Abstract
The aim of this study was to evaluate the biopharmaceutical characteristics of three fluoroquinolones (FQs), ciprofloxacin (CIP), moxifloxacin (MXF), and grepafloxacin (GRX), after delivery via a nebulized aerosol to rats. Bronchoalveolar lavages (BAL) were conducted 0.5, 2, 4, and 6 h after FQ intravenous administration and nebulized aerosol delivery to estimate epithelial lining fluid (ELF) drug concentrations. Plasma drug concentrations were also measured, and profiles of drug concentrations versus time after intravenous administration and nebulized aerosol delivery were virtually superimposable, attesting for rapid and complete systemic absorption of FQs. ELF drug concentrations were systematically higher than corresponding plasma drug concentrations, whatever the route of administration, and average ELF-to-unbound plasma drug concentration ratios post-distribution equilibrium did not change significantly between the ways of administration and were equal: 4.0 ± 5.3 for CIP, 12.6 ± 7.3 for MXF, and 19.1 ± 10.5 for GRX (means ± standard deviations). The impact of macrophage lysis on estimated ELF drug concentrations was significant for GRX but reduced for MXF and CIP; therefore, simultaneous pharmacokinetic modeling of plasma and ELF drug concentrations was only performed for the latter two drugs. The model was characterized by a fixed volume of ELF (VELF), passive diffusion clearance (QELF), and active efflux clearance (CLout) between plasma and ELF, indicating active efflux transport systems. In conclusion, this study demonstrates that ELF drug concentrations of these three FQs are several times higher than plasma drug concentrations, probably due to the presence of efflux transporters at the pulmonary barrier level, but no biopharmaceutical advantage of FQ nebulization was observed compared with intravenous administration.
INTRODUCTION
Aerosol delivery of antimicrobial agents is receiving increasing interest, as it could provide an advantage over other routes of administration for the treatment of pulmonary infections by achieving higher drug concentrations at the infection site and lower systemic exposure and toxicity (1). Several antibiotics are administered as aerosols, including tobramycin, colistin methanesulfonate, and aztreonam (2, 3), to cystic fibrosis patients (4) and to critically ill patients (5). Aerosol delivery of fluoroquinolones (FQs), such as ciprofloxacin (CIP) or levofloxacin, is currently under development (6).
An important issue with aerosol administration is the ability of pulmonary cells to form a barrier that controls drug diffusion to maintain high sustained antibiotic concentrations within the epithelial lining fluid (ELF) (7). FQs diffuse passively, and recent in vitro studies using a Calu-3 lung epithelial cell line model showed that the apparent passive permeability (Papp) of an FQ is related to its lipophilicity (8, 9). But active transport is another important issue to consider. Indeed, P-glycoprotein (P-gp), an efflux pump constitutive in various biological barriers, in particular the blood-brain barrier (10), has been identified at the apical membrane of human and rat alveolar epithelial cells (11), and we have shown that in Calu-3 cells, FQs are also actively transported by P-gp (8, 9), suggesting that their unbound drug concentrations at steady state in lung ELF should be higher than in plasma (12).
The objective of this study was to investigate in vivo the biopharmaceutical characteristics of various FQs, previously compared in vitro using Calu-3 cells, after administration to rats via nebulized aerosol.
MATERIALS AND METHODS
Chemicals.
CIP was purchased from Sigma and was used to prepare CIP solutions in 0.9% NaCl for intravenous (i.v.) administration and nebulization, respectively. Moxifloxacin hydrochloride (MXF) and grepafloxacin hydrochloride (GRX) were provided by Bayer Healthcare (Leverkusen, Germany) and Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan), respectively, and both were used to prepare GRX and MXF solutions in 5% glucose for i.v. administration and nebulization. All chemicals used were of analytical grade, and solvents were of high-performance liquid chromatography (HPLC) grade.
Animals.
This work was carried out in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals (13) under agreement 86.051. Male Sprague-Dawley rats (n = 147) from Janvier Laboratories (Le Genest-St.-Isle, France), weighing between 300 and 350 g, were used for the in vivo pharmacokinetic investigations. Two extra rats (male, Sprague-Dawley) weighing between 450 and 560 g were used for obtaining alveolar macrophages to perform FQ uptake experiments. All animals were acclimatized for 5 days after their arrival and before experiments, as previously described (14).
FQ uptake in rat alveolar macrophages.
Rats were deeply anesthetized via intraperitoneal injection of ketamine and xylazine (90 and 2 mg/kg of body weight, respectively). The trachea was cannulated and the rib cage opened. Lungs were flushed with 10 separate 10-ml volumes of phosphate-buffered saline (PBS) solution containing 1% (wt/vol) penicillin and streptomycin. The lavage fluid was centrifuged at 1,000 × g at 4°C for 10 min, and the pellet was resuspended in culture medium, Dulbecco's modified Eagle's medium (DMEM) and Ham's F-12 (1:1) supplemented with l-glutamine (2 mM), fetal calf serum (10% [vol/vol]), and 1% (wt/vol) penicillin and streptomycin. Purity of macrophages was assessed by May-Grunwald-Giemsa staining and was about 95%. An total of 250,000 cells per well were seeded in 24-well plates at 37°C. After 1 h of incubation in order to allow the cells to attach to the bottom of the wells, culture medium was replaced with Hank's buffered salt solution (HBSS) with HEPES (pH 7.4) containing 25 μM (about 10 μg ml−1; the extracellular drug concentration [Cextra]) of GRX, MFX, or CIP. Following 90 min of incubation at 37°C, cells were washed 3 times with ice-cold PBS, and the wells were let to dry overnight at 4°C. Cells were then lysed by addition of 100 μl water for 1 h. FQ concentrations were assessed by HPLC. The macrophage-associated concentration (CAM) of an FQ was calculated according to equation 1:
| (1) |
where Xcell is the amount of FQ in 100 μl of cell lysate, nAM is the number of macrophages per well, and VAM is the volume of alveolar macrophages, set at 1,200/μm3, as reported in the literature (15). The value of K, related to FQ accumulation in alveolar marcrophages, was then determined as the ratio between intracellular (CAM) and extracellular concentration (Cextra) (equation 2).
| (2) |
FQ administration and collection of samples for the plasma PK study.
