Abstract
Daptomycin exhibits clinical activity in the treatment of infections with Gram-positive organisms, including infections due to methicillin-resistant Staphylococcus aureus. However, little is known about its penetration into bone and synovial fluid. The aim of our study was to assess the penetration of daptomycin into bone and synovial fluid after a single intravenous administration. This study was conducted in 16 patients who underwent knee or hip replacement and received a single intravenous dose of 8 mg of daptomycin per kg of body weight prior to surgery. Plasma daptomycin concentrations were measured 1 h after the end of daptomycin infusion and when bone fragments were removed. Daptomycin concentrations were also measured on bone fragments and synovial fluid collected at the same time during surgery. All samples were analyzed with a diode array–high-performance liquid chromatography (HPLC) method. After a single-dose intravenous infusion, bone daptomycin concentrations were above the MIC of daptomycin for Staphylococcus aureus in all subjects, and the median bone penetration percentage was 9.0% (interquartile range [IQR], 4.4 to 11.4). These results support the use of daptomycin in the treatment of Staphylococcus aureus bone and joint infections.
INTRODUCTION
Staphylococcal osteoarticular infections are difficult to treat (1, 2). They are caused mainly by Staphylococcus aureus and coagulase-negative staphylococci (1–5). Antibiotics are cornerstone of their treatment, but optimal regimens have not been identified, since few controlled trials have been performed to assess and compare different regimens. The high prevalence of methicillin-resistant Staphylococcus aureus (MRSA) and the emergence of strains with decreased susceptibility to vancomycin make it necessary to evaluate newer options in such situations (6).
Daptomycin has many interesting characteristics relative to bone and joint infections, with or without foreign bodies.
First, daptomycin is as a cyclic lipopeptide antibiotic with an spectrum of activity against Gram-positive organisms, including MRSA. Until now, there have been few instances of acquired resistance and rare resistance in vancomycin-heteroresistant Staphylococcus aureus (7). Second, daptomycin has a high and very rapid concentration-dependent bactericidal activity (8–11), not affected by decreased susceptibility to vancomycin (12). Third, daptomycin shows an antimicrobial activity in an in vivo model of acute osteomyelitis (13) and penetrates rapidly into biofilms (14, 15); also, due to its unique mechanism of action on cell membrane, daptomycin retains antimicrobial activity against both stationary-phase cultures of staphylococci within the biofilm and bacteria in the multiplication phase (16–20). Finally, the safety and tolerability of daptomycin have been established (21–23). Therefore, daptomycin could be a relevant treatment for bone and joint infection, provided that its penetration into bone is satisfactory. The European Cubicin Outcomes Registry and Experience (EU-CORE) database, a large database on real-world daptomycin use, contains data for 220 patients treated for osteomyelitis with daptomycin. It is a quite heterogeneous group of patients that includes those with permanent prosthetic joint infection and those with temporary prosthetic-related infection (i.e., spacer infection) and non-prosthetic-related osteomyelitis. Overall clinical success was achieved in 165/220 patients, who were mostly treated with a daptomycin infusion of 6 mg per kg of body weight per day, and safety and tolerability were good. However, this high rate of clinical success should be viewed in light of the fact that the hindsight was short and clinical success was used to collectively describe patients with an outcome of cure or improvement (24). Despite these clinical results, data on the penetration of daptomycin in human bone are lacking.
The aim of our study was to assess the penetration of daptomycin into bone and synovial fluid after a single intravenous administration of this antibiotic.
MATERIALS AND METHODS
Patients.
Healthy adults were invited to participate in the study during the preoperative phase of a planned knee or hip surgery. They gave informed written consent to their participation.
Excluded from the study were subjects with either an elevated (≥5× upper limit of normal [ULN]) serum creatinine phosphokinase before surgery, a serum C-reactive protein concentration of >10 mg/liter, a creatinine clearance of <30 ml/min (measured by the Cockcroft-Gault equation), Child-Pugh class C liver failure, a body mass index of >40 kg/m2, chronic limb ischemia (stage II or higher), chronic osteomyelitis, treatment with statins, fibrates, or cyclosporine, or a known intolerance to daptomycin.
