Abstract
Using an established nonhuman primate model, rhesus macaques were infected intravenously with a chimeric simian immunodeficiency virus (SIV) consisting of SIVmac239 with the human immunodeficiency virus type 1 (HIV-1) reverse transcriptase from clone HXBc2 (RT-SHIV). The impacts of two enhanced (four- and five-drug) highly active antiretroviral therapies (HAART) on early viral decay and rebound were determined. The four-drug combination consisted of an integrase inhibitor, L-870-812 (L-812), together with a three-drug regimen comprising emtricitabine [(−)-FTC], tenofovir (TFV), and efavirenz (EFV). The five-drug combination consisted of one analog for each of the four DNA precursors {using TFV, (−)-FTC, (−)-β-d-(2R,4R)-1,3-dioxolane-2,6-diaminopurine (amdoxovir [DAPD]), and zidovudine (AZT)}, together with EFV. A cohort treated with a three-drug combination of (−)-FTC, TFV, and EFV served as treated controls. Daily administration of a three-, four-, or five-drug combination of antiretroviral agents was initiated at week 6 or 8 after inoculation and continued up to week 50, followed by a rebound period. Plasma samples were collected routinely, and drug levels were monitored using liquid chromatography-tandem mass spectrometry (LC–MS-MS). Viral loads were monitored with a standard TaqMan quantitative reverse transcriptase PCR (qRT-PCR) assay. Comprehensive analyses of replication dynamics were performed. RT-SHIV infection in rhesus macaques produced typical viral infection kinetics, with untreated controls establishing persistent viral loads of >104 copies of RNA/ml. RT-SHIV loads at the start of treatment (V0) were similar in all treated cohorts (P > 0.5). All antiretroviral drug levels were measureable in plasma. The four-drug and five-drug combination regimens (enhanced HAART) improved suppression of the viral load (within 1 week; P < 0.01) and had overall greater potency (P < 0.02) than the three-drug regimen (HAART). Moreover, rebound viremia occurred rapidly following cessation of any treatment. The enhanced HAART (four- or five-drug combination) showed significant improvement in viral suppression compared to the three-drug combination, but no combination was sufficient to eliminate viral reservoirs.
INTRODUCTION
The current treatment for AIDS is highly active antiretroviral therapy (HAART), which includes a combination of antiretroviral drugs, usually three or more from two or more classes. HAART serves as a means to achieve long-term control of replication of human immunodeficiency virus type 1 (HIV-1) (1–6). Effective HAART can reduce viremia to below the detectable limits of conventional clinical assays (<50 viral-RNA [vRNA] copies/ml) in people living with HIV-1 who are able to adhere to the treatment regimen. However, viremia inevitably rebounds following cessation of HAART, likely due to established viral reservoirs (7–9). In addition, with more sensitive quantitative reverse transcriptase PCR (qRT-PCR) assays now available, it is clear that low-level viremia persists (10). Therefore, HIV-1 is not eradicated with current HAART.
Attempts to address mechanisms of viral persistence have been limited in people living with HIV-1, partly because comprehensive tissue sampling during suppressive HAART is not feasible, and HIV-1 eradication cannot be proven unless individuals can be removed from HAART without viral rebound (11). However, ethical concerns surround structured treatment interruptions (12). Nonhuman primate models, particularly simian immunodeficiency virus (SIV) infection of macaques, have provided robust animal models for AIDS, contributing significantly to the understanding of important aspects of pathogenesis, viral diversity, and long-lived reservoirs (13–17). Macaques infected with SIV often exhibit a fatal immunodeficiency disease similar to that in humans infected with HIV-1, but the disease course is accelerated, permitting more rapid experimentation (18). A limitation to SIV as a model for HAART is that SIV is not susceptible to the nonnucleoside reverse transcriptase (RT) inhibitors (NNRTIs) that are widely used in current HAART. Nonhuman primate models that can utilize NNRTIs have been developed by us and others (18–21). One rhesus macaque model uses a virus consisting of the backbone of the pathogenic molecular clone SIVmac239 with the HIV-1 RT from clone HXBc2 (RT-SHIV) (21). RT-SHIV is sensitive to several nucleoside RT inhibitors (NRTIs), protease inhibitors (PIs), and NNRTIs (22–24). Studies in RT-SHIV-infected macaques support the relevance of this animal model for identifying potential reservoirs of latency/persistence during HAART.
Efforts toward eradication of HIV have taken two approaches. One type, a sterilizing cure, requires that HIV be eradicated from the body of the infected person. The second, a functional cure, is less stringent in that it requires that the infected individual be able to stop taking antiretroviral therapy without suffering any adverse consequences from the HIV that remains in the body. Attempts to reactivate latent virus during suppressive HAART to develop a sterilizing cure have had limited success (25). The alternative approach to achieve a functional cure is focused on more effective suppression of viral replication (26). In this study, we compare two enhanced-HAART regimens (a four- and a five-drug combination) to a current three-drug HAART in our animal model. Our comprehensive approach utilized the well-controlled RT-SHIV model of AIDS for evaluating antiviral strategies that aim to eliminate virus from reservoirs.
