Abstract
The amikacin-fosfomycin inhalation system (AFIS) is a combination of 2 antibiotics and an in-line nebulizer delivery system that is being developed for adjunctive treatment of pneumonia caused by Gram-negative organisms in patients on mechanical ventilation. AFIS consists of a combination of amikacin and fosfomycin solutions at a 5:2 ratio (amikacin, 3 ml at 100 mg/ml; fosfomycin, 3 ml at 40 mg/ml) and the PARI Investigational eFlow Inline System. In this antibiotic potentiation study, the antimicrobial activities of amikacin and fosfomycin, alone and in a 5:2 combination, were assessed against 62 Gram-negative pathogens from a worldwide antimicrobial surveillance collection (SENTRY). The amikacin MICs for 62 isolates of Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae were ≥32 μg/ml (intermediate or resistant according to the Clinical and Laboratory Standards Institute [CLSI]; resistant according to the European Committee on Antimicrobial Susceptibility Testing [EUCAST]). Each isolate was tested against amikacin (0.25 to 1,024 μg/ml), fosfomycin (0.1 to 409.6 μg/ml), and amikacin-fosfomycin (at a 5:2 ratio) using CLSI reference agar dilution methods. The median MIC values for amikacin and fosfomycin against the 62 isolates each decreased 2-fold with the amikacin-fosfomycin (5:2) combination from that with either antibiotic alone. Interactions between amikacin and fosfomycin differed by isolate and ranged from no detectable interaction to high potentiation. The amikacin-fosfomycin (5:2) combination reduced the amikacin concentration required to inhibit all 62 isolates from >1,024 to ≤256 μg/ml and reduced the required fosfomycin concentration from 204.8 to 102.4 μg/ml. These results support continued development of the amikacin-fosfomycin combination for aerosolized administration, where high drug levels can be achieved.
INTRODUCTION
The amikacin-fosfomycin inhalation system (AFIS), a combination of 2 antibiotics and an in-line nebulizer delivery system, is being developed for adjunctive treatment of pneumonia caused by Gram-negative organisms in patients on mechanical ventilation. Subtherapeutic antibiotic concentrations are often obtained in the respiratory tract with systemic administration (1); thus, adjunctive therapy with aerosolized antibiotics is being investigated. Treatment with these products generally results in higher antibiotic concentrations in tracheal aspirate (sputum) samples than a maximum dose delivered systemically, with reduced systemic exposure (2). Results from studies of aerosolized antibiotics for the treatment or prevention of pneumonia in mechanically ventilated patients have indicated benefits such as lower rates of pneumonia at the end of treatment, reduced usage of systemic antibiotics, and earlier weaning of patients from the ventilator, leading to shorter stays in the intensive care unit (3, 4). In the United States, more than 250,000 cases of pneumonia occur in mechanically ventilated patients each year, representing approximately 800 cases per million for the overall U.S. population (5). Nosocomial pneumonia is the primary cause of morbidity and mortality among patients with respiratory failure (6), with an attributed mortality rate as high as 50% (7).
It is challenging to empirically prescribe suitable antibacterial agents for the initial treatment of an individual patient with clinical signs, symptoms, and chest radiographs indicating pneumonia. Gram stains of tracheal secretions can be obtained rapidly but lack sensitivity and specificity, and it takes as long as 3 days to obtain the more-accurate microbiological culture results (8–10). Inappropriate therapy during the first 48 h has been associated with approximately 90% mortality among mechanically ventilated patients, even when the inappropriate therapy was followed by empirically correct therapy (11). Therefore, the choice of the initial antibiotic must take into account the possible presence of many different organisms, including highly resistant Gram-negative pathogens and methicillin-resistant Staphylococcus aureus (MRSA). Intravenous (i.v.) antibiotics are the standard treatment for ventilator-associated pneumonia (VAP), and currently available i.v. antibiotics may not be effective in treating this range of bacteria with a safety profile that is acceptable for widespread empirical use while one is waiting for the results from microbiological cultures.