This phase I, open-label, nonrandomized study was carried out at the university hospital in Besançon, France. It was approved by an independent ethics committee (CPP Est-II, Besançon, France) at the session on 25 March 2010 and by the French health authority (AFSSAPS) under the number B100319-77.
Drug administration.
Patients received a single dose of daptomycin intravenously between 4 and 12 h before surgery. The delay took into account the linear pharmacokinetics of daptomycin, the half-time of elimination of the drug, around 8 h, and the availability of the operating room.
Because of the uncertainty of optimal daptomycin dosing for adequate bone penetration, we decided to start the study using an 8-mg/kg dose and designed a two-step study that is summarized in Fig. 1. Daptomycin was administered intravenously over 30 min at 8 or 10 mg/kg. In any case of poor tolerance of the infusion and/or an allergic reaction, daptomycin infusion was stopped.
FIG 1.
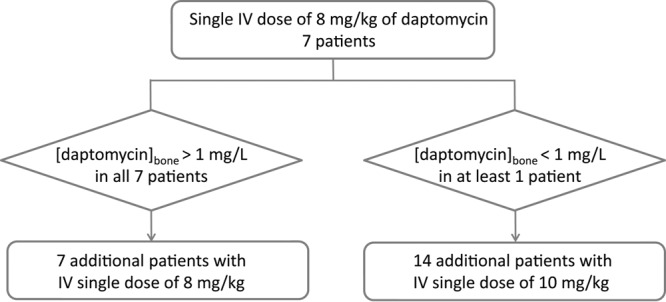
The two-step study design depending on the daptomycin concentrations in bone in the first seven patients.
Patients were given a standard intravenous dose of cefuroxime (or clindamycin if they had an allergy to beta-lactams) before surgery, according to the French guidelines for antibiotic prophylaxis in orthopedic surgery (25).
Sample collection.
Two blood samples were collected from each patient. In order to minimize the variability of the first rapid phase of distribution of the daptomycin, the first sample (used to determine the concentration at 1 h [C1HOUR]) was taken 1 h after the end of the daptomycin infusion. The second one (used to determine the concentration during surgery [COP]) was collected during surgery, around the time of bone removal.
For each patient, bone tissues were collected to determine the daptomycin concentration in shinbone and thighbone in cases of knee replacement and in thighbone in cases of hip replacement. Synovial fluid was also collected at the time of bone removal provided that a volume of at least 5 ml was available. Samples were transported within 10 min to the laboratory at room temperature. After treatment, plasma, bone, and synovial fluid samples were frozen at −80°C.
For a total knee replacement, a tourniquet was applied around the thigh immediately after induction of anesthesia.
Daptomycin concentration measurements. (i) Blood and synovial fluids. (a) Collection.
Blood samples were collected in heparin-containing tubes (volume, 7 ml). Plasma was separated by centrifugation at 1,500 × g for 10 min at 4°C, transferred into propylene vials, and stored at −80°C until analysis. Samples of synovial fluid were collected in heparin-containing tubes (volume, 7 ml) and were stored at −80°C until analysis. The daptomycin stability in plasma samples is acceptable with storage at −20°C (26).
(b) Extraction.
For extraction, 100 μl of plasma (or synovial fluid) were added with ethylparaben (10 μl of a 0.25 g/liter solution) as internal standard. Proteins precipitation was performed by adding 400 μl of methanol. After 30 s of shaking (Vortex), the solution was centrifuged 3 min and 10,000 × g. Forty microliters of supernatant was injected into the HPLC system.
(c) HPLC quantification.
All samples (plasma and synovial fluid) were analyzed with validated photodiode array–high-performance liquid chromatography (PDA-HPLC). Chromatography was carried out with an OmniSphere 5 C18 column (150 by 4.6 mm; Agilent France, Les Ulis, France). The chromatographic system included a 717 Plus injector (Waters France, Guyancourt, France), a SpectraSeries P100 pump, and a UV6000LP detector (Thermo Scientific Waltham, MA, USA). The mobile phase was a phosphate buffer (pH 3.8)-acetonitrile (65/35 [vol/vol]) solution. The flow rate was 1 ml/min with an injection volume of 40 μl. The wavelength used for detection was 224 nm. Each sample took 10 min to run.
