Abstract
Artilysins constitute a novel class of efficient enzyme-based antibacterials. Specifically, they covalently combine a bacteriophage-encoded endolysin, which degrades the peptidoglycan, with a targeting peptide that transports the endolysin through the outer membrane of Gram-negative bacteria. Art-085, as well as Art-175, its optimized homolog with increased thermostability, are each composed of the sheep myeloid 29-amino acid (SMAP-29) peptide fused to the KZ144 endolysin. In contrast to KZ144, Art-085 and Art-175 pass the outer membrane and kill Pseudomonas aeruginosa, including multidrug-resistant strains, in a rapid and efficient (∼5 log units) manner. Time-lapse microscopy confirms that Art-175 punctures the peptidoglycan layer within 1 min, inducing a bulging membrane and complete lysis. Art-175 is highly refractory to resistance development by naturally occurring mutations. In addition, the resistance mechanisms against 21 therapeutically used antibiotics do not show cross-resistance to Art-175. Since Art-175 does not require an active metabolism for its activity, it has a superior bactericidal effect against P. aeruginosa persisters (up to >4 log units compared to that of the untreated controls). In summary, Art-175 is a novel antibacterial that is well suited for a broad range of applications in hygiene and veterinary and human medicine, with a unique potential to target persister-driven chronic infections.
INTRODUCTION
During the so-called golden age of antibiotics in the 1960s, many thought the war against bacterial infections was won, but this confidence was proven to be premature. More than ever, the increasing emergence of multidrug-resistant bacterial infections and a drying pipeline of new antibiotics call for the development of novel classes of antibiotics and new antibacterial strategies that can help address this global health care threat (1). However, only four new classes of antibiotics have been approved to the market in the last decade: (i) oxazolidones (linezolid), (ii) lipopeptides (daptomycin), (iii) pleuromutilins (retapamulin), and (iv) macrocycles (fidaxomicin). All of these target Gram-positive infections, and none of these drugs have novel mechanisms of action (2). In fact, these four chemical classes were all reported or patented prior to 1987, and no novel classes have been discovered since then. Hence, the discovery void is without precedent (3, 4). Governmental incentives, like the New Drugs 4 Bad Bugs (ND4BB) program, a core element of the European public-private Innovative Medicines Initiative, and the Generating Antibiotic Incentives Now (GAIN) act of the FDA further underline this alarming situation. The overuse of antibiotics in medicine (50% of all antibiotics prescribed for people are not needed or not optimally effective [5]) and the misuse for nonmedical purposes as growth promoters in livestock and poultry have now been addressed by European Union legislation (6) and U.S. FDA guidelines (see the FDA's strategy on antimicrobial resistance [http://www.fda.gov/animalveterinary/guidancecomplianceenforcement/guidanceforindustry/ucm216939.htm]).
Among all resistant pathogens, the situation is most critical for Gram-negative pathogens, which often show more extensive resistance to the available therapeutic options than do Gram-positive pathogens. One of the prevailing reasons is the presence of the protective outer membrane in Gram-negative pathogens, which represents a formidable barrier for antibiotics to overcome (7, 8). This not only eliminates the susceptibility of Gram-negative pathogens to many existing antibiotics, but also means that the current development pipeline of novel antibiotics has been directed toward resistant Gram-positive pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA). Therefore, the difficulty in discovering new antibiotics that are active against Gram-negative species is not a paucity of targets per se, but rather lies in the impermeability of the outer membrane (3). Persisters, an antibiotic-tolerant subpopulation, pose an additional challenge. They are proposed to be the cause of the rising number of chronic infections, simply because surviving persisters may repopulate the infection site once the periodic application of antibiotics stops (8). Therefore, novel effective drugs against persisters are of utmost importance.
The introduction of bacterial genome sequencing heralded a new era of hope for antibiotic discovery. However, the genomics approach for delivering novel targets for traditional small-molecule screening yielded a disappointing and financially unsustainable outcome (2, 9). Alternative antibacterial approaches, such as those involving cationic antimicrobial peptides and endolysins, emerged and are highly promising routes of investigation for controlling antibiotic-resistant bacteria (10–12).
Antimicrobial peptides are produced by a wide variety of organisms in the animal and plant kingdoms, and they play a fundamental role in nonspecific innate immunity. Although diverse with respect to their amino acid sequences, they share an amphipathic conformation, with positively charged amino acids distributed on one side and hydrophobic amino acids on the other side (13). The overall positive charge of these peptides allows them to accumulate at the polyanionic cell surface, which contains acidic polymers, such as lipopolysaccharide (LPS) and teichoic acids, in Gram-negative and Gram-positive bacteria, respectively (10). In Gram-negative bacteria, the peptides pass the outer membrane via a self-promoted uptake pathway: first, they interact with the cation binding sites of LPS molecules by competitive displacement of the divalent cations Mg2+ or Ca2+, and then they disrupt the barrier function of the membrane due to their bulkiness; transient cracks in the LPS layer subsequently permit uptake not only of themselves but also of other antibiotics or small proteins (14). The peptides kill the bacterium by either disrupting the cytoplasmic membrane or by crossing this membrane and acting on intracellular targets (10). Unfortunately, such peptides are often associated with cytotoxic properties (15).
Endolysins are enzymes produced by bacterial viruses (bacteriophages) at the end of their lytic cycle. They enzymatically degrade the peptidoglycan (PG) layer, the major structural component of a bacterium, from within the infected host cell. As such, they induce a sudden lysis of the infected bacterial cell in order to dissipate the newly formed phage particles (16). Exogenous application of purified endolysins to Gram-positive pathogens quickly kills them by lysis due to turgor pressure. Their efficacy as antibacterials has been shown for the treatment of mucosal and systemic Gram-positive infections in diverse animal models and for food applications (for reviews, see references 17 to 19). No resistant strains have been isolated so far in spite of a number of attempts to elicit resistance to endolysins against Gram-positive bacteria (20–22). The specificity of an endolysin in general is determined by the susceptibility of a certain PG chemotype for the enzymatically active domain (EAD) and the recognition of the cell wall or associated ligand by the cell wall binding domain (CBD), limiting the antibacterial effect to a certain genus, species, serovar, or strain (12, 18).
A major hurdle in the development of endolysins as antibacterials is the expansion to Gram-negative pathogens, since the outer membrane shields access to the PG from outside. This has partially been overcome by combining endolysins with outer membrane-permeabilizing agents, like EDTA (23). In this study, we combined the self-promoted uptake mechanism of a cationic antimicrobial peptide and the PG-degrading activity of an endolysin to tackle this hurdle. Specifically, we fused the sheep myeloid antimicrobial peptide of 29 amino acids (SMAP-29) (24) to the N terminus of the endolysin KZ144 (25) to create a so-called Artilysin. SMAP-29 is an α-helical cathelicidin found in sheep leukocytes and possesses potent antimicrobial activity against a broad spectrum of pathogens, including Pseudomonas aeruginosa, the target in this study. SMAP-29 is amphipathic and has two LPS binding sites (26). KZ144 is a modular endolysin with an N-terminal peptidoglycan binding domain and a C-terminal catalytic domain with transglycosylase activity, encoded by bacteriophage ϕKZ, which infects P. aeruginosa (25, 27). We show that an Artilysin composed of SMAP-29 fused to KZ144 is a highly potent antibacterial that kills virtually all P. aeruginosa strains, acts quickly (within minutes), is recalcitrant to resistance development, and is also able to kill metabolically inactive persisters.
MATERIALS AND METHODS
Cloning and mutagenesis.
The coding sequence for Artilysin 085 (Art-085) was constructed by fusing the sequence of SMAP-29 (24) to the 5′ end of the open reading frame of KZ144. Therefore, the SMAP-29 gene fragment was inserted between the NdeI and BamHI restriction sites of pET21a (Novagen, Madison, WI) and the fragment encoding KZ144 between the BamHI and XhoI sites. For proper selection, ampicillin (100 μg/ml) was used. A C-terminal His6 tag is present for affinity purification. Three Cys residues at positions 45, 54, and 81 of Art-085 were changed to Ser residues using the Quick Change mutagenesis (Q5 site-directed mutagenesis kit; New England BioLabs, Ipswich, MA), according to the manufacturer's instructions, resulting in Art-175. The same kit was used to change the catalytic residue of KZ144 in Art-175 (Glu115 of KZ144) to alanine.
