Abstract
We assessed the activity of tigecycline (TGC) combined with colistin (COL) against carbapenem-resistant enterobacteria. Synergy occurred in vitro against the majority of isolates, with the exception of Serratia marcescens. In a simple animal model (Galleria mellonella), TGC-COL was superior (P < 0.01) in treating Escherichia coli, Klebsiella pneumoniae, and Enterobacter infections, including those with TGC-COL resistance. Clinical studies are needed to determine whether TGC-COL regimens may be a viable option.
TEXT
Resistance to antimicrobial agents is an ongoing problem that consistently undermines our ability to treat bacterial infections (1). The emergence of multidrug-resistant (MDR) Enterobacteriaceae producing extended-spectrum β-lactamases (ESBLs) has led to the increased use of carbapenems. Carbapenem resistance in Enterobacteriaceae (CRE) may be mediated by mutations affecting membrane permeability (porin loss), overexpression of intrinsic β-lactamases (AmpC), and/or broad-spectrum resistance-nodulation-division (RND)-type efflux pumps (2) but also via the acquisition of carbapenem-hydrolyzing enzymes, including members of the KPC, IMP, VIM, OXA-48, and NDM families (3, 4). Although CRE strains are often reported to be susceptible to polymyxins (polymyxin B, colistin) and tigecycline (TGC) (5–7), there are concerns with the use of these drugs for treatment with debate over how to safely dose colistin (COL) (8) and warnings over the efficacy of TGC in the treatment of bloodstream and other serious infections (9). Rapid emergence of resistance has also been documented with these two agents if either one is used alone in the treatment of MDR Gram-negative infections (10). Due to the lack of alternatives, clinicians are increasingly using antimicrobials in combination. The mechanism of action of COL is not entirely clear, although it has been previously shown to disrupt the integrity of the Gram-negative outer membrane (11), thereby increasing its permeability by drugs that are typically excluded (12). This may improve the activity of a number of antibiotics which would otherwise have little effect (13). This approach is validated by reports of better clinical outcomes with unorthodox therapies for an increasing range of carbapenem-resistant bacteria (14–16). In this study, we assessed the activity of TGC in combination with COL against a range of CRE both in vitro and in vivo by using standard checkerboard and time-kill assays and a simple invertebrate model (Galleria mellonella) of infection and therapeutics (17–19) (we do acknowledge that currently the G. mellonella model lacks the required validation with regard to comparability with human therapy).
Enterobacteriaceae isolates used in this study (n = 18) consisted of susceptible type strains and clinical isolates exhibiting resistance to β-lactams (including carbapenems), TGC, or COL (Table 1). MICs of TGC and COL were determined by Etest (bioMérieux, France), and mechanisms of resistance were confirmed by genetic and phenotypic tests as previously described (20).
TABLE 1.
Characteristics of bacterial strains investigated, including resistance determinants (10, 20), MICs of TGC and COL, FICIs and SBPIs for TGC/COL combinations, and results from time-kill assays
| Strain | Feature(s) | TGC MIC (μg/ml) | COL MIC (μg/ml) | FICIa | SBPI | Time-kill assay resultsa |
|---|---|---|---|---|---|---|
| E. coli NCTC 12241 | Antibiotic-susceptible type strain | 0.064 | 0.75 | 0.625 (I) | 40 | |
| E. coli EC5 | ST131 CTX-M-15 producer | 0.064 | 1 | 0.5 (S) | 39.95 | S |
| E. coli EC204 | Carbapenem-resistant NDM-1 producer | 0.25 | 0.38 | 0.374 (S) | 24.13 | S |
| E. coli EC421 | Carbapenem-resistant NDM-1 producer | 0.16 | 8 | 0.748 (I) | 20.13 | |
| E. coli EC405 | Carbapenem-resistant NDM-5 producer | 0.16 | 0.75 | 1 (I) | 10 | |
| E. aerogenes NCTC 9753 | Antibiotic-susceptible type strain | 0.16 | 0.75 | 0.56 (I) | 40 | |
| E. aerogenes EA2 | Carbapenem-susceptible CTX-M-1 producer | 0.25 | 3 | 0.188 (S) | 39.95 | S |
| E. aerogenes EA1 | Carbapenem -resistant (porin loss, AmpC hyperproducer); in vivo derivative of EA2 | 0.125 | 0.38 | 0.75 (I) | 24.13 | |
