Abstract
Recent Food and Drug Administration (FDA) guidance endorses the use of an early clinical response endpoint as the primary outcome for community-acquired bacterial pneumonia (CABP) trials. While antibiotics will now be approved for CABP, in practice they will primarily be used to treat patients with community-acquired pneumonia (CAP). More importantly, it is unclear how achievement of the new FDA CABP early response endpoint translates into clinically applicable real-world outcomes for patients with CAP. To address this, a retrospective cohort study was conducted among adult patients who received ceftriaxone and azithromycin for CAP of Pneumonia Outcomes Research Team (PORT) risk class III and IV at an academic medical center. The clinical response was defined as clinical stability for 24 h with improvement in at least one pneumonia symptom and with no symptom worsening. A classification and regression tree (CART) was used to determine the delay in response time, measured in days, associated with the greatest risk of a prolonged hospital length of stay (LOS) and adverse outcomes (in-hospital mortality or 30-day CAP-related readmission). A total of 250 patients were included. On average, patients were discharged 2 days following the achievement of a clinical response. In the CART analysis, adverse clinical outcomes were higher among day 5 nonresponders than those who responded by day 5 (22.4% versus 6.9%, P = 0.001). The findings from this study indicate that time to clinical response, as defined by the recent FDA guidance, is a reasonable prognostic indictor of real-world effectiveness outcomes among hospitalized PORT risk class III and IV patients with CAP who received ceftriaxone and azithromycin.
INTRODUCTION
There have been considerable debates in defining the most appropriate efficacy endpoint for antimicrobials evaluated in phase III trials for community-acquired pneumonia (CAP) (1, 2). The Food and Drug Administration (FDA) has historically recommended clinical response assessments at a test-of-cure (TOC) visit as the primary efficacy outcome measure in phase III CAP trials. However, the ability of TOC assessments to determine efficacy in phase III CAP trials has been called into question due to the lack of data that demonstrate that this endpoint is clinically meaningful and adequate for capturing the treatment effect (1, 2). The criteria for assessing the clinical response at a TOC visit were also criticized for being loosely defined and somewhat subjective in nature. A more recent FDA briefing endorsed the use of an early clinical response endpoint between study days 3 and 5 on the basis of clinical symptom improvement and stabilization of vital signs rather than TOC assessments (1). The recent recommendation to assess the response in the first 3 to 5 days of therapy is predicated on the basis of data from the early antibiotic era which suggested that the response to antibiotic therapy is evident early in treatment (3, 4). The recent FDA guidance also emphasizes that early clinical response assessments should, ideally, be limited to patients with culture-confirmed community-acquired bacterial pneumonia (CABP) (1, 2). The FDA made this strong recommendation to better identify patients who are most likely to have pneumonia of bacterial etiology and who, therefore, would benefit from antimicrobial therapy (1, 2). In the past, efficacy assessments involved patients with CAP. This is a critical distinction, since the etiology of CAP is often unknown in both clinical trials and clinical practice (5–11). In clinical trials, a bacterial pathogen is identified in only 25% of cases (7–11). A 2009 report from the Centers for Medicare and Medicaid Services (CMS) of a study that included over 17,000 hospitalized patients with CAP indicated that a microbiological diagnosis was identified for only 7.6% of cases (12).
Despite the recent change in the FDA guidance for CABP, several critical questions regarding the clinical applicability of the new FDA early clinical response endpoint remain. As previously mentioned, the FDA guidance now focuses on the treatment of patients with CABP and not the broader population with CAP (1, 2). While antibiotics will now be approved for CABP, in practice they will primarily be used to treat patients with CAP (6, 12). Thus, it is of the upmost importance to determine if achievement of the new FDA CABP early response endpoint is meaningful to patients with CAP. In particular, it is unclear how this new FDA CABP surrogate response endpoint translates into clinically applicable real-world treatment metrics like hospital length of stay (LOS), mortality, and readmission for patients with CAP. Given the considerable clinical and economic sequelae associated with CAP (6, 13–16), the utility of the early clinical response endpoint to discern risk for these outcomes merits close investigation.
The intent of our analysis was to explore how time to clinical response, as defined in the 2011 FDA briefing documents (1) and post hoc examination of data from the phase III trials of ceftaroline fosamil versus ceftriaxone (10), translates into real-world measures of treatment success in hospitalized adults with CAP of Pneumonia Outcomes Research Team (PORT) risk class III and IV (17). Specifically, we evaluated the relationship between time to clinical response and hospital LOS, in-hospital mortality, and 30-day readmission. Since the recent FDA briefing documents specified an assessment of the response only between days 3 and 5 (1), we sought to quantify the delay in clinical response, measured in days, associated with an increased incidence of adverse outcomes among patients with CAP. Given the potentially subjective nature of the symptoms assessment component of the FDA's early clinical response definition (1, 2), we performed a secondary analysis examining the relationship between time to clinical stability, as defined in the American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) CAP treatment guidelines (6).
In light of the recent phase III CAP FOCUS trials (7–10), ceftriaxone was the subject of our analysis. One of the criticisms of the FOCUS trials was the restricted use of concomitant macrolide therapy, which was permitted only in the FOCUS 1 trial and was limited to 24 h (7–9). This is inconsistent with the empirical regimens currently recommended by the Joint Commission on Accreditation of Healthcare Organizations (JCAHO), CMS, and ATS/IDSA (6, 18). Furthermore, the day 4 analysis in the FOCUS trials was restricted to patients with a documented bacterial etiology consistent with CABP (10). Thus, in addition to quantifying the relationship between time to clinical response and real-world effectiveness metrics for CAP, we also sought to address the external generalizability concerns of the day 4 CABP analyses in the FOCUS trials (7–10) by capturing the outcomes associated with ceftriaxone and azithromycin among hospitalized PORT risk class III and IV patients with CAP.
MATERIALS AND METHODS
Study design and population.
A retrospective cohort study was conducted among patients aged ≥18 years who received ceftriaxone and azithromycin for CAP between January 2008 and February 2012 at the Albany Medical Center Hospital (AMCH; Albany, NY). Empirical therapy with ceftriaxone and azithromycin is the standard of care for CAP at AMCH. This study was approved by the AMCH Institutional Review Board committee (protocol 3216), and a waiver of informed consent was obtained. The study criteria were modeled after those of the ceftaroline fosamil versus ceftriaxone CAP phase III (FOCUS) trials (7–10). Patients were included if they had the presence of a new or increasing pulmonary infiltrate(s) on chest radiograph or other imaging technique along with at least 3 of the following clinical features: new or increased cough, purulent sputum or change in sputum character, auscultatory findings consistent with pneumonia (e.g., rales, egophony, consolidation), dyspnea, tachypnea or hypoxemia, an oral temperature of >38°C (>38.5°C rectally or tympanically) or hypothermia (<35°C), a white blood cell count of >10,000 cells/mm3 or <4,500 cells/mm3, and >15% immature neutrophils (bands) (6). Additional inclusion criteria were (i) hospitalization, (ii) receipt of at least 24 h of therapy with ceftriaxone and azithromycin beginning within 24 h of hospitalization, and (iii) CAP of PORT risk class III or IV (17).
