICs (≥64 μg/ml) and a range of MICs of the ceftazidime-avibactam combination.
Regimen 1.
In the regimen 1 experiments (data not shown), hollow-fiber studies simulating a single dose of ceftazidime-avibactam (1 g plus 250 mg) showed that the combination was rapidly microbicidal against all of the test organisms, reducing the viable count from ∼106 CFU/ml to below the level of detection (<102 CFU/ml). With the exception of E. cloacae 293HT96, growth was held below detectable levels for the 8 h of the experiment. In the case of E. cloacae 293HT96, slight growth (103 to 104 CFU/ml was detected at between 6 and 8 h) postdosing. With the higher-dose simulation (2 g plus 500 mg), the growth of all of the organisms was held below the limit of detection for the 8-h period.
Regimen 2.
In the regimen 2 experiments, each organism was studied against one to six dosing regimens. In experiments where both ceftazidime and avibactam were continuously infused and with ceftazidime at 8 or 16 μg/ml, avibactam at a 1-μg/ml or higher concentration resulted in a growth suppression window of 24 h against all of the isolates (data not shown).
In the paired experiments, where a continuous infusion of ceftazidime (8 or 16 μg/ml) was administered and the concentration of avibactam rose to a peak and then declined biexponentially, rapid killing of all of the strains was again observed, with growth suppression windows of 10 to 24 h for all of the avibactam profiles with a 12-μg/ml or higher Cmax (Table 3).
In the presence of adequate inhibition of β-lactamases, the PD of a β-lactam–β-lactamase inhibitor combination is predicted to revert to the PD of the β-lactam partner (20). With simulated bolus doses of avibactam, the variable growth suppression windows observed indicate that β-lactamase inhibition is inadequate when the avibactam concentration falls below a certain critical CT. This was the basis of the measurements described in Table 2. From the limited data available from the regimen 2 studies, the CT was ≤0.5 μg/ml but to investigate the critical threshold under different avibactam and ceftazidime exposure conditions, a further series of studies was conducted.
Regimen 3.
In the regimen 3 experiments (Table 4), instead of the critical concentration of avibactam being estimated after the attainment of its Cmax, the critical concentration was investigated on the basis of short periods at a constant concentration. Using roughly the equivalent of a 1-g dose of ceftazidime q8h in adults (Cmax, 52 μg/ml), a constant 0.25 μg/ml avibactam for 24 h gave a growth suppression window of 4 to 6 h, while a constant 0.5 μg/ml avibactam gave a growth suppression window of >24 h (Table 4). The equivalent of 2 g of ceftazidime q8h (Cmax, 105 μg/ml) gave a growth suppression window of 6 h with 0.1 μg/ml avibactam and 8 h with 0.25 μg/ml avibactam infused for 24 h. On the basis of these data, the CTQ8 (where the superscript indicates this critical concentration of avibactam estimated in the background of rising-and-falling ceftazidime concentrations from the CT obtained during the exponential decline in the avibactam concentration following a peak in the background of a constant ceftazidime concentration) must be >0.25 μg/ml but ≤0.5 μg/ml.
TABLE 4.
Ceftazidime peaka and exponential declineb cycled q8h for 24 h with avibactam infused at fixed concentrations for various time periods
| Strain | Ceftazidime Cmax (μg/ml) | Avibactam infusion concn (μg/ml) | Avibactam infusion time (h) | Growth suppression windowc (h) |
|---|---|---|---|---|
| E. cloacae 293HT96d | 52 | 0.25 | 24 | 4–6 |
| 0.5 | 3 | 8 | ||
| 4.5 | 7–11 | |||
| 6 | 6–8 | |||
| 24 | >24 | |||
| 105 | 0.1 | 24 | 6 | |
| 0.25 | 24 | 8 | ||
| K. pneumoniae 5761e | 105 | 0.5 | 4.5 | 12 |
| 1 | 4.5 | >24 |
t = 30 min.
t½ of 2 h.