It is well established that the activity of aminoglycosides against Gram-negative pathogens is potentiated by the addition of fosfomycin (12–15). Fosfomycin enhanced the antibacterial activity of amikacin against Pseudomonas aeruginosa, decreasing the MIC90 (MIC required to inhibit the growth of 90% of isolates) of amikacin for 20 clinical isolates by 64-fold, and an aminoglycoside (isepamicin)-fosfomycin combination had greater therapeutic effect in a rat model of P. aeruginosa biofilm infection than either antibiotic alone (12). Synergy between fosfomycin and amikacin was also observed against multidrug-resistant Acinetobacter baumannii isolates (13). A 4:1 combination of fosfomycin and tobramycin demonstrated high activity and good postantibiotic effect against multiple respiratory pathogens in vitro and in a rat pneumonia model (14). This combination also exhibited enhanced bactericidal activity against P. aeruginosa in the presence of mucin over that of either antibiotic alone; this enhanced activity was attributed to increased uptake of tobramycin in the presence of fosfomycin (15).
AFIS consists of amikacin (100 mg/ml in 3 ml) and fosfomycin (40 mg/ml in 3 ml) solutions and the PARI investigational eFlow inline nebulizer system (16). The antibiotic mixture is administered using the inline system in standard ventilator circuits. The ratio and doses of amikacin and fosfomycin were chosen on the basis of the maximal doses that patients can tolerate in a short (≤15-min) aerosolized-antibiotic administration time, after consideration of the need to achieve (i) a high level of an aminoglycoside in tracheal aspirates, (ii) a fosfomycin concentration sufficient for synergy with amikacin, to allow the treatment of emerging highly resistant Gram-negative pathogens and common concomitant Gram-positive pathogens (including MRSA) without increasing the osmolality of the solution to a level that induces involuntary coughing, and (iii) a product volume that can be delivered safely in a relatively short administration time.
The in vitro microbiology studies described here were conducted to evaluate the antimicrobial activities of amikacin and fosfomycin, alone and in a 5:2 combination, against selected amikacin-nonsusceptible Gram-negative respiratory tract pathogens. To confirm that the levels of antimicrobial activity observed were clinically relevant, they were compared to the tracheal aspirate pharmacokinetics in a recently completed phase 1 study of AFIS (16). An accompanying paper describes AFIS resistance selection rates for pathogens that are representative of those commonly associated with VAP and interaction of AFIS with antibiotics commonly used intravenously to treat VAP (29).
MATERIALS AND METHODS
Bacterial isolates.
Sixty-two clinical isolates of Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae were selected from a worldwide antimicrobial surveillance collection (SENTRY) that contains >35,000 organisms collected in 2011 from >100 medical centers on 6 continents (JMI Laboratories, North Liberty, IA). Isolates were chosen to include at least 20 per species(A. baumannii, P. aeruginosa, and K. pneumoniae) and to consist of amikacin-nonsusceptible (R) strains that would be either susceptible (S) or nonsusceptible (R) to gentamicin and/or tobramycin. This resulted in four phenotypic patterns with respect to amikacin, gentamicin, and tobramycin: R/R/R, R/S/S, R/S/R, and R/R/S (Table 1). Due to the limited number of some phenotypic patterns in the 2011 global collection, the numbers of isolates with each phenotype for each species were not all equal. Six isolates were selected from earlier years (2 isolates from 2006, 1 from 2005, 1 from 2004, 1 from 2003, and 1 from 2000) due to the scarcity of strains matching the desired phenotypes in the 2011 collection.
TABLE 1.
Characteristics of the 62 amikacin-nonsusceptible pathogens
| Species (no. of isolates) | Beta-lactamase(s) produceda | No. of isolates with the following pattern of susceptibility or resistanceb to amikacin, gentamicin, and tobramycin: |
|||
|---|---|---|---|---|---|
| R/R/R | R/S/S | R/S/R | R/R/S | ||
| A. baumannii (21) | Any or none | 9 | 5 | 0 | 7 |
| OXA-23 and OXA-51 | 2 | 2 | 1 | ||
| OXA-24 and OXA-51 | 1 | 1 | |||
| OXA-51 | 1 | ||||
| OXA-51 and OXA-58 | 1 | ||||
| P. aeruginosa (21) | Any or none | 15 | 0 | 3 | 3 |
| GES-1 | 1 | ||||
| OXA-2, OXA-10, and VIM-2 | 1 | ||||
| OXA-14 | 1 | ||||
| VIM-2 | 2 | 1 | 1 | ||
| VIM-4 | 1 | ||||
| K. pneumoniae (20) | Any or none | 13 | 0 | 3 | 4 |
| KPC-2 | 3 | 1 | |||
| KPC-3 | 1 | 2 | |||
GES, Guiana extended-spectrum β-lactamase; KPC, Klebsiella pneumoniae carbapenemase; OXA, oxacillin-hydrolyzing extended-spectrum β-lactamase; VIM, Verona integron-encoded metallo-β-lactamase.