Patients were excluded from the study if they had (i) CAP of PORT risk class I, II, or V; (ii) confirmed respiratory tract infection due to a pathogen known to be resistant to ceftriaxone; (iii) a noninfectious cause of pulmonary infiltrates or pleural empyema; (iv) previous antimicrobial therapy for CAP for ≥24 h within 96 h prior to hospital admission (except where unequivocal evidence for treatment failure existed); (v) receipt of chronic concomitant systemic corticosteroids of >40 mg of prednisone equivalent; (vi) hematologic disease (current or anticipated neutropenia defined as <500 neutrophils/mm3 or thrombocytopenia with a platelet count of <60,000 cells/mm3); or (vii) immunologic disease (known HIV infection and either a CD4 count of ≤200 cells/mm3 at the most recent measurement or a current diagnosis of an AIDS-defining illness) or (viii) if they were pregnant or nursing (for females).
Patient data.
Data were extracted from the patients' medical records by trained reviewers using a structured data collection instrument. Data elements included age, sex, weight, height, physical exam and laboratory findings, medical history and comorbid diseases, episodes of pneumonia in the past 180 days, severity of illness upon admission (calculated by means of the PORT scoring system [17] and the CURB-65 [confusion, uremia, elevated respiratory rate, low blood pressure, age greater than 65] score [19]), antibiotic treatment data, microbiological data, and hospital disposition.
The PORT and CURB-65 scores were calculated from the worst physiological scores within 24 h of admission (17, 19). Treatment data included all antibiotics administered to the patient throughout the hospitalization. Microbiological data included the organisms recovered from positive cultures of respiratory and blood specimens within the first 48 h of admission, susceptibility testing was done by the Kirby-Bauer method, and susceptibility testing results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) guidelines (20, 21).
Patients' vital signs and symptoms were collected on the day of admission and each subsequent day for up to 10 days of hospitalization. The most extreme measurement of temperature, heart rate, systolic blood pressure, respiratory rate, oxygen saturation, and arterial partial pressure of oxygen in arterial blood (PaO2) on each day was recorded (22). The presence of cough, shortness of breath, chest pain, and sputum production was also documented. Following the day of admission, these symptoms, as well as the overall clinical response, were assessed as improved, the same, or worsened relative to those on both admission and the previous day. The patient's mental status and ability to take oral medications were also recorded. When data were missing, the measurement from the day prior was carried forward.
Outcome assessment.
Time to clinical response was determined using a standardized definition consistent with the recent FDA briefing documents (1, 2), ATS/IDSA guidelines (6), FOCUS trials (10), and the study of Halm et al. (22). Clinical response was defined as clinical stability (temperature, ≤37.8°C; heart rate, ≤100 beats per min; systolic blood pressure, ≥90 mm Hg; respiratory rate, ≤24 breaths per min; oxygen saturation, ≥90%; arterial PaO2, ≥60 mm Hg; normal mental status; no receipt of supplemental oxygen by face mask or mechanical ventilation; and able to take oral medications) with improvement in at least one symptom of pneumonia (cough, shortness of breath, chest pain, or sputum production) and with no symptom worsening that was sustained for at least 24 h. Information on hospital LOS, in-hospital mortality, readmission up to 30 days after discharge (all causes), readmission up to 30 days after discharge that was CAP related, and overall adverse clinical outcomes (composite of in-hospital mortality and 30-day CAP-related readmission) was also collected.
Statistical analysis.
The primary analyses focused on examining the relationship between time to overall clinical response and outcomes. The secondary analyses examined the relationship between time to clinical stability (6) and outcomes. In the univariate analyses, the cumulative clinical response and clinical stability over time were calculated using the product-limit formula, which censored patients following the date of death or discharge. In the bivariate analyses, time to clinical response and time to clinical stability were modeled as both categorical and continuous variables. When modeled as categorical variables, the relationships between time to overall clinical response and time to clinical stability and hospital LOS were evaluated using the Kruskal-Wallis test and analysis of variance (ANOVA). Linear regression was employed when time to response was modeled as a continuous variable. Hospital LOS was log transformed to more closely approximate a normal distribution for ANOVA and linear regression. The Mantel-Haenszel test for trends was used to assess the relationships between times to response (overall clinical response and clinical stability) and dichotomous outcomes variables. Breakpoints in the distribution of time to response variables where adverse clinical outcomes were distinctly different between groups were also sought by classification and regression tree (CART) analysis (23). When evaluating the relationships between times to response (overall clinical response and clinical stability) and outcomes, patients who were discharged or expired prior to achieving a response were considered responders after day 5. To evaluate the impact that this assumption had on the observed results, sensitivity analyses were performed where (i) patients discharged without a response were classified as responders on the day of discharge and (ii) patients who died prior to the analysis time point were excluded.
A series of multivariate analyses (Poisson regression with robust variance estimates [24, 25] and linear regression) were performed to quantify the relationships between time to response, adverse clinical outcomes, and hospital length of stay after adjustment for potential confounding baseline variables. Both continuous and CART-derived dichotomous expressions of the time to clinical response variables were considered and evaluated in separate multivariate models. Baseline covariates associated with the outcome of interest at a P value of <0.2 were considered potential confounders in the multivariate analyses at model entry, and a stepwise process was used to identify variables independently associated with the outcome of interest. All calculations were performed with SAS, version 9.3 (SAS, Cary, NC), SPSS, version 12.0.1 (SPSS, Chicago, IL), and CART, version 6.0 ProEX (Salford Systems, San Diego, CA).
RESULTS
A total of 1,111 patients received ceftriaxone and azithromycin at AMCH between January 2008 and February 2012; of these, 250 patients were included. Common reasons for exclusion were PORT class of I/II (21.0%) or V (12.4%), failure to meet ATS/IDSA criteria for CABP (15.5%), and an age of <18 years (14.0%). Patient demographics, clinical characteristics, and outcomes are summarized in Table 1. The median durations of therapy with ceftriaxone and azithromycin were 3 days (interquartile range [IQR], 2 to 5 days) and 4 days (IQR, 2 to 5 days), respectively. The median hospital LOS was 6 days (IQR, 4 to 10 days), and 29 patients (11.6%) experienced an adverse clinical outcome. Fourteen patients (5.6%) died during their hospitalization. Among the 14 patients who died during their hospitalization, 1 died on day 2, 2 died on day 3, and 11 died after day 7 of hospitalization. Twenty-nine patients (11.6%) were readmitted within 30 days of discharge. Of the 29 readmissions, 15 were CAP related.
TABLE 1.