| E. cloacae NCTC 13380 | Antibiotic-susceptible type strain | 0.16 | 0.5 | 0.56 (I) | 39 | |
| E. cloacae TGC-R | TGC-resistant efflux mutant | 8 | 1.5 | 0.5 (S) | 8.5 | S |
| K. pneumoniae NCTC 9633 | Antibiotic-susceptible type strain | 0.125 | 0.38 | 0.125 (S) | 12 | I |
| K. pneumoniae KP52 | Carbapenem-resistant OXA-48 producer | 0.5 | 1 | 0.375 (S) | 12 | S |
| K. pneumoniae KPC-3 | Carbapenem-resistant KPC-3 producer | 1 | 0.5 | 0.75 (I) | 9 | |
| K. pneumoniae KP51 | Carbapenem-susceptible CTX-M-15 producer | 4 | 0.5 | 0.313 (S) | 9 | S |
| K. pneumoniae KP96 | Carbapenem-resistant VIM-1 and KPC-2 producer | 0.5 | 0.38 | 0.531 (I) | 39.95 | |
| S. marcescens NCTC 13382 | Antibiotic-susceptible type strain | 0.125 | >256 | 9 (A) | 0.125 | |
| S. marcescens | Carbapenem-resistant NDM-1 producer | 1.5 | >256 | 9 (A) | 0.09 | |
| S. marcescens SM346 | TGC-resistant efflux mutant | 6 | 2 | 5 (A) | 0.125 |
S, synergy; I, intermediate or additive; A, antagonism.
Synergy testing was conducted in IsoSensitest broth in 96-well microtiter plates. Assays were set up in checkerboard style with 2-fold-decreasing concentrations of COL (16 to 0 μg/ml) and TGC (32 to 0 μg/ml) and bacterial inocula of 105 CFU per well. Plates were read after 24 h of incubation at 37°C. Synergy between TGC and COL was quantified by calculation of the fractional inhibitory concentration index (FICI) and the susceptibility breakpoint index (SBPI) (21, 22). A FICI of ≤0.5 was defined as synergy, a FICI of 0.5 to ≤4.0 as indifferent or additive, and values of ≥4.0 as antagonistic. An SBPI of >2 was deemed to represent therapeutically useful synergy. Time-kill assays were performed for each isolate with a FICI of ≤0.5 by using starting inocula of 1 × 106 CFU/ml TGC (1 μg/ml) and COL (2 μg/ml) alone and in combination. Time-kill curves were plotted from serial viable counts collected over 24 h, and synergy between TGC and COL was defined as a difference of ≥2 log10 CFU/ml between single and combination therapies (23). All isolates for which synergy was observed in time-kill assays were then used for treatment assays with G. mellonella.
To establish the optimal inocula (50% lethal dose [LD50]) required for staggered killing of G. mellonella over 96 h, 10 caterpillars (KJ Reptile Supplies, Nuneaton, United Kingdom) were inoculated with bacterial suspensions containing final concentrations of 102 to 105 CFU/larva. Suspensions were injected directly into the G. mellonella hemocoel, and larvae were incubated at 37°C for 96 h. Treatment assays with TGC and COL alone and in combination were assessed using 16 animals as previously described (24). Drug doses were selected to be representative of those used to treat human infection and consisted of TGC at 1 mg/kg and COL at 2.5 mg/kg. Phosphate-buffered saline (PBS) injections (10 μl) were used to control for both inoculation injury and no antimicrobial treatment. Survival curves were plotted over 96 h and analyzed using the log rank test. All in vitro and in vivo experiments were performed three times on separate occasions to ensure reproducibility.
The combination of TGC and COL was active against susceptible and resistant strains of Escherichia coli, Klebsiella pneumoniae, and Enterobacter spp., with synergy (FICI, ≤0.5) noted against 7 of 15 (47%) strains and an indifferent or additive effect (FICI, >0.5 and <4.0) produced against a further 8 strains (53%). In contrast, marked antagonism (FICI, ≥4.0) was observed against all Serratia marcescens strains (Table 1). Synergy was largely independent of resistance profiles and was seen against producers of CTX-M-1 (strain EA2), CTX-M-15 (EC5 and KP51), NDM-1 (EC204), and OXA-48 (KP52) enzymes and a TGC-resistant efflux mutant (Encl TGC-R). Indifferent or additive effects seen using the FICI as the definition of synergy contrasted with the SBPI data for E. coli, K. pneumoniae, and Enterobacter isolates with carbapenem resistance due to KPC (strains KPC-3 and KP96), VIM-1 (KP96), hyper-AmpC (EA1), NDM-5 (EC405), and/or NDM-1 (EC421) production, whereby values of 8.5 to 40 indicated that the interaction may still be clinically relevant.