MATERIALS AND METHODS
Animals, virus stocks, and inoculations.
Young adult rhesus macaques (Macaca mulatta; 1 to 3 years old, weighing 2 to 5 kg) were used for these studies. All macaques were from the type D retrovirus-free and SIV-free colony at the California National Primate Research Center (CNPRC) at the University of California (UC) Davis and were handled in accordance with the American Association for Accreditation of Laboratory Animal Care Standards. All protocols strictly adhered to the Guide for the Care and Use of Laboratory Animals prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Research Council. This study was approved by the UC Davis Institutional Animal Care and Use Committee.
RT-SHIV has an open nef reading frame, and the RT-SHIV stocks we use have the T-to-C substitution at position 8 of the SIV tRNA primer binding site, which is necessary for rapid replication of RT-SHIV in vitro and in vivo (27). Virus stocks were prepared by propagating viruses in CEMx174 cells (a T/B-cell hybrid tumor line), and concentrations were determined as previously described (18, 28). Importantly, levels of plasma vRNA in animals infected with RT-SHIV and not treated with antiretroviral drugs were comparable to the viral loads in untreated HIV-1-infected patients (18). Peak viremia in RT-SHIV-infected animals occurred 2 to 3 weeks postinoculation, and by 8 weeks, plasma viral loads approached set points.
HAART regimens in RT-SHIV-infected rhesus macaques.
A standard three-drug combination regimen consisting of 2 NRTIs and 1 NNRTI was selected. In comparison, a four-drug regimen included the addition of a third class, an integrase inhibitor (INI), to the 2 NRTI and 1 NNRTI combination. A novel five-drug combination was designed to include 4 NRTIs (containing one nucleoside analog for each of the 4 natural nucleosides A, T, C, and G) together with 1 NNRTI. Specifically, the three-drug combination consisted of tenofovir (TFV) (30 mg/kg of body weight, subcutaneously [SQ], once a day [QD]; provided by Gilead Sciences, Inc., Foster City, CA), emtricitabine [(−)-FTC] (16 mg/kg SQ, QD; supplied by ST Pharm Co., Ltd., Seoul, South Korea), and efavirenz (EFV) (200 mg per day, orally [p.o.], QD; purchased from the Veterans Administration [VA] pharmacy, Atlanta, GA) and was initiated in a cohort of macaques (n = 8) at 6 weeks postinoculation, as previously reported (29). Similarly, a four-drug combination [using the identical three-drug combination of TFV, (−)-FTV, and EFV (98% pure; provided by Raymond Schinazi's Laboratory of Biochemical Pharmacology, Emory University, Atlanta, GA) with the addition of an INI, L-812 (provided by Merck, White House Station, NJ)] was initiated at 6 weeks post-RT-SHIV inoculation (n = 8). In addition, a five-drug combination {using the identical three-drug combination of TFV, (−)-FTC, and EFV with the addition of zidovudine (AZT) (30 mg/kg, SQ, twice a day [BID]; supplied by ST Pharm Co., Ltd., Seoul, South Korea), and amdoxovir (DAPD) (85 mg per day, p.o., QD; 98% pure; supplied by ST Pharm Co., Ltd., Seoul, South Korea)} was initiated at 8 weeks post-RT-SHIV inoculation in a third cohort of macaques (n = 7). The dosages of drugs were selected based on previous experience with the drugs and/or on previously published pharmacokinetic studies in rhesus macaques (18, 24, 30–34). Of note, the INI, L-812, has been previously reported to have potent antiviral activity in vitro against both HIV-1 and SIV (250 and 350 nM, respectively) (34). Untreated controls were averaged from RT-SHIV-infected macaques from previous studies. The combination regimens are summarized in Table 1.
TABLE 1.
HAART treatment scheme
| Cohort | No. of animals | Drugsa | ART classes | Treatment initiation (wk) |
|---|---|---|---|---|
| 3-drug | 8 | (−)-FTC, TFV, EFV | 2 NRTI, 1 NNRTI | 6 |
| 4-drug | 4 | (−)-FTC, TFV, EFV, L-812 | 2 NRTI, 1 NNRTI, 1 INI | 6 |
| 5-drug | 7 | (−)-FTC, TFV, EFV, AZT, DAPD | 4 NRTI, 1 NNRTI | 8 |
EFV, L-812, and DAPD were compounded into drug paste (1 dose/ml) from bulk powder using sucrose solution (63%).