Patient demographics, clinical characteristics, and outcomesa
| Characteristic | Value |
|---|---|
| Demographics | |
| Mean (SD) age (yr) | 65.9 (14.5) |
| No. (%) ≥65 yr of age | 138 (55.0) |
| No. (%) male | 148 (59) |
| No. (%) of the following race: | |
| White | 192 (76.5) |
| Black | 37 (14.7) |
| Asian | 4 (1.6) |
| Other | 18 (7.2) |
| Clinical characteristics | |
| No. (%) with the following comorbid conditions: | |
| Diabetes | 84 (33.5) |
| COPD | 90 (35.9) |
| Asthma | 15 (6.0) |
| CAP in past 180 days | 19 (7.6) |
| HIV infection | 14 (5.6) |
| Neoplastic disease | 45 (17.9) |
| Liver disease | 16 (6.4) |
| CHF | 71 (28.3) |
| Cerebrovascular disease | 20 (12.0) |
| Renal disease | 64 (25.5) |
| Alcoholism | 12 (4.8) |
| Smoking | 153 (61.2) |
| No. (%) with positive respiratory specimen culture for the following in first 48 h of admission: | 14 (5.6) |
| Staphylococcus aureus | 5 (2.0) |
| Streptococcus pneumoniae | 4 (1.6) |
| Klebsiella pneumoniae | 2 (0.8) |
| Haemophilus influenzae | 2 (0.8) |
| Enterobacter aerogenes | 1 (0.4) |
| No. (%) with bacteremia caused by the following in first 48 h of admission: | 9 (3.6) |
| Staphylococcus aureus | 2 (0.8) |
| Streptococcus pneumoniae | 4 (1.6) |
| Klebsiella pneumoniae | 1 (0.4) |
| Escherichia coli | 1 (0.4) |
| Enterococcus faecalis | 1 (0.4) |
| Median (IQR) duration of therapy (days) with: | |
| Ceftriaxone | 3 (2, 4.25) |
| Azithromycin | 4 (2, 5) |
| No. (%) with the following physical exam findings: | |
| Altered mental status | 19 (7.6) |
| Respiratory rate ≥ 30/min | 51 (20.3) |
| Systolic blood pressure < 90 mm Hg | 25 (10.0) |
| Temp of <35°C or ≥40°C | 8 (3.2) |
| Heart rate ≥ 125/min | 34 (13.5) |
| No. (%) with the following laboratory/radiologic findings: | |
| pH < 7.35 | 23 (9.2) |
| Blood urea nitrogen concn > 10.7 mmol/liter | 47 (18.7) |
| Sodium concn < 135 meq/liter | 15 (6.0) |
| Glucose concn > 13.9 mmol/liter | 27 (10.8) |
| Hematocrit < 30% | 32 (12.7) |
| PaO2 < 60 mm Hg | 26 (10.4) |
| Pleural effusion | 68 (27.1) |
| No. (%) with the following PORT risk classb: | |
| III | 103 (41) |
| IV | 148 (59) |
| No. (%) with the following CURB-65 scoreb: | |
| 0 | 37 (14.7) |
| 1 | 109 (43.4) |
| 2 | 82 (32.7) |
| 3 | 21 (8.4) |
| 4 | 2 (0.8) |
| No. (%) with ICU admission | 42 (16.8) |
| Outcomes | |
| Median (IQR) hospital LOS (days) | 6 (4,10) |
| No. (%) with: | |
| Adverse clinical outcome | 29 (11.6) |
| In-hospital mortality | 14 (4.6) |
| 30-day readmission | 29 (11.6) |
| 30-day CAP-related readmission | 15 (6.0) |
Data are for 250 patients. COPD, chronic obstructive pulmonary disease; CAP, community-acquired pneumonia; CHF, congestive heart failure; PORT, Pneumonia Outcomes Research Team; ICU, intensive care unit.
Calculated on the basis of the worst physiological scores derived from physical exam and laboratory findings collected within 24 h of admission.
Relationship between time to overall clinical response and outcomes: primary analyses.
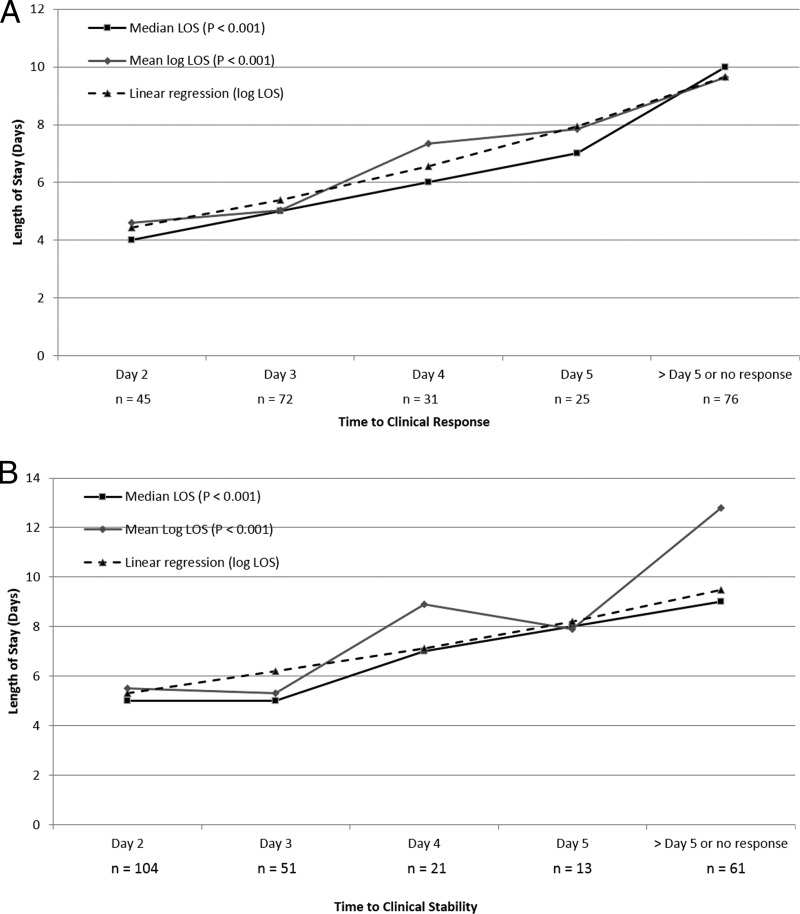
The results of the time to clinical response analysis are displayed in Fig. 1. Of the 250 included patients, 48.0%, 61.3%, and 72.3% responded by days 3, 4, and 5, respectively. The relationship between time to clinical response and hospital LOS is displayed in Fig. 2A. Overall, a significant relationship was noted in both the ANOVA and the Kruskal-Wallis test. In the post hoc pairwise comparisons, the mean log LOS was significantly different between days of response (data not shown). Time to clinical response was found to be associated with the hospital log LOS in the linear regression (β = 0.19, 95% confidence interval = 0.14 to 0.25, P < 0.001). Time to response was nearly identically associated with hospital log LOS (β = 0.19, 95% confidence interval = 0.14 to 0.24, P < 0.001) in the multivariate linear regression analysis that adjusted for baseline variables associated with log LOS at a P value of <0.2 (age, PORT score, congestive heart failure, a pH of <7.35, and partial O2 pressure [PO2] of <60). In the sensitivity analysis, the relationship between time to clinical response and hospital log LOS persisted (P < 0.001).
FIG 1.