The growth suppression window is the period of time for which the viable count remained at least 103-fold lower than the starting inoculum. Ceftazidime 52-μg/ml growth suppression windows are averages of two or three results; ceftazidime 105-μg/ml growth suppression windows are single datum points.
Produces AmpC; ceftazidime-avibactam MIC = 4 μg/ml.
Produces KPC-3; ceftazidime-avibactam MIC = 4 μg/ml.
When ceftazidime was administered at the equivalent of 1 g q8h and avibactam was infused for part of each dose interval at a fixed concentration of 0.5 μg/ml against AmpC-producing E. cloacae, the growth suppression window was 8 h with a 3-h avibactam infusion, 7 to 11 h with a 4.5-h avibactam infusion, and 6 to 8 h with a 6-h avibactam infusion. On the basis of these data, the in vitro continuous-infusion CTQ8 was >0.5 μg/ml when the background dose of ceftazidime was equivalent to 1 g q8h.
When ceftazidime was administered at the equivalent of 2 g q8h and avibactam was infused for 4.5 h at 0.5 or 1 μg/ml at the start of each dose interval (Table 4), the growth suppression window was 12 to 24 h. On the basis of these data, it appears that the in vitro intermittent continuous-infusion CTQ8 in the background of a dose of ceftazidime of 2 g q8h would be judged to be ≤0.5 μg/ml.
DISCUSSION
The correlation between the two methods of determining combination MICs in vitro was fairly poor (Table 1), but the 4:1 ratio data showed that lower inhibitor concentrations mostly resulted in higher ceftazidime MICs for this collection of isolates. These in vitro assays report the results of testing fixed concentrations of agents over a 24-h period, whereas the hollow-fiber studies reported here were intended to give a better view of the pharmacodynamic properties of the combination.
For β-lactams, the PK-PD index that best defines efficacy is the fT>MIC (14, 29). This is defined as the amount of time during the period between doses for which the unbound concentration of the drug is at or above the MIC. fT>MIC is expressed as a percentage of the interdose period, and an ∼50% fT>MIC is usually considered an appropriate target for achieving antibacterial efficacy with a cephalosporin in vivo (30, 31). For ceftazidime, a 50% fT>MIC has proved effective clinically in the treatment of Gram-negative nosocomial pneumonia (19). When the β-lactam is coadministered with an adequate concentration of a β-lactamase inhibitor, the PD of the combination revert to the PD of the β-lactam partner (20). Avibactam has no meaningful antibacterial activity alone against the organisms under test, so the MIC cannot be used to determine what constitutes an adequate concentration. We infer that there must be some CT of avibactam below which the inhibitor is unable to protect the β-lactam from hydrolysis. On the basis of hollow-fiber experiments, Louie et al. (32) reported that time above the CT was the avibactam variable dynamically linked with cell killing and resistance suppression when combined with the cephalosporin ceftaroline against β-lactamase-producing E. cloacae and K. pneumoniae. That would fit with the observation in the present study that avibactam, when not replenished by repeated dosing (or continuous infusion), appears to allow the return of β-lactamase activity and growth in the presence of “unprotected” ceftazidime (Fig. 1 and 2).
In a few of the studies reported here, the growth suppression window was smaller for the avibactam bolus dose than for the avibactam infusion, consistent with the findings of Louie et al. (32) with ceftaroline, that T>[threshold] rather than AUC/[threshold] is the critical PD variable. In the growth suppression window experiments (summarized in Tables 3 and 4), regrowth was clearly occurring when avibactam dropped below a critical CT. In the present studies, the avibactam CT was ≤0.3 μg/ml (Table 2) and the CTQ8 (in the background of ceftazidime PK representative of the human dose of 2 g) was <0.5 μg/ml, demonstrating good agreement. Furthermore, some recent data reported by Berkhout et al. from neutropenic mouse lung and thigh P. aeruginosa infection models confirmed that the PK-PD index that best correlated with the avibactam effect was 20 to 40% of the time above a CT of 1 μg/ml (33, 34).
Although not explored in the work reported here, the β-lactam combined with avibactam is assumed to be critical and the magnitude of CT below which the protection of ceftazidime is lost may be different for other β-lactams.