Recombinant expression and purification.
The recombinant expression of both Artilysins was performed in 2 liters lysogeny broth (LB) with exponentially growing Escherichia coli strain BL21(DE3) pLysS cells (optical density at 600 nm [OD600], 0.45 to 0.55) upon induction with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C for 3 to 4 h. E. coli cells were harvested by centrifugation (3,900 × g for 30 min at 4°C), and the obtained cell pellet was resuspended in 40 ml lysis buffer (20 mM HEPES, 1,000 mM NaCl, 20 mM imidazole, and 1 mM MgCl2 [pH 7.4] supplemented with 40 μg DNase I). Cell disruption was done by sonication (Bandelin Sonopuls HD3200; conditions, 45% for 6 min with 10-s pulse/20-s break steps) on ice. Cell debris was separated by centrifugation at 15,000 × g for 20 min, and the supernatant was then filtered (0.2 μm) before application to the column. Purification of the His6-tagged fusion proteins was performed on an Aktä fast protein liquid chromatography (FPLC) system (GE Healthcare, Little Chalfont, United Kingdom) controlled by UNICORN 5.1 software with Ni2+-charged immobilized-metal affinity chromatography (IMAC) columns (1 ml HiPrep IMAC FF; GE Healthcare). The proteins were bound to the column using buffer A (20 mM HEPES, 1,000 mM NaCl, 20 mM imidazole [pH 7.4]) and eluted with a linear gradient to 100% buffer B (20 mM HEPES, 500 mM NaCl, 500 mM imidazole [pH 7.4]). The fractions containing the purified Artilysins were pooled and adjusted to a concentration of approximately 0.5 mg/ml with buffer C (20 mM HEPES, 500 mM NaCl [pH 7.4]). Next, ammonium sulfate was added using a 3.8 M stock solution at room temperature, with stirring, up to 850 mM. The prepared protein solution was added to a 5-ml or 20-ml GE Healthcare Phe-HP Sepharose column and eluted with a 60% buffer C step (20 mM HEPES, 500 mM NaCl [pH 7.4]). After dialysis against an appropriate buffer, the protein concentration was determined spectrophotometrically in silica cuvettes at a wavelength of 280 nm (Jasco V-650; Jasco Corporation, Tokyo, Japan). The endotoxin content of the purified Art-175 was measured with the Pyrogent Plus LAL kit (catalog no. N294-125; sensitivity, 0.125 endotoxin unit [EU]/ml; Lonza, Basel, Switzerland), according to the manufacturer's instructions, and was ≤0.325 EU/ml.
CD spectroscopy.
To measure the melting temperature (Tm), the ellipticity of the proteins was recorded at 220 nm in a Jasco model J-815 circular dichroism (CD) spectrometer (Jasco Corporation). The protein melting temperatures were determined with a heating rate of 1°C/min, an incubation time of 3 s, and a volume of 410 μl in a 1-mm light path Hellma quartz cuvette. All measurements were performed in 50 mM sodium phosphate buffer-300 mM NaCl at pH 7.4. The midpoint of the unfolding transition was determined by fitting to a sigmoid unfolding model using JASCO analysis software. The proteins were measured at concentrations of 5.4 μM (Art-085) and 5.3 μM (Art-175). Far-UV spectra were recorded at protein concentrations of 5.56 μM (KZ144), 5.60 μM (Art-085), and 6.25 μM (Art-175) in 50 mM sodium phosphate buffer-300 mM NaCl (pH 7.4) at a temperature of 20°C.
Thermal aggregation.
The thermally induced aggregation was measured in a V-650 UV/VIS spectrometer (Jasco Corporation) with temperature control. For all measurements, silica cuvettes were used, with stirring. The aggregation was measured at 360 nm within a temperature range of 20°C to 60°C, with a constant heating rate of 0.5°C/min. The protein concentration was 0.1 mg/ml in 20 mM HEPES-500 mM NaCl (pH 7.4).
Bacterial strains and culture conditions.
The P. aeruginosa strains used in this study were collected in the Queen Astrid Military Hospital in Brussels, Belgium (28, 29), or provided by A. Gessner from the Institute of Medical Microbiology University of Regensburg, Germany, P. Cornelis from the Free University of Brussels, Belgium (strain PA14wt), F. Van Bambeke from the Université Catholique de Louvain, Belgium, O. Denis from Hôpital Erasme, Belgium (strain PA1255), K. Tateda from the Toho University in Tokyo, Japan, or M. Schobert from the Technical University of Braunschweig, Braunschweig, Germany. Other bacteria used in this study were from DSMZ (German Collection of Microorganisms and Cell Cultures), the Robert Koch Institute, Wernigerode, Germany (A. Fruth), the Centre of Microbial and Plant Genetics, Leuven, Belgium (R. De Mot), or A. Gessner. All strains were grown in LB, except Enterococcus faecalis, Clostridium perfringens, and Bacteroides fragilis, which were grown in liquid Reinforced Clostridial Medium (RCM) (Oxoid, Basingstoke, United Kingdom) without shaking or on Columbia blood agar plates at 37°C. E. faecalis was grown under microaerophilic conditions (85% N2, 10% CO2, 5% O2). C. perfringens and B. fragilis were grown anaerobically (AnaeroGen [Oxoid]; oxygen level, <1% within 30 min; resulting CO2 level, 9 to 30%).
Antibacterial assay.
P. aeruginosa strain PAO1 or S. aureus strain DSM 346 was grown to the mid-exponential phase (OD600, 0.6), harvested by centrifugation (16,000 × g for 5 min), washed, and 10-fold diluted in 10 mM HEPES (pH 7.4) containing 0, 0.25, or 1 mM EDTA to a final density of ∼108 cells/ml. Fifty microliters of the bacterial cell dilution was incubated at 25°C, with shaking, with 50 μl of a protein solution containing either 10 μg KZ144, Art-085, or Art-175 or 1 μg of SMAP-29 (Sigma-Aldrich, St. Louis, MO) in 20 mM HEPES buffer (pH 7.4). After a 60-min incubation, appropriate dilutions of the cell suspensions were plated on LB agar in triplicate. Colonies were counted after overnight incubation at 37°C. The antibacterial activity was quantified as the relative inactivation in log units (log10[N0/Ni] with N0 as the initial number of untreated cells and Ni as the number of residual cells counted after treatment).
Cytotoxicity.
In compliance with EN ISO 10993-5:2009, Art-175 (5.17 mg/ml in phosphate-buffered saline [PBS]-0.01% Tween 20) was tested in vitro for cytotoxicity by Zwisler Laboratorium GmbH (Constance, Germany). Therefore, L-929 mouse connective tissue fibroblasts were cultured as a monolayer (6th split of L-929 DSM ACC2 culture, clone of strain L). The culture medium (RPMI 1640) was supplemented with 10% (vol/vol) fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. All compounds were purchased from Life Technologies (Grand Island, NY). The sample was diluted 1:5, 1:10, 1:20, and 1:40 in cell culture medium and was incubated with the cells for 2 days. Thereafter, the degree of cell destruction was evaluated using microscopy and subsequent viability staining using the tetrazole 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) in a colorimetric assay. The suitability of the test system was confirmed by positive and negative controls. A sample with a resulting cell viability of >90% was considered noncytotoxic.
MIC determination.