R, resistance; S, susceptibility. In each pattern, the first letter refers to amikacin, the second to gentamicin, and the third to tobramycin. The “R” designation for amikacin includes 13 isolates classified as intermediate (MIC, 32 μg/ml) and 49 isolates classified as resistant (MIC, ≥64 μg/ml) by CLSI standards (17). All 62 isolates were classified as resistant by EUCAST standards (MIC, >16 μg/ml) (18). These strains are referred to as “nonsusceptible” in the text.
The 62 isolates each had an amikacin MIC of ≥32 μg/ml. Thirteen of the 62 isolates were classified as intermediate (MIC, 32 μg/ml) and 49/62 as resistant (MIC, ≥64 μg/ml) to amikacin according to the Clinical and Laboratory Standards Institute (CLSI) (17), while all 62 were classified as resistant (MIC, >16 μg/ml) according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (18). These strains are referred to as “nonsusceptible” here. There are no CLSI or EUCAST interpretive criteria for fosfomycin against P. aeruginosa or A. baumannii. According to CLSI criteria for K. pneumoniae (17), 18/20 isolates were susceptible to fosfomycin (MIC, ≤64 μg/ml), no isolates were intermediate (MIC, 128 μg/ml) and 2/20 isolates were resistant (MIC, ≥256 μg/ml). According to EUCAST criteria for K. pneumoniae (18), 14/20 isolates were susceptible (MIC, ≤32 μg/ml) and 6/20 isolates were resistant (MIC, >32 μg/ml).
Control strains.
Control strains were obtained from ATCC (Manassas, VA) and covered a range of MIC values: 0.25 to 1,024 μg/ml for amikacin and 0.1 to 409.6 μg/ml for fosfomycin. Control stains included P. aeruginosa (ATCC 27853), Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 29213), and Enterococcus faecalis (ATCC 29212). The dilutions tested matched the product specifications of a 5:2 ratio of amikacin to fosfomycin, and the concentration of the primary stock solution was 20 times the highest concentration tested (1,024 μg/ml of amikacin and 409.6 μg/ml of fosfomycin).
Three replicate MICs were determined for each of the 4 control strains and were compared with the ranges published by the CLSI (17).
Potentiation testing.
Each of the 62 isolates was tested against amikacin (0.25 to 1,024 μg/ml), fosfomycin (0.1 to 409.6 μg/ml), and amikacin-fosfomycin (at a 5:2 ratio; the same concentration ranges as those for the single-agent tests were used) by CLSI methods (19). Isolates were tested on Mueller-Hinton agar plates. Plates used for testing fosfomycin or amikacin-fosfomycin were supplemented with 25 μg/ml of glucose-6-phosphate. Amikacin and fosfomycin were obtained from Ercros Industrial S.A. (Madrid, Spain) and glucose-6-phosphate from Sigma-Aldrich (St. Louis, MO).
Comparison to clinical data.
MIC values for the 62 isolates were compared with amikacin and fosfomycin concentrations in tracheal aspirate samples that had been collected in a phase 1 trial of AFIS (16). The viscosity of tracheal aspirate samples makes measurements of the volume both inaccurate and nonreproducible; therefore, drug concentrations were measured per gram of sputum instead of per milliliter. To compare the MIC values, expressed in micrograms per milliliter, with the drug concentrations, expressed in micrograms per gram, we adopted the convention commonly used in studies of cystic fibrosis, in which tracheal aspirate samples are weighed and comparisons are conducted under the assumption that 1 g of sputum equals 1 ml (20, 21).
RESULTS
MICs for control strains.
For control strains, 100% of amikacin and 91.7% of fosfomycin MIC values were within published ranges; 1 of the 3 replicate fosfomycin MICs for P. aeruginosa ATCC 27853 (12.8 μg/ml) exceeded the published reference range (1 to 8 μg/ml) (17).
MICs for amikacin-nonsusceptible isolates.
For the 62 amikacin-nonsusceptible Gram-negative pathogens, combining amikacin at a 5:2 ratio with fosfomycin reduced the median amikacin MIC to 64 μg/ml from 128 μg/ml with amikacin alone (Table 2; note that the median MIC is equal to the MIC at which 50% of isolates are inhibited [MIC50]). The highest amikacin MIC observed for any of the 62 isolates was reduced from >1,024 to 256 μg/ml by the addition of fosfomycin. Combining amikacin in a 5:2 ratio with fosfomycin also reduced the median fosfomycin MIC to 25.6 μg/ml from 51.2 μg/ml with fosfomycin alone (Table 3). The highest fosfomycin MIC observed for any of the 62 isolates was reduced from 204.8 to 102.4 μg/ml by the addition of amikacin.