Plasma samples were collected at weeks 1, 2, 3, and 4 postinoculation and then, in general, at 2-week intervals thereafter. For the three-drug HAART regimen, drug therapy ceased at week 39, and viral rebound was measured up to week 64. For the four-drug enhanced-HAART regimen, drug therapy ceased at week 55 postinoculation, and viral rebound was measured in plasma up to week 74. For the five-drug enhanced-HAART regimen, drug therapy ceased at week 50 postinoculation, and viral rebound was measured in plasma up to week 66. The time to rebound, defined as the time from cessation of therapy to reach a viral load of >500 copies of vRNA/ml, was documented, and areas under the curve (AUC) of a plot of the viral load versus time were calculated.
Viral load.
RT-SHIV RNA quantification, targeting the p27 gag region, was performed by real-time qRT-PCR utilizing TaqMan (Applied Biosystems) hydrolysis probes (35). For each cohort, the mean viral load at each time point was determined as vRNA copies/ml ± standard deviation (SD).
Drug extraction and quantification in plasma from RT-SHIV-inoculated rhesus macaques.
Plasma aliquots were stored at −80°C. Antiretroviral drug levels were detected by liquid chromatography-tandem mass spectrometry (LC–MS-MS) (31, 32). Calibration curves were prepared using serially diluted standards added to plasma of drug-free donors. Briefly, acetonitrile was used to precipitate the plasma for (−)-FTC, DAPD, ZDV, and TFV detection, and methanol was used for EFV. In addition, a metabolite of DAPD, (−)-β-d-dioxolane guanosine (DXG), resulting from deamination by adenosine deaminase, was also quantified.
Viral-load dynamics.
To simplify analysis, viral-load curves (logV) were normalized by subtracting the starting logV0 from each logV at subsequent weekly intervals. This yielded curves of change in logV for a time range identified for each animal from treatment initiation (time zero) to undetectable values. Undetectable values were assumed when two consecutive samples were <50 vRNA copies/ml. The magnitudes of areas of displacement in logV [AUC log(V0/V) versus time] of each animal were computed using a cubic spline routine (MESS Package; R Statistical Foundation, Vienna, Austria).
Statistics.
Nonparametric AUC, box plots, and statistical tests were computed using the multcomp and ggplot2 packages in R (www.r-project.org; R statistical Foundation, Vienna, Austria). Other graphics were performed using GraphPad Prism (Graphpad Software, San Diego, CA, USA). Comparisons (t tests) between groups were performed using the Tukey correction for multiple comparisons, with a sandwich operator to provide consistent estimation of the covariance matrix, given apparent differences in variation (heteroscedascity) between groups.
RESULTS
Efficacy of the enhanced-HAART regimen in rhesus macaques.
Distinct cohorts of young adult macaques were each inoculated intravenously with cell-free RT-SHIV, as described previously (18). All animals became persistently infected (with viral loads reaching a peak at week 2), and antiviral drug combinations were initiated at 6 to 8 weeks postinoculation (Fig. 1). All of the treated macaque cohorts in these studies showed normal weight gains with moderate side effects that were reversible with early detection and dose modifications. Data from control (no antiretroviral drugs) RT-SHIV-inoculated animals from two other studies were included in this study as historic controls. The mean viral load ± SD for this group of RT-SHIV-infected control animals is shown in Fig. 1. In contrast to the drug-treated animals, many of the untreated RT-SHIV-inoculated control animals developed severe complications of simian AIDS and were euthanized during the experimental time course.
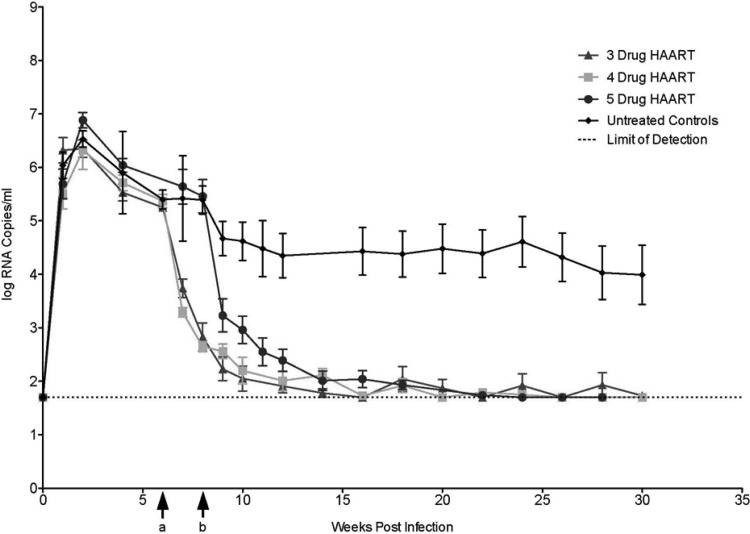
FIG 1.