The magnitude of CT is likely to be dependent on the MIC for the organism, the inoculum, and the dose of β-lactam used. These studies used a starting inoculum of about 106 CFU/ml, representing a total bacterial burden of approximately 3 × 107 CFU/ml, which could be regarded as representative of a moderate rather than a severe infection, where an inoculum of ≥108 may be more clinically relevant. On the other hand, the hollow-fiber system employed for these studies was impermeable to the β-lactamases produced by the bacteria, resulting in an unnatural accumulation of enzyme over the course of the study, whereas the enzyme produced in a clinical environment might reasonably be expected to be cleared from the site of infection. Thus, although the low inoculum concentration might suggest that the CT and CTQ8 reported here are underestimated for mimicking infections characterized by a high total human bacterial load, the unnatural accumulation of β-lactamase would tend to cause overestimation of the CT and CTQ8 for the test inoculum.
The idea of defining a critical CT below which adequate inhibition of β-lactamase is lost is rather different from another approach to PD that attempts to reconstruct the MIC as an “instantaneous MIC” dependent on the concentration of the β-lactamase inhibitor (35). In that case, one could revert to calculating target attainment in order to help guide dosing by just considering the time during which the free drug concentration exceeds the instantaneous MIC, as long as there is a good correlation between an in vitro measured MIC and the instantaneous MIC. The alternative approach of the critical-concentration concept would be to calculate achievement of the critical-concentration β-lactamase inhibitor PD target simultaneously with the achievement of the β-lactam target. In both cases, as is the case with the fT>MIC for a β-lactam, the PD “target” would be a compromise to cover as many organisms as possible on the basis of simple in vitro MIC testing but could not practically be tailored to one isolate at a time.
In many (but not all) of these studies, the organism was examined at the end of the test to determine whether reduced-susceptibility variants had developed. In all of the cases, the susceptibility of the organism was unchanged from that of the starting culture, but longer incubation times and/or higher starting inoculum concentrations are needed to fully evaluate the development of resistance to this combination.
ACKNOWLEDGMENTS
We thank Celine Dietrich and Eline Rouault-Hardoin for their technical assistance during this study. We also thank R. Bonomo and G. Arlet for supplying some of the strains used in this study.
This study was funded by Novexel. Ceftazidime-avibactam is now being developed by AstraZeneca and Forest-Cerexa. K.C. and P.L. are ex-employees of Novexel and received compensation fees for services in relation to preparing the manuscript, which was funded by AstraZeneca. M.B., C.M., and H.M. are ex-employees of Novexel and W.W.N. is an employee of AstraZeneca.
Footnotes
Published ahead of print 31 March 2014
REFERENCES
- 1.Coleman K. 2011. Diazabicyclooctanes (DBOs): a potent new class of non-β-lactam β-lactamase inhibitors. Curr. Opin. Microbiol. 14:550–555. 10.1016/j.mib.2011.07.026 [DOI] [PubMed] [Google Scholar]
- 2.Stachyra T, Levasseur P, Pechereau MC, Girard AM, Claudon M, Miossec C, Black MT. 2009. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J. Antimicrob. Chemother. 64:326–329. 10.1093/jac/dkp197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mushtaq S, Warner M, Livermore DM. 2010. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J. Antimicrob. Chemother. 65:2376–2381. 10.1093/jac/dkq306 [DOI] [PubMed] [Google Scholar]
- 4.Bonnefoy A, Dupuis-Hamelin C, Steier V, Delachaume C, Seys C, Stachyra T, Fairley M, Guitton M, Lampilas M. 2004. In vitro activity of AVE1330A, an innovative broad-spectrum non-β-lactam β-lactamase inhibitor. J. Antimicrob. Chemother. 54:410–417. 10.1093/jac/dkh358 [DOI] [PubMed] [Google Scholar]