TABLE 2.
MICs of amikacin, alone or in combination with fosfomycin
| Species | No. of isolates | Mediana (range) amikacin MIC (μg/ml) |
|
|---|---|---|---|
| Amikacin alone | Amikacin-fosfomycin (5:2) | ||
| A. baumannii | 21 | 64 (32–>1,024) | 64 (32–256) |
| P. aeruginosa | 21 | 128 (32–>1,024) | 64 (8–256) |
| K. pneumoniae | 20 | 256 (32–>1,024) | 32 (16–128) |
| All isolates | 62 | 128 (32–>1,024) | 64 (8–256) |
Median MIC, MIC50.
TABLE 3.
MICs of fosfomycin, alone or in combination with amikacin
| Species | No. of isolates | Mediana (range) fosfomycin MIC (μg/ml) |
|
|---|---|---|---|
| Fosfomycin alone | Amikacin-fosfomycin (5:2) | ||
| A. baumannii | 21 | 204.8 (204.8) | 25.6 (12.8–102.4) |
| P. aeruginosa | 21 | 51.2 (3.2–102.4) | 25.6 (3.2–102.4) |
| K. pneumoniae | 20 | 25.6 (12.8–204.8) | 12.8 (6.4–51.2) |
| All isolates | 62 | 51.2 (3.2–204.8) | 25.6 (3.2–102.4) |
Median MIC, MIC50.
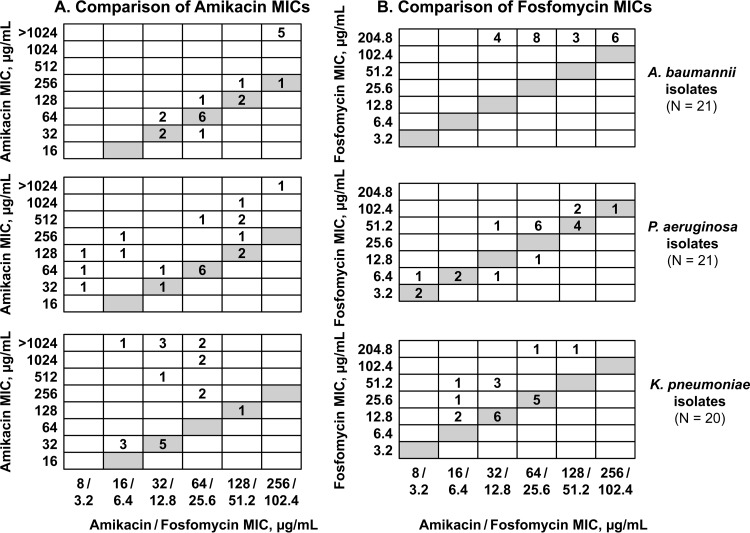
The magnitude of the amikacin-fosfomycin potentiation differed by organism and by isolate (Tables 2 and 3; Fig. 1). For the 21 A. baumannii isolates, the addition of fosfomycin did not change the median amikacin MIC value (Table 2). However, when individual isolates were considered, a pronounced effect was observed for isolates with high amikacin resistance (MIC, >1,024 μg/ml). The amikacin MIC values for these 5 isolates were reduced to ≤256 μg/ml with the amikacin-fosfomycin (5:2) combination (Fig. 1A, top). For the 21 P. aeruginosa isolates, the addition of fosfomycin decreased the median amikacin MIC value from 128 to 64 μg/ml (Table 2). For 11 of 21 P. aeruginosa isolates, amikacin MIC values remained stable (±1 log2 dilution step) with the addition of fosfomycin (Fig. 1A, center). For the other 10 isolates, amikacin MIC values decreased ≥4-fold with the addition of fosfomycin. For the 20 K. pneumoniae isolates, the addition of fosfomycin decreased the median amikacin MIC value from 256 to 32 μg/ml (Table 2). For 9 of 20 K. pneumoniae isolates, amikacin MIC values remained stable (±1 log2 dilution step) with the addition of fosfomycin (Fig. 1A, bottom). For the other 11 isolates, amikacin MIC values decreased ≥4-fold with the addition of fosfomycin, with decreases of ≥32-fold observed for 6 of the 11 isolates. The addition of amikacin to fosfomycin also decreased the fosfomycin MICs for some isolates (Fig. 1B).