The MICs of Art-085 and Art-175 were determined by the broth microdilution method in 96-well microtiter plates. The respective bacterial overnight culture was diluted 1:5,000 to a concentration of 2 × 105 to 8 × 105 CFU/ml in non-cation-adjusted Mueller-Hinton (MH) broth (Difco, BD, Franklin Lakes, NJ) and split into the required amount of wells. Reinforced Clostridial Medium (Oxoid) was used for C. perfringens and B. fragilis. Art-085 and Art-175 were added in different concentrations (determined as final concentration [μg/ml] in MH broth). EDTA was added to a final concentration of 0 or 0.5 mM, as indicated in Tables S1 and S2 in the supplemental material. The MIC assays with C. perfringens and B. fragilis were prepared in the microaerophilic chamber prior to anaerobic incubation. The mixtures were incubated overnight (18 h) at 37°C, except for P. aeruginosa strains Colja 7 (33 h), 06071 (48 h), and va32872_1 (48 h). P. aeruginosa strains BT73 and 11159 were measured after incubation for 4 days at room temperature. Bacterial growth was determined by turbidity measurements in an enzyme-linked immunosorbent assay (ELISA) reader (Tecan Infinite M 200 Pro; Tecan, Männedorf, Switzerland). All measurements were performed in comparison to a negative control (MH broth without bacteria) or a positive control (without Artilysin). The MIC was defined as the minimum concentration at which no bacterial growth was observed in the well.
Time-lapse microscopy.
Time-lapse microscopy experiments were performed with a temperature-controlled (Okolab, Ottaviano, Italy) Eclipse Ti-E inverted microscope (Nikon, Champigny-sur-Marne, France) equipped with a Ti-CT-E motorized condenser and a CoolSNAP HQ2 FireWire CCD camera, as described previously (30). P. aeruginosa PAO1 cells were grown to mid-exponential phase (OD600, 0.6), washed three times with PBS (pH 7.4), and finally concentrated five times by resuspension in PBS–0.5 mM EDTA-Na2. Art-175 was added to a final concentration of 0.1 mg/ml, corresponding to 25× MIC. For imaging, the samples were placed between PBS agar pads and a cover glass, essentially as described previously (30), and incubated at 37°C. The time between the addition of Art-175 to the cells and recording was kept as short as possible (60 s). Images were acquired using NIS-Elements (Nikon), and the resulting pictures were further handled with open-source software, ImageJ (http://rsbweb.nih.gov/ij/).
Time-kill curve.
Mid-exponentially growing cells (OD600, 0.6) of P. aeruginosa PAO1 in LB medium were harvested by centrifugation (16,000 × g for 5 min), washed twice with 2.5 ml PBS (pH 7.4), and resuspended in 1 ml PBS to a final density of ∼109 cells/ml. A suspension with a total volume of 800 μl containing 576 μl of the bacterial cell dilution, 0.5 mM EDTA-Na2 (final concentration), and 80 μg Art-175 in 20 mM HEPES buffer-500 mM NaCl (pH 7.4) was prepared. This stock solution was incubated at 25°C with shaking for the times indicated. At the respective time points, 50 μl was taken, and appropriate dilutions of the cell suspensions were plated on LB agar in triplicate. Colonies were counted after overnight incubation at 37°C. The antibacterial activity was quantified as the relative inactivation in log units (log10[N0/Ni], with N0 as the initial number of untreated cells and Ni as the number of residual cells counted after treatment).
Resistance development.
The MICs of Art-175 and ciprofloxacin (Sigma-Aldrich) against P. aeruginosa strains PAO1, Br257, and Br776 were determined. Two-fold dilution series of Art-175 were prepared in 20 mM HEPES-100 mM NaCl in a round-bottom 96-well microtiter plate (Nunc; Thermo Fisher Scientific, Waltham, MA). The bacterial inoculum was prepared from an 18-h Mueller-Hinton (MH) agar plate, and the suspension was adjusted to reach a turbidity equivalent to a 0.5 McFarland standard (31). This suspension was further diluted 100× in 2× MH medium (with 1 mM EDTA-Na2 for Art-175). Fifty microliters of bacterial suspension was mixed with 50 μl Art-175 and incubated for 24 h at 37°C. Controls with MH medium (0.5 mM EDTA-Na2) only and MH medium (0.5 mM EDTA-Na2) with cells were included. On the next day, the MIC was recorded as the minimal concentration that completely inhibited growth. A new MIC assay was prepared using 1,000×-diluted cells grown at MIC/2 (occasionally MIC/4 if growth in MIC/2 was too limited). This was repeated for 20 passages.
Persister killing.
Planktonic persister cells were isolated from a stationary-phase culture as described previously (32), with minor modifications. An overnight culture of P. aeruginosa PA14 or PA1255 (a cystic fibrosis isolate) was inoculated in 50 ml 1:20 Trypticase soy broth (TSB). After 48 h of growth at 37°C, the culture was treated with ofloxacin at a final concentration of 10 μg/ml (5× MIC) for 5 h at 37°C, while shaking at 200 rpm. The persister cells surviving this antibiotic treatment were isolated by centrifugation (5,250 × g for 15 min at 4°C), and the cell pellet was washed twice with 20 mM HEPES (pH 7.4). Prior to treatment, the cells were resuspended in the same volume of 20 mM HEPES (pH 7.4). A persister killing assay was performed as described previously (32), with minor modifications. A volume of 100 μl of the isolated persister fraction was mixed with either Art-175 or ciprofloxacin (final concentrations of 10× or 30× MIC against the respective strains), in the absence or presence of 0.5 mM EDTA-Na2 (final concentration) up to a final volume of 200 μl. Control treatments with 20 mM HEPES (pH 7.4), 0.5 mM EDTA, or ofloxacin (30× and 10× MIC) were performed in parallel. The mixtures were shaken (200 rpm) for 1 h at 37°C. The treated persisters were washed twice with 20 mM HEPES (pH 7.4), and the appropriate dilutions were plated on TSB plates and incubated at 37°C. The number of persisters was determined after 72 h of incubation to allow for complete resuscitation of all surviving persisters. Each experiment was independently repeated ≥3 times.
RESULTS
Art-085 and its optimized Art-175 homolog efficiently kill P. aeruginosa.
Initially, the SMAP-29 peptide was fused to the N terminus of full-length KZ144, named Art-085. A His6 tag at the C terminus allowed for purification by metal affinity chromatography. Since Art-085 forms oligomers due to (an) intermolecular disulfide bridge(s), similar to wild-type KZ144, a triple mutant of Art-085 was constructed by exchanging three cysteines (Cys14, Cys23, and Cys50) of KZ144 for serine (Art-175). Circular dichroism (CD) spectra show a mainly α-helical composition and do not show conformational differences among KZ144, Art-085, and Art-175 (see Fig. S1A in the supplemental material). SDS-PAGE of reduced and nonreduced Art-085 and Art-175 (see Fig. S1B in the supplemental material) and gel filtration chromatography confirm that no oligomers are present in Art-175 in contrast to nonreduced Art-085 (data not shown). The unfolding of Art-085 and Art-175 was monitored as a function of temperature by measuring the ellipticity at 222 nm, an indicator for changes in helicity (see Fig. S1C in the supplemental material). The unfolding of Art-175 occurs more cooperatively and at a significantly higher temperature (Tm = 50.3°C) than that of Art-085 (Tm = 44.2°C), indicating a more stable conformation for Art-175. In addition, we analyzed the aggregation of Art-085 and Art-175 by measuring UV absorbance at 360 nm with increasing temperature (see Fig. S1D in the supplemental material). The aggregation of Art-085 starts at a lower temperature than for Art-175, and the corresponding peak is wider. This confirms the increased thermostability and rapid cooperative unfolding of Art-175 (only monomer) compared to those of Art-085 (mono- and oligomers).