Summary of RT-SHIV infection and decay kinetics in rhesus macaques following a HAART (three-drug) or enhanced-HAART (four- or five-drug) regimen. Cohorts were treated with a three-drug [EFV, TFV, and (−)-FTC] (n = 8), four-drug [EFV, TFV, (−)-FTC, and L-812] (n = 8), or five-drug [EFV, TFV, (−)-FTC, AZT, and DAPD] (n = 7) regimen. Viral loads were monitored using qRT-PCR in macaques inoculated with RT-SHIV (week 0), followed by combination HAART initiated at 6 weeks (a) (three drugs and four drugs) or 8 weeks (b) (five drugs) after inoculation. The average viral load for each cohort, including an untreated control group (pooled historic data), is summarized as log10 vRNA copies/ml ± SD (error bars) for each time point (shown from weeks 0 to 30), with a limit of detection of ≥50 copies/ml.
Following initiation of the enhanced five-drug combination, drug concentrations in plasma were measured using LC–MS-MS. As expected, all five antiretroviral drugs were detected in plasma (data not shown). In addition, a major metabolite of DAPD (DXG) was also detected (data not shown). Plasma viral loads rapidly declined in all macaque cohorts following initiation of HAART or enhanced HAART (Fig. 1), with a pattern similar to that observed with HAART in HIV-1-infected humans (36). However, the four- and five-drug cohorts showed a more rapid decline than the three-drug cohort (as shown by the individual-animal curves for each group). By 22 weeks postinoculation, plasma viral loads were below the level of detection of the standard assay (<50 vRNA copies/ml) in all drug-treated animals, and the viral loads remained suppressed for the duration of therapy, with only occasional blips (Fig. 1). Although plasma vRNA was not detected (<50 vRNA copies/ml) with our standard viral-load assay during prolonged enhanced-HAART administration, persistent low-level viremia was detected with a more sensitive viral-load assay that used larger volumes of plasma available at necropsy (data not shown). Treatment was stopped after 18 to 49 weeks of combination drug administration, depending on the experimental design of the cohort. Upon cessation of therapy, some of the animals in each cohort were maintained for an additional 16- to 25-week period of observation to measure viral-rebound dynamics.
Dynamics of viral suppression of enhanced HAART.
The viral decay kinetics of the four- and five-drug regimens appeared multiphasic, as has previously been reported for rhesus macaques treated with the three-drug regimen (29). Due to the complex and often oscillatory viral decay profiles and limited permissible samplings (restricted to weekly intervals), the data in this study were analyzed using nonparametric approaches to avoid overfitting of data (Fig. 2). Viral response curves for the first 10 weeks in cohorts administered enhanced HAART (a four- or five-drug combination) were compared to previously reported data from HAART (three-drug) administration (29), now expressed as log10 copies of SIV RNA/ml plasma. Overall, viral dynamic curves appeared multiphasic and sometimes oscillated (Fig. 2A). Therefore, we did not assume linear functions. Instead, AUC were computed using cubic spline curves. To ensure a dynamic range of >4 log units, we restricted inclusion of animals with initial viral loads (logV0) of >5 × 104 vRNA copies/ml prior to treatment. Median starting viral loads (logV0) at treatment initiation (week 0 on HAART) were similar for all three treatment groups (Fig. 2B) (P > 0.5; Tukey multiple-comparison test), and therefore, it was presumed that any changes in viral-load dynamics following antiretroviral administration could be ascribed to differences in the treatment regimens (i.e., enhanced HAART versus HAART).
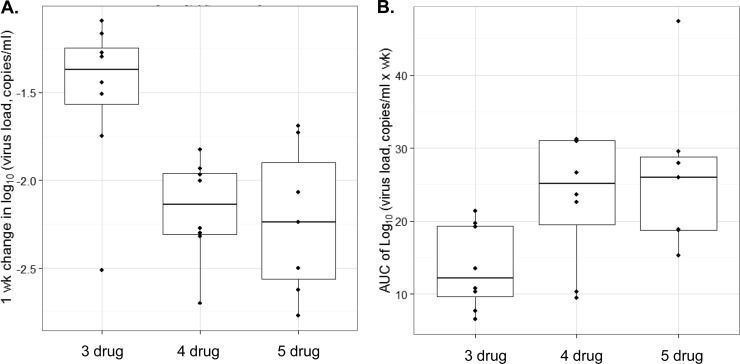
FIG 2.
Comparative kinetics of RT-SHIV in rhesus macaques was assessed following enhanced HAART (four- or five-drug combination HAART) versus three-drug combination HAART. (A) Viral loads for individual animals within each treatment cohort (log vRNA copies/ml) over 10 weeks, monitored using RT-PCR. (B) AUC values computed for starting viral loads (logV0) prior to initiation of each HAART regimen. The median logV0 values for each treatment cohort were similar (P > 0.5). Horizontal lines and boxes represent median and interquartile ranges (IQR). Observations >1.5× IQR from the median are not linked with horizontal lines.