FIG 1.
Comparisons of MIC values. (A) Comparison of amikacin MIC values for amikacin alone with those for amikacin-fosfomycin (5:2). (B) Comparison of fosfomycin MIC values for fosfomycin alone with those for amikacin-fosfomycin (5:2). Comparisons are shown for A. baumannii (top), P. aeruginosa (center), and K. pneumoniae (bottom) isolates. Each number in a cell is the number of isolates with the indicated combination of MIC values. Shaded cells indicate amikacin or fosfomycin MIC values that are the same for amikacin or fosfomycin alone and amikacin-fosfomycin (5:2).
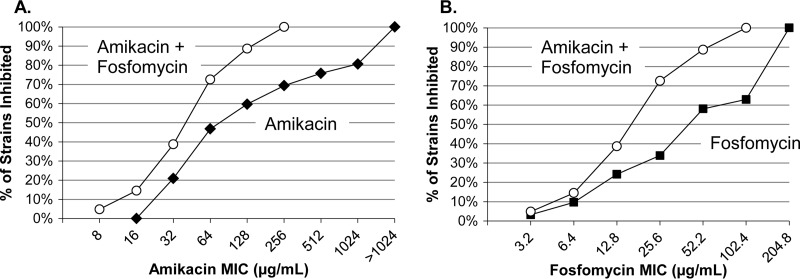
The cumulative percentages of inhibition of the 62 amikacin-nonsusceptible isolates in the presence of amikacin or fosfomycin alone were compared to those in the presence of amikacin-fosfomycin (5:2) (Fig. 2). The addition of fosfomycin reduced the amikacin concentration required to inhibit all 62 isolates from >1,024 to ≤256 μg/ml (Fig. 2A; Table 2). The addition of amikacin reduced the fosfomycin concentration required to inhibit all 62 isolates from 204.8 to 102.4 μg/ml (Fig. 2B; Table 3).
FIG 2.
Cumulative percentages of inhibition of 62 Gram-negative bacterial pathogens tested with amikacin (with or without fosfomycin) (A) or fosfomycin (with or without amikacin) (B). The amikacin-fosfomycin combination had a 5:2 ratio of amikacin to fosfomycin.
Comparison to clinical data.
In a previously conducted phase 1 randomized, double-blind, placebo-controlled dose escalation study of patients with VAP or ventilator-associated tracheobronchitis (VAT), amikacin and fosfomycin concentrations in tracheal aspirate samples were analyzed (16). Seven patients received single 6-ml doses with AFIS (300 mg amikacin and 120 mg fosfomycin). Median maximum concentrations (Cmax) of drug in tracheal aspirates were 11,400 μg/g sputum (range, 6,910 to 17,000 μg/g) for amikacin and 6,650 μg/g sputum (range, 2,930 to 10,000 μg/g) for fosfomycin. The Cmax observed in tracheal aspirates after AFIS treatment (11,400 μg/g sputum for amikacin and 6,650 μg/g sputum for fosfomycin) (16) were larger than the median and maximal MIC values for amikacin-fosfomycin (5:2) observed for the isolates described here, which were 64 and 256 μg/ml for amikacin and 25.6 and 102.4 μg/ml for fosfomycin (Tables 2 and 3).
DISCUSSION
The combination of amikacin and fosfomycin at a 5:2 ratio had significantly greater potency against 62 amikacin-nonsusceptible Gram-negative pathogens than either amikacin or fosfomycin alone. The amikacin-fosfomycin (5:2) combination reduced the amikacin concentration required to inhibit all 62 isolates from >1,024 to ≤256 μg/ml and reduced the required fosfomycin concentration from 204.8 to 102.4 μg/ml.
The highest amikacin MIC observed for amikacin-fosfomycin (5:2) among the 62 pathogens was 256 μg/ml, more than 44-fold lower than the median peak amikacin concentration observed in tracheal aspirates in the phase 1 study of AFIS (11,400 μg/g) (16). Amikacin is inhibited by sputum in airways with a high concentration of mucus. It is estimated that amikacin concentrations 25-fold higher than the MIC values are necessary to provide bactericidal activity (22, 23). The 44-fold difference observed between the highest amikacin MIC for the 62 pathogens and the peak concentrations in the phase 1 study exceeded the 25-fold multiple, suggesting that the amikacin concentrations achieved after administration of AFIS were clinically relevant and would be high enough to kill the 62 nonsusceptible isolates.