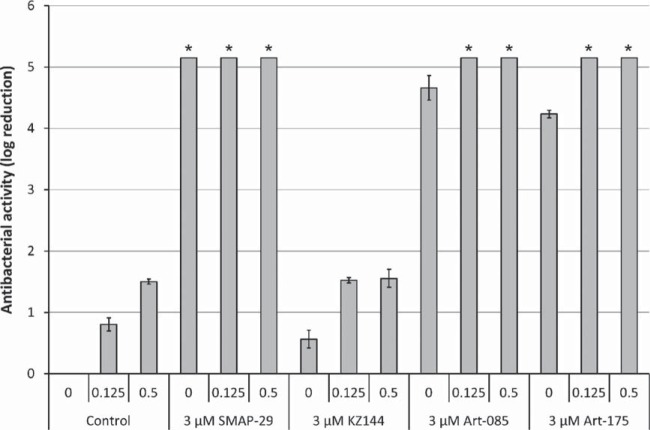
We compared the antibacterial activities of 3 μM SMAP-29, KZ144, Art-085, and Art-175 against P. aeruginosa PAO1 in the presence of 0, 0.125, or 0.5 mM EDTA (Fig. 1). SMAP-29 is highly bactericidal, with complete killing without EDTA (≥5.15 log units) in 60 min. The endolysin KZ144 cannot efficiently permeate the outer membrane to hydrolyze the PG layer and therefore shows only limited antibacterial activity (0.57 ± 0.14 log units) in the absence of EDTA. At 125 μM EDTA, the bacterial reduction is improved slightly from 0.80 ± 0.11 log (125 μM EDTA alone) to 1.52 ± 0.04 log units. Art-085 and Art-175 have bactericidal activities similar to that of SMAP-29, with almost complete eradication (4.66 ± 0.20 log and 4.23 ± 0.26 log units, respectively) in the absence of EDTA; this is further enhanced in the presence of 125 μM EDTA, resulting in complete killing (≥5.15 log units compared to the untreated controls). Since SMAP-29 shows cytotoxicity to mammalian cells (15), the cytotoxicity of Art-175 was analyzed using L-929 mouse connective tissue fibroblasts. A sample of 5.2 mg/ml Art-175 was tested in 1:5, 1:10, 1:20, and 1:40 dilutions. After 2 days of exposure, 93% ± 1.4% fibroblasts were viable, and no more than 10% of the cells showed cell lysis or discrete intracytoplasmic granules. Art-175 is therefore considered to be noncytotoxic, in compliance with EN ISO 10993-5:2009 (see Fig. S2 in the supplemental material).
FIG 1.
Bactericidal effects of SMAP-29, KZ144, Art-085, and Art-175 against P. aeruginosa PAO1. A cell suspension was treated with equimolar amounts of SMAP-29, KZ144, Art-085, and Art-175 (3 μM) in the absence and presence of 0.125 or 0.5 mM EDTA. Bacterial reduction after 1 h is expressed in log10 units relative to the untreated control. Mean values (± standard deviation [SD]) are shown. An asterisk indicates when the detection limit (5.15 log units) was reached.
Art-175 kills all P. aeruginosa strains, including multidrug-resistant isolates.
The MICs of Art-085 and Art-175 against P. aeruginosa PAO1 were 18 and 10 μg/ml, respectively (Table 1). Expressed as a molar concentration, these correspond to 0.5 and 0.3 μM, respectively, which are approximately the same as those for ciprofloxacin against the same strain (0.5 μM) and endolysins against Gram-positive pathogens (0.3 μM) (34). In the presence of EDTA, the MICs decrease in a nonlinear EDTA-dependent way to 4 and 2 μg/ml, respectively. Art-085 and Art-175 are strongly inhibitory against all tested P. aeruginosa strains (n = 74 and 79, respectively) with an MIC50 and an MIC90 of 8 and 15 μg/ml for Art-085 and 4 and 10 μg/ml for Art-175, respectively, with no differences in susceptibility among the environmental, clinical, and multidrug-resistant strains (see Table S1 in the supplemental material). In fact, the growth of multidrug-resistant P. aeruginosa strains covering resistance mechanisms to several therapeutically used antibiotics is fully inhibited by Art-175 (see Table S2 in the supplemental material).
TABLE 1.
MICs of Art-085 and Art-175a
| EDTA concn (mM) or parameter | MIC (μg/ml) for: |
|
|---|---|---|
| Art-085 | Art-175 | |
| 0 | 18 | 10 |
| 0.125 | 20 | 10 |
| 0.25 | 16 | 6 |
| 0.5 | 10 | 4 |
| 1 | 6 | 4 |
| 2 | 4 | 2 |
| 4 | 4 | 2 |
| MIC50b | 8 | 4 |
| MIC90 | 15 | 10 |
Art-175 (10 μg/ml) has an improved inhibitory activity against P. aeruginosa PAO1 compared to that of Art-085 (18 μg/ml). From 0.25 mM EDTA, MICs further decrease to 4 and 2 μg/ml for Art-085 and Art-175, respectively.
The MICs at which growth of 50% (MIC50) or 90% (MIC90) of the isolates are inhibited, are shown.
The activity spectra of Art-085 and Art-175 were further analyzed on a broad panel of bacterial species, including Gram-negative organisms (Pseudomonadaceae, Enterobacteriaceae, and Bacteroidaceae), Gram-positive species, and a single yeast species (Candida albicans) (see Table S3 in the supplemental material) in the absence of EDTA. Art-085 and Art-175 showed the highest inhibitory activities against P. aeruginosa and the plant pathogen Pseudomonas syringae. Also, some reduced activity was observed against the soil bacterium Pseudomonas putida. All other species tested were not susceptible to ≥20 μg/ml Art-085 or Art-175, with the exception of Klebsiella pneumoniae (7.5 and 10 μg/ml for Art-085 and Art-175, respectively) and Salmonella enterica serovar Enteritidis (14 μg/ml for Art-085).
Art-175 rapidly kills bacteria through cell lysis.
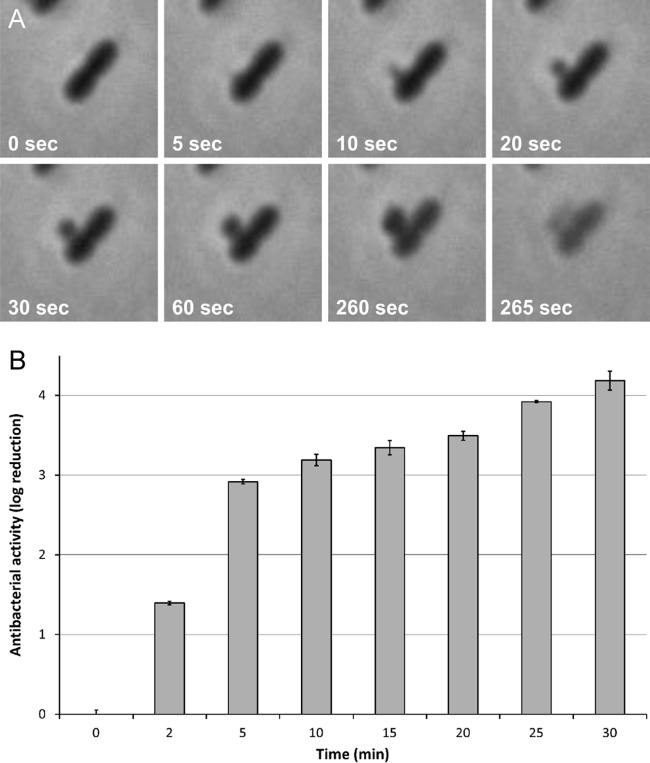
The working mechanism of Art-175 was visualized using time-lapse microscopy (see Movie S1 in the supplemental material). A concentration corresponding to 25× MIC in the presence of 0.5 mM EDTA was mixed with P. aeruginosa PAO1 cells. Within 20 min, all cells within the microscopic field were lysed. A detailed view (Fig. 2A; see also Movie S2 in the supplemental material) shows that complete lysis was mostly preceded by cytoplasmic membrane bulging through a hole created in the cell wall. When a slightly higher concentration of Art-175 (30× MIC) was added, all cells had already adopted a pleomorphic shape before recording could start (∼1 min), resembling spheroplasts that lacked an intact PG sacculus. A complete microscopic field was cleared within 6 min. To quantify the rapid mode of action of Art-175, a time-kill curve of Art-175 was determined under conditions identical to those of the time-lapse series (25× MIC) (Fig. 2B). In the first 5 min, a time-dependent bactericidal effect up to an approximately 3-log reduction was observed. Between 5 and 30 min, the bacterial reduction further increased from about 3 to 4 log units. Art-175 thus conserves a rapid action similar to those described for SMAP-29 and endolysins in general (18, 35).
FIG 2.
Rapid mode of action of Art-175. (A) P. aeruginosa PAO1 is rapidly lysed upon contact with Art-175 (25× MIC). Shortly after adding Art-175, a hole is the cell wall is punctured and the cytoplasmic membrane increasingly bulges until the cell undergoes abrupt lysis (see also Movie S1 in the supplemental material). (B) A 30-min time course shows that P. aeruginosa PAO1 treated with 25× MIC Art-175 is reduced by approximately 3 log units in the first 5 min, increasing to 4 log units after 30 min. Mean values (± SD) are shown.