For the plasma PK study, catheters were introduced as previously described (14) into the femoral vein and artery the day before the experiment for drug administration and plasma collection.
Intravenous bolus administration of FQs (n = 19).
The i.v. bolus administration of CIP (n = 7), MXF (n = 6), and GRX (n = 6) at doses of 7.5, 5, and 5 mg kg−1, respectively, was performed via the left femoral vein. Arterial blood samples were collected before administration and at 0.083 (5 min), 0.25, 0.5, 1, 2, 4, 6, and 8 h postdosing via the left femoral artery catheter. Plasma was separated by centrifugation and frozen at −20°C until analysis.
Intratracheal administration of nebulized aerosols of FQs (n = 16).
FQ doses for intratracheal administration of nebulized aerosols of CIP (n = 6), MXF (n = 5), and GRX (n = 5) were 7.5, 7.5, and 5 mg kg−1, respectively, which corresponded to approximate nebulized aerosol volumes of 150 μl for CIP and 225 μl for MXF and GRX. The nebulization was performed using a MicroSprayer IA-1B apparatus (Penn Century Inc., Philadelphia, PA) inserted between the vocal cords of anesthetized rats, as previously described (14). After administration, arterial blood samples were collected before administration and at 0.083, 0.25, 0.5, 1, 2, 4, 6, and 8 h postnebulization.
FQ administrations and collection of samples for determination of local concentrations by BAL.
CIP (n = 57), MXF (n = 33), and GRX (n = 22) as nebulized aerosols were administered intratracheally under isoflurane anesthesia or were administered i.v. in freely moving rats at respective doses of 7.5, 7.5, and 5 mg kg−1. BAL fluid collection was carried out according to the method of Marchand et al. (14) at 2 h and 4 h after administration (4 to 7 rats per group) for all FQs. Extra BAL sampling was performed at 0.5 h for MXF and CIP and at 6 h for CIP. BAL fluid samples were obtained after tracheal administration of 1 ml of 0.9% NaCl solution at 37°C, and a maximum volume was aspirated.
Analytical assays. (i) CIP analysis in plasma and BAL.
Determination of CIP concentrations in plasma and BAL was performed by the liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. Reversed-phase chromatography was performed on a security guard cartridge (Gemini C18; 4 by 2 mm; Phenomenex, California) and a C18 Jupiter C18 300A column (50 by 2 mm, internal diameter, with 5-μm pore size; Phenomenex, California). The mobile phase was 0.1% (vol/vol) formic acid in acetonitrile and 0.1% formic acid in water (25:75 [vol/vol]), and the pump flow rate was 0.18 ml min−1. The LC-MS/MS system consisted of a Waters Alliance 2695 separation module, equipped with a binary pump with an autosampler with the thermostat set at 4°C, along with a Waters Micromass Quattro micro API tandem mass spectrometer. The mass spectrometer was operated in the positive/ion mode. Ions were analyzed by multiple reactions monitoring (MRM). Transition ions were m/z 332.2/314.2 for CIP and 402.2/384.2 for MXF, the internal standard.
For CIP concentration determinations in plasma, seven-point calibration standards with concentrations between 0.01 and 2 μg ml−1 and 3 levels of the control were prepared. For the preparation of CIP calibration standards, controls, and samples, 200 μl of internal standard solution containing 0.25 μg ml−1 of MXF in acetonitrile was added to 50 μl of plasma or sample and then centrifuged at 1,500 × g during 5 min at 4°C. After centrifugation, 200 μl of the mixture was added to 400 μl of water.
For CIP analysis in BAL fluid, seven-point calibration standards in NaCl (0.9%) with drug concentrations between 0.004 and 2 μg ml−1 and 3 levels of control (0.01, 0.2, and 1.8 μg ml−1) were prepared. A volume (50 μl) of BAL sample was mixed with 50 μl of internal standard solution (0.25 μg ml−1 of MXF).
(ii) MXF and GRX analyses in plasma and BAL fluid.
Samples containing MXF and GRX were analyzed by reversed-phase HPLC with fluorescence detection (λex, 290 nm; λem, 460 nm). The analysis was based on a method previously described by Hemanth Kumar et al. (16). Briefly, seven-point calibration standards in plasma (between 0.02 and 2 μg ml−1 for GRX and 0.03 and 3 μg ml−1 for MXF) and three levels of controls were prepared for both FQs. The calibration standards, controls, and samples were prepared with 50 μl of plasma added to 100 μl of 7% perchloric acid, and then the mixture was centrifuged at 1,500 × g for 10 min at 4°C.
For MXF and GRX analysis in BAL fluid, six-point calibration standards in NaCl (0.9%) with concentrations between 0.004 and 0.1 μg ml−1 for MXF and 0.004 and 0.2 μg ml−1 for GRX and 3 levels of controls were prepared.
For all media and chromatographic systems used in this study, intra- and interday variabilities for FQs were characterized at three levels of drug concentration (low, medium, and high), with a precision and accuracy always lower than 15%. Furthermore, no experimental measurements were outside the standard curves.
(iii) Urea analysis in BAL fluid and plasma.
Urea concentrations were determined in BAL fluid by using LC-MS/MS, and the analysis was adapted from a previously described method (14). Eight-point calibration standards were made in NaCl (0.9%) between 1.25 and 100 μg ml−1. The limit of quantification (LOQ) for urea determination in BAL fluid was estimated at 1.25 μg ml−1, and no experimental measurements were outside the standard curves. Intra- and interday variabilities were characterized at these four concentrations with precision and accuracy lower than 15% for 75, 25, and 2.5 μg ml−1 concentrations and lower than 20% for the LOQ.
Urea concentrations in plasma were measured by photometric detection using a modular automatic analyzer (Roche, France).
Determination of FQ concentrations in ELF.
In order to estimate ELF FQ concentrations, FQ concentrations measured in BAL aspirates were corrected by a dilution factor obtained from urea measurements in plasma and BAL fluid, according to equation 3:
| (3) |
where [CELF(estimated)] is the estimated concentration of CIP, MXF, and GRX in ELF; CBAL corresponds to the FQ concentration measured in BAL fluid; UreaBAL and Ureaplasma are the urea concentrations determined in BAL fluid and plasma.