Three-drug treatment (HAART) produced a 1.4-fold reduction in the median log(V/V0) after only 1 week. In comparison, the four-drug or five-drug (enhanced-HAART) treatment regimens produced 2.15- and 2.25-fold reductions, respectively, in the median log(V/V0) during the same time interval (Fig. 3A). These data suggested improved viral-load reduction dynamics with four- and five-drug enhanced HAART compared to three-drug HAART (P < 0.01). Of note, there was no statistical difference in the viral decay dynamics between the two enhanced-HAART regimens. Similarly, the median displacement AUC of log(V0/V) versus time following 10 weeks of enhanced HAART indicated >2-fold viral suppression (P < 0.03) compared with the three-drug treatment (Fig. 3B). In short, the greater AUC demonstrated a more pronounced decrease in viral-load dynamics over the initial 10-week time interval, suggesting that enhanced HAART is more effective at reducing the initial RT-SHIV load in macaques upon initiation of antiretroviral treatment.
FIG 3.
Comparison of RT-SHIV dynamics following a HAART (three-drug) or enhanced-HAART (four- or five-drug) regimen in rhesus macaques. Nonparametric analysis was performed on the viral-load data sets from each treatment cohort, assuming nonlinear decay kinetics. The AUC was determined for each individual animal and normalized by subtracting the initial viral load (logV0) from each subsequent viral load during treatment (logV). (A) Box plots of the median log decrease in the viral load after 1 week of treatment for each cohort (enhanced HAART or HAART). Both enhanced-HAART regimens resulted in significantly greater reduction in the viral load than HAART (P < 0.01). (B) Median AUC of log(V0/V) versus the time from the start of treatment (time zero) to the point at which two consecutive samples were at undetectable levels (<50 copies/ml). Again, both enhanced-HAART regimens resulted in significant increase in the AUC compared to HAART (P < 0.05).
Viral rebound.
For each of the treatment groups, the plasma viral loads, in general, remained undetectable (<50 vRNA copies/ml) during the continued administration of drug regimens, with occasional blips, as mentioned above. Following cessation of therapy, we evaluated viral-load rebound in selected animals from each regimen group for an additional period (16 to 25 weeks). As expected, rebound occurred immediately (within 1 to 3 weeks) after cessation of the three-drug regimen. However, rebound also occurred with both the four- and five-drug regimens (also within 1 to 3 weeks), suggesting even enhanced HAART alone was unable to eliminate viral reservoirs. As a limited number of animals were available (as some were used for other studies) for the rebound phase, statistical analyses were limited. Again, all animals rebounded, with equivalent peaks within 3 weeks. These results are in agreement with our previous study of viral rebound after cessation of HAART (37).
DISCUSSION
In this study, we compared two enhanced-HAART regimens to the widely used three-drug HAART [(−)-FTC, TFV, and EFV] to determine whether enhanced-HAART regimens can provide more rapid and/or more complete suppression during therapy in an animal model system. The enhanced regimens were a four-drug [(−)-FTC, TFV, and EFV plus L-812] and a five-drug [(−)-FTC, TFV, and EFV plus AZT and DAPD] combination. We demonstrated that enhanced HAART does provide improved results, with more rapid suppression of virus (29). Despite the enhanced suppression of these regimens, virus eradication was not achieved over the time studied, as virus was detectable at the end of the experiments in all animals.
In this study, we observed multiphasic viral decay curves similar to what has been reported in persons living with HIV-1 and receiving HAART. In HIV-1 decay, the first phase primarily occurs in productively infected CD4+ T cells, typically with a half-life of 1 to 2 days. The second phase may reflect decay of HIV-infected macrophages and related cells or activation of preintegration latency with a half-life of 2 to 3 weeks (38). Additionally, we found a third phase of decay that is less pronounced, as low-level viremia persists in RT-SHIV-inoculated macaques despite a HAART regimen commonly used in humans (10, 29). Therefore, eradication of long-lasting or latently infected cells may require decades of suppressive therapy (39).
In these studies, we found more rapid viral decay kinetics following enhanced HAART (four- or five-drug regimen) than with the three-drug regimen. Viral loads for each of the treatment groups (HAART or enhanced HAART) were reduced to below 50 vRNA copies/ml by 10 weeks of treatment. A reduction in the viral load corresponded to confirmed detection of systemic drug concentrations of each antiretroviral drug following the enhanced five-drug HAART regimen. Despite improved viral decay kinetics in the cohorts receiving enhanced HAART (either the four- or five-drug regimen), rebound rapidly occurred following treatment cessation. Therefore, increasing the combination of drugs administered may not be sufficient to eradicate viral reservoirs. Recently, a randomized open-label study of three- versus five-drug PI-based combination HAART in newly HIV-1-infected individuals also found no significant long-term impact on virologic or immunologic responses at 48 weeks beyond those achieved with standard three-drug PI-based HAART (40). Unlike this recent clinical study, which was limited to monitoring the viral load after 12 weeks of treatment, our studies demonstrated an immediate impact on viral decay kinetics during the first 10 weeks of treatment, which arguably could be of benefit to the infected individuals in preserving their functional immunity.