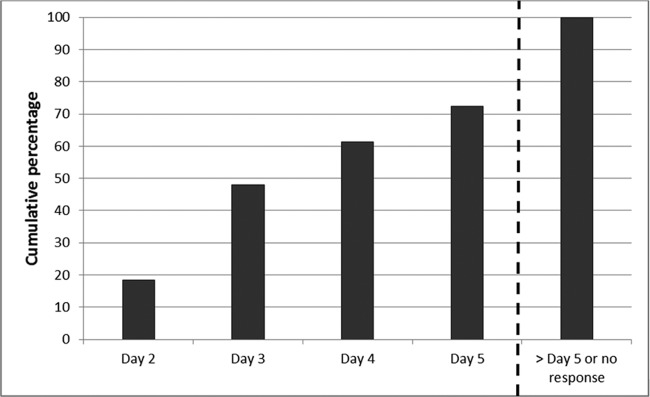
Cumulative clinical response over time.
FIG 2.
(A) Relationship between time to clinical response and hospital LOS; (B) relationship between time to clinical stability and hospital LOS.
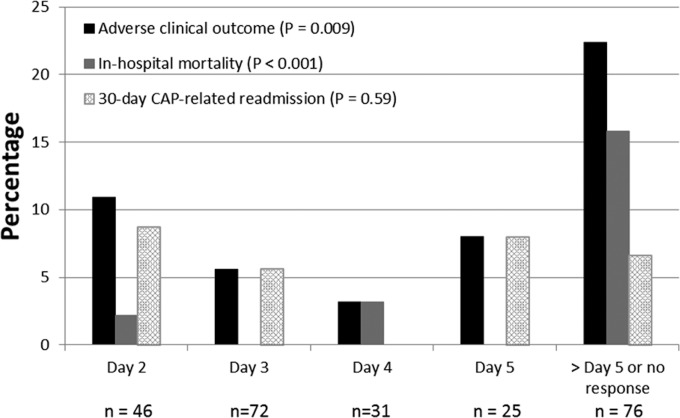
The results of the relationship between time to clinical response and adverse outcomes are displayed in Fig. 3. The test-for-trend analysis demonstrated a significant relationship between time to response and adverse clinical outcomes (in-hospital mortality or 30-day CAP-related readmission). The relationship between time to clinical response and in-hospital mortality was significant. All-cause and CAP-related 30-day readmissions did not vary by day of response. Time to clinical response was independently associated with the adverse clinical outcome endpoint in the Poisson regression analysis (relative risk [RR] for each 1-day change in time to clinical response = 1.33, 95% confidence interval = 1.04 to 1.72, P = 0.02) that adjusted for the baseline covariates associated with adverse clinical outcomes (age, respiratory rate of >30, CURB-65 score, blood urea nitrogen [BUN] concentration of >10.7 mmol/liter, presence of pleural effusion, chronic obstructive pulmonary disease [COPD], and smoking).
FIG 3.
Relationship between time to clinical response and outcomes.
Using CART, a delay in clinical response of >5 days was identified to be the optimal breakpoint for adverse clinical outcomes (Table 2). A failure to respond by day 5 was associated with a greater than 3-fold increase in the incidence of adverse clinical outcomes. In-hospital mortality was nearly 14-fold higher among patients who failed to respond by day 5 than those who responded by day 5. The median hospital LOS was significantly longer among patients who failed to achieve a clinical response by day 5. Similar to the test-for-trend analyses, no significant relationships between achievement of a clinical response by day 5 and all-cause and CAP-related 30-day readmissions were observed. In the Poisson regression analysis that adjusted for baseline covariates associated with adverse clinical outcomes (age, respiratory rate of >30 breaths per min, CURB-65 score, BUN concentration of >10.7 mmol/liter, presence of pleural effusion, COPD, and smoking), a failure to respond by day 5 was independently associated with the adverse clinical outcome composite endpoint (RR = 4.36, 95% confidence interval = 2.27 to 8.39, P < 0.001). Similarly, a failure to respond by day 5 was independently associated with hospital log LOS (β = 0.63, 95% confidence interval = 0.42 to 0.84, P < 0.001) after adjustment for baseline variables associated with log LOS at a P value of <0.2 in the multivariate linear regression analysis (age, PORT score, congestive heart failure, a pH of <7.35, and PO2 of <60). The results of the sensitivity analyses were nearly identical to those of the primary analysis (Table 2).
TABLE 2.
Relationship between clinical response by day 5 and outcomes
| Analysis and outcome | No response by day 5 | Response by day 5 | RR (95% CIc) | P value |
|---|---|---|---|---|
| Primary analysisa | ||||
| Median (IQR) hospital LOS (days) | 10 (6, 17) | 5 (4, 8) | <0.001 | |
| No. (%) with: | ||||
| Adverse clinical outcome | 17 (22.4) | 12 (6.9) | 3.3 (1.64–6.49) | 0.001 |
| In-hospital mortality | 12 (15.8) | 2 (1.1) | 13.8 (3.17–60.2) | <0.001 |
| 30-day readmission | 7 (9.2) | 22 (12.6) | 0.7 (0.33–1.64) | 0.52 |
| 30-day CAP related readmission | 5 (6.6) | 10 (5.7) | 1.2 (0.41–3.26) | 0.78 |
| Sensitivity analysisb | ||||
| Median (IQR) hospital LOS (days) | 12 (8, 18) | 5 (4, 7.5) | <0.001 | |
| No. (%) with: | ||||
| Adverse clinical outcome | 14 (23.7) | 12 (6.3) | 3.74 (1.83–7.63) | <0.001 |
| In-hospital mortality | 9 (15.3) | 2 (1.1) | 14.42 (3.20–64.9) | <0.001 |
| 30-day readmission | 7 (11.9) | 22 (11.6) | 1.02 (0.46–2.27) | 1 |
| 30-day CAP-related readmission | 5 (8.5) | 10 (5.3) | 1.60 (0.57–4.50) | 0.36 |
Death prior to day 5 or discharge without a response prior to day 5 is considered no response by day 5. Data are for 76 patients for no response by day 5 and 174 patients for a response by day 5.
Deaths prior to day 5 were excluded; discharge without a response prior to day 5 was considered to have achieved a response upon discharge. Data are for 59 patients for no response by day 5 and 188 patients for a response by day 5.
CI, confidence interval.
Relationship between time to clinical stability and outcomes: secondary analyses.
The relationships between time to clinical stability and outcomes were largely consistent with those found in the primary analyses. Of the 250 included patients, 62.8%, 72.1%, and 77.9% responded by days 3, 4, and 5, respectively. Although the relationships were not as monotonic as those in the overall clinical response-LOS analyses, significant relationships between both the mean and median times to clinical stability and outcomes were observed (Fig. 2B). Time to clinical stability was also found to be associated with hospital log LOS in the linear regression (β = 0.14, 95% confidence interval = 0.09 to 0.19, P < 0.001). A significant relationship between time to clinical stability and adverse clinical outcomes was noted in the test-for-trend analysis. Similar to the primary analyses, the relationship between time to clinical stability and in-hospital mortality was significant, and all-cause and CAP-related 30-day readmissions did not vary by day of achievement of clinical stability among survivors. Using CART, a delay in clinical stability of >4 days was identified to be the optimal breakpoint for adverse clinical outcomes. Compared to those who responded by day 4, those who did not respond by day 4 had a >2.5-fold increase in adverse clinical outcomes (20.3% versus 8.0%, P = 0.006), a 6-fold increase in in-hospital mortality (12.2% versus 2.8%, P = 0.003), and similar incidences of all-cause and CAP-related readmissions.