Urea as a marker of dilution was also used to estimate the volume of ELF according to equation 4 (17):
| (4) |
where VBAL corresponds to the aspirated BAL volume.
Impact of alveolar macrophage lysis.
A correction for the lysis effect on alveolar macrophages was determined by using equation 5, according to the methods described by Kiem and Schentag (18):
| (5) |
where CELF(estimated) is the estimated (experimental) FQ concentration in ELF, Vlysis is the volume of lysed cells (macrophages), VELF is the volume of ELF, CELF(actual) is the actual concentration in ELF that would be measured in the absence of cell lysis, and Cintracellular is the FQ concentration in alveolar macrophages.
The Cintracellular in equation 5 was replaced by the product of CELF(actual) and K (see equation 6), where K represents the FQ accumulation determined in in vitro uptake experiments in rat alveolar macrophages.
| (6) |
Equation 7 was obtained by combining equation 5 and equation 6 to evaluate the impact of macrophage lysis.
| (7) |
Simultaneous PK modeling of plasma and ELF drug concentrations.
For CIP and MXF, drug concentrations in plasma and ELF versus time were analyzed simultaneously by using a nonlinear mixed-effects method with the S-ADAPT software (version 1.52) and the MC-PEM (Monte-Carlo parametric expectation maximization) estimation algorithm within the S-ADAPT TRAN translator (19). Modeling was initiated from a generic PK hybrid model that included an ELF compartment that was characterized by a fixed physiological ELF volume (VELF) set at 30 μl kg−1 and connected to a traditional compartment model by a reversible first-order process. Whether the PK in plasma was mono-, bi-, or tricompartmental was determined, while the FQ PK in ELF was assumed to be monocompartmental. Likelihood ratio tests were used to compare models, using a P value of 0.01 required for statistical significance. Only the unbound drug in plasma was assumed to cross the membrane to penetrate within ELF, and unbound fractions in plasma were fixed at 59% and 63% for CIP and MXF, respectively, according to the published data (20, 21). The interindividual variability (IIV) of PK parameters was modeled assuming a log-normal distribution, and the residual variabilities in plasma and ELF were estimated with a combined additive plus proportional error model. Plasma drug concentrations below the limit of quantification were handled by using the Beal M3 method (22). Typical values and interindividual variabilities of PK parameters are reported below in Table 2.
TABLE 2.
PK parameters estimated for CIP and MXF based on simultaneous analyses of plasma and ELF drug concentrations following i.v. administration or intratracheal administration of nebulized drug
| Parametera (units) | CIP value (CVb [%]) | MXF value (CVb [%]) |
|---|---|---|
| Vc (liters/kg) | 2.39 (36) | 0.844 (38) |
| VP1 (liters/kg) | 2.09 | 1.22 |
| VP2 (liters/kg) | 1.18 | |
| VELF (liters/kg) | 30 × 10−6 (fixed) | 30 × 10−6 (fixed) |
| Faero (%) | 98 | 98 |
| CLT (liters/h/kg) | 3.42 (27) | 1.60 (24) |
| Q1 (liters/h/kg) | 3.56 (9) | 5.71 |
| Q2 (liters/h/kg) | 0.632 (39) | |
| QELF (liters/h/kg) | 0.634 × 10−3 (7) | 6.15 × 10−3 (74) |
| CLout (liters/h/kg) | 0.700 × 10−3 (134) | 57.1 × 10−3 |
| Residual errors | ||
| Plasma | ||
| Additive (μg/ml) | 0.004 | 0.004 |
| Proportional (%) | 12 | 6 |
| ELF | ||
| Proportional (%) | 7 | 12 |
Vc, volume of distribution in central compartment; VP1 and VP2, volumes of distribution in peripheral compartments; VELF, fixed volume of distribution in ELF compartment (30 × 10−6 liters/kg); Faero, systemic bioavailability after nebulization; CLT, total systemic clearance; Q1 and Q2, equilibrium distribution clearances between central compartment and peripheral compartments; QELF and CLout, transfer clearances between central compartment and ELF compartment.
CV, interindividual variability, expressed as the coefficient of variation.
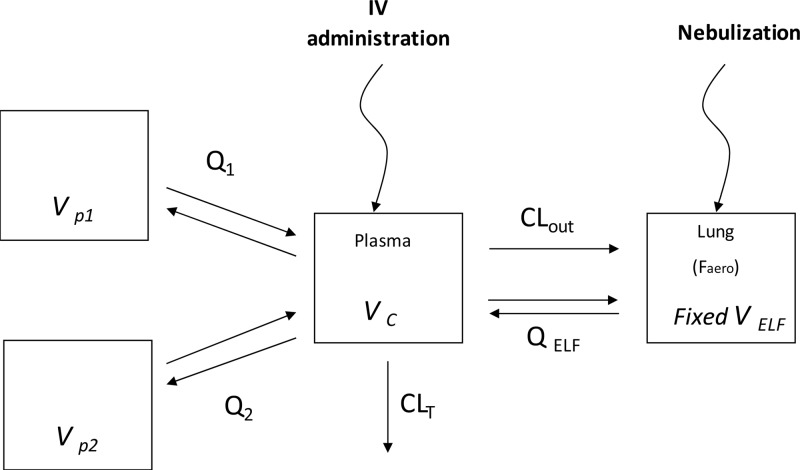
The final selected PK model (Fig. 1) was characterized by a central compartment (Vc) and one (CIP) or two (MOX) peripheral compartments (VP1 and VP2), with corresponding equilibrium distribution clearances (Q1 and Q2), and by a total systemic clearance (CLT) plus a bioavailability coefficient (Faero) after delivery of the nebulized form. Drug exchanges between plasma and ELF were described by two-way diffusion clearance (QELF) and by efflux clearance (CLout) from the plasma to ELF compartment (Fig. 1).
FIG 1.
Structural model used for simultaneous PK analysis of plasma and ELF drug concentrations following i.v. administration or intratracheal administration in nebulized form of MXF, where the Vc is the volume of the central compartment, VELF is the volume of the ELF compartment, which was fixed at 30 × 10−6 liters kg−1, and VP1 and VP2 are the volumes of peripheral compartments. CLT corresponds to total plasma clearance, Q1 and Q2 are distribution clearances between the central compartment and peripheral compartments, QELF is the diffusion clearance between the central compartment and ELF, CLout is the transfer efflux clearance between the central compartment and ELF compartment, and Faero is the systemic bioavailability after aerosol administration. The same model was used for CIP, except for the absence of peripheral compartment 2.