The HPLC method was validated on a range of daptomycin concentrations from 0.15 to 80 μg/ml. The linearity was validated (R2 = 0.9976). Three internal controls were used (concentrations of 0.2 μg/ml, 20 μg/ml, and 80 μg/ml). The within-run precision was 14.8%, 5.8%, and 0.2%, respectively. The within-run accuracy was 14.6%, 5.2%, and 5.8%, respectively. The between-run accuracy between day 1 and day 5 was 11.2%, 3.7%, and 3.3%, respectively. The limit of quantification was 0.15 mg/liter (the lowest concentration for which the coefficient of variation was above 20%).
(ii) Bone. (a) Collection.
Bone samples were collected in dry and appropriate packaging. After resection of the bone samples, adhering blood, bone marrow, and soft tissues were removed from the specimen by swabbing, scraping, or rinsing, and samples were immediately stored at −80°C until analysis.
(b) Extraction.
For extraction, approximately 700 mg (the precise weight of bone sample used for extraction was measured) of cancellous bone was manually crushed and immersed in 1.2 ml of cold phosphate buffer (pH 6). Samples were agitated for 30 s (Vortex) and then ultrasonicated for 10 min. Samples were then stored 2 h at 4°C. They were then shaken for 30 s and centrifuged for 10 min at 10,000 × g. This procedure was repeated three times for each bone sample. The 3 supernatants were pooled, and 1,000 μl of supernatant (with addition of 10 µl of a solution of ethylparaben [0.25 mg/liter]) was cleared on Agilent SPE Bond Elud columns with 1,000 μl of methanol. This clear extract was recovered and injected into the HPLC system.
(c) HPLC quantification.
After extraction of daptomycin from bone, 40 μl of clear extract was injected, with the same HPLC parameters as for plasma and synovial extracts. For each bone sample, the amount of daptomycin (μg) was determined then reported relative to the exact quantity of crushed bone (g) to get the concentration of daptomycin in bone (μg/g).
The HPLC method was validated on a range of daptomycin amounts from 0.15 to 2.4 μg. The linearity was validated (R2 = 0.99). The within-run precision was 1.7%, 1.4%, and 4.7% for amounts of 0.2 μg, 1.2 μg and 2.4 μg, respectively. The within-run accuracy was 9.0%, 4.5%, and 2.1% for amounts of 0.2 μg, 1.2 μg, and 2.4 μg, respectively. The between-run accuracy was 4.7%, 8.5%, and 4.2% between day 1 and day 5 for amounts of 0.2 μg, 1.2 μg, and 2.4 μg, respectively. The limit of quantification was of 0.15 μg (first small amount for which the coefficient of variation was above 20%).
Statistical analysis.
We describe the demographic data (age and sex) and surgical site, the results of the pharmacological dosages of daptomycin in bones (CTHIGHBONE and CSHINBONE), synovial fluid (CSF), and plasma (C1HOUR and COP). Statistical analysis was performed using a dependent t test for paired samples with a fixed statistical significance at a 5% level.
We calculated the percentage of penetration of daptomycin into bone and synovial fluid by calculating for each patient the ratio of the bone drug concentration to COP: CSF/COP for the daptomycin penetration in synovial fluid, CTIGHBONE/COP for the daptomycin penetration in thighbone, and CSHINBONE/COP for the daptomycin penetration in shinbone.
The concentrations of daptomycin and the bone penetration percentages are presented as medians and interquartile ranges (IQR), and the time variables are presented as means and ranges.
RESULTS
At the end of the first step of the study, bone concentrations of daptomycin were found to be above the cutoff threshold of 1 μg/ml in all 7 patients. Consequently, the same dose of 8 mg/kg of daptomycin was used in the patients enrolled in the second step of the study.
Data were obtained from 16 patients. There were six men and 10 women. The median age was 69 years (IQR, 55 to 91). Ten knee and six hip replacements were performed. All the biological samples scheduled were obtained, except that synovial fluid was missing for three patients due to an insufficient volume of synovial fluid.