Predictors of all-cause and CAP-related readmissions among survivors.
Given the lack of association between time to overall clinical response and readmission and time to clinical stability and readmission, a series of post hoc analyses was performed to identify baseline covariates associated with all-cause and CAP-related readmissions among survivors (Table 3). The most pronounced bivariate predictors of both types of readmission were a history of smoking and COPD. To assess the relationship between the cumulative effect of baseline covariates and incidences of readmissions (all cause and CAP related), all bivariate predictors (P < 0.25) associated with each readmission classification were consolidated into an ordinal variable with the following four rank-ordered categories: 0, 1, 2, and ≥3 (Fig. 4). As the cumulative number of predictors increased, the incidences of both all-cause and CAP-related readmissions increased in a monotonic fashion, with a 25% readmission rate being found among patients with at least 3 predictors.
TABLE 3.
Bivariate comparison of baseline demographics and clinical characteristics between readmissions and nonreadmissions
| Variable | Overall 30-day readmission |
30-day CAP-related readmission |
||||
|---|---|---|---|---|---|---|
| Readmission (n = 29) | No readmission (n = 207) | RR (95% CI)a | Readmission (n = 15) | No readmission (n = 221) | RR (95% CI) | |
| Demographics and clinical characteristics | ||||||
| Age ≥ 65 yr | 12 (41.4) | 117 (56.5) | 0.59 (0.29–1.17) | 8 (53.3) | 121 (54.8) | 0.95 (0.36–2.53) |
| Male | 14 (48.3) | 122 (58.9) | 0.69 (0.35–1.36) | 7 (46.7) | 129 (58.4) | 0.64 (0.24–1.72) |
| White | 20 (69.0) | 160 (77.3) | 0.69 (0.33–1.43) | 13 (86.7) | 178 (75.6) | 2.02 (0.47–8.69) |
| Black | 5 (17.2) | 31 (15.0) | 1.16 (0.47–2.84) | 2 (13.3) | 34 (15.4) | 0.86 (0.20–3.63) |
| Asian | 0 | 4 (1.9) | 0 | 4 (1.8) | ||
| Otherb | 4 (13.8) | 12 (5.8) | 2.20 (0.87–5.55) | 0 | 16 (7.2) | |
| Diabetes | 10 (34.5) | 71 (34.3) | 1.01 (0.49–2.06) | 5 (33.3) | 76 (34.4) | 0.96 (0.34–2.71) |
| COPDb,c | 15 (51.7) | 71 (34.3) | 1.87 (0.95–3.68) | 11 (73.3) | 75 (33.9) | 4.80 (1.58–14.6) |
| Asthma | 0 | 14 (6.8) | 0 | 14 (6.3) | ||
| CAP in past 180 days | 1 (3.4) | 17 (8.2) | 0.43 (0.06–3.00) | 0 | 18 (8.1) | |
| HIV infection | 1 (3.4) | 13 (6.3) | 0.57 (0.08–3.86) | 0 | 14 (6.3) | |
| Neoplastic disease | 4 (13.8) | 37 (17.9) | 0.76 (0.28–2.1) | 3 (20.0) | 38 (17.2) | 1.19 (0.35–4.03) |
| Liver diseaseb | 4 (13.8) | 12 (5.8) | 2.20 (0.87–5.55) | 2 (13.3) | 14 (6.3) | 2.12 (0.52–8.57) |
| CHFb | 12 (41.4) | 55 (26.6) | 1.78 (0.90–3.52) | 6 (40.0) | 61 (27.6) | 1.68 (0.62–4.54) |
| Cerebrovascular disease | 1 (3.4) | 25 (12.1) | 0.29 (0.04–2.03) | 0 | 26 (11.8) | |
| Renal disease | 8 (27.6) | 55 (26.6) | 1.05 (0.49–2.24) | 3 (20.0) | 60 (27.1) | 0.68 (0.20–2.35) |
| Alcoholismb,c | 4 (13.8) | 5 (2.4) | 4.04 (1.78–9.15) | 2 (13.3) | 7 (3.2) | 3.88 (1.03–14.7) |
| Smokingb,c | 24 (82.8) | 121 (58.5) | 3.01 (1.19–7.61) | 14 (93.3) | 131 (59.3) | 8.79 (1.18–65.7) |
| Altered mental status | 1 (3.4) | 18 (8.7) | 0.41 (0.06–2.84) | 1 (6.7) | 18 (8.1) | 0.82 (0.11–5.87) |
| Respiratory rate ≥ 30/min | 3 (10.3) | 46 (22.2) | 0.44 (0.14–1.40) | 1 (6.7) | 48 (21.7) | 0.27 (0.04–2.02) |
| Systolic blood pressure < 90 mm Hg | 2 (6.9) | 21 (10.1) | 0.69 (0.17–2.70) | 1 (6.7) | 22 (10.0) | 0.66 (0.09–4.80) |
| Temp of <35°C or ≥40°C | 1 (3.4) | 7 (3.4) | 1.02 (0.16–6.58) | 0 | 8 (3.6) | |
| Heart rate ≥ 125/min | 2 (6.9) | 28 (13.5) | 0.51 (0.13–2.03) | 2 (13.3) | 28 (12.7) | 1.06 (0.25–4.45) |
| pH < 7.35 | 2 (6.9) | 18 (8.7) | 0.80 (0.21–3.12) | 1 (6.7) | 19 (8.6) | 0.77 (0.11–5.57) |
| Blood urea nitrogen concn > 10.7 mmol/literb | 9 (31.0) | 33 (15.9) | 2.08 (1.02–4.24) | 4 (26.7) | 38 (17.2) | 1.68 (0.56–5.02) |
| Sodium concn < 130 meq/liter | 1 (3.4) | 12 (5.8) | 0.61 (0.09–4.16) | 1 (6.7) | 12 (5.4) | 1.23 (0.17–8.61) |
| Glucose concn > 13.9 mmol/liter | 0 | 26 (12.6) | 0 | 26 (11.8) | ||
| Hematocrit < 30%b,c | 6 (20.7) | 25 (12.1) | 1.73 (0.76–3.90) | 4 (26.7) | 27 (12.2) | 2.41 (0.82–7.08) |
| PaO2 < 60 mm Hg | 1 (3.4) | 21 (10.1) | 0.35 (0.05–2.43) | 0 | 22 (10.0) | |
| Pleural effusion | 10 (34.7) | 51 (24.6) | 1.51 (0.74–3.07) | 5 (33.3) | 56 (25.3) | 1.43 (0.51–4.03) |
| ICUd admission | 4 (13.8) | 33 (15.9) | 0.86 (0.32–2.33) | 3 (20.0) | 34 (15.4) | 1.35 (0.40–4.53) |
| Positive culture of respiratory specimen in first 48 h | 0 | 12 (5.8) | 0 | 12 (5.4) | ||
| Bacteremia in first 48 hb | 2 (6.9) | 5 (2.4) | 2.42 (0.71–8.24) | 1 (6.7) | 6 (2.7) | 2.34 (0.36–15.4) |
| PORT risk class | ||||||
| PORT III | 15 (51.7) | 86 (41.5) | 1.43 (0.73–2.83) | 8 (53.3) | 93 (42.1) | 1.53 (0.57–4.07) |
| PORT IV | 14 (48.3) | 121 (58.5) | 0.70 (0.35–1.38) | 7 (46.7) | 128 (57.9) | 0.66 (0.25–1.75) |
| CURB-65 score | ||||||
| 0 | 7 (24.1) | 27 (13.0) | 1.89 (0.88–4.08) | 3 (20.0) | 31 (14.0) | 1.49 (0.44–4.99) |
| 1 | 11 (37.9) | 95 (45.9) | 0.75 (0.37–1.52) | 6 (40.0) | 100 (45.2) | 0.82 (0.30–2.22) |
| 2 | 11 (37.9) | 65 (31.4) | 1.29 (0.64–2.59) | 6 (40.0) | 70 (31.7) | 1.40 (0.52–3.80) |
| 3 | 0 | 18 (8.7) | 0 | 18 (8.1) | ||
| 4 | 0 | 2 (1.0) | 0 | 2 (0.8) | ||
The relative risk (95% confidence interval [CI]) reflects the ratio of the incidence of readmission in the exposed (variable present) versus the incidence of readmission in the nonexposed (variable absent).