Statistical analysis.
ELF drug concentrations and ELF versus unbound plasma drug concentration ratios between routes of administration were analyzed for each compound by using the Kruskal-Wallis test followed by Dunn's multiple comparison test for each time of sampling (Prism5; GraphPad, La Jolla, CA). Differences were considered significant at a P level of <0.05.
RESULTS
K values estimated in the uptake experiments to characterize FQ accumulation in alveolar macrophages were 1.5 ± 0.1 for CIP, 4.3 ± 0.7 for MXF, and 25.2 ± 0.9 for GRX (means ± standard deviations).
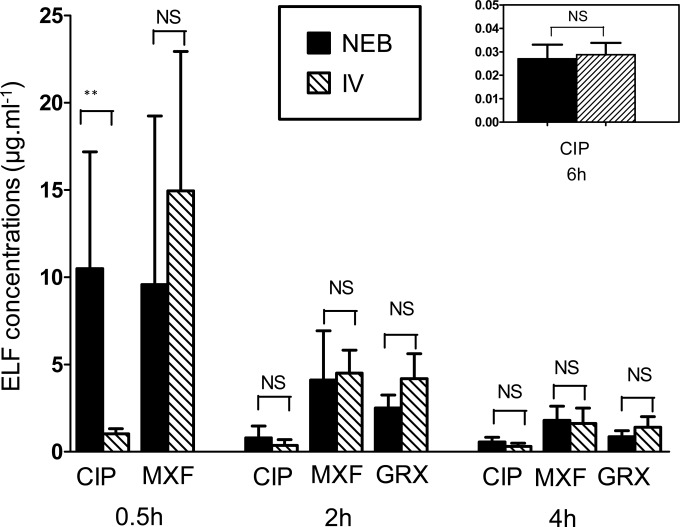
ELF drug concentrations estimated at 0.5 h, 2 h, 4 h, and 6 h after i.v. administration or administration of nebulized aerosol of each FQ are shown in Fig. 2. The mean ELF concentration of CIP 0.5 h after administration of the nebulized aerosol was 10 times higher (10.5 ± 6.3 μg ml−1) than the corresponding ELF drug concentration after i.v. administration (1.03 ± 0.29 μg ml−1). These differences in ELF drug concentrations as a function of the route of administration were not observed for CIP at other sampling times (2, 4, and 6 h) (Fig. 2). For MXF and GRX, no significant difference was shown between estimated ELF drug concentrations after i.v. versus nebulized aerosol administration at any time (Fig. 2). However, FQ ELF concentrations were systematically higher than corresponding plasma drug concentrations, whatever the route of administration, as illustrated for CIP and MXF in Fig. 3. At postdistribution equilibrium (2, 4, and 6 h), ratios of estimated ELF versus unbound plasma drug concentrations [CELF(estimated)/C(unbound plasma)] did not vary significantly with the route of administration (P > 0.05) (Table 1). Therefore, after pooling the data, ELF-to-unbound plasma drug concentration ratios in the postdistribution phase were equal to 4.0 ± 5.3 for CIP, 12.6 ± 7.3 for MXF, and 19.1 ± 10.5 for GRX.
FIG 2.
Mean ± SD concentrations of CIP, MXF, and GRX estimated in ELF [CELF(estimated)] at 0.5 h, 2 h, 4 h, and 6 h after i.v. administration or administration intratracheally of nebulized forms at doses of 7.5 mg kg−1 for CIP and MXF and 5 mg kg−1 for GRX. NS, not significantly different; **, P < 0.001.
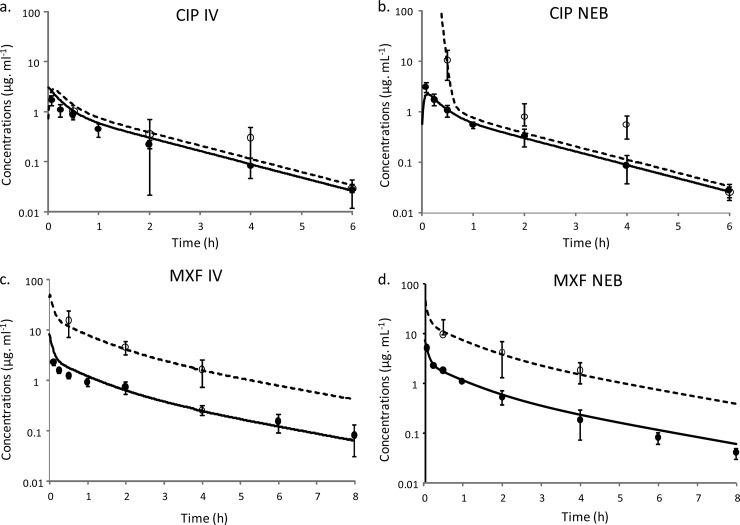
FIG 3.
Concentration-time profiles of CIP and MXF following i.v. administration (a and c) and administraion of the nebulized form (b and d) in plasma (closed symbols and solid line) and in ELF (open symbols and dashed line), predicted from simultaneous PK modeling of plasma and ELF data. Symbols represent means ± SD concentrations measured in plasma and ELF.
TABLE 1.
C(ELF estimated)/C(unbound plasma) ratios for CIP, MXF, and GRX at 0.5, 2, 4, and 6 h after i.v. administration or intratracheal administration of nebulized drugs
| Time postdosing (h) |
C(ELF estimated)/C(unbound plasma) ratioa |
|||||
|---|---|---|---|---|---|---|
| CIP |
MXF |
GXF |
||||
| i.v. | NEB | i.v. | NEB | i.v. | NEB | |
| 0.5 | 1.9 ± 0.5 | 19.2 ± 16.0b | 15.1 ± 9.1 | 8.1 ± 7.7 | NA | NA |
| 2 | 2.4 ± 2.1 | 3.2 ± 2.2 | 10.1 ± 3.7 | 13.1 ± 9.8 | 29.9 ± 11.0 | 12.7 ± 3.7 |
| 4 | 5.1 ± 3.8 | 10.9 ± 10.2 | 9.7 ± 4.2 | 18.1 ± 8.7 | 21.9 ± 10.9 | 12.7 ± 4.4 |
| 6 | 1.9 ± 0.4 | 1.3 ± 0.4 | NA | NA | NA | NA |
CIP, MXF, and GRX C(ELF estimated)/C(unbound plasma) ratios were not significantly different postdistribution (2, 4, and 6 h). NEB, nebulized form; NA, not available.