The persistence of HIV infection despite HAART is a major challenge. Animal models for viral persistence during antiviral therapy may be important and necessary for HIV eradication strategies. We and others have achieved long-term viral-load suppression in the RT-SHIV models (16, 19, 41). Shytaj et al. have also achieved impressive long-term suppression using a highly intensified multidrug ART in SIVmac251-infected rhesus macaques. Their regimen consisted of TFV, (−)-FTC, and raltegravir, initially for 1.5 months and then intensified with the protease inhibitor darunavir (pharmacokinetically enhanced by ritonavir) for 80 days and, lastly, reinforced with the addition of the CCR5 antagonist maraviroc (42). Likewise, Kline et al. monitored RT-SHIVmne in rhesus macaques over 20 weeks and found persistence of viral reservoirs in lymphoid tissues, despite undetectable plasma viremia at the time of necropsy (43). Unfortunately, eradication was not achieved in any of these studies. In all cases where therapy was terminated, the viral load rebounded after cessation of therapy. Although eradication was not achieved, these models are valuable to evaluate strategies for HIV eradication.
ACKNOWLEDGMENTS
We thank Daria Hazuda for her contributions to the studies and her editorial suggestions. Special thanks are due to the UC Davis Lucy Whittier Molecular and Diagnostic Core Facility for performing some of the viral-load assays. We also thank K. Van Rompay, L. Hirst, T. Dearman, A. Spinner, R. Tarara, D. Canfield, and others on the veterinary staff, Colony Services and Clinical Laboratory of the California National Primate Research Center, for expert technical assistance.
The studies were supported by NIH 8R01OD011094 (T.W.N. and R.F.S.) and 1RO1MH100999 (R.F.S.), by the Department of Veterans Affairs (R.F.S.), and in part by the Emory Center for AIDS Research (P30 AI050409).
Footnotes
Published ahead of print 28 April 2014
REFERENCES
- 1.Schinazi RF, Cannon DL, Arnold BH, Martino-Saltzman D. 1988. Combinations of isoprinosine and 3′-azido-3′-deoxythymidine in lymphocytes infected with human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 32:1784–1787. 10.1128/AAC.32.12.1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma PL, Nurpeisov V, Hernandez-Santiago B, Beltran T, Schinazi RF. 2004. Nucleoside inhibitors of human immunodeficiency virus type 1 reverse transcriptase. Curr. Top. Med. Chem. 4:895–919. 10.2174/1568026043388484 [DOI] [PubMed] [Google Scholar]
- 3.Humphreys EH, Chang LW, Harris J. 16 June 2010. Antiretroviral regimens for patients with HIV who fail first-line antiretroviral therapy. Cochrane Database Syst. Rev. 10.1002/14651858.CD006517.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKinnon JE, Mellors JW, Swindells S. 2009. Simplification strategies to reduce antiretroviral drug exposure: progress and prospects. Antivir. Ther. 14:1–12 [PMC free article] [PubMed] [Google Scholar]
- 5.Hammer SM, Eron JJ, Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA. 2008. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-U. S. A. panel. JAMA 300:555–570. 10.1001/jama.300.5.555 [DOI] [PubMed] [Google Scholar]
- 6.De Clercq E. 2010. Antiretroviral drugs. Curr. Opin. Pharmacol. 10:507–515. 10.1016/j.coph.2010.04.011 [DOI] [PubMed] [Google Scholar]
- 7.Chun TW, Davey RT, Jr, Engel D, Lane HC, Fauci AS. 1999. Re-emergence of HIV after stopping therapy. Nature 401:874–875. 10.1038/44755 [DOI] [PubMed] [Google Scholar]
- 8.Press N, Tyndall MW, Wood E, Hogg RS, Montaner JS. 2002. Virologic and immunologic response, clinical progression, and highly active antiretroviral therapy adherence. J. Acquir. Immune Defic. Syndr. 31(Suppl 3):S112–S117. 10.1097/00126334-200212153-00005 [DOI] [PubMed] [Google Scholar]
- 9.Costiniuk CT, Kovacs C, Routy JP, Singer J, Gurunathan S, Sekaly RP, Angel JB. 2013. Short communication: human immunodeficiency virus rebound in blood and seminal plasma following discontinuation of antiretroviral therapy. AIDS Res. Hum. Retroviruses 29:266–269. 10.1089/AID.2011.0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer S, Maldarelli F, Wiegand A, Bernstein B, Hanna GJ, Brun SC, Kempf DJ, Mellors JW, Coffin JM, King MS. 2008. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc. Natl. Acad. Sci. U. S. A. 105:3879–3884. 10.1073/pnas.0800050105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun TW, Fauci AS. 1999. Latent reservoirs of HIV: obstacles to the eradication of virus. Proc. Natl. Acad. Sci. U. S. A. 96:10958–10961. 10.1073/pnas.96.20.10958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeks SG, Wrin T, Liegler T, Hoh R, Hayden M, Barbour JD, Hellmann NS, Petropoulos CJ, McCune JM, Hellerstein MK, Grant RM. 2001. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N. Engl. J. Med. 344:472–480. 10.1056/NEJM200102153440702 [DOI] [PubMed] [Google Scholar]