In comparison, the peak concentrations achieved in serum after intramuscular or i.v. administration of 7.5 mg of amikacin/kg of body weight were reported as 21 and 38 μg/ml, respectively (24); these amikacin concentrations would not be high enough to kill most of the 62 amikacin-nonsusceptible isolates described here, which had amikacin MIC values ranging from 32 to >1,024 μg/ml (median, 128 μg/ml). In addition, the concentrations of antibiotics in the airway after intramuscular or i.v. administration would be even lower than the antibiotic concentrations in serum.
The high antibiotic concentrations in the airway achieved after aerosol administration of AFIS in the phase 1 study were accompanied by low systemic concentrations (≤1.4 μg/ml amikacin and ≤0.8 μg/ml fosfomycin in plasma) (16), which should reduce systemic side effects from those with systemic administration of amikacin or fosfomycin alone. Further, any efficacy observed with AFIS in future studies will likely be due to the high airway antibiotic concentrations, since the systemic amikacin and fosfomycin concentrations are subtherapeutic (24, 25).
The aerosolized amikacin-fosfomycin combination is planned for use as adjunctive therapy with i.v. antibiotics, so it is not anticipated that the systemic sub-MIC concentrations of amikacin and fosfomycin will be relevant if appropriate i.v. antibiotics are being used. However, with highly resistant bacteria, a scenario could be envisioned in which all available systemic antibiotics are ineffective and the patient has bacteremia or an additional nonpulmonary infection. The likely outcome for this patient is not good, and resistant organisms could be created. However, it is also likely that the bacteria would already be resistant to aminoglycosides, and since fosfomycin is available as an i.v. drug in only five countries worldwide, there would be no loss of a valuable i.v. antibiotic with the aerosol use of amikacin and fosfomycin. In a rather bold study, this question was addressed by Lu et al., who compared aerosol and i.v. administration of ceftazidime and amikacin to patients with VAP caused by P. aeruginosa (26). While the clinical outcomes for the aerosol and i.v. arms were similar, bacterial resistance developed exclusively in the i.v. arm, suggesting that the aerosol therapy may actually prevent, rather than induce, the development of resistance.
One limitation of the current study is that isolates were selected on the basis of amikacin resistance and thus do not represent the most fosfomycin resistant pathogens. However, one can compare the results of this study with published studies of fosfomycin-resistant pathogens. Since Greece is one of the five countries that use i.v. fosfomycin, the MICs there are likely representative of the highest MICs observed in the clinical setting. The highest fosfomycin MIC reported in a study of 90 multidrug-resistant clinical isolates from a hospital in Greece was 512 μg/ml (27). This is more than 10-fold lower than the peak fosfomycin concentrations observed in tracheal aspirates in the phase 1 study (6,650 μg/g of sputum) (16), suggesting that the fosfomycin concentrations achieved after administration of AFIS are clinically relevant. Further, the bactericidal activity of fosfomycin is dependent on the time above the MIC (28), which should be increased by the observed 2-fold reduction in the fosfomycin MIC provided by the amikacin-fosfomycin (5:2) combination.
In conclusion, combining amikacin and fosfomycin at a 5:2 ratio enhanced the potency of both amikacin and fosfomycin against 62 amikacin-nonsusceptible Gram-negative pathogens. Comparison with results from a phase 1 study of AFIS (16) indicated that the antibiotic concentrations achieved in tracheal aspirates after AFIS inhalation are clinically relevant; they were more than 44-fold higher than the highest amikacin MIC value for the 62 amikacin-nonsusceptible pathogens included in this study. These results support continued development of the amikacin-fosfomycin combination for aerosolized administration, which achieves high drug levels in the airways. Based on the data on the susceptibility of Gram-negative pathogens to the amikacin-fosfomycin (5:2) combination, the tracheal aspirate drug concentrations measured in the phase 1 study, and the ability to target drug delivery to the site of infection, adjunctive treatment with AFIS may improve clinical outcomes for mechanically ventilated patients with pneumonia caused by Gram-negative bacteria, including those infected with multidrug-resistant Gram-negative bacteria.
ACKNOWLEDGMENTS
This work was funded by Cardeas Pharma Corp.
Kate Loughney provided medical writing assistance under the sponsorship of Cardeas Pharma.