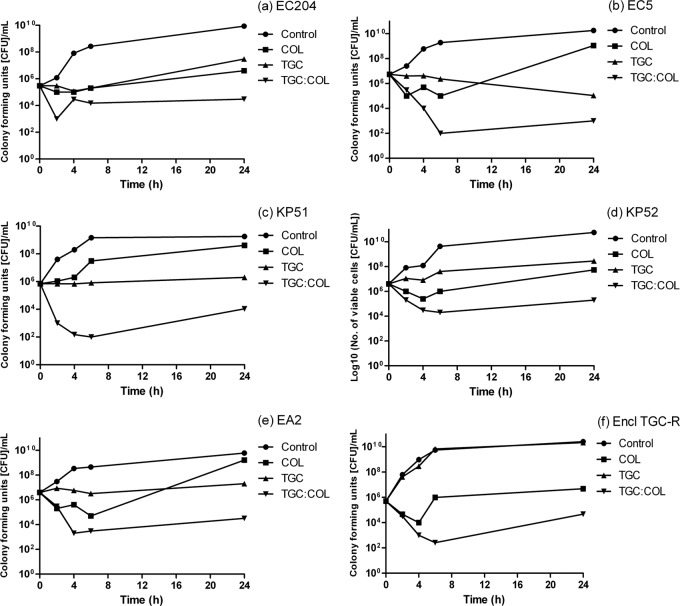
Synergy in checkerboard analysis was confirmed in time-kill assays for all isolates except K. pneumoniae NCTC 9633 (Fig. 1; see also Fig. S1 in the supplemental material). TGC alone was bacteriostatic against all 7 strains, as expected for a glycylcycline. Although COL was initially bactericidal, regrowth was observed with COL at 2 μg/ml against isolates NCTC 12241, EC5, NCTC 9633, KP51, KP52, and Enterobacter cloacae TGC-R. Previous studies have shown that regrowth after COL exposure can occur at concentrations many times above the MIC and may be indicative of a heteroresistant phenotype, detectable when investigated by population analysis profiling (25). MICs of colistin sulfate can also be overestimated due to its affinity to bind to laboratory plastic and glassware used in in vitro susceptibility tests (26). Although regrowth with the TGC-COL combination was also seen with 5 isolates, TGC-COL was still significantly more effective than either agent alone (≤3 log different from the control), supporting other reports of sustained bactericidal synergy with the TGC-COL combination in vitro (27, 28).
FIG 1.
Time-kill graphs showing antimicrobial synergy between TGC and COL against MDR isolates of Enterobacteriaceae, namely EC204 (a), EC5 (b), KP51 (c), KP52 (d), EA2 (e), and Encl TGC-R (f).
Of interest, TGC-COL was not effective against S. marcescens, with marked antagonism of the combination observed against all the strains tested. The mechanism of synergy between the two antibiotics against other species tested is likely to be linked to COL-mediated membrane permeabilization, allowing entry of TGC into bacterial cells. The intrinsic colistin resistance of S. marcescens indicates that its membrane is not permeabilized in this fashion. The mechanism of antagonism in S. marcescens remains to be elucidated, although it was not abolished by the addition of broad-spectrum inhibitors of resistance-nodulation-division (RND) pumps (para-aminobenzoic acid [PaβA]) (unpublished data), suggesting that dysregulation of antibiotic efflux is unlikely to be involved.