- 5.Lagacé-Wiens PR, Tailor F, Simner P, DeCorby M, Karlowsky JA, Walkty A, Hoban DJ, Zhanel GG. 2011. Activity of NXL104 in combination with β-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum β-lactamases and class C β-lactamases. Antimicrob. Agents Chemother. 55:2434–2437. 10.1128/AAC.01722-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stachyra T, Pechereau MC, Bruneau JM, Claudon M, Frère JM, Miossec C, Coleman K, Black MT. 2010. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-β-lactam β-lactamase inhibitor. Antimicrob. Agents Chemother. 54:5132–5138. 10.1128/AAC.00568-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehmann DE, Jahic H, Ross PL, Gu R-F, Hu J, Kern G, Walkup GK, Fisher SL. 2012. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc. Natl. Acad. Sci. U. S. A. 109:11663–11668. 10.1073/pnas.1205073109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 55:390–394. 10.1128/AAC.00756-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endimiani A, Choudhary Y, Bonomo RA. 2009. In vitro activity of NXL104 in combination with β-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob. Agents Chemother. 53:3599–3601. 10.1128/AAC.00641-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endimiani A, Hujer KM, Hujer AM, Pulse ME, Weiss WJ, Bonomo RA. 2011. Evaluation of ceftazidime and NXL104 in two murine models of infection due to KPC-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 55:82–85. 10.1128/AAC.01198-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crandon JL, Schuck VJ, Banevicius MA, Beaudoin ME, Nichols WW, Tanudra MA, Nicolau DP. 2012. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 56:6137-6146. 10.1128/AAC.00851-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. 2013. Comparative study of the efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, phase II trial. J. Antimicrob. Chemother. 68:1183–1192. 10.1093/jac/dks523 [DOI] [PubMed] [Google Scholar]
- 13.Vazquez JA, González Patzán LD, Stricklin D, Duttaroy DD, Kreidly Z, Lipka J, Sable C. 2012. Efficacy and safety of ceftazidime—avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr. Med. Res. Opin. 28:1921–1931. 10.1185/03007995.2012.748653 [DOI] [PubMed] [Google Scholar]
- 14.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North Am. 17:479–501. 10.1016/S0891-5520(03)00065-5 [DOI] [PubMed] [Google Scholar]
- 15.Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug'. Nat. Rev. Microbiol. 2:289–300. 10.1038/nrmicro862 [DOI] [PubMed] [Google Scholar]
- 16.Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261–268. 10.1016/S0924-8579(02)00022-5 [DOI] [PubMed] [Google Scholar]
- 17.Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. 2008. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 61:235–237. 10.1093/jac/dkm476 [DOI] [PubMed] [Google Scholar]
- 18.Mouton JW, Punt N, Vinks AA. 2007. Concentration-effect relationship of ceftazidime explains why the time above the MIC is 40 percent for a static effect in vivo. Antimicrob. Agents Chemother. 51:3449–3451. 10.1128/AAC.01586-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller AE, Punt N, Mouton JW. 2013. Optimal exposures of ceftazidime predict the probability of microbiological and clinical outcome in the treatment of nosocomial pneumonia. J. Antimicrob. Chemother. 68:900–906. 10.1093/jac/dks468 [DOI] [PubMed] [Google Scholar]
- 20.Dudley MN. 1995. Combination β-lactam and β-lactamase-inhibitor therapy: pharmacokinetic and pharmacodynamic considerations. Am. J. Health Sys. Pharm. 52:23–28 [DOI] [PubMed] [Google Scholar]