- 13.Weed MR, Steward DJ. 2005. Neuropsychopathology in the SIV/macaque model of AIDS. Front. Biosci. 10:710–727. 10.2741/1566 [DOI] [PubMed] [Google Scholar]
- 14.Ansari AA, Mayne AE, Onlamoon N, Pattanapanyasat K, Mori K, Villinger F. 2004. Use of recombinant cytokines for optimized induction of antiviral immunity against SIV in the nonhuman primate model of human AIDS. Immunol. Res. 29:1–18. 10.1385/IR:29:1-3:001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rausch DM, Murray EA, Eiden LE. 1999. The SIV-infected rhesus monkey model for HIV-associated dementia and implications for neurological diseases. J. Leukoc. Biol. 65:466–474 [DOI] [PubMed] [Google Scholar]
- 16.Deere JD, Schinazi RF, North TW. 2011. Simian immunodeficiency virus macaque models of HIV latency. Curr. Opin. HIV AIDS 6:57–61. 10.1097/COH.0b013e32834086ce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearney M, Spindler J, Shao W, Maldarelli F, Palmer S, Hu SL, Lifson JD, KewalRamani VN, Mellors JW, Coffin JM, Ambrose Z. 2011. Genetic diversity of simian immunodeficiency virus encoding HIV-1 reverse transcriptase persists in macaques despite antiretroviral therapy. J. Virol. 85:1067–1076. 10.1128/JVI.01701-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.North TW, Van Rompay KK, Higgins J, Matthews TB, Wadford DA, Pedersen NC, Schinazi RF. 2005. Suppression of virus load by highly active antiretroviral therapy in rhesus macaques infected with a recombinant simian immunodeficiency virus containing reverse transcriptase from human immunodeficiency virus type 1. J. Virol. 79:7349–7354. 10.1128/JVI.79.12.7349-7354.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambrose Z, KewalRamani VN, Bieniasz PD, Hatziioannou T. 2007. HIV/AIDS: in search of an animal model. Trends Biotechnol. 25:333–337. 10.1016/j.tibtech.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 20.Hatziioannou T, Ambrose Z, Chung NP, Piatak M, Jr, Yuan F, Trubey CM, Coalter V, Kiser R, Schneider D, Smedley J, Pung R, Gathuka M, Estes JD, Veazey RS, KewalRamani VN, Lifson JD, Bieniasz PD. 2009. A macaque model of HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 106:4425–4429. 10.1073/pnas.0812587106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uberla K, Stahl-Hennig C, Bottiger D, Matz-Rensing K, Kaup FJ, Li J, Haseltine WA, Fleckenstein B, Hunsmann G, Oberg B. 1995. Animal model for the therapy of acquired immunodeficiency syndrome with reverse transcriptase inhibitors. Proc. Natl. Acad. Sci. U. S. A. 92:8210–8214. 10.1073/pnas.92.18.8210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balzarini J, Weeger M, Camarasa MJ, De Clercq E, Uberla K. 1995. Sensitivity/resistance profile of a simian immunodeficiency virus containing the reverse transcriptase gene of human immunodeficiency virus type 1 (HIV-1) toward the HIV-1-specific non-nucleoside reverse transcriptase inhibitors. Biochem. Biophys. Res. Commun. 211:850–856. 10.1006/bbrc.1995.1890 [DOI] [PubMed] [Google Scholar]
- 23.Giuffre AC, Higgins J, Buckheit RW, Jr, North TW. 2003. Susceptibilities of simian immunodeficiency virus to protease inhibitors. Antimicrob. Agents Chemother. 47:1756–1759. 10.1128/AAC.47.5.1756-1759.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofman MJ, Higgins J, Matthews TB, Pedersen NC, Tan C, Schinazi RF, North TW. 2004. Efavirenz therapy in rhesus macaques infected with a chimera of simian immunodeficiency virus containing reverse transcriptase from human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 48:3483–3490. 10.1128/AAC.48.9.3483-3490.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durand CM, Blankson JN, Siliciano RF. 2012. Developing strategies for HIV-1 eradication. Trends Immunol. 33:554–562. 10.1016/j.it.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewin SR, Rouzioux C. 2011. HIV cure and eradication: how will we get from the laboratory to effective clinical trials? AIDS 25:885–897. 10.1097/QAD.0b013e3283467041 [DOI] [PubMed] [Google Scholar]