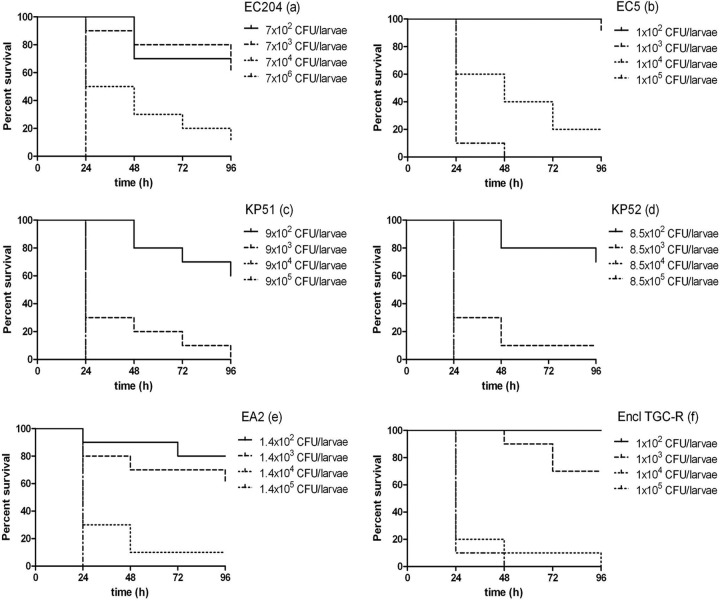
All of the isolates tested were pathogenic to G. mellonella when inocula of ≥102 CFU/larva were used (Fig. 2). The optimal inoculum able to promote staggered killing of >50% of larvae over 96 h for use in treatment assays varied from 103 to 104 CFU/larva (Fig. 3). Monotherapy with TGC was superior to COL in the treatment of EC204, EC5, EA2, and K. pneumoniae (KP52) infections (P < 0.01) but not against K. pneumoniae (KP51). TGC monotherapy of caterpillars infected with the E. cloacae TGC-R strain was ineffective, as predicted from in vitro susceptibility tests (Fig. 3).
FIG 2.
Kill kinetics of strains EC204 (a), EC5 (b), KP51 (c), KP52 (d), EA2 (e), and Encl TGC-R (f) at various numbers of CFU/ml in G. mellonella over 96 h. Curves were plotted from single experiments using 10 insect larvae.
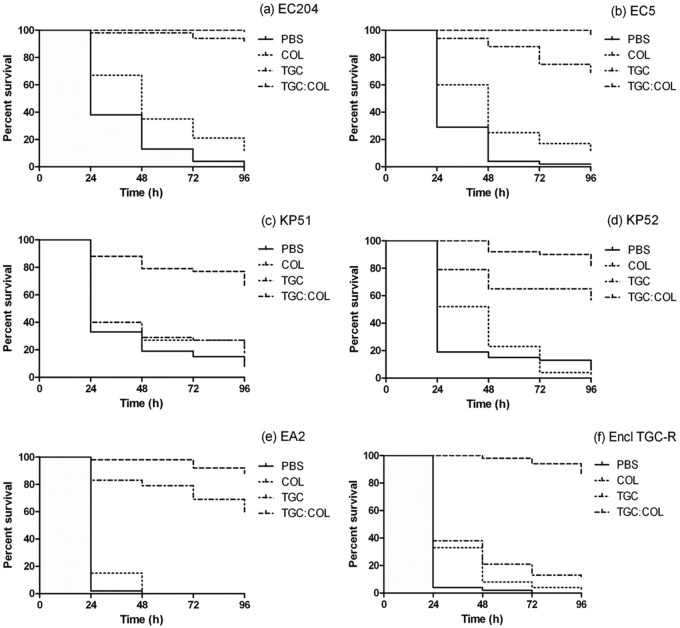
FIG 3.
G. mellonella survival curves over 96 h when larvae were infected with strains EC204 (a), EC5 (b), KP51 (c), KP52 (d), EA2 (e), and Encl TGC-R (f) and treated with PBS (control), TGC, COL, or the TGC-COL combination at humanized doses (mg/kg). Inoculum for strains KP51 and KP52 was 103 CFU/larva; that for strains EC204, EC5, EA2, and Encl TGC-R was104 CFU/larva.
Overall, the TGC-COL combination was significantly more effective (P < 0.01) than monotherapy against all of the CRE isolates studied in vivo. Treatment with TGC-COL resulted in survival of 99% (±1 percentage point [pp]) and 96% of EC204- and EC5-infected larvae (Fig. 3a and b), respectively, and was significantly more effective than TGC alone (P < 0.01). The TGC-COL combination was also significantly (P < 0.01) more effective against K. pneumoniae KP51 (67% survival ± 33 pp), KP52 (81% survival ± 19 pp) (Fig. 3c and d), E. aerogenes EA2 (88% ±13 pp), and the E. cloacae (85% ± 4 pp) TGC-R strain than either agent given as monotherapy (P < 0.01) (Fig. 3e and f).
In vitro data on the potential for TGC-COL regimens to be beneficial in treatment were correlated by the in vivo studies using G. mellonella larvae. In Galleria organisms, TGC and COL given together at humanized doses significantly improved survival against infections with E. coli, K. pneumoniae, and E. cloacae, including those producing KPC and NDM-1 carbapenemases. Although the relevance of an invertebrate model to human therapeutics can be questioned, there is increasing evidence that virulence, therapeutic outcomes, and pharmacokinetic parameters (29) can be predicted in this system. However, it should be noted that recent studies using murine models of sepsis (30), pneumonia (31), and soft tissue infection (32) have not found TGC-COL combination treatment to be consistently superior to treatment with either drug alone against carbapenemase-producing K. pneumoniae and A. baumannii isolates.