Predictive of overall 30-day readmission with a P value of <0.25.
Predictive of 30-day CAP-related readmission with a P value of <0.25.
ICU, intensive care unit.
FIG 4.
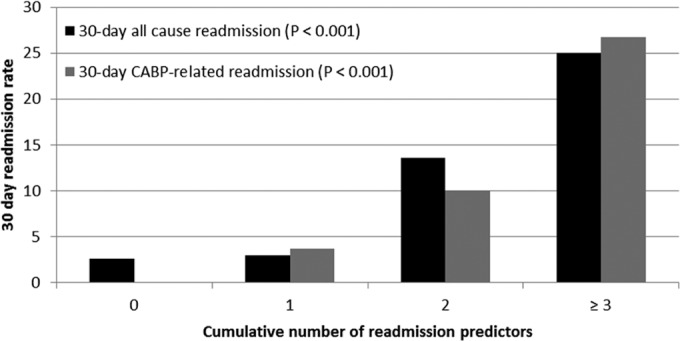
Relationship between the cumulative number of readmission predictors and all-cause and CAP-related 30-day readmissions.
DISCUSSION
There has been considerable discussion regarding the appropriate efficacy endpoint in CABP registration trials (1, 2). In the past, efficacy in CAP registration trials was based on clinical response assessments at the TOC visit. However, there is much debate if clinical response assessments at TOC visits can adequately capture the treatment effect due to the lack of historical control data demonstrating the significance of this endpoint (1, 2). More recently, a report from the Institute of Medicine (IOM) of the National Academies Committee on Qualification of Biomarkers and Surrogate Endpoints explicitly states that clinical endpoints in registrational trials should capture how a patient feels, functions, and survives (26). In light of this and the uncertainty regarding the clinical meaningfulness of TOC assessments in defining treatment effect, the FDA changed its guidance and now advocates the use of an early clinical response endpoint based on symptomatic improvement and stabilization of vital signs (1). The FDA now also recommends that early efficacy assessments be limited to patients who had a confirmed bacterial pathogen consistent with CABP at the baseline since this is the group that would benefit from antimicrobial therapy (1). While this guidance change aligns with the recommendations from IOM and focuses the efficacy analyses on patients who would most likely benefit from antimicrobial therapy, it is unclear how the early CABP endpoint relates to later outcomes, such as hospital LOS, mortality, and readmission, in patients with CAP in the clinical arena. This is an important consideration, given that most antimicrobials will now be approved for CABP, although in clinical practice they will be used primarily for CAP, given the low number of patients with CAP who ultimately have a confirmed bacteriological diagnosis (12). Given the critical need for these data, this study sought to evaluate the relationship between time to clinical response, as defined by the FDA, and later clinical outcomes in CAP that are of great interest to providers and payers.
Overall, the results of the primary analyses indicated that time to achievement of clinical response was predictive of both hospital LOS and the incidence of adverse clinical outcomes (in-hospital mortality or 30-day CAP-related readmission). There was a monotonic relationship between time to achievement of clinical response and hospital LOS. On average, patients were discharged 2 days following the achievement of clinical response. We also observed a strong relationship between time to clinical response and the incidence of adverse clinical outcomes in both the primary and sensitivity analyses. Most notably, patients who failed to respond by day 5 were at the greatest risk for deleterious outcomes. Patients who failed to respond by day 5 were more than three times as likely to experience an adverse clinical outcome as those who responded by day 5. Moreover, hospital LOS was significantly longer among nonresponders by day 5. Collectively, our findings suggest that time to clinical response, as defined by the new FDA guidance, was useful in discerning patients with CAP at the greatest risk for prolonged hospital stays and adverse clinical outcomes.
While a relationship between time to clinical response and adverse clinical outcomes was demonstrated, this was largely due to an increased incidence of in-hospital mortality among patients with delays in clinical response. No clear relationships between time to clinical response and all-cause and CAP-related readmissions were observed. Surprisingly, the cumulative incidences of readmissions were comparable between patients who achieved an early response and those who did not. In an effort to delineate patient types at the greatest risk for readmission, we performed a series of post hoc analyses and found a number of baseline covariates to be associated with all-cause and CAP-related readmissions (Table 3). Smoking and COPD were two of the strongest predictors of both all-cause and CAP-related readmission classifications, and the risk of both readmission types escalated in a monotonic fashion as the number of predictors increased (Fig. 4). Patients with 3 or more predictive characteristics had overall and CAP-related readmission rates in excess of 25%. These findings highlight the need to develop strategies to better identify patients at risk for readmission. For risk stratification studies, it is of paramount importance to determine if the readmission rates among patients deemed at the greatest risk are modifiable through medical treatments or other interventions. This is especially relevant in light of the Patient Protection and Affordable Care Act (27), which triggers withholding of reimbursement as a penalty for higher-than-expected readmission rates among Medicaid patients with pneumonia.
Another notable finding was the reported cumulative overall clinical response rates. One of the major concerns regarding the FOCUS trials was the external validity of the findings due to the limited use of concomitant macrolide therapy and a small number of sites in the United States (7–10). The lack of macrolide therapy is consistent with FDA guidance, which discourages the concomitant use of antibiotics that can potentially confound interpretation of the investigational drug's treatment effect in noninferiority trials (28). However, dual beta-lactam–macrolide therapy is the standard of care for CAP in the United States, as recommended by JCAHO, CMS, and ATS/IDSA (6, 18), and it is unclear whether the observed day 4 response rate of ceftriaxone in the FOCUS trials (10) would be improved with the addition of a macrolide. Several studies have shown that the macrolides possess many immunomodulatory properties and can reduce the proinflammatory response to infectious stimuli, potentially contributing to the clinical response (29, 30). Interestingly, the day 4 response rate of our cohort (61.3%) was nearly identical to that of the ceftriaxone arm of the FOCUS trials (59.4%) (10). Further investigation is necessary to draw definitive conclusions regarding the effectiveness of combination ceftriaxone-macrolide therapy. However, the concordance between the ceftriaxone day 4 response rates in this real-world effectiveness study and the phase III efficacy trials does not refute the external generalizability of the day 4 findings for the ceftriaxone group in the FOCUS trials to patients with CAP (10).