A similar time-lapse microscopic experiment was performed with an equimolar amount of SMAP-29 (see Movie S3 in the supplemental material). The observations, however, differ significantly from those with Art-175: first, the cells gradually lost their dense cellular content, as indicated by the formation of blebs on the surface and obvious contrast reduction, but intact PG sacculi remained and no cell lysis was observed; second, the morphological changes were slower, with visible changes taking place during a 1-h period; third, some unaffected cells continued to grow and multiply. These observations correspond to the proposed mode of action of SMAP-29, i.e., permeabilizing the outer and cytoplasmic membrane (24). The differences between killing by Art-175 and SMAP-29 clearly indicate a different mode of action, with a pivotal role for cell lysis through cell wall degradation by Art-175. These observations are consistent with a peptide-assisted uptake of the endolysin KZ144 through the outer membrane, followed by osmotic lysis through PG degradation.
To analyze possible remaining bactericidal activity of the SMAP-29 moiety through cytoplasmic membrane disruption, we also analyzed the bactericidal activities of SMAP-29, KZ144, Art-085, and Art-175 against S. aureus DSM 346 (see Fig. S3 in the supplemental material). SMAP-29 has a broad antibacterial spectrum against both Gram-positive and Gram-negative species (25), whereas KZ144 is enzymatically active only on PG with chemotype A1γ, conserved among Gram-negative bacteria (25). The PG of S. aureus has chemotype A3α (36) and is thus not susceptible to KZ144 (25). Whereas SMAP-29 kills S. aureus (∼3.7 log units) independently of EDTA concentration, KZ144, Art-085, and Art-175 have no significant antibacterial activity (<0.5 log units) under any conditions. This indicates that (i) the SMAP-29 moiety in Art-085/Art-175 has lost its cytoplasmic membrane-disrupting activity and (ii) efficient lytic activity of KZ144 is required for successful killing by Art-085/Art-175. We also prepared an enzymatically inactive mutant of Art-175 by exchanging the Glu115 of KZ144 for alanine. The antibacterial activity of this mutant against P. aeruginosa PAO1 dropped from 4.23 ± 0.05 to 1.97 ± 0.06 log units in the absence of EDTA, showing the essential role of active KZ144 in Art-175 and possible residual activity of the SMAP-29 moiety against P. aeruginosa.
Art-175 is refractory to resistance development.
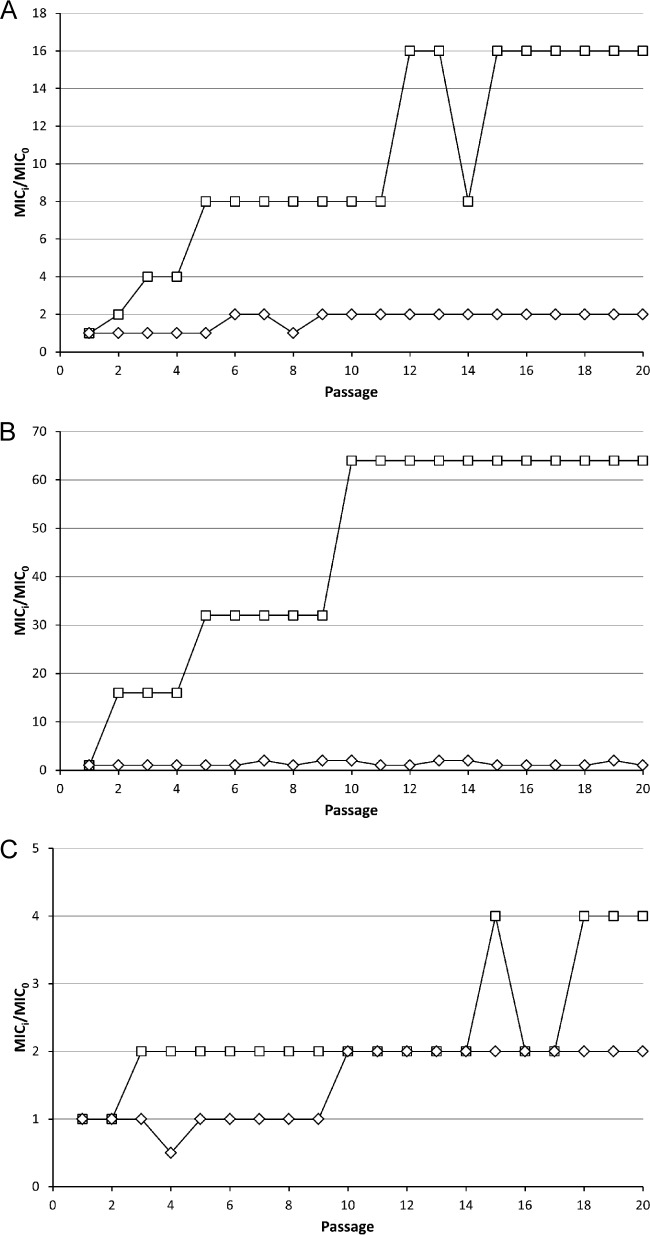
Serial exposure of three different P. aeruginosa strains (PAO1, a laboratory strain of clinical origin; Br257, isolated from the environment; Br776, a multidrug-resistant clinical isolate) to subinhibitory doses of Art-175 did not lead to the recovery of resistant strains after 20 passages (Fig. 3A to C). A maximum 2-fold increase of MIC was observed for all strains. A similar experiment with ciprofloxacin yielded 16- and 64-fold-increased MICs for PAO1 and Br257, respectively. Multidrug-resistant Br776, which showed initial high resistance against ciprofloxacin (16 μM versus 0.5 and 0.25 μM for PAO1 and Br257, respectively), showed a further 4-fold increase in MIC. Art-175 is thus highly refractory to resistance development.
FIG 3.
Resistance development against Art-175 and ciprofloxacin. A laboratory strain of clinical origin (P. aeruginosa PAO1) (A), an environmental strain (P. aeruginosa Br257) (B), and a multidrug-resistant strain (P. aeruginosa Br776) (C) were serially exposed to subinhibitory concentrations to select for decreased susceptibility. Over 20 passages, the MIC of Art-175 (◊) increased 2-fold, whereas the MIC of ciprofloxacin (☐) increased 16- (PAO1), 64- (Br257), or 4-fold (Br776).
Art-175 has a superior bactericidal effect against P. aeruginosa persisters.
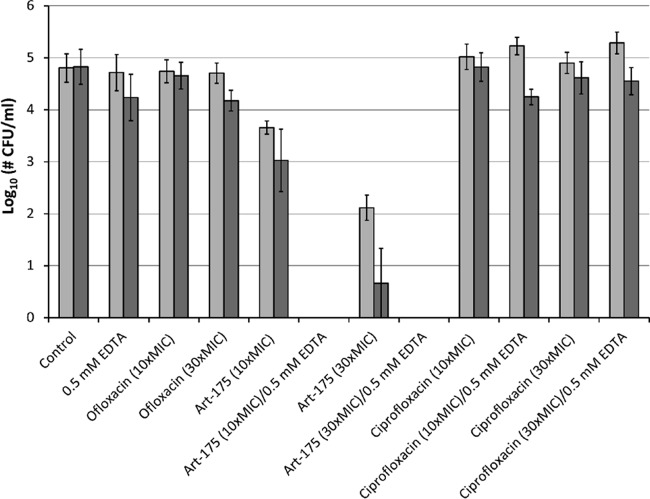
Persisters are a subpopulation of antibiotic-tolerant cells and have been linked to chronic and recurrent infections (8). The bactericidal activity of Art-175 was tested against this dormant subpopulation (Fig. 4). Persister fractions of two different strains (PA14 and PA1255, an isolate of a cystic fibrosis patient) were collected after 5-h exposure to 5× MIC ofloxacin. The lack of killing after further exposure of the isolated fractions to increased ofloxacin concentrations (10× and 30× MIC) confirmed the persistent (antibiotic-tolerant) nature of both isolated persister fractions. The persisters were incubated with different concentrations of Art-175 or ciprofloxacin (10× MIC and 30× MIC) in the absence or presence of 0.5 mM EDTA. Art-175 (10× and 30× MIC) completely eradicated the remaining persisters (approximately 5-log reduction) in the presence of 0.5 mM EDTA, whereas similar doses of ciprofloxacin (with and without EDTA) or 0.5 mM EDTA alone did not affect the persisters. In the absence of EDTA, a 30×-MIC dose of Art-175 reduced the persister fractions of PA14 and PA1255 by 2.16 ± 0.42 log and 4.35 ± 1.15 log units, respectively. In summary, the overall activity of Art-175 against these persister fractions is extremely pronounced.