In summary, a synergistic or additive effect between TGC and COL was observed in vitro and in vivo against a number of MDR Enterobacteriaceae isolates, although not against S. marcescens. For TGC-COL combination therapy to be employed effectively and safely, further work on the pharmacokinetic parameters of TGC-COL in vivo and its effects on clinical outcomes from MDR Gram-negative infections will be needed.
Supplementary Material
ACKNOWLEDGMENT
We gratefully acknowledge the financial support of ASPIRE, an independent research program funded by Pfizer.
Footnotes
Published ahead of print 31 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02449-14.
REFERENCES
- 1.Wareham DW, Gordon NC, Hornsey M. 2011. In vitro activity of teicoplanin combined with colistin versus multidrug-resistant strains of Acinetobacter baumannii. J. Antimicrob. Chemother. 66:1047–1051. 10.1093/jac/dkr069 [DOI] [PubMed] [Google Scholar]
- 2.Lavigne JP, Sotto A, Nicolas-Chanoine MH, Bouziges N, Bourg G, Davin-Regil A, Pages JM. 2012. Membrane permeability, a pivotal function involved in antibiotic resistance and virulence in Enterobacter aerogenes clinical isolates. Clin. Microbiol. Infect. 18:539–545. 10.1111/j.1469-0691.2011.03607.x [DOI] [PubMed] [Google Scholar]
- 3.Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnam P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodword N. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan and the UK: a molecular, biological and epidemiological study. Lancet Infect. Dis. 10:597–602. 10.1016/S1473-3099(10)70143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carattoli A, Villa L, Poirel L, Bonnin RA, Nordmann P. 2012. Evolution of IncA/C blaCMY-2-carrying plasmids by acquisition of the blaNDM-1 carbapenemase gene. Antimicrob. Agents Chemother. 56:783–786. 10.1128/AAC.05116-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Docobo-Perez F, Nordmann P, Dominguez-Herrera J, Lopez-Rojas R, Smani Y, Poirel L, Pachon J. 2012. Efficacies of colistin and tigecycline in mice with experimental pneumonia due to NDM-1-producing strains of Klebsiella pneumoniae and Escherichia coli. Int. J. Antimicrob. Agents 39:251–254. 10.1016/j.ijantimicag.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Karageorgopoulos DE, Nordmann P. 2011. Therapeutic options for infections with Enterobacteriaceae producing carbapenem-hydrolyzing enzymes. Future Microbiol. 6:653–666. 10.2217/fmb.11.49 [DOI] [PubMed] [Google Scholar]
- 7.Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N. 2011. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int. J. Antimicrob. Agents 37:415–419. 10.1016/j.ijantimicag.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 8.Bergen PJ, Li J, Nation RL. 2011. Dosing of colistin—back to basic PK/PD. Curr. Opin. Pharmacol. 11:464–469. 10.1016/j.coph.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yahav D, Lador A, Paul M, Leibovici I. 2011. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J. Antimicrob. Chemother. 66:1963–1971. 10.1093/jac/dkr242 [DOI] [PubMed] [Google Scholar]
- 10.Hornsey M, Ellington MJ, Doumith M, Thomas CP, Gordon NC, Wareham DW, Quinn J, Lolans K, Livermore DM, Woodford N. 2010. AdeABC-mediated efflux and tigecycline MICs for epidemic clones of Acinetobacter baumannii. J. Antimicrob. Chemother. 65:1589–1593. 10.1093/jac/dkq218 [DOI] [PubMed] [Google Scholar]
- 11.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. 2005. Evaluation of colistin as an agent against mult-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 25:11–25. 10.1016/j.ijantimicag.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 12.Vaara M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon NC, Png K, Wareham DW. 2010. Potent synergy and sustained bactericidal activity of a vancomycin-colistin combination versus multidrug-resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 54:5316–5322. 10.1128/AAC.00922-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. 2014. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob. Agents Chemother. 58: 654–663. 10.1128/AAC.01222-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrosillo N, Giannella M, Antonelli M, Antonini M, Barsic B, Belancic L, Cadkan Inkaya A, De Pascale G, Grilli E, Tumbarello M, Akova M. 25 November 2013. Colistin-glycopeptide combination in critically ill patients with Gram-negative infection: the clinical experience. Antimicrob. Agents Chemother. 10.1128/AAC.00871-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob. Agents Chemother. 56:2108-2113. 10.1128/AAC.06268-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown SE, Howard A, Kasprzak AB, Gordon KH, East PD. 2009. A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect Biochem. Mol. Biol. 39:792–800. 10.1016/j.ibmb.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 18.Champion OL, Cooper IA, James SL, Ford D, Karlyshev A, Wren BW, Duffield M, Oyston PC, Titball RW. 2009. Galleria mellonella as an alternative infection model for Yersinia pseudotuberculosis. Microbiology 155:1516–1522. 10.1099/mic.0.026823-0 [DOI] [PubMed] [Google Scholar]