As part of this investigation, we also examined the relationship between time to clinical stability (1, 6) and outcomes. Although the definition of the clinical response at the TOC visit has often been criticized for being somewhat subjective in nature, the symptoms assessment component of the FDA's early clinical response definition can be viewed similarly (1, 2). The criteria employed for the definition of clinical stability in this study are identical to the criteria set forth by ATS/IDSA (6) to define patients eligible for hospital discharge. Not surprisingly, as clinical stability is a component of the overall clinical response, a greater proportion of patients more readily achieved clinical stability before meeting the overall clinical response criteria. Interestingly, a similar proportion of patients did not meet the clinical stability (22.1%) or full clinical response (28%) criteria by day 5. Overall, the relationships between time to clinical stability and outcomes were largely consistent with the relationships between time to overall response and outcomes. In particular, time to clinical stability was associated with both hospital LOS and in-hospital mortality. Since symptom assessments, the other component of the overall clinical response criteria (1), are often difficult to quantify consistently in patients over the course of their hospital stay, these data suggest that it may be reasonable to track the clinical stability criteria set forth by ATS/IDSA (6) as a tangible, objective, and consistent way to monitor patients and gauge their likelihood of a poor outcome or readiness for discharge.
There are several considerations to note when interpreting these results. This analysis evaluated time to clinical response among a strictly defined cohort of patients at a single United States academic medical center. We purposefully restricted the study to hospitalized PORT risk class III and IV patients with CAP. Although the ATS/IDSA CAP treatment guidelines (6) recommend hospitalization in a medical ward for PORT risk class III and IV patients, there is considerable variability in hospital admission rates for CAP among institutions and individual physicians (31). Cognizant of this, we restricted the analysis to include only patients designated to be treated on a hospital ward by the use of ATS/IDSA guidelines in an effort to maximize the internal validity. By doing so, we also made it clear to whom these findings can be applied or generalized. However, it is uncertain if results from the analyses can be applied to other populations with CAP.
We only examined patients who received ceftriaxone and azithromycin. It is unclear if the study findings can be generalized to other antibiotic regimens for CAP. Furthermore, this study lacked a control group. The addition of a control group would have provided us the opportunity to address additional study aims. For example, the inclusion of an inadequately treated control group would enable one to determine if time to response varied by appropriateness of treatment. It would also enable one to determine if time to response leads to similar downstream outcomes, independently of the initial treatment.
The low number of bacteriologically confirmed cases of CABP in this study limited our ability to draw inferences regarding the clinical response by pathogen. As mentioned above, this limitation also prevented us from considering an alternate control group. Our inability to identify a larger number of documented cases of bacterial infection is consistent with the findings in the literature (12) and represents a more realistic look at the clinical scenarios in which antimicrobials are deployed in clinical practice. Thus, we believe that our study offers a unique perspective on the predictive performance of the FDA endpoint in the real world among patients with CAP. However, this finding does highlight the need to evaluate the relationship between time to response and subsequent outcomes among patients with culture-confirmed CABP, as the response may vary by organism.
Since this was a retrospective observational cohort study, our findings are subject to the caveats associated with this design. One of the major concerns in studies of this nature is information bias due to missing data. While AMCH is a major academic medical center, a small portion of the patients included were transferred from an outside hospital, and they may have been readmitted to their local hospital within 30 days of discharge. Despite this, any missed readmissions were just as likely to occur in responders as nonresponders and thus would not significantly alter our findings. As stated above, symptomology is often inconsistently coded across patients. However, it is unlikely that symptom coding would alter the reported findings. The high degree of concordance between the time to overall response and time to clinical stability outcomes analyses provides credence to the time to overall response analyses since the variables in the clinical stability criteria were readily available for >95% of patients. More importantly, the similar findings between the time to overall response and time to clinical stability outcomes analyses suggest that it may be more straightforward to track clinical stability as a method to discern a patient's risk for a negative outcome.
We did not collect 30-day mortality. Because these data were not readily available from the medical records and the national death index was incomplete for the period studied at the time of analysis, we chose to use a more accurate endpoint of in-hospital mortality. Lastly, a limited number of patients in this study were discharged prior to achieving a clinical response. While it was unclear how to characterize these patients, the sensitivity analysis in which these patients were classified as responders on the day of discharge did not significantly alter the findings.
In conclusion, time to clinical response, as defined in the recent FDA guidance for CABP, was found to be associated with adverse clinical outcomes and increased hospital LOS among patients with CAP of PORT risk class III and IV receiving ceftriaxone and azithromycin. Delays in the achievement of a clinical response were associated with prolonged hospital stays and the incidence of adverse clinical outcomes. A failure to respond by day 5 was the most strongly associated with poor outcomes and provided the most robust delineation of those at higher risk. Interestingly, the relationships between time to clinical stability and outcomes were largely consistent with the relationships between time to overall response and outcomes. Symptoms constitute the other component of the overall clinical response criteria (1) and are often subjective, making them difficult to quantify consistently in patients over the course of their hospital stay. Therefore, these data suggest that it may be reasonable to use the clinical stability criteria set forth by ATS/IDSA (6) as a tangible, objective, and consistent way to monitor patients and gauge their likelihood of a poor outcome and discharge readiness. As with all retrospective, single-site studies, our findings should be interpreted cautiously and verified with a multicenter, prospective study.
ACKNOWLEDGMENTS
This article has greatly benefited from the thoughtful editing of Allison Krug.
This work was supported by an investigator-initiated research grant from Forest Laboratories, Inc. T.P.L. was the principal investigator for this grant.
Forest Laboratories, Inc., provided support only to complete the project and was not involved in the following: design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation or review of the manuscript.
T.P.L. is also a consultant for Forest Laboratories, Inc. No other conflicts of interest exist for any of the authors.