The CIP C(ELF estimated)/C(unbound plasma) ratio after nebulization was significantly different from the corresponding ratio after i.v. administration (P < 0.05).
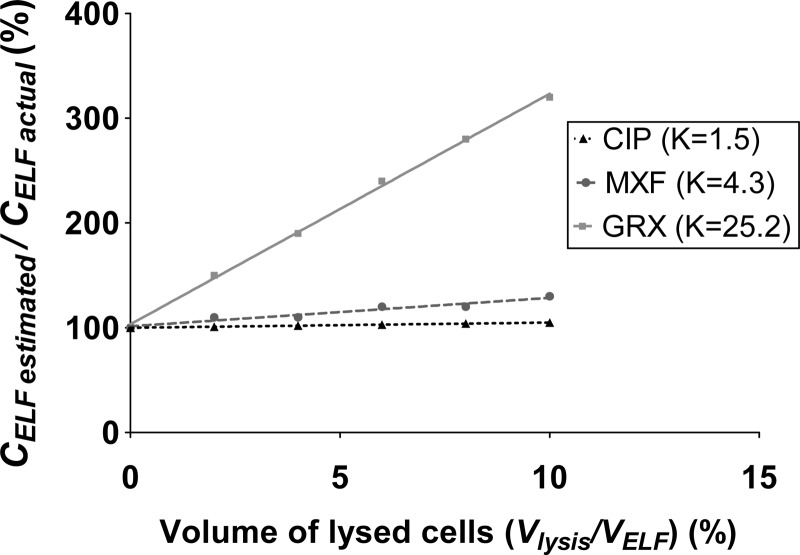
Overestimation of the ELF drug concentration due to macrophage lysis, predicted from equation 7, is presented in Fig. 4. For CIP, which is characterized by the lowest intracellular accumulation (K = 1.5 ± 0.1), the maximum lysis (Vlysis/VELF, 10%) would only overestimate the actual ELF concentration by 5%, which is within the analytical error and therefore negligible (Fig. 4). For MXF, which is characterized by a greater intracellular accumulation than CIP (K = 4.3 ± 0.7), the estimated ELF drug concentration would overestimate the actual value by 30%, for a maximum Vlysis/VELF of 10%, but only by 15% when the Vlysis/VELF is 5% (Fig. 4). For GRX, which presents the greatest intracellular penetration (K = 25.2 ± 0.9), a difference of more than 300% between the estimated and actual ELF drug concentrations was observed when lysis was maximum (Vlysis/VELF, 10%) (Fig. 4).
FIG 4.
CELF(estimated)/CELF(actual) ratio predicted from equation 7, as a function of volume of lysed cells during BAL and K values determined for CIP, MXF, and GRX.
For this reason, no further investigations were conducted for GRX. For CIP and MXF, plasma drug concentrations-versus-time curves resulting after i.v. administration or administration via nebulized aerosol were almost superimposable (Fig. 3), and maximum plasma drug concentrations were observed at the first sampling time (5 min) after administration of the nebulized aerosol.
No distinction was made between FQs or route of administration to estimate the ELF volume, leading to an average value equal to 28.8 ± 22.3 × 10−6 liters kg−1 (28.8 ± 22.3 μ1 kg−1), close to the 30 µl kg−1 value used for the modeling.
Simultaneous PK modeling provided an adequate fitting of MXF plasma and ELF data (Fig. 3). Parameters estimated from the model are presented in Table 2. Modeling was not as good with CIP, due to greater data scattering (especially at 4 h) (Fig. 3), in accordance with a high estimated interindividual variability of the CLout (coefficient of variation [CV], 134%) (Table 2). Passive clearance by diffusion (QELF) was higher for MXF than for CIP (Table 1), and the presence of an extra efflux transport between plasma and ELF compartments (CLout) (Table 2) in addition to passive diffusion accounted for higher ELF than unbound plasma drug concentrations (Fig. 3). The CLout-to-QELF ratio was close to 10 for MXF and to 1 for CIP (Table 2), in accordance with higher ELF drug concentrations and with higher ELF-to-unbound plasma drug concentration ratios for MXF than for CIP, as illustrated in Fig. 3 and summarized in Table 1.
DISCUSSION
The assessment of the intrapulmonary distribution of antibiotics in vivo is not an easy task. Whole-tissue drug concentration measurement is not recommended (23), and more sophisticated methods, such as lung microdialysis (24), are not routinely applicable (25). Therefore, BAL is the most common and probably the most relevant technique for the in vivo investigation of antibiotic lung distribution (18). However, Kiem and Schentag have recently suggested that erroneous data could result from uncontrolled lysis of alveolar macrophages occurring during BAL, especially for antibiotics with an extensive intracellular distribution (18). Using retrospective data, those authors concluded that this phenomenon could be responsible for an artificially high ELF-to-unbound plasma drug concentration ratio, depending on the FQ, and they have suggested a classification based on the ELF-to-unbound plasma drug concentration ratio (18). For the present study, we selected three representative FQs belonging to these different groups. CIP represents a group for which the ratio between ELF and unbound plasma drug concentrations is moderately higher than unity (1 to 5) and may be explained by macrophage lysis. MXF represents a group for which the ratio between ELF and unbound plasma drug concentrations is much higher than unity (>5), but this cannot be explained solely by macrophage lysis. GRX was described as an outlier in the FQ classification, with characteristics similar to macrolides, meaning that even limited lysis could explain high ELF-to-unbound plasma drug concentration ratios. But the BAL technique raises a number of issues. First, the volume of NaCl solution used for BAL should be selected appropriately. Volumes ranging between 5 and 10 ml are frequently used for BAL in rats (26–28). However, these volumes are high compared to the tidal volume, which is close to 1 ml for a rat weighing 300 g (29). Therefore, in the present study the volume was limited to 1 ml in order to preserve the integrity of the pulmonary barrier as much as possible. A second issue during the BAL procedure is the use of urea as a marker of dilution (17). Urea is an endogenous molecule that freely crosses the pulmonary barrier. In a normal physiological state and therefore before BAL, the urea concentration is similar in plasma and ELF, which provides the rationale for using urea as a marker of dilution (17). However, during BAL, urea may diffuse quickly out of plasma, as urea concentrations tend to reequilibrate, increasing artificially the urea concentration in the BAL aspirate. According to Rennard et al. (17), with a short-duration aspiration (20 s), most of the recovered urea comes from ELF prior to lavage, and in this case urea is an appropriate marker of dilution to predict ELF drug concentrations. Therefore, in the present study, saline aspiration was performed only once and for no longer than 15 to 20 s in order to avoid a significant “dwelling time.” In order to further optimize the whole procedure, a sensitive LC-MS/MS assay was developed and validated for urea quantification in BAL samples (14). All these limits contribute to the high variability observed in ELF drug concentrations (Fig. 2).