During the study, one adverse event occurred. It consisted of a bullous pemphigoid diagnosed 28 days after surgery. It was an expected serious adverse event. According to the evaluation of imputability using the WHO global introspection method, the causality assessment of the reported adverse event and daptomycin was classified as possible.
For the whole study, bone tissue, blood for determining COP, and synovial fluid were collected at the same time from any one patient. For the whole study, bone tissue, blood for COP, and synovial fluid were taken after a mean of 7.3 h, 7.4 h, and 7.3 h after daptomycin infusion, respectively. For each patient, bone tissues, blood for COP, and synovial fluid were collected in a median time of 27 min (IQR, 19 to 34). Mean times between the inflation of the tourniquet and the bone samplings were short: 21 min (range, 12 to 43 min) for thighbone and 39 min (range, 27 to 54 min) for shinbone.
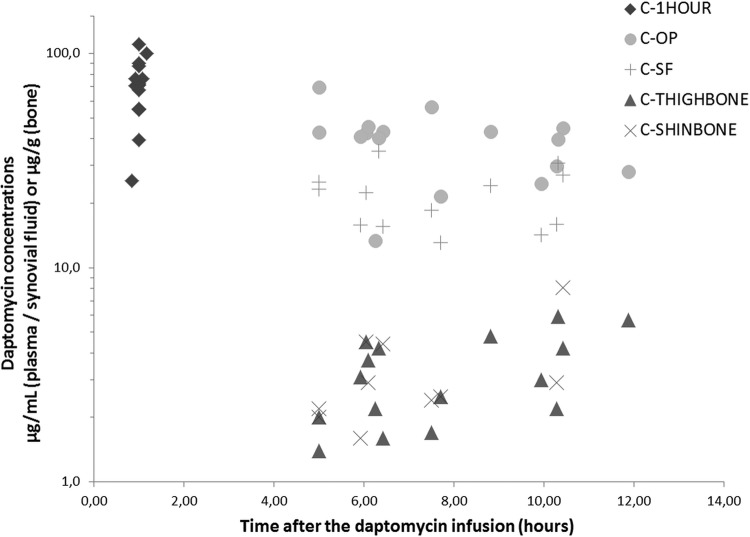
Figure 2 presents the concentrations of daptomycin determined in plasma (C1HOUR and COP), in synovial fluid (CSF) and in bones (CTHIGHBONE and CSHINBONE) plotted against the time of sampling. Table 1 displays the concentrations of daptomycin determined for each patient. The mean values (± standard deviation [SD]) were 70.3 (±21.5), 39.3 (±13.6), and 21.6 (±6.8) μg/ml for C1HOUR, COP, and CSF, respectively. In bone samples, the mean values (± SD) were 3.3 (±1.5) and 3.4 (±1.9) μg/g for CTHIGHBONE and CSHINBONE, respectively. CSF and COP were statistically significantly different (P < 0.0001) (Table 2).
FIG 2.
Representation of the daptomycin concentrations in plasma (C1HOUR and COP) or in synovial fluid (CSF) (μg/ml) and in bones (CTHIGHBONE and CSHINBONE) (μg/g) versus the sampling time.
TABLE 1.