Footnotes
Published ahead of print 21 April 2014
REFERENCES
- 1.Pennington JE. 1981. Penetration of antibiotics into respiratory secretions. Rev. Infect. Dis. 3:67–73. 10.1093/clinids/3.1.67 [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld M, Cohen M, Ramsey B. 1997. Aerosolized antibiotics for bacterial lower airway infections: principles, efficacy, and pitfalls. Clin. Pulm. Med. 4:101–112. 10.1097/00045413-199703000-00006 [DOI] [Google Scholar]
- 3.Palmer LB, Smaldone GC, Chen JJ, Baram D, Duan T, Monteforte M, Varela M, Tempone AK, O'Riordan T, Daroowalla F, Richman P. 2008. Aerosolized antibiotics and ventilator-associated tracheobronchitis in the intensive care unit. Crit. Care Med. 36:2008–2013. 10.1097/CCM.0b013e31817c0f9e [DOI] [PubMed] [Google Scholar]
- 4.Wood GC, Boucher BA, Croce MA, Hanes SD, Herring VL, Fabian TC. 2002. Aerosolized ceftazidime for prevention of ventilator-associated pneumonia and drug effects on the proinflammatory response in critically ill trauma patients. Pharmacotherapy 22:972–982. 10.1592/phco.22.12.972.33596 [DOI] [PubMed] [Google Scholar]
- 5.Soo Hoo GW, Wen YE, Trung VN, Goetz MB. 2005. Impact of clinical guidelines in the management of severe hospital-acquired pneumonia. Chest 128:2778–2787. 10.1378/chest.128.4.2778 [DOI] [PubMed] [Google Scholar]
- 6.Kollef MH, Silver P, Murphy DM, Trovillion E. 1995. The effect of late-onset ventilator-associated pneumonia in determining patient mortality. Chest 108:1655–1662. 10.1378/chest.108.6.1655 [DOI] [PubMed] [Google Scholar]
- 7.Koenig SM, Truwit JD. 2006. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin. Microbiol. Rev. 19:637–657. 10.1128/CMR.00051-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albert M, Friedrich JO, Adhikari NK, Day AG, Verdant C, Heyland DK, Canadian Critical Care Trials Group 2008. Utility of Gram stain in the clinical management of suspected ventilator-associated pneumonia. Secondary analysis of a multicenter randomized trial. J. Crit. Care 23:74–81. 10.1016/j.jcrc.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 9.Goldberg AE, Malhotra AK, Riaz OJ, Aboutanos MB, Duane TM, Borchers CT, Martin N, Ivatury RR. 2008. Predictive value of broncho-alveolar lavage fluid Gram's stain in the diagnosis of ventilator-associated pneumonia: a prospective study. J. Trauma 65:871–876. 10.1097/TA.0b013e31818481e0 [DOI] [PubMed] [Google Scholar]
- 10.O'Horo JC, Thompson D, Safdar N. 2012. Is the Gram stain useful in the microbiologic diagnosis of VAP? A meta-analysis. Clin. Infect. Dis. 55:551–561. 10.1093/cid/cis512 [DOI] [PubMed] [Google Scholar]
- 11.Luna CM, Vujacich P, Niederman MS, Vay C, Gherardi C, Matera J, Jolly EC. 1997. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest 111:676–685. 10.1378/chest.111.3.676 [DOI] [PubMed] [Google Scholar]
- 12.Cai Y, Fan Y, Wang R, An MM, Liang BB. 2009. Synergistic effects of aminoglycosides and fosfomycin on Pseudomonas aeruginosa in vitro and biofilm infections in a rat model. J. Antimicrob. Chemother. 64:563–566. 10.1093/jac/dkp224 [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Martinez L, Rodriguez G, Pascual A, Suarez AI, Perea EJ. 1996. In-vitro activity of antimicrobial agent combinations against multiresistant Acinetobacter baumannii. J. Antimicrob. Chemother. 38:1107–1108. 10.1093/jac/38.6.1107 [DOI] [PubMed] [Google Scholar]