Another difficulty with drugs that penetrate extensively within cells, such as FQs, consists of assessing the effect of cell lysis occurring during BAL on estimated ELF concentrations. Consequently, the in vivo study was completed by in vitro uptake experiments in freshly isolated rat alveolar macrophages to determine FQ accumulation in these cells and to characterize K values. The experiment duration (90 min) was longer than necessary, to reach equilibration (30). FQ accumulation differed between drugs: it was limited for CIP (K = 1.5 ± 0.1), intermediate for MXF (K = 4.3 ± 0.7), and quite large for GRX (K = 25.2 ± 0.9). These results are consistent with data in the literature, since K values of 3 for CIP and 16 for MXF were reported in experiments using J774 murine macrophages (30), 7 for CIP and 8 for MXF when using human macrophage cell line THP-1 cells (31), and 6.6 for CIP and 24.1 for GRX when using the same human macrophages from cell line THP-1 (32). Differences in apparent cell accumulation between these three FQs may be related to their lipophilicity, with the highest cell accumulation for GRX and the lowest for CIP, in agreement with log(D) values at physiological pH equal to 0.03, −0.28, and −0.93 for GRX, MFX, and CIP respectively (8). Moreover, these three representative FQs also differ in terms of P-gp affinity (8, 9).The actual extent of lysis was previously estimated based on cell counting in human fluid. It was determined that in 100 μl of ELF, 4 to 10 μl may correspond to the alveolar macrophage volume; hence, up to 10% of the total ELF volume may correspond to intracellular lysate (18). This potential bias due to macrophage lysis was greater for MXF than for CIP (Fig. 4). However, it was still relatively modest and it will be considered for the rest of this study that estimated ELF concentrations of MXF are only slightly overestimated and sufficiently close to the actual values for modeling purposes. But this was not the case for GRX, which presents the greatest intracellular penetration (K = 25.2 ± 0.9), leading to a difference of more than 300% between the estimated and actual ELF drug concentrations when lysis is maximum (Vlysis/VELF = 10%) (Fig. 4). Although the average ELF-to-unbound plasma drug concentration ratios estimated during the present study in rats exhibited relatively large interindividual variabilities, they were remarkably consistent with those previously obtained in studies in humans. In fact, at distribution equilibrium in rats, ratios for CIP, MXF, and GRX were equal to 4.0 ± 5.3, 12.6 ± 7.3, and 19.1 ± 10.5, which are close to the results obtained in humans 4 to 24 h after oral administrations, with ratio values lower than 4 for CIP (33–35), between 7.0 and 14.6 for MXF (36, 37), and close to 23 for GRX (38). However, as ELF drug concentrations are probably not reliable due to uncontrolled and unavoidable cell lysis, GRX data were not submitted for the further experiments.
Simple visual inspection of experimental data clearly showed that in this experimental setting, the route of administration had no major effect on FQ distribution within ELF, as opposed to what was observed with colistin (39). Yet, in order to better quantify this distribution process and then conduct precise comparisons between compounds, a hybrid PK model was developed. ELF volume (VELF) was fixed to a best guess estimate of its physiological value derived from urea concentration determinations and consequently was independent of the drug administered. We decided to set this parameter in the PK model at 30 × 10−6 liters kg−1, corresponding to 30 μl kg−1. This value was very close to that estimated in the present study (28.8 ± 22.3 × 10−6 liters kg−1) and consistent with a previous estimate made by our group (7.6 ± 5.3 × 10−6 liters, corresponding to 24.3 ± 19.2 × 10−6 liters kg−1) (14). Noticeably, these VELF estimates for rats are close to the initial estimate for humans, performed by Rennard et al. in 1986 (1.0 ± 0.1 ml, corresponding to 14 × 10−6 liters kg−1, assuming a body weight of 70 kg) (17), and also to more recent estimates of 0.99 ± 0.62 ml (12.5 × 10−6 liters kg−1) and 0.77 ± 0.50 ml (11 × 10−6 liters kg−1) used for measurements of MXF and CIP ELF drug concentrations, respectively, in humans (36, 40).
The estimated passive distribution clearance (QELF) was higher for MXF (6.15 × 10−3 liters h−1 kg−1) than for CIP (0.634 × 10−3 liters h−1 kg−1) (Table 1), in agreement with respective log(D) values at physiological pH for these two drugs (−0.28 and −0.93) (8). The addition of a CLout term from ELF to plasma provided a statistically significant improvement of the modeling and reflects the higher ELF than unbound plasma drug concentrations observed, independent of the route of administration (Fig. 1). This CLout term could characterize an active efflux transport (12), possibly mediated by P-gp, which is present in alveolar epithelial cells at apical sides (11). This observation is also consistent with our previous in vitro observations in Calu-3 cells (8, 9). Yet, in vitro, the relative contribution of P-gp efflux was greater with CIP than with MXF (efflux ratios equal to 4 and 2.1, respectively), whereas in vivo the CLout-to-QELF ratio was 10 times lower for CIP than for MXF (Table 2). However, the higher CLout-to-QELF ratio for MXF than for CIP is in accordance with ELF-to-unbound plasma drug concentration ratios for these two compounds (Tables 1 and 2).