Daptomycin concentrations in plasma (C1HOUR and COP), in bones (CTHIGHBONE and CSHINBONE), and in synovial fluid (CSF)
| Patient | Replacement | C1HOUR (μg/ml) | COP (μg/ml) | Time between C1HOUR and COP (h:min) | CTHIGHBONE (μg/g bone) | CSHINBONE (μg/g bone) | CSF (μg/ml) |
|---|---|---|---|---|---|---|---|
| 1 | Knee | 71.3 | 43.0 | 04:00 | 1.4 | 2.0 | 23.3 |
| 2 | Knee | 110.3 | 69.9 | 04:00 | 2.0 | 2.2 | 25.0 |
| 3 | Knee | 67.8 | 43.4 | 05:25 | 1.6 | 4.4 | 15.6 |
| 4 | Knee | 72.6 | 42.5 | 05:03 | 4.5 | 4.5 | 22.4 |
| 5 | Knee | 100.3 | 56.4 | 06:20 | 1.7 | 2.4 | 18.5 |
| 6 | Knee | 39.4 | 21.6 | 06:42 | 2.5 | 2.5 | 13.1 |
| 7 | Hip | 76.2 | 40.4 | 05:15 | 4.2 | 35.0 | |
| 8 | Hip | 87.6 | 43.4 | 07:49 | 4.8 | 24.1 | |
| 9 | Knee | 70.5 | 44.8 | 09:30 | 4.2 | 8.1 | 27.0 |
| 10 | Hip | 90.3 | 39.9 | 09:19 | 5.9 | 30.8 | |
| 11 | Hip | 55.0 | 24.7 | 08:56 | 3.0 | 14.2 | |
| 12 | Hip | 54.6 | 28.2 | 10:53 | 5.7 | ||
| 13 | Knee | 71.3 | 41.0 | 04:55 | 3.1 | 1.6 | 15.8 |
| 14 | Knee | 76.4 | 45.6 | 05:11 | 3.7 | 2.9 | |
| 15 | Knee | 55.3 | 29.8 | 09:17 | 2.2 | 2.9 | 15.9 |
| 16 | Hip | 25.4 | 13.4 | 05:25 | 2.2 |
TABLE 2.
Daptomycin concentrations in plasma (C1HOUR and COP), in bones (CTHIGHBONE and CSHINBONE), and in synovial fluid (CSF)
| Measurementa | C1HOUR (μg/ml) | COP (μg/ml) | CTHIGHBONE (μg/g bone) | CSHINBONE (μg/g bone) | CSF (μg/ml) |
|---|---|---|---|---|---|
| Mean | 70.3 | 39.3 | 3.3 | 3.4 | 21.6 |
| SD | 21.5 | 13.6 | 1.5 | 1.9 | 6.8 |
| Median | 71.3 | 41.8 | 3.1 | 2.7 | 22.4 |
| Minimum | 25.4 | 13.4 | 1.4 | 1.6 | 13.1 |
| Q1 | 55.2 | 29.4 | 2.2 | 2.3 | 15.8 |
| Q3 | 79.2 | 43.8 | 4.3 | 4.0 | 25.0 |
| Maximum | 110.3 | 69.9 | 5.9 | 8.1 | 35.0 |
Q1 and Q3, first and third quartiles, respectively.
The median synovial fluid penetration percentage was 54% (IQR, 38 to 60%). In thighbone, it was 9.5% (IQR, 6.5 to 11.7%), and in shinbone, it was 8.2% (IQR, 4.4 to 10.5%) (Table 3).
TABLE 3.
Percentage of daptomycin penetration into bone (thighbone and shinbone) and synovial fluid sampled in a mean time of 7.3 h after the perfusion of 8 mg/kg of daptomycin. The results are expressed by the mean, standard deviation (SD), median, first quartile (Q1), third quartile (Q3); min and max
| Measurementa | Penetration (%) in: |
|||
|---|---|---|---|---|
| Thighbone (n = 16) | Shinbone (n = 10) | Bone (n = 26) | Synovial fluid (n = 13) | |
| Mean | 9.5 | 8.2 | 14.1 | 53.9 |
| SD | 5.0 | 4.7 | 11.9 | 15.9 |
| Median | 9.9 | 8.0 | 11.1 | 54.2 |
| Minimum | 2.9 | 3.1 | 3.0 | 32.8 |
| Q1 | 6.5 | 4.4 | 6.6 | 38.5 |
| Q3 | 11.7 | 10.5 | 17.3 | 60.3 |
| Maximum | 20.2 | 18.1 | 50.4 | 86.6 |
Q1 and Q3, first and third quartiles, respectively.
DISCUSSION
Our single-center pilot study showed that daptomycin penetrates synovial fluid well and that it also penetrates cancellous bone in healthy volunteers.