FIG 4.
Persister killing by Art-175. The bactericidal effects of Art-175 at 10× and 30× MIC on isolated persister fractions of P. aeruginosa PA14 (light gray) and PA1255 (dark gray) are compared to those of ciprofloxacin at 10× MIC and 30× MIC, in both the absence and the presence of 0.5 mM EDTA. Controls include 0.5 mM EDTA and ofloxacin (10× MIC and 30× MIC). Mean values (± standard error of the mean [SEM]) are shown for at least three independent replicates.
DISCUSSION
To address the ongoing struggle between the emergence of antibiotic resistance and the lagging development of new antibiotics, drastically novel strategies for antibacterial development are required. This is particularly true for Gram-negative pathogens, which are protected by a largely impermeable outer membrane, thinning out the number of potential antibacterials that are effective against them. Nevertheless, Gram-negative pathogens, such as P. aeruginosa, are often the primary cause of chronic infections. Artilysins feature essential properties of such a novel strategy, as they are innovative because of the unique combination of two novel classes of antibacterials, exert a rapid bactericidal effect, are active on multidrug-resistant strains, are highly refractory to resistance development, and kill persisters.
In spite of major advances in endolysin research in recent years (12, 18), Gram-negative pathogens have remained out of reach. Artilysins now expand the potential of endolysins to reach Gram-negative species. The conceptual idea of Artilysins relies on a peptide-assisted uptake of the endolysin moiety through the outer membrane, followed by enzymatic degradation of the PG layer and osmotic lysis. Our microscopic observations are consistent with this model, showing cytosol release, followed by complete cell lysis, in contrast to SMAP-29, which works more slowly and does not induce lysis. This demonstrates that the endolysin must be transferred to the periplasm due to the outer membrane-disrupting capacity of the SMAP-29 moiety. The bulging cytoplasmic membrane prior to complete lysis is identical to the lytic effect of endolysins targeting Gram-positive species (20), indicating that local holes are created in the PG layer. The critical pore size in the Gram-negative PG layer to initiate bulging has been calculated as approximately 20 nm (37), meaning that only several adjacent glycan chains have to be hydrolyzed to induce irreversible release of the cytosol. Hence, the capacity of SMAP-29 to induce local outer membrane disruption is conserved within the fusion protein. In contrast, the bactericidal effect of the SMAP-29 moiety through cytoplasmic membrane permeabilization appears to be lost or limited within the Artilysins. The inactivity of Art-175 against Gram-positive S. aureus DSM 346, from which the PG layer is not a suitable substrate for KZ144, points to a complete loss of this effect. However, the limited antibacterial activity of an enzymatically inactive mutant of Art-175 may suggest some remaining effect of the SMAP-29 moiety against P. aeruginosa. An alternative explanation might be that the binding of the mutant to the PG layer upon crossing the outer membrane interferes with PG homeostasis even without catalytic activity, resulting in limited cell death through the disturbed balance of PG degradation and synthesis. In addition, we cannot exclude minor residual muralytic activity of the mutant due to a secondary catalytic residue, which falls below the sensitivity of the assay.
Importantly, Art-175 lacks any cytotoxicity on L-929 mouse connective tissue fibroblasts, an international standard cell culture line used to evaluate acute adverse biological effects (ISO 10993-5:2009). Hence, Art-175 has a high bactericidal effect but differs from SMAP-29 and antimicrobial peptides in general, which often show cytotoxicity to mammalian cells (15).
Alternative approaches that translocate a lytic enzyme across the outer membrane have been reported. We have shown the efficacy of high hydrostatic pressure combined with endolysins against Gram-negative species (approximately 4 log units with 200 MPa), a technique applicable as a hurdle technology in food conservation (38). Protein engineering-based methods rely on the transport of T4 lysozyme via the TonB-dependent outer membrane transporter FyuA through a fusion of the enzyme to the FyuA binding domain of pesticin (39), or the modification of eukaryotic lysozymes with diverse peptides or chemical substituents to increase hydrophobicity (for a review, see reference 50). Both methods yielded fusion proteins that successfully killed Gram-negative bacteria, but the bactericidal effect was generally limited (<1-log reduction) compared to that of the Artilysins presented in this study.
Art-085 and its improved homolog Art-175 have been developed to target P. aeruginosa infections. Within the family Pseudomonadaceae, nonhuman pathogens (P. syringae and P. putida) are susceptible to Art-085 and Art-175 as well, but Enterobacteriaceae (except K. pneumoniae) are excluded. Commensal flora, such as Staphylococcus epidermidis, S. aureus, Streptococcus pneumoniae (skin and nasopharynx), C. perfringens, C. albicans (skin), B. fragilis, and E. coli (gut) are unaffected (>20 μg/ml). A large screen of inhibitory activities against a diverse panel of P. aeruginosa isolates (n = 79), comprising both environmental and clinical strains, showed the broad applicability of both Artilysins. Clinical isolates from samples from (burn) wounds, sputum of cystic fibrosis patients, throat, urinary tract, and lung infections, and otitis in dogs are all susceptible, irrespective of their source or extent of multidrug resistance. The MIC is also independent of the relative biofilm formation capacity of a strain (data not shown). In general, Art-175 has improved MIC50 (4 μg/ml) and MIC90 (10 μg/ml) values compared to those of Art-085 (8 and 15 μg/ml, respectively), most likely due to its purely monomeric form. In addition, Art-175 has a more stable conformation and improved thermostability.
Novel classes of antibacterials should display limited resistance development to avoid rapid and wide distribution of resistance against novel antibacterials in the future. Art-175 meets this criterion, since it features a low probability of resistance development, in contrast to ciprofloxacin, a first-line antibiotic in health care. Highly selective exposure to subinhibitory concentrations of Art-175 does not elicit resistance development through genetic modifications in any of three P. aeruginosa strains tested. Similarly, no resistance has been reported to endolysins acting on Gram-positive pathogens in experimental settings to identify such resistant mutants (40), and microbial resistance against antimicrobial peptides is said to be rare as well (41). In future studies, mutator strains could be used to stress resistance development by mechanisms requiring multiple mutations against antimicrobial peptides, endolysins, and Artilysins (42). However, the immutable nature of PG will certainly contribute to the refractory nature of endolysins and Artilysins in resistance development.
In addition, drug-resistant P. aeruginosa isolates, which are resistant against any of 21 different antibiotics tested, are susceptible to Art-175 to the same extent as antibiotic-sensitive strains. This is also true for the most resistant strains that are not susceptible to 19 out of 21 antibiotics tested. Hence, existing resistance mechanisms against those therapeutically used antibiotics do not give cross-resistance to Art-175. Cystic fibrosis isolates undergo physiological adaptations of their LPS structure in chronic infections, generally decreasing the net charge of LPS molecules and the susceptibility to cationic peptides (43). However, we did not note a difference in susceptibility among those isolates (see Table S1 in the supplemental material; MICs, 4 to 12 μg/ml).
Unmetabolized antibiotic substances have been detected in hospital effluents, sewage systems, manure, soil, and surface and ground water, and most of them persist in the environment. These antimicrobials exert a selection pressure and select for resistant mutants in resident microbial communities, particularly when bacteria are exposed to subtherapeutic levels of the antimicrobials. In addition, resistance genes can be transferred by horizontal transfer (44). Due to the proteinaceous nature of Artilysins and their consequent biodegradability, Artilysins are expected not to persist in the environment and therefore to have a low environmental impact. This property further reduces their risk of developing resistance.