- 27.Soderberg K, Denekamp L, Nikiforow S, Sautter K, Desrosiers RC, Alexander L. 2002. A nucleotide substitution in the tRNA(Lys) primer binding site dramatically increases replication of recombinant simian immunodeficiency virus containing a human immunodeficiency virus type 1 reverse transcriptase. J. Virol. 76:5803–5806. 10.1128/JVI.76.11.5803-5806.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marthas ML, Ramos RA, Lohman BL, Van Rompay KK, Unger RE, Miller CJ, Banapour B, Pedersen NC, Luciw PA. 1993. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed by using attenuated and pathogenic molecular clones of SIVmac. J. Virol. 67:6047–6055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deere JD, Higgins J, Cannavo E, Villalobos A, Adamson L, Fromentin E, Schinazi RF, Luciw PA, North TW. 2010. Viral decay kinetics in the highly active antiretroviral therapy-treated rhesus macaque model of AIDS. PLoS One 5:e11640. 10.1371/journal.pone.0011640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cretton EM, Schinazi RF, McClure HM, Anderson DC, Sommadossi JP. 1991. Pharmacokinetics of 3′-azido-3′-deoxythymidine and its catabolites and interactions with probenecid in rhesus monkeys. Antimicrob. Agents Chemother. 35:801–807. 10.1128/AAC.35.5.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Rompay KK, Durand-Gasselin L, Brignolo LL, Ray AS, Abel K, Cihlar T, Spinner A, Jerome C, Moore J, Kearney BP, Marthas ML, Reiser H, Bischofberger N. 2008. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob. Agents Chemother. 52:3144–3160. 10.1128/AAC.00350-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boudinot FD, Schinazi RF, Gallo JM, McClure HM, Anderson DC, Doshi KJ, Kambhampathi PC, Chu CK. 1990. 3′-Azido-2′,3′-dideoxyuridine (AzddU): comparative pharmacokinetics with 3′-azido-3′-deoxythymidine (AZT) in monkeys. AIDS Res. Hum. Retroviruses 6:219–228. 10.1089/aid.1990.6.219 [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Anaya A, Unadkat JD, Schumann LA, Smith AL. 1990. Pharmacokinetics of zidovudine (azidothymidine). II. Development of metabolic and renal clearance pathways in the neonate. J. Acquir. Immune Defic. Syndr. 3:1052–1058 [PubMed] [Google Scholar]
- 34.Hazuda DJ, Young SD, Guare JP, Anthony NJ, Gomez RP, Wai JS, Vacca JP, Handt L, Motzel SL, Klein HJ, Dornadula G, Danovich RM, Witmer MV, Wilson KA, Tussey L, Schleif WA, Gabryelski LS, Jin L, Miller MD, Casimiro DR, Emini EA, Shiver JW. 2004. Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques. Science 305:528–532. 10.1126/science.1098632 [DOI] [PubMed] [Google Scholar]
- 35.Leutenegger CM, Higgins J, Matthews TB, Tarantal AF, Luciw PA, Pedersen NC, North TW. 2001. Real-time TaqMan PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantitation of simian immunodeficiency virus RNA. AIDS Res. Hum. Retroviruses 17:243–251. 10.1089/088922201750063160 [DOI] [PubMed] [Google Scholar]
- 36.Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho DD. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188–191. 10.1038/387188a0 [DOI] [PubMed] [Google Scholar]
- 37.North TW, Higgins J, Deere JD, Hayes TL, Villalobos A, Adamson L, Shacklett BL, Schinazi RF, Luciw PA. 2010. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J. Virol. 84:2913–2922. 10.1128/JVI.02356-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray JM, Emery S, Kelleher AD, Law M, Chen J, Hazuda DJ, Nguyen BY, Teppler H, Cooper DA. 2007. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS 21:2315–2321. 10.1097/QAD.0b013e3282f12377 [DOI] [PubMed] [Google Scholar]
- 39.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5:512–517. 10.1038/8394 [DOI] [PubMed] [Google Scholar]
- 40.Markowitz M, Evering TH, Garmon D, Caskey M, La Mar M, Rodriguez K, Sahi V, Palmer S, Prada N, Mohri H. 21 January 2014. A randomized open-label study of three- versus five-drug combination antiretroviral therapy in newly HIV-1 infected individuals. J. Acquir. Immune Defic. Syndr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apetrei C, Pandrea I, Mellors JW. 2012. Nonhuman primate models for HIV cure research. PLoS Pathog. 8:e1002892. 10.1371/journal.ppat.1002892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shytaj IL, Norelli S, Chirullo B, Della Corte A, Collins M, Yalley-Ogunro J, Greenhouse J, Iraci N, Acosta EP, Barreca ML, Lewis MG, Savarino A. 2012. A highly intensified ART regimen induces long-term viral suppression and restriction of the viral reservoir in a simian AIDS model. PLoS Pathog. 8:e1002774. 10.1371/journal.ppat.1002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kline C, Ndjomou J, Franks T, Kiser R, Coalter V, Smedley J, Piatak M, Jr, Mellors JW, Lifson JD, Ambrose Z. 2013. Persistence of viral reservoirs in multiple tissues after antiretroviral therapy suppression in a macaque RT-SHIV model. PLoS One 8:e84275. 10.1371/journal.pone.0084275 [DOI] [PMC free article] [PubMed] [Google Scholar]