Even though there is currently no validated method to measure bone concentrations of any antibiotics, the method we used resembles in part the current state-of-the-art methods described by Landersdorfer et al. (27). The daptomycin concentrations were determined in only one type of bone, the cancellous bone. The bone samples were rather large operating specimens of noninfected bone (around 700 g), and the samples used for extraction were taken, whenever possible, from the center of the bone. The bone samples were finely homogenized using a mortar and then vortexed and ultrasonicated. These extraction conditions ensured stability of the drug and the achievement of the best extraction possible. Finally, we used a validated HPLC-UV (photodiodes array) method for the quantification of the daptomycin. Our results showed that daptomycin penetrates synovial fluid well, with a median penetration percentage of 54% (IQR, 38 to 60%). But most of all, our results showed that daptomycin also penetrates cancellous bone in healthy volunteers. The median penetration of daptomycin into cancellous bone was 9.0% around 8 h after an 8-mg/kg infusion. Regardless of the samples and the sampling times, daptomycin concentrations have always been found to be above 1 μg/g. We had chosen the value of 1 μg/g, which is equivalent to the MIC breakpoint for clinical isolates of Staphylococcus aureus for daptomycin (1 μg/ml) (28), and this was achieved after an unique intravenous single dose of 8 mg/kg daptomycin.
However, there are limitations to our study. First, it was a single-center pilot study. Second, noninfected bone was used, and one cannot be sure that bone penetration would be the same in infected bone. Moreover, we could not study the antimicrobial activity of daptomycin in the bone samples and correlate this activity to the bone concentration of daptomycin. Third, the study design (one bone sample per patient taken during joint replacement surgery) did not allow us to determine the kinetics of daptomycin in bone, in contrast to the microdialysis technique or animal models. Finally, in knee replacement, the tourniquet was applied to the leg to be operated on just before surgery but at least 4 h after the daptomycin infusion and a few minutes before the plasma and bone samples were taken. Even if the tourniquet could decrease blood circulation to the site of surgery and therefore could affect the rate of bone penetration (29, 30), it certainly had a small impact in our study.
To our knowledge, only one study assessed the penetration of daptomycin into bone tissue of diabetic patients with bacterial foot infections requiring surgical debridement (31). But this study used a different design and assay method. Indeed, Traunmüller et al. (31) used the microdialysis technique with a probe inserted during surgery into cancellous healthy metatarsal bone and a reference probe inserted into an unaffected region of subcutaneous adipose tissue of the same lower limb. Daptomycin concentrations in microdialysates were measured by HPLC with UV detection. Thanks to the microdialysis technique, many samples were collected for each patient, and thus the authors were able to calculate the area under the curve of the daptomycin concentration in bone and plasma. Unlike our study, in which uninfected patients received only one infusion of daptomycin at 8 mg/kg, the nine patients with diabetic foot infections received 4 to 5 daptomycin infusions at 6 mg/kg/day before surgery. The degree of bone penetration was assessed by calculating the ratio of the area under the concentration-time curve (fAUC) of free daptomycin in bone to the fAUC of free daptomycin in plasma. The mean ratio was 1.08 for infected bone. Thus, although the results of the study by Traunmüller et al. (31) cannot be directly compared with ours, both studies suggest good penetration of daptomycin into bone tissue.
Our data show that concentrations of daptomycin in noninfected bone and synovial fluid are above 1 μg/g and 10 μg/ml, respectively, after a unique dose of 8 mg/kg of daptomycin. Considering the daptomycin MIC breakpoint (1 μg/ml) defined by EUCAST for Staphylococcus aureus, daptomycin may be a useful agent in the management of prosthetic joint infection and could be an alternative to glycopeptides. But it should be interesting to do more studies in infected patients to correlate the antimicrobial efficacy and the concentrations of daptomycin in bone tissues and to explore the daptomycin concentrations in bone after numerous administrations.
ACKNOWLEDGMENTS
This work was supported in part with an unrestricted grant from Novartis, France, who supported the research but had no access to the data.
We acknowledge A. Denis for her technical assistance.