Although chronic infections are primarily caused by drug-sensitive pathogens, they are hard to treat with antibiotics. Persisters are responsible for this apparent paradox. These cells constitute a small fraction of the bacterial population that is transiently drug tolerant without having acquired resistance through genetic modification. Instead, their insensitivity to antibiotics is mostly explained by a shutdown of metabolic processes (45). In a model of relapsing biofilm infections (8), it has been proposed that persisters hidden in the biofilm matrix are shed from the surrounding tissue and bloodstream. Hence, these persisters are protected from elimination by the immune system and can repopulate the biofilm when the antibiotic concentration drops. Artilysins do not require an active bacterial metabolism to exert their bactericidal effect, as they actively degrade the PG layer, resulting in immediate osmotic lysis. Unlike traditional antibiotics, Art-175 kills persisters of both strains tested (PA14 and PA1255) in a rapid (≤1 h) and efficient (from >2 log units to complete eradication) way. Despite the clinical relevance of persisters and their role in treatment failures being increasingly recognized, there are currently no practical means for eradicating them (46), or no such molecules have been/are being tested in clinical trials (see clinicaltrialsregister.eu and clinicaltrials.gov). Current developments of antipersister therapies focus on screening large libraries to identify molecules that kill persisters (47), the resuscitation of dormant persisters to resensitize them to conventional antibiotics (48), or the prevention/reduction of persister formation (45). Treatment with the Trp-/Arg-containing peptide (RW)4-NH2 reduced viable E. coli persister cells by 2.5 log units in 30 min; however, a higher concentration of (RW)4-NH2 (80 μM) was needed than that needed for Art-175 (1.2 to 3.6 μM) (32). Art-175 thus shows promise not only for killing of metabolically active vegetative cells but also as a potential treatment option for chronic infections caused by ever-repopulating persisters surviving antibiotic treatment, e.g., in cystic fibrosis (CF) patients. The majority of CF patients succumb to chronic lung infections caused by P. aeruginosa, such as the PA1255 strain used in this study, and the recalcitrance of CF infections has been linked to an increased level of persister formation, particularly in isolates recovered during the late stages of infection (49). Art-175 inhibits the growth of all cystic fibrosis isolates tested (see Table S1 in the supplemental material), has a low probability of developing resistance, and kills both replicating and nonreplicating P. aeruginosa cells. Art-175 is thus well suited for further development to protect or treat chronically infected CF patients.
In summary, Artilysins represent a class of novel antibacterials that expand the applicability of endolysins to include Gram-negative species by merging the benefits of an antimicrobial peptide and a highly active endolysin. Art-175 combines superior features, as it has a quick onset of action, low risk for developing resistance, expected low environmental impact, and bactericidal activity against both drug-resistant strains and persisters. Therefore, Art-175 holds promise in a wide range of applications in hygiene and veterinary and human medicine, with a special emphasis on chronic infections (in human medicine), such as cystic fibrosis-associated lung infections.
Supplementary Material
ACKNOWLEDGMENTS
We are particularly thankful for the technical assistance of I. Anosova, S. Dancker, S. Deml, and E. Olbrich. We also acknowledge M. Schobert (Technical University of Braunschweig, Germany), A. Fruth (Robert Koch Institute, Wernigerode, Germany), A. Gessner (University of Regensburg, Germany), K. Tateda (University of Toho, Japan), P. Cornelis (Free University of Brussels, Belgium), F. Van Bambeke (Université Catholique de Louvain, Belgium), O. Denis (Hôpital Erasme, ULB), and R. De Mot (University of Leuven, Belgium) for providing the many different P. aeruginosa isolates used in this study.
Artilysin is a registered trademark in the European Union, United States, and other countries.
This work was supported by the KU Leuven Industrial Research Fund (IOF/HB/12/014), KU Leuven Research Fund (IDO/10/012), and the Research Foundation of Vlaanderen (FWO-Vlaanderen) (grants G.0599.11, G.0413.10, and G.0471.12N).
V.D. holds a Ph.D. grant from the Agency for Innovation by Science and Technology (IWT), S.M. and M.B. are employees of Lisando GmbH, and R.L. acts as a scientific advisor to Lisando GmbH.
Footnotes
Published ahead of print 21 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02668-14.
REFERENCES
- 1.Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, Bartlett JG, Edwards J, Jr, Infectious Diseases Society of America 2008. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 46:155–164. 10.1086/524891 [DOI] [PubMed] [Google Scholar]
- 2.Xu ZQ, Flavin MT, Flavin J. 2014. Combating multidrug-resistant Gram-negative bacterial infections. Expert Opin. Invest. Drugs 23:163–182. 10.1517/13543784.2014.848853 [DOI] [PubMed] [Google Scholar]
- 3.Silver LL. 2011. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 24:71–109. 10.1128/CMR.00030-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancaster JW, Matthews SJ. 2012. Fidaxomicin: the newest addition to the armamentarium against Clostridium difficile infections. Clin. Ther. 34:1–13. 10.1016/j.clinthera.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf [Google Scholar]
- 6.European Parliament. 2011. Resolution of 27 October 2011 on the public health threat of antimicrobial resistance. European Union, Strasbourg, France: http://www.europarl.europa.eu/sides/getDoc.do?pubRef=−//EP//TEXT+TA+P7-TA-2011-0473+0+DOC+XML+V0//EN [Google Scholar]
- 7.Delcour AH. 2009. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 1794:808–816. 10.1016/j.bbapap.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis K. 2010. Persister cells. Annu. Rev. Microbiol. 64:357–372. 10.1146/annurev.micro.112408.134306 [DOI] [PubMed] [Google Scholar]
- 9.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. 2007. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6:29–40. 10.1038/nrd2201 [DOI] [PubMed] [Google Scholar]
- 10.Hancock RE, Sahl HG. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557. 10.1038/nbt1267 [DOI] [PubMed] [Google Scholar]
- 11.Parisien A, Allain B, Zhang J, Mandeville R, Lan CQ. 2008. Novel alternatives to antibiotics: bacteriophages, cell wall hydrolases and antimicrobial peptides. J. Appl. Microbiol. 104:1–13. 10.1111/j.1365-2672.2007.03498.x [DOI] [PubMed] [Google Scholar]
- 12.Nelson DC, Schmelcher M, Rodriguez-Rubio L, Klumpp J, Pritchard DG, Dong S, Donovan DM. 2012. Endolysins as antimicrobials. Adv. Virus Res. 83:299–365. 10.1016/B978-0-12-394438-2.00007-4 [DOI] [PubMed] [Google Scholar]
- 13.Baltzer SA, Brown MH. 2011. Antimicrobial peptides: promising alternatives to conventional antibiotics. J. Mol. Microbiol. Biotechnol. 20:228–235. 10.1159/000331009 [DOI] [PubMed] [Google Scholar]
- 14.Hancock RE. 1997. Peptide antibiotics. Lancet 349:418–422. 10.1016/S0140-6736(97)80051-7 [DOI] [PubMed] [Google Scholar]
- 15.Dawson RM, Liu CQ. 2009. Cathelicidin peptide SMAP-29: comprehensive review of its properties and potential as a novel class of antibiotics. Drug Dev. Res. 70:481–498. 10.1002/ddr.20329 [DOI] [Google Scholar]
- 16.Young R, Wang I, Roof WD. 2000. Phages will out: strategies of host cell lysis. Trends Microbiol. 8:120–128. 10.1016/S0966-842X(00)01705-4 [DOI] [PubMed] [Google Scholar]