Unlike colistin, for which a pronounced effect of the route of administration was observed, with ELF drug concentrations significantly higher after delivery in the nebulized form than after i.v. administration (39), these biopharmaceutical characteristics do not suggest any advantage of FQs nebulization compared with i.v. administration. One may only expect ELF drug concentrations to be transiently higher after administration of the nebulized form than after i.v. administration, which may be beneficial for these concentration-dependent antibiotics. However, the impact of infection on ELF FQ distributions was not evaluated in the present study, and this presents a major limit for our study. In fact, a superior antimicrobial effect of levofloxacin aerosol over i.v. administration was observed in a mouse model of lung infection due to Pseudomonas aeruginosa (41). However, the reduction of systemic exposure and toxicity, which is an expected advantage of aerosol delivery (1), is not supported by these new data. Notably, in clinical practice, FQ nebulization should be compared with oral instead of i.v. administration.
In conclusion, CIP and MXF as representative FQs present similar biopharmaceutical characteristics after nebulization, with a rapid equilibration between lung and plasma and drug concentration profiles in ELF and plasma that were virtually superimposable whatever the route of administration. FQ ELF concentrations were also higher than corresponding plasma drug concentrations whatever the route of administration, likely due to the presence of transporters with an apical localization at alveolar epithelial cells. Yet, this study failed to demonstrate any biopharmaceutical advantage of FQ nebulization compared with i.v. administration, at least in healthy rats.
ACKNOWLEDGMENT
A.V.L. Gontijo is supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil.
Footnotes
Published ahead of print 5 May 2014
REFERENCES
- 1.Hagerman JK, Hancock KE, Klepser ME. 2006. Aerosolised antibiotics: a critical appraisal of their use. Expert Opin. Drug Deliv. 3:71–86. 10.1517/17425247.3.1.71 [DOI] [PubMed] [Google Scholar]
- 2.Michalopoulos AS. 2012. Aerosolized antibiotics: the past, present and future, with a special emphasis on inhaled colistin. Expert Opin. Drug Deliv. 9:493–495. 10.1517/17425247.2012.676039 [DOI] [PubMed] [Google Scholar]
- 3.Michalopoulos A, Papadakis E. 2010. Inhaled anti-infective agents: emphasis on colistin. Infection 38:81–88. 10.1007/s15010-009-9148-6 [DOI] [PubMed] [Google Scholar]
- 4.Hewer SL. 2012. Inhaled antibiotics in cystic fibrosis: what's new? J. R. Soc. Med. 105(Suppl 2):S19–S24. 10.1258/jrsm.2012.12s004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michalopoulos AS, Falagas ME. 2014. Inhaled antibiotics in mechanically ventilated patients. Minerva Anestesiol. 80:236–244 [PubMed] [Google Scholar]
- 6.Dudley MN, Loutit J, Griffith DC. 2008. Aerosol antibiotics: considerations in pharmacological and clinical evaluation. Curr. Opin. Biotechnol. 19:637–643. 10.1016/j.copbio.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 7.Bosquillon C. 2010. Drug transporters in the lung: do they play a role in the biopharmaceutics of inhaled drugs? J. Pharm. Sci. 99:2240–2255. 10.1002/jps.21995 [DOI] [PubMed] [Google Scholar]
- 8.Brillault J, De Castro WV, Couet W. 2010. Relative contributions of active mediated transport and passive diffusion of fluoroquinolones with various lipophilicities in a Calu-3 lung epithelial cell model. Antimicrob. Agents Chemother. 54:543–545. 10.1128/AAC.00733-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brillault J, De Castro WV, Harnois T, Kitzis A, Olivier JC, Couet W. 2009. P-glycoprotein-mediated transport of moxifloxacin in a Calu-3 lung epithelial cell model. Antimicrob. Agents Chemother. 53:1457–1462. 10.1128/AAC.01253-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westerhout J, Danhof M, De Lange EC. 2011. Preclinical prediction of human brain target site concentrations: considerations in extrapolating to the clinical setting. J. Pharm. Sci. 100:3577–3593. 10.1002/jps.22604 [DOI] [PubMed] [Google Scholar]
- 11.Campbell L, Abulrob A-NG, Kandalaft LE, Plummer S, Hollins AJ, Gibbs A, Gumbleton M. 2003. Constitutive expression of P-glycoprotein in normal lung alveolar epithelium and functionality in primary alveolar epithelial cultures. J. Pharmacol. Exp. Ther. 304:441–452. 10.1124/jpet.102.042994 [DOI] [PubMed] [Google Scholar]
- 12.Dahyot C, Marchand S, Bodin M, Debeane B, Mimoz O, Couet W. 2008. Application of basic pharmacokinetic concepts to analysis of microdialysis data: illustration with imipenem muscle distribution. Clin. Pharmacokinet. 47:181–189. 10.2165/00003088-200847030-00004 [DOI] [PubMed] [Google Scholar]
- 13.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 14.Marchand S, Gobin P, Brillault J, Baptista S, Adier C, Olivier JC, Mimoz O, Couet W. 2010. Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats. Antimicrob. Agents Chemother. 54:3702–3707. 10.1128/AAC.00411-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krombach F, Munzing S, Allmeling AM, Gerlach JT, Behr J, Dorger M. 1997. Cell size of alveolar macrophages: an interspecies comparison. Environ. Health Perspect. 105(Suppl 5):S1261–S1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemanth Kumar AK, Ramachandran G. 2009. Simple and rapid liquid chromatography method for determination of moxifloxacin in plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877:1205–1208. 10.1016/j.jchromb.2009.02.042 [DOI] [PubMed] [Google Scholar]
- 17.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532–538 [DOI] [PubMed] [Google Scholar]