Footnotes
Published ahead of print 5 May 2014
REFERENCES
- 1.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645–1654. 10.1056/NEJMra040181 [DOI] [PubMed] [Google Scholar]
- 2.Calhoun JH, Manring MM. 2005. Adult osteomyelitis. Infect. Dis. Clin. North Am. 19:765–786. 10.1016/j.idc.2005.07.009 [DOI] [PubMed] [Google Scholar]
- 3.Lazzarini L, Lipsky BA, Mader JT. 2005. Antibiotic treatment of osteomyelitis: what have we learned from 30 years of clinical trials? Int. J. Infect. Dis. 9:127–138. 10.1016/j.ijid.2004.09.009 [DOI] [PubMed] [Google Scholar]
- 4.Cunha BA. 2002. Osteomyelitis in elderly patients. Clin. Infect. Dis. 35:287–293. 10.1086/341417 [DOI] [PubMed] [Google Scholar]
- 5.Akins RL, Rybak MJ. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454–459. 10.1128/AAC.45.2.454-459.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simor AE. 2001. Containing methicillin-resistant S aureus. Surveillance, control, and treatment methods. Postgrad. Med. 110:43–48 [DOI] [PubMed] [Google Scholar]
- 7.van Hal SJ, Paterson DL, Gosbell IB. 2011. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureus bacteraemia in a daptomycin-naïve patient—a review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 30:603–610. 10.1007/s10096-010-1128-3 [DOI] [PubMed] [Google Scholar]
- 8.Fuchs PC, Barry AL, Brown SD. 2002. In vitro bactericidal activity of daptomycin against staphylococci. J. Antimicrob. Chemother. 49:467–470. 10.1093/jac/49.3.467 [DOI] [PubMed] [Google Scholar]
- 9.Wootton M, Walsh T, MacGowan A. 2005. Evidence for reduction in breakpoints used to determine vancomycin susceptibility in Staphylococcus aureus. Antimicrob. Agents Chemother. 49:3982–3983. 10.1128/AAC.49.9.3982-3983.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y-T, Liao C-H, Teng L-J, Hsueh P-R. 2008. Comparative bactericidal activities of daptomycin, glycopeptides, linezolid and tigecycline against blood isolates of Gram-positive bacteria in Taiwan. Clin. Microbiol. Infect. 14:124–129. 10.1111/j.1469-0691.2007.01870.x [DOI] [PubMed] [Google Scholar]
- 11.Kanafani ZA, Corey GR. 2007. Daptomycin: a rapidly bactericidal lipopeptide for the treatment of Gram-positive infections. Expert Rev. Anti Infect. Ther. 5:177–184. 10.1586/14787210.5.2.177 [DOI] [PubMed] [Google Scholar]
- 12.Sader HS, Fritsche TR, Jones RN. 2006. Daptomycin bactericidal activity and correlation between disk and broth microdilution method results in testing of Staphylococcus aureus strains with decreased susceptibility to vancomycin. Antimicrob. Agents Chemother. 50:2330–2336. 10.1128/AAC.01491-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefebvre M, Jacqueline C, Amador G, Le Mabecque V, Miegeville A, Potel G, Caillon J, Asseray N. 2010. Efficacy of daptomycin combined with rifampicin for the treatment of experimental meticillin-resistant Staphylococcus aureus (MRSA) acute osteomyelitis. Int. J. Antimicrob. Agents 36:542–544. 10.1016/j.ijantimicag.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 14.Stewart PS, Davison WM, Steenbergen JN. 2009. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 53:3505–3507. 10.1128/AAC.01728-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominguez-Herrera J, Docobo-Pérez F, Lopez-Rojas R, Pichardo C, Ruiz-Valderas R, Lepe JA, Pachon J. 2012. Efficacy of daptomycin versus vancomycin in an experimental model of foreign-body and systemic infection caused by biofilm producers and methicillin-resistant Staphylococcus epidermidis. Antimicrob. Agents Chemother. 56:613–617. 10.1128/AAC.05606-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tedesco KL, Rybak MJ. 2003. Impact of high inoculum Staphylococcus aureus on the activities of nafcillin (NAF), vancomycin (VAN), linezolid (LZD), gentamicin (GEN), and daptomycin DAP in an in vitro pharmacodynamic model (IVD), abstr. A-1151. 