Footnotes
Published ahead of print 21 April 2014
REFERENCES
- 1.Food and Drug Administration, Division of Anti-Infective Products. Office of Antimicrobial Products. 2011. Briefing document. Endpoints and clinical trial issues in community-acquired bacterial pneumonia. Food and Drug Administration, Division of Anti-Infective Products, Office of Antimicrobial Products, Washington, DC: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/anti-infectivedrugsadvisorycommittee/ucm275823.pdf Accessed 2 January 2013 [Google Scholar]
- 2.Food and Drug Administration, Center for Drug Evaluation and Research. 2009. Guidance for industry. Community-acquired bacterial pneumonia: developing drugs for treatment, draft guidance. Food and Drug Administration, Center for Drug Evaluation and Research, Washington, DC: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm123686.pdf Accessed 2 January 2013 [Google Scholar]
- 3.Flippin HF, Lockwood JS. 1939. The treatment of pneumococcic pneumonia with sulfapyridine: a progress report on observations in 100 cases. JAMA 112:529–534. 10.1001/jama.1939.02800060045009 [DOI] [Google Scholar]
- 4.Osler W. 1910. Specific infectious diseases: lobar pneumonia, p 164–192 In The principles and practice of medicine. D. Appleton and Company, New York, NY [Google Scholar]
- 5.Mandell LA, Bartlett JG, Dowell SF, File TM, Jr, Musher DM, Whitney C. 2003. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin. Infect. Dis. 37:1405–1433. 10.1086/380488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, Torres A, Whitney CG. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44(Suppl 2):S27–S72. 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.File TM, Jr, Low DE, Eckburg PB, Talbot GH, Friedland HD, Lee J, Llorens L, Critchley I, Thye D. 2010. Integrated analysis of FOCUS 1 and FOCUS 2: randomized, doubled-blinded, multicenter phase 3 trials of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in patients with community-acquired pneumonia. Clin. Infect. Dis. 51:1395–1405. 10.1086/657313 [DOI] [PubMed] [Google Scholar]
- 8.File TM, Jr, Low DE, Eckburg PB, Talbot GH, Friedland HD, Lee J, Llorens L, Critchley I, Thye D. 2011. FOCUS 1: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J. Antimicrob. Chemother. 66(Suppl 3):iii19–iii32. 10.1093/jac/dkr096 [DOI] [PubMed] [Google Scholar]
- 9.Low DE, File TM, Jr, Eckburg PB, Talbot GH, Friedland HD, Lee J, Llorens L, Critchley I, Thye D. 2011. FOCUS 2: a randomized, double-blinded, multicentre, phase III trial of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in community-acquired pneumonia. J. Antimicrob. Chemother. 66(Suppl 3):iii33–iii44. 10.1093/jac/dkr097 [DOI] [PubMed] [Google Scholar]
- 10.Eckberg PB, Friedland DH, Llorens L, Smith A, Witherell GW, Laudano JB, Thye D. 2012. Day 4 clinical response of ceftaroline fosamil versus ceftriaxone for community-acquired bacterial pneumonia. Infect. Dis. Clin. Pract. 20:254–260. 10.1097/IPC.0b013e318255d65f [DOI] [Google Scholar]
- 11.Pertel PE, Bernardo P, Fogarty C, Matthews P, Northland R, Benvenuto M, Thorne GM, Luperchio SA, Arbeit RD, Alder J. 2008. Effects of prior effective therapy on the efficacy of daptomycin and ceftriaxone for the treatment of community-acquired pneumonia. Clin. Infect. Dis. 46:1142–1151. 10.1086/533441 [DOI] [PubMed] [Google Scholar]
- 12.Bartlett JG. 2011. Diagnostic tests for agents of community-acquired pneumonia. Clin. Infect. Dis. 52(Suppl 4):S296–S304. 10.1093/cid/cir045 [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. 2010. Mortality data. Centers for Disease Control and Prevention, Atlanta, GA: www.CDC.gov/nchs/dataaccess/vitalsstatsonline.htm Accessed 31 May 2013 [Google Scholar]
- 14.Bartlett JG, Dowell SF, Mandell LA, File TM, Jr, Musher DM, Fine MJ. 2000. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin. Infect. Dis. 31:347–382. 10.1086/313954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.File TM, Jr, Marrie TJ. 2010. Burden of community-acquired pneumonia in North American adults. Postgrad. Med. 122:130–141. 10.3810/pgm.2010.03.2130 [DOI] [PubMed] [Google Scholar]
- 16.Niederman MS. 2009. Community-acquired pneumonia: the U.S. perspective. Semin. Respir. Crit. Care Med. 30:179–188. 10.1055/s-0029-1202937 [DOI] [PubMed] [Google Scholar]
- 17.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. 1997. A prediction rule to identify low-risk patients with community-acquired pneumonia. N. Engl. J. Med. 336:243–250. 10.1056/NEJM199701233360402 [DOI] [PubMed] [Google Scholar]
- 18.Centers for Medicare and Medicaid Services, The Joint Commission. 2013. Specification manual for national hospital quality measures. Centers for Medicare and Medicaid Services. The Joint Commission, Washington, DC [Google Scholar]
- 19.Capelastegui A, Espana PP, Quintana JM, Areitio I, Gorordo I, Equrrola M, Bilbao A. 2006. Validation of a predictive rule for the management of community-acquired pneumonia. Eur. Respir. J. 27:151–157. 10.1183/09031936.06.00062505 [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement (June 2010 update). CLSI document M100-S20-U. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 9th ed. CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 22.Halm EA, Fine MJ, Marrie TJ, Coley CM, Kapoor WN, Obrosky DS, Singer DE. 1998. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA 279:1452–1457. 10.1001/jama.279.18.1452 [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Burthon S. 1999. Recursive partitioning in the health sciences. Springer, New York, NY [Google Scholar]
- 24.McNutt LA, Wu C, Xue X, Hafner JP. 2003. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 157:940–943. 10.1093/aje/kwg074 [DOI] [PubMed] [Google Scholar]
- 25.Spiegelman D, Hertzmark E. 2005. Easy SAS calculations for risk or prevalence ratios and differences. Am. J. Epidemiol. 162:199–200. 10.1093/aje/kwi188 [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine. 2011. Perspectives on biomarker and surrogate endpoint evaluation: discussion forum summary. National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- 27.U.S. Congress. 2010. H.R. 3590. Patient protection and affordable care act. U.S. Congress, Washington, DC: http://www.gpo.gov/fdsys/pkg/BILLS-111hr3590enr/pdf/BILLS-111hr3590enr.pdf Accessed 2 January 2013 [Google Scholar]
- 28.Food and Drug Administration, Center for Drug Evaluation and Research. 2010. Guidance for industry. Hospital-acquired and ventilator-associated bacteria pneumonia: developing drugs for treatment, draft guidance. Food and Drug Administration, Center for Drug Evaluation and Research, Washington, DC: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM234907.pdf Accessed 16 February 2014 [Google Scholar]
- 29.Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombola L, Carnuccio R, Luvone T, D'Acquisto F, Di Rosa M. 2000. Anti-inflammatory activity of macrolide antibiotics. J. Pharmacol. Exp. Ther. 292:156–163 [PubMed] [Google Scholar]
- 30.Parnham MJ. 2005. Immunomodulatory effects of antimicrobials in the therapy of respiratory tract infections. Curr. Opin. Infect. Dis. 18:125–131. 10.1097/01.qco.0000160901.71813.fe [DOI] [PubMed] [Google Scholar]
- 31.Gilbert K, Gleason PP, Singer DE, Marrie TJ, Coley CM, Obrosky DS, Lave JR, Kapoor WN, Fine MJ. 1998. Variations in antimicrobial use and cost in more than 2,000 patients with community-acquired pneumonia. Am. J. Med. 104:17–27. 10.1016/S0002-9343(97)00274-X [DOI] [PubMed] [Google Scholar]