- 18.Kiem S, Schentag JJ. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 52:24–36. 10.1128/AAC.00133-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulitta JB, Bingolbali A, Shin BS, Landersdorfer CB. 2011. Development of a new pre- and post-processing tool (SADAPT-TRAN) for nonlinear mixed-effects modeling in S-ADAPT. AAPS J. 13:201–211. 10.1208/s12248-011-9257-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roosendaal R, Bakker-Woudenberg IA, van den Berghe-van Raffe M, Vink-van den Berg JC, Michel MF. 1987. Comparative activities of ciprofloxacin and ceftazidime against Klebsiella pneumoniae in vitro and in experimental pneumonia in leukopenic rats. Antimicrob. Agents Chemother. 31:1809–1815. 10.1128/AAC.31.11.1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siefert HM, Domdey-Bette A, Henninger K, Hucke F, Kohlsdorfer C, Stass HH. 1999. Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: a comparison in humans and other mammalian species. J. Antimicrob. Chemother. 43:69–76. 10.1093/jac/43.suppl_2.69 [DOI] [PubMed] [Google Scholar]
- 22.Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481–504. 10.1023/A:1012299115260 [DOI] [PubMed] [Google Scholar]
- 23.Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. 2008. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 61:235–237. 10.1093/jac/dkm476 [DOI] [PubMed] [Google Scholar]
- 24.Marchand S, Frasca D, Dahyot-Fizelier C, Breheret C, Mimoz O, Couet W. 2008. Lung microdialysis study of levofloxacin in rats following intravenous infusion at steady state. Antimicrob. Agents Chemother. 52:3074–3077. 10.1128/AAC.00242-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhanani J, Roberts JA, Chew M, Lipman J, Boots RJ, Paterson DL, Fraser JF. 2010. Antimicrobial chemotherapy and lung microdialysis: a review. Int. J. Antimicrob. Agents 36:491–500. 10.1016/j.ijantimicag.2010.08.013 [DOI] [PubMed] [Google Scholar]
- 26.Deguchi Y, Sun J, Tauchi Y, Sakai S, Morimoto K. 2003. Distribution characteristics of grepafloxacin, a fluoroquinolone antibiotic, in lung epithelial lining fluid and alveolar macrophage. Drug Metab. Pharmacokinet. 18:319–326. 10.2133/dmpk.18.319 [DOI] [PubMed] [Google Scholar]
- 27.Hamishehkar H, Emami J, Najafabadi AR, Gilani K, Minaiyan M, Hassanzadeh K, Mahdavi H, Koohsoltani M, Nokhodchi A. 2010. Pharmacokinetics and pharmacodynamics of controlled release insulin loaded PLGA microcapsules using dry powder inhaler in diabetic rats. Biopharm. Drug Dispos. 31:189–201. 10.1002/bdd.702 [DOI] [PubMed] [Google Scholar]
- 28.Togami K, Chono S, Morimoto K. 2011. Distribution characteristics of clarithromycin and azithromycin, macrolide antimicrobial agents used for treatment of respiratory infections, in lung epithelial lining fluid and alveolar macrophages. Biopharm. Drug Dispos. 32:389–397. 10.1002/bdd.767 [DOI] [PubMed] [Google Scholar]
- 29.Strohl KP, Thomas AJ, St Jean P, Schlenker EH, Koletsky RJ, Schork NJ. 1997. Ventilation and metabolism among rat strains. J. Appl. Physiol. 82:317–323. 10.1063/1.365814 [DOI] [PubMed] [Google Scholar]
- 30.Michot JM, Seral C, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. 2005. Influence of efflux transporters on the accumulation and efflux of four quinolones (ciprofloxacin, levofloxacin, garenoxacin, and moxifloxacin) in J774 macrophages. Antimicrob. Agents Chemother. 49:2429–2437. 10.1128/AAC.49.6.2429-2437.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carryn S, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. 2002. Comparative intracellular (THP-1 macrophage) and extracellular activities of β-lactams, azithromycin, gentamicin, and fluoroquinolones against Listeria monocytogenes at clinically relevant concentrations. Antimicrob. Agents Chemother. 46:2095–2103. 10.1128/AAC.46.7.2095-2103.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hara T, Takemura H, Kanemitsu K, Yamamoto H, Shimada J. 2000. Comparative uptake of grepafloxacin and ciprofloxacin by a human monocytic cell line, THP-1. J. Infect. Chemother. 6:162–167. 10.1007/s101560070016 [DOI] [PubMed] [Google Scholar]
- 33.Gotfried MH, Danziger LH, Rodvold KA. 2001. Steady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjects. Chest 119:1114–1122. 10.1378/chest.119.4.1114 [DOI] [PubMed] [Google Scholar]
- 34.Schuler P, Zemper K, Borner K, Koeppe P, Schaberg T, Lode H. 1997. Penetration of sparfloxacin and ciprofloxacin into alveolar macrophages, epithelial lining fluid, and polymorphonuclear leucocytes. Eur. Respir. J. 10:1130–1136. 10.1183/09031936.97.10051130 [DOI] [PubMed] [Google Scholar]
- 35.Baldwin DR, Wise R, Andrews JM, Gill M, Honeybourne D. 1993. Comparative bronchoalveolar concentrations of ciprofloxacin and lomefloxacin following oral administration. Respir. Med. 87:595–601. 10.1016/S0954-6111(05)80262-8 [DOI] [PubMed] [Google Scholar]
- 36.Capitano B, Mattoes HM, Shore E, O'Brien A, Braman S, Sutherland C, Nicolau DP. 2004. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest 125:965–973. 10.1378/chest.125.3.965 [DOI] [PubMed] [Google Scholar]
- 37.Soman A, Honeybourne D, Andrews J, Jevons G, Wise R. 1999. Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 44:835–838. 10.1093/jac/44.6.835 [DOI] [PubMed] [Google Scholar]
- 38.Cook PJ, Andrews JM, Wise R, Honeybourne D, Moudgil H. 1995. Concentrations of OPC-17116, a new fluoroquinolone antibacterial, in serum and lung compartments. J. Antimicrob. Chemother. 35:317–326. 10.1093/jac/35.2.317 [DOI] [PubMed] [Google Scholar]
- 39.Gontijo AVL, Grégoire N, Lamarche I, Gobin P, Couet W, Marchand S. 2014. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 2. Colistin. Antimicrob. Agents Chemother. 58:3950–3956. 10.1128/AAC.02819-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conte JE, Jr, Golden J, Duncan S, McKenna E, Lin E, Zurlinden E. 1996. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob. Agents Chemother. 40:1617–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabet M, Miller CE, Nolan TG, Senekeo-Effenberger K, Dudley MN, Griffith DC. 2009. Efficacy of aerosol MP-376, a levofloxacin inhalation solution, in models of mouse lung infection due to Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 53:3923–3928. 10.1128/AAC.00268-09 [DOI] [PMC free article] [PubMed] [Google Scholar]