43rd Annu. Intersci. Conf. Antimicrob. Agents Chemother [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaPlante KL, Woodmansee S. 2009. Activities of daptomycin and vancomycin alone and in combination with rifampin and gentamicin against biofilm-forming methicillin-resistant Staphylococcus aureus isolates in an experimental model of endocarditis. Antimicrob. Agents Chemother. 53:3880–3886. 10.1128/AAC.00134-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raad I, Hanna H, Jiang Y, Dvorak T, Reitzel R, Chaiban G, Sherertz R, Hachem R. 2007. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob. Agents Chemother. 51:1656–1660. 10.1128/AAC.00350-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith K, Perez A, Ramage G, Gemmell CG, Lang S. 2009. Comparison of biofilm-associated cell survival following in vitro exposure of meticillin-resistant Staphylococcus aureus biofilms to the antibiotics clindamycin, daptomycin, linezolid, tigecycline and vancomycin. Int. J. Antimicrob. Agents 33:374–378. 10.1016/j.ijantimicag.2008.08.029 [DOI] [PubMed] [Google Scholar]
- 20.Leonard SN, Vidaillac C, Rybak MJ. 2009. Activity of telavancin against Staphylococcus aureus strains with various vancomycin susceptibilities in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 53:2928–2933. 10.1128/AAC.01544-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dvorchik BH, Brazier D, DeBruin MF, Arbeit RD. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318–1323. 10.1128/AAC.47.4.1318-1323.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figueroa DA, Mangini E, Amodio-Groton M, Vardianos B, Melchert A, Fana C, Wehbeh W, Urban CM, Segal-Maurer S. 2009. Safety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical program. Clin. Infect. Dis. 49:177–180. 10.1086/600039 [DOI] [PubMed] [Google Scholar]
- 23.Benvenuto M, Benziger DP, Yankelev S, Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245–3249. 10.1128/AAC.00247-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seaton RA, Malizos KN, Viale P, Gargalianos-Kakolyris P, Santantonio T, Petrelli E, Pathan R, Heep M, Chaves RL. 2013. Daptomycin use in patients with osteomyelitis: a preliminary report from the EU-CORESM database. J. Antimicrob. Chemother. 68:1642–1649. 10.1093/jac/dkt067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Société Française d'Anesthésie et de Réanimation 2011. Antibioprophylaxis in surgery and interventional medicine (adult patients). Actualization 2010. Ann. Fr. Anesth. Reanim. 30:168–190. 10.1016/j.annfar.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 26.Verdier M, Bentué-Ferrer D, Tribut O, Collet N, Revest M, Bellissant E. 2011. Determination of daptomycin in human plasma by liquid chromatography-tandem mass spectrometry. Clinical application. Clin. Chem. Lab. Med. 49:69–75. 10.1515/CCLM.2011.005 [DOI] [PubMed] [Google Scholar]
- 27.Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sörgel F. 2009. Penetration of antibacterials into bone—pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin. Pharmacokinet. 48:89–124. 10.2165/00003088-200948020-00002 [DOI] [PubMed] [Google Scholar]
- 28.EUCAST. 2011. Breakpoint tables for interpretation of MICs and zone diameters, p 1–68 EUCAST, Växjö, Sweden [Google Scholar]
- 29.de Lalla F, Novelli A, Pellizzer G, Milocchi F, Viola R, Rigon A, Stecca C, Dal Pizzol V, Fallani S, Periti P. 1993. Regional and systemic prophylaxis with teicoplanin in monolateral and bilateral total knee replacement procedures: study of pharmacokinetics and tissue penetration. Antimicrob. Agents Chemother. 37:2693–2698. 10.1128/AAC.37.12.2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rana B, Butcher I, Grigoris P, Murnaghan C, Seaton RA, Tobin CM. 2002. Linezolid penetration into osteo-articular tissues. J. Antimicrob. Chemother. 50:747–750. 10.1093/jac/dkf207 [DOI] [PubMed] [Google Scholar]
- 31.Traunmüller F, Schintler MV, Metzler J, Spendel S, Mauric O, Popovic M, Konz KH, Scharnagl E, Joukhadar C. 2010. Soft tissue and bone penetration abilities of daptomycin in diabetic patients with bacterial foot infections. J. Antimicrob. Chemother. 65:1252–1257. 10.1093/jac/dkq109 [DOI] [PubMed] [Google Scholar]