- 17.Callewaert L, Walmagh M, Michiels CW, Lavigne R. 2011. Food applications of bacterial cell wall hydrolases. Curr. Opin. Biotechnol. 22:164–171. 10.1016/j.copbio.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 18.Schmelcher M, Donovan DM, Loessner MJ. 2012. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 7:1147–1171. 10.2217/fmb.12.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pastagia M, Schuch R, Fischetti VA, Huang DB. 2013. Lysins: the arrival of pathogen-directed anti-infectives. J. Med. Microbiol. 62(Pt 10):1506–1516. 10.1099/jmm.0.061028-0 [DOI] [PubMed] [Google Scholar]
- 20.Loeffler JM, Nelson D, Fischetti VA. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170–2172. 10.1126/science.1066869 [DOI] [PubMed] [Google Scholar]
- 21.Schuch R, Nelson D, Fischetti VA. 2002. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884–889. 10.1038/nature01026 [DOI] [PubMed] [Google Scholar]
- 22.Pastagia M, Euler C, Chahales P, Fuentes-Duculan J, Krueger JG, Fischetti VA. 2011. A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus strains. Antimicrob. Agents Chemother. 55:738–744. 10.1128/AAC.00890-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briers Y, Walmagh M, Lavigne R. 2011. Use of bacteriophage endolysin EL188 and outer membrane permeabilizers against Pseudomonas aeruginosa. J. Appl. Microbiol. 110:778–785. 10.1111/j.1365-2672.2010.04931.x [DOI] [PubMed] [Google Scholar]
- 24.Skerlavaj B, Benincasa M, Risso A, Zanetti M, Gennaro R. 1999. SMAP-29: a potent antibacterial and antifungal peptide from sheep leukocytes. FEBS Lett. 46:58–62 [DOI] [PubMed] [Google Scholar]
- 25.Briers Y, Volckaert G, Cornelissen A, Lagaert S, Michiels CW, Hertveldt K, Lavigne R. 2007. Muralytic activity and modular structure of the endolysins of Pseudomonas aeruginosa bacteriophages phiKZ and EL. Mol. Microbiol. 65:1334–1344. 10.1111/j.1365-2958.2007.05870.x [DOI] [PubMed] [Google Scholar]
- 26.Tack BF, Sawai MV, Kearney WR, Robertson AD, Sherman MA, Wang W, Hong T, Boo LM, Wu H, Waring AJ, Lehrer RI. 2002. SMAP-29 has two LPS-binding sites and a central hinge. Eur. J. Biochem. 269:1181–1189. 10.1046/j.0014-2956.2002.02751.x [DOI] [PubMed] [Google Scholar]
- 27.Briers Y, Schmelcher M, Loessner MJ, Hendrix J, Engelborghs Y, Volckaert G, Lavigne R. 2009. The high-affinity peptidoglycan binding domain of Pseudomonas phage endolysin KZ144. Biochem. Biophys. Res. Commun. 383:187–191. 10.1016/j.bbrc.2009.03.161 [DOI] [PubMed] [Google Scholar]
- 28.Pirnay JP, De Vos D, Cochez C, Bilocq F, Vanderkelen A, Zizi M, Ghysels B, Cornelis P. 2002. Pseudomonas aeruginosa displays an epidemic population structure. Environ. Microbiol. 4:898–911. 10.1046/j.1462-2920.2002.00321.x [DOI] [PubMed] [Google Scholar]
- 29.Pirnay JP, De Vos D, Mossialos D, Vanderkelen A, Cornelis P, Zizi M. 2002. Analysis of the Pseudomonas aeruginosa oprD gene from clinical and environmental isolates. Environ. Microbiol. 4:872–882. 10.1046/j.1462-2920.2002.00281.x [DOI] [PubMed] [Google Scholar]
- 30.Cenens W, Mebrhatu MT, Makumi A, Ceyssens PJ, Lavigne R, Van Houdt R, Taddei F, Aertsen A. 2013. Expression of a novel P22 ORFan gene reveals the phage carrier state in Salmonella Typhimurium. PLoS Genet. 9:e1003269. 10.1371/journal.pgen.1003269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CLSI. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—9th ed. CLSI M07–A9. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 32.Chen X, Zhang M, Zhou C, Kallenbach NR, Ren D. 2011. Control of bacterial persister cells by Trp/Arg-containing antimicrobial peptides. Appl. Environ. Microbiol. 77:4878–4885. 10.1128/AEM.02440-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Groote VN, Verstraeten N, Fauvart M, Kint CI, Verbeeck AM, Beullens S, Cornelis P, Michiels J. 2009. Novel persistence genes in Pseudomonas aeruginosa identified by high-throughput screening. FEMS Microbiol. Lett. 297:73–79. 10.1111/j.1574-6968.2009.01657.x [DOI] [PubMed] [Google Scholar]
- 34.Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, Rotolo JA, Horiuchi Y, Couto DE, Raz A, Fischetti VA, Huang DB, Nowinski RC, Wittekind M. 2013. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J. Infect. Dis. 10.1093/infdis/jit637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson RC, Hancock REW, Yu PL. 2004. Antimicrobial activity and bacterial-membrane interaction of ovine-derived cathelicidins. Antimicrob. Agents Chemother. 48:673–676. 10.1128/AAC.48.2.673-676.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schleifer H, Kandler O. 1972. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 36:407–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daly KE, Huang KC, Wingreen NS, Mukhopadhyay R. 2011. Mechanics of membrane bulging during cell-wall disruption in Gram-negative bacteria. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 83:041922. 10.1103/PhysRevE.83.041922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briers Y, Cornelissen A, Aertsen A, Hertveldt K, Michiels CW, Volckaert G, Lavigne R. 2008. Analysis of outer membrane permeability of Pseudomonas aeruginosa and bactericidal activity of endolysins KZ144 and EL188 under high hydrostatic pressure. FEMS Microbiol. Lett. 280:113–119. 10.1111/j.1574-6968.2007.01051.x [DOI] [PubMed] [Google Scholar]
- 39.Lukacik P, Barnard TJ, Keller PW, Chaturvedi KS, Seddiki N, Fairman JW, Noinaj N, Kirby TL, Henderson JP, Steven AC, Hinnebusch BJ, Buchanan SK. 2012. Structural engineering of a phage lysin that targets Gram-negative pathogens. Proc. Natl. Acad. Sci. U. S. A. 109:9857–9862. 10.1073/pnas.1203472109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischetti VA. 2005. Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol. 13:491–496. 10.1016/j.tim.2005.08.007 [DOI] [PubMed] [Google Scholar]
- 41.Peschel A, Sahl H-G. 2006. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4:529–536. 10.1038/nrmicro1441 [DOI] [PubMed] [Google Scholar]
- 42.Cabot G, Bruchmann S, Mulet X, Zamorano L, Moyà B, Juan C, Haussler S, Oliver A. 2014. Pseudomonas aeruginosa ceftolozane-tazobactam resistance development requires multiple mutations leading to overexpression and structural modification of AmpC. Antimicrob. Agents Chemother. 10.1128/AAC.02462-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ernst RK, Moskowitz SM, Emerson JC, Kraig GM, Adams KN, Harvey MD, Ramsey B, Speert DP, Burns JL, Miller SI. 2007. Unique lipid A modifications in Pseudomonas aeruginosa isolated from the airways of patients with cystic fibrosis. J. Infect. Dis. 196:1088–1092. 10.1086/521367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kümmerer K. 2003. Significance of antibiotics in the environment. J. Antimicrob. Chemother. 52:5–7. 10.1093/jac/dkg293 [DOI] [PubMed] [Google Scholar]
- 45.Fauvart M, De Groote VN, Michiels J. 2011. Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 60:699–709. 10.1099/jmm.0.030932-0 [DOI] [PubMed] [Google Scholar]
- 46.Allison KR, Brynildsen MP, Collins J. 2011. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220. 10.1038/nature10069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bryk R, Gold B, Venugopal A, Singh J, Samy R, Pupek K, Cao H, Popescu C, Gurney M, Hotha S, Cherian J, Rhee K, Ly L, Converse PJ, Ehrt S, Vandal O, Jiang X, Schneider J, Lin G, Nathan C. 2008. Selective killing of nonreplicating mycobacteria. Cell Host Microbe 3:137–145. 10.1016/j.chom.2008.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dworkin J, Shah IM. 2010. Exit from dormancy in microbial organisms. Nat. Rev. Microbiol. 8:890–896. 10.1038/nrmicro2453 [DOI] [PubMed] [Google Scholar]
- 49.Mulcahy LR, Burns JL, Lory S, Lewis K. 2010. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 192:6191–6196. 10.1128/JB.01651-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masschalck B, Michiels CW. 2003. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit. Rev. Microbiol. 29:191–214. 10.1080/713610448 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.