- 19.Jander G, Rahme LG, Ausubel FM. 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 182:3843–3845. 10.1128/JB.182.13.3843-3845.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornsey M, Phee L, Stubbings W, Wareham DW. 2013. In vitro activity of the novel monosulfactam BAL30072 alone and in combination with meropenem versus a diverse collection of important Gram-negative pathogens. Int. J. Antimicrob. Agents 42:343–346. 10.1016/j.ijantimicag.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 21.Wareham DW, Bean DC. 2006. In vitro activities of polymyxin B, imipenem, and rifampin against multi-drug resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 50:825–826. 10.1128/AAC.50.2.825-826.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milne KEN, Gould IM. 2010. Combination testing of a multidrug-resistant cystic fibrosis isolates of Pseudomonas aeruginosa: use of a new parameter, the susceptible breakpoint index. J. Antimicrob. Chemother. 65:82–90. 10.1093/jac/dkp384 [DOI] [PubMed] [Google Scholar]
- 23.NCCLS. 1999. Methods for determining bactericidal activity of antimicrobial agents, vol. 19, no. 18 Approved guideline M26-A. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 24.Hornsey M, Wareham DW. 2011. In vivo efficacy of glycopeptide-colistin combination therapies in a Galleria mellonella model of Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 55:3534–3537. 10.1128/AAC.00230-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen RJ, Li J, Nation RL, Spelman D. 2007. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J. Antimicrob. Chemother. 59:473–477. 10.1093/jac/dkl512 [DOI] [PubMed] [Google Scholar]
- 26.Hindler JA, Humphries RM. 2013. Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant Gram-negative bacilli. J. Clin. Microbiol. 51:1678–1684. 10.1128/JCM.03385-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albur M, Noel A, Bowker K, MacGowen A. 2012. Bactericidal activity of multiple combinations of tigecycline and colistin against NDM-1-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 56:3441–3443. 10.1128/AAC.05682-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pournaras S, Vironi G, Neou E, Dendrinos J, Dimitroulia E, Poulou A, Tsakris A. 2011. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumonia carbapenemase (KPC)-producing Enterobacteriaceae by time-kill assay. Int. J. Antimicrob. Agents 37:244–247. 10.1016/j.ijantimicag.2010.10.031 [DOI] [PubMed] [Google Scholar]
- 29.Hill L, Veli N, Coote PJ. 2014. Evaluation of Galleria mellonella larvae for measuring the efficacy and pharmacokinetics of antibiotic therapies against Pseudomonas aeruginosa infection. Int. J. Antimicrob. Agents. 43:254–261. 10.1016/j.ijantimicag.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 30.Demirasian H, Dinc G, Ahmed SS, Elmali F, Metan G, Alp E, Doganay M. 25 September 2013. Carbapenem-resistant Klebsiella pneumoniae sepsis in corticosteroid receipt mice: tigecycline or colistin monotherapy versus tigecycline/colistin combination. J. Chemother. 10.1179/1973947813Y.0000000143 [DOI] [PubMed] [Google Scholar]
- 31.Mutlu Yilmaz E, Sunbul M, Aksoy A, Yilmaz H, Guney AK, Guvenc T. 2012. Efficacy of tigecycline/colistin combinations in a pneumonia model caused by extensively drug-resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents 40:332–336. 10.1016/j.ijantimicag.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 32.Michail G, Labrou M, Pitiriga V, Manousaka S, Sakellaridis N, Tskris A, Pournaras S. 2013. Activity of tigecycline in combination with colistin, meropenem, rifampin, or gentamicin against KPC-producing Enterobacteriaceae in a murine thigh model. Antimicrob. Agents Chemother. 57:6028–6033. 10.1128/AAC.00891-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.