- 21.Lister PD, Prevan AM, Sanders CC. 1997. Importance of β-lactamase inhibitor pharmacokinetics in the pharmacodynamics of inhibitor-drug combinations: studies with piperacillin-tazobactam and piperacillin-sulbactam. Antimicrob. Agents Chemother. 41:721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsch EB, Ledesma KR, Chang KT, Schwartz MS, Motyl MR, Tam VH. 2012. In vitro activity of MK-7655, a novel β-lactamase inhibitor, in combination with imipenem against carbapenem-resistant Gram-negative bacteria. Antimicrob. Agents Chemother. 56:3753–3757. 10.1128/AAC.05927-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borgonovi M, Merdjan H, Girard AM, Levasseur P, Quernin MH, Lowther J, Miossec C, Schlaes D, Drusano GL. 2008. Importance of NXL104 pharmacokinetics (PK) in the pharmacodynamics (PD) of ceftazidime+NXL104 combinations in an in vitro hollow fibre infection model, abstr A-023 48th Interscience Congress of Antimicrobial Agents and Chemotherapy, Washington, DC [Google Scholar]
- 24.Levasseur P, Girard AM, Lavallade L, Merdjan H, Quernin MH, Drusano GL. 2009. Use of the hollow fibre infection model in the pharmacodynamic evaluation of the β-lactamase inhibitor NXL104, abstr P-1463 19th European Congress of Clinical Microbiology & Infectious Diseases, Helsinki, Finland [Google Scholar]
- 25.Nichols W, Levasseur P, Li J, Das S. 2012. A threshold concentration of avibactam (AVI) during the pharmacokinetic decline phase, below which β-lactamase inhibition in Enterobacteriaceae becomes ineffective, abstr A-1760 Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 9th ed. CLSI, Wayne, PA [Google Scholar]
- 27.Tam VH, Louie A, Deziel MR, Liu W, Leary R, Drusano GL. 2005. Bacterial-population responses to drug-selective pressure: examination of garenoxacin's effect on Pseudomonas aeruginosa. J. Infect. Dis. 192:420–428. 10.1086/430611 [DOI] [PubMed] [Google Scholar]
- 28.Merdjan H, Tarral A, Girard AM, Levasseur P, Lowther J, Miossec C, Chassard D, Rangaraju M. 2007. Safety, single dose pharmacokinetics, and pharmacodynamics of β-lactamase inhibitor NXL104 in healthy young male adults, poster A-809 Abstr 47th Intersci. Conf. Antimicrob. Agents Chemother [Google Scholar]
- 29.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. 2005. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J. Antimicrob. Chemother. 55:601–607. 10.1093/jac/dki079 [DOI] [PubMed] [Google Scholar]
- 30.DeRyke C, Nicolau D. 2007. Is all free time above the minimum inhibitory concentration the same: implications for β-lactam in vivo modelling. Int. J. Antimicrob. Agents 29:341–343. 10.1016/j.ijantimicag.2006.10.006 [DOI] [PubMed] [Google Scholar]
- 31.Andes D, Craig WA. 2005. Treatment of infections with ESBL-producing organisms: pharmacokinetic and pharmacodynamic considerations. Clin. Microbiol. Infect. 11:10–17. 10.1111/j.1469-0691.2005.01265.x [DOI] [PubMed] [Google Scholar]
- 32.Louie A, Castanheira M, Liu W, Grasso C, Jones RN, Williams G, Critchley I, Thye D, Brown D, Vanscoy B, Kulawy R, Drusano GL. 2012. Pharmacodynamics of β-lactamase inhibition by NXL104 in combination with ceftaroline: examining organisms with multiple types of β-lactamases. Antimicrob. Agents Chemother. 56:258–270. 10.1128/AAC.05005-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berkhout J, Melchers MJ, Van Mil CH, Seyedmousavi S, Lagarde CM, Schuck V, Nichols WW, Mouton JW. 2013. Pharmacodynamics of ceftazidime and avibactam in a neutropenic mouse kung model, abstr A-1022 Abstr. 53rd Intersci. Conf. Antimicrobial Agents Chemother [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkhout J, Melchers MJ, Van Mil CH, Seyedmousavi S, Lagarde CM, Schuck V, Nichols WW, Mouton JW. 2013. Exposure response relationships of ceftazidime and avibactam in a neutropenic thigh model, abstr A-1023 Abstr. 53rd Intersci. Conf. Antimicrob. Agents Chemother [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhagunde P, Chang KT, Hirsch EB, Ledesma KR, Nikolaou M, Tam VH. 2012. Novel modeling framework to guide design of optimal dosing strategies for β-lactamase inhibitors. Antimicrob. Agents Chemother. 56:2237–2240. 10.1128/AAC.06113-11 [DOI] [PMC free article] [PubMed] [Google Scholar]