- 14.MacLeod DL, Barker LM, Sutherland JL, Moss SC, Gurgel JL, Kenney TF, Burns JL, Baker WR. 2009. Antibacterial activities of a fosfomycin/tobramycin combination: a novel inhaled antibiotic for bronchiectasis. J. Antimicrob. Chemother. 64:829–836. 10.1093/jac/dkp282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacLeod DL, Velayudhan J, Kenney TF, Therrien JH, Sutherland JL, Barker LM, Baker WR. 2012. Fosfomycin enhances the active transport of tobramycin in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 56:1529–1538. 10.1128/AAC.05958-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montgomery AB, Vallance S, Abuan T, Tservistas M, Davies A. 2 January 2014. A randomized double-blind placebo-controlled dose-escalation phase 1 study of aerosolized amikacin and fosfomycin delivered via the PARI Investigational eFlow® Inline Nebulizer System in mechanically ventilated patients. J. Aerosol Med. Pulm. Drug Deliv. 10.1089/jamp.2013.1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CLSI. 2013. M100-S23. Performance standards for antimicrobial susceptibility testing: 23rd informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 18.European Committee on Antimicrobial Susceptibility Testing. 2013. Breakpoint tables for interpretations of MICs and zone diameters, version 3.1. http://www.eucast.org/clinical_breakpoints/ [Google Scholar]
- 19.CLSI. 2012. M07-A9. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—9th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 20.Retsch-Bogart GZ, Burns JL, Otto KL, Liou TG, McCoy K, Oermann C, Gibson RL, AZLI Phase II Study Group 2008. A phase 2 study of aztreonam lysine for inhalation to treat patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatr. Pulmonol. 43:47–58. 10.1002/ppul.20736 [DOI] [PubMed] [Google Scholar]
- 21.Smith AL, Ramsey BW, Hedges DL, Hack B, Williams-Warren J, Weber A, Gore EJ, Redding GJ. 1989. Safety of aerosol tobramycin administration for 3 months to patients with cystic fibrosis. Pediatr. Pulmonol. 7:265–271. 10.1002/ppul.1950070413 [DOI] [PubMed] [Google Scholar]
- 22.Niederman MS, Chastre J, Corkery K, Fink JB, Luyt CE, García MS. 2012. BAY41-6551 achieves bactericidal tracheal aspirate amikacin concentrations in mechanically ventilated patients with Gram-negative pneumonia. Intensive Care Med. 38:263–271. 10.1007/s00134-011-2420-0 [DOI] [PubMed] [Google Scholar]
- 23.Mendelman PM, Smith AL, Levy J, Weber A, Ramsey B, Davis RL. 1985. Aminoglycoside penetration, inactivation, and efficacy in cystic fibrosis sputum. Am. Rev. Respir. Dis. 132:761–765 [DOI] [PubMed] [Google Scholar]
- 24.Hospira, Inc. 2011. Amikacin sulfate injection, solution. U.S. package insert. Hospira, Inc, Lake Forest, IL [Google Scholar]
- 25.Forest Pharmaceuticals, Inc. 2011. Fosfomycin tromethamine (Monurol). U.S. package insert. Forest Pharmaceuticals, Inc, Earth City, MO [Google Scholar]
- 26.Lu Q, Yang J, Liu Z, Gutierrez C, Aymard G, Rouby JJ, Nebulized Antibiotics Study Group 2011. Nebulized ceftazidime and amikacin in ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 184:106–115. 10.1164/rccm.201011-1894OC [DOI] [PubMed] [Google Scholar]
- 27.Falagas ME, Kanellopoulou MD, Karageorgopoulos DE, Dimopoulos G, Rafailidis PI, Skarmoutsou ND, Papafrangas EA. 2008. Antimicrobial susceptibility of multidrug-resistant Gram negative bacteria to fosfomycin. Eur. J. Clin. Microbiol. Infect. Dis. 27:439–443. 10.1007/s10096-007-0456-4 [DOI] [PubMed] [Google Scholar]
- 28.Sauermann R, Karch R, Langenberger H, Kettenbach J, Mayer-Helm B, Petsch M, Wagner C, Sautner T, Gattringer R, Karanikas G, Joukhadar C. 2005. Antibiotic abscess penetration: fosfomycin levels measured in pus and simulated concentration-time profiles. Antimicrob. Agents Chemother. 49:4448–4454. 10.1128/AAC.49.11.4448-4454.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montgomery AB, Rhomberg PR, Abuan T, Walters K-A, Flamm RK. 2014. Amikacin-fosfomycin at a five-to-two ratio: characterization of mutation rates in microbial strains causing ventilator-associated pneumonia and interactions with commonly used antibiotics. Antimicrob. Agents Chemother. 58:3708–3713. 10.1128/AAC.02779-13 [DOI] [PMC free article] [PubMed] [